Abstract
Objectives
Many European countries have engaged in awareness campaigns to decrease outpatient antibiotic use and several measures have been proposed, e.g. the number of defined daily doses (DDDs) or packages per 1000 inhabitants per day, producing conflicting findings. Therefore, we set out to explore what measure is most appropriate.
Methods
Outpatient data on each dispensed and reimbursed medicinal package in Belgium between 2002 and 2009 were aggregated at the level of the active substance in accordance with the Anatomical Therapeutic Chemical classification and expressed as the numbers of DDDs (WHO, version 2010), packages, treatments and insured individuals per 1000 inhabitants, insured individuals and patient contacts, per day, and in July–June years. Using these measures, time trends of outpatient antibiotic use were compared and explored in detail.
Results
Expressed per 1000 inhabitants per day, outpatient antibiotic use increased between 2002–03 and 2008–09 in DDDs, whereas in packages, treatments and insured individuals it decreased. The same was true for use expressed per 1000 insured individuals or when allowing for the decreasing number of patient contacts. Increasing numbers of DDDs per package (more items per package and higher doses per unit for amoxicillin and co-amoxiclav) explain these discrepancies.
Conclusions
The number of packages is a more appropriate measure than the number of DDDs when assessing outpatient antibiotic use over time and the impact of awareness campaigns in countries dispensing ‘complete packages’. We recommended the use of different complementary measures or caution when interpreting trends based only on DDDs.
Keywords: antibacterial agents, surveillance, awareness campaigns, antibiotic resistance, ambulatory care
Introduction
Antibiotic resistance is a major health problem that is mainly caused by antibiotic consumption.1,2 The highest volumes of antibiotics are being prescribed and consumed in ambulatory care.3,4 Interventions to combat resistance have therefore targeted general practitioners (GPs) and the general public and focused on improving antibiotic prescribing and antibiotic consumption in ambulatory care to reduce antibiotic selective pressure.5–8 Several measures have been proposed to assess outpatient antibiotic use,4 which in this paper refers to the whole of antibiotic prescribing, buying, dispensing, reimbursing and consuming.
When launched in 2001 to complement the European Antimicrobial Resistance Surveillance System (EARSS), the European Surveillance of Antimicrobial Consumption (ESAC) project adopted the most widely recommended measure, i.e. the number of defined daily doses (DDDs). Countries with high antibiotic use in DDDs per thousand inhabitants per day (DID) have indeed shown higher rates of antibiotic resistance.3 More recently, measures other than the DDD have been proposed to measure outpatient antibiotic consumption, e.g. the number of packages, the number of prescriptions and the number of treated individuals.4 In France, a national campaign launched in 2002 decreased the total number of reimbursed antibiotic prescriptions per 100 inhabitants by 26.5% over 5 years.9 In Belgium, public awareness campaigns launched in the 2000–01 winter decreased outpatient antibiotic use by 36% over 7 years when expressed as the number of reimbursed packages per 1000 inhabitants per day (PID).10 The longitudinal trend in PID, however, differed substantially from that in DID, as did the ranking of Belgium among other European countries,4 and to date these contradictions confuse all stakeholders, i.e. policy makers, researchers, clinicians and the general public, in Belgium and beyond.
Nevertheless, the successes in France and Belgium inspired the European Centre for Disease Prevention and Control (ECDC) to introduce the European Antibiotic Awareness Day (EAAD) in 2008,8 and meanwhile many European countries have engaged in interventions to decrease antibiotic use. In Sweden, a target was set using prescriptions as the measure.11,12 Whereas the 2010 prescribing rate in Sweden was 390 antibiotic prescriptions per 1000 inhabitants per year, the target is set at 250 for each county. In Belgium, the government even made an increase in the GP fee for service conditional on a significant decrease in antibiotic prescribing.13 Consequently, we should be able to assess the impact of such interventions on antibiotic prescribing and consumption, and subsequently on antimicrobial resistance, to inform all stakeholders. This requires valid measures as well as valid data.
General practice prescribing data are not available at a national level in most countries, and >30% non-adherence to primary care clinicians' antibiotic prescriptions has been found in Europe.14 National data on antibiotic use are available and have been collected within ESAC.4,15–19 Currently, data on antibiotic dispensing (reimbursement or sales data) are considered to be the best routinely and nationally available proxy for both antibiotic prescribing and antibiotic consumption.
We used total Belgian reimbursement data to explore the various measures described above in order to identify the most appropriate one. The overall aim of settling this debate is to improve international surveillance of outpatient antibiotic use and associated resistance for the sake of all stakeholders, and especially to inform policy makers about the impact of their antibiotic awareness campaigns.
Methods
Detailed data on antibiotic use in ambulatory care was extracted from the database of the Intermutualistic Agency (IMA), based on reimbursement claims data of the seven non-profit sickness funds in charge of managing compulsory health insurance in Belgium. The IMA database contains data on each dispensed and reimbursed medicinal package in Belgium from 2002 onwards, including information on the prescriber (encoded), the insured individual (encoded) and the medicinal package. In Belgium, community pharmacists dispense complete medicinal packages of antibiotics (‘complete package’ dispensing).
The use data were aggregated at the level of the active substance in accordance with the Anatomical Therapeutic Chemical (ATC) classification DDD measurement unit (WHO, version 2010).20 Within the ATC subgroup J01 (i.e. antibacterials for systemic use, excluding topical antibiotics), 229 unique chemical substances were listed for antibiotics or their combinations (e.g. J01CA04, i.e. amoxicillin), aggregated into 33 chemical subgroups (e.g. J01CA, i.e. penicillins with extended spectrum) and subsequently into 10 pharmacological subgroups (e.g. J01C, i.e. β-lactam antibacterials, penicillins).
The IMA data allow antibiotic use to be expressed as the number of DDDs, packages, treatments and insured individuals reimbursed. The number of treatments was calculated from the number of dispensed and reimbursed packages prescribed by the same prescriber to the same insured individual on the same day. To control for changes in the number of inhabitants, insured individuals and contacts with ambulatory care prescribers, use data were expressed per 1000 inhabitants, per 1000 insured individuals and per 1000 physician contacts per day (DID, PID, TID and I3D, DIID, PIID, TIID and I4D, and DCD, PCD, TCD and IICD, respectively; see Table S1, available as Supplementary data at JAC Online). In Belgium, ambulatory care prescribers include GPs, paediatricians, dentists and other specialists.
Since antibiotic use is linked to influenza epidemics with peaks that can occur in early winter in one year and late winter in another, one can observe no, one or two influenza winter peaks in one calendar year. To include only one influenza winter peak per 12 month period, instead of years from January to December we used years from July to June to express annual antibiotic use data.
The IMA database covers the total population of insured individuals in Belgium and hence provides census data. Therefore, we can draw conclusions using descriptive rather than inferential statistics. The proportion of self-employed individuals was negligible until 2008, when they became covered by compulsory health insurance. They were excluded from all analyses to avoid substantial bias in per-capita comparisons over time.
Permission to study these detailed data was granted by the Sector Committee of Social Security and Health of the Commission for the Protection of Privacy (CPP), better known as the Belgian Privacy Commission (SCSZ/10/098 and SCSZ/10/103).
Results
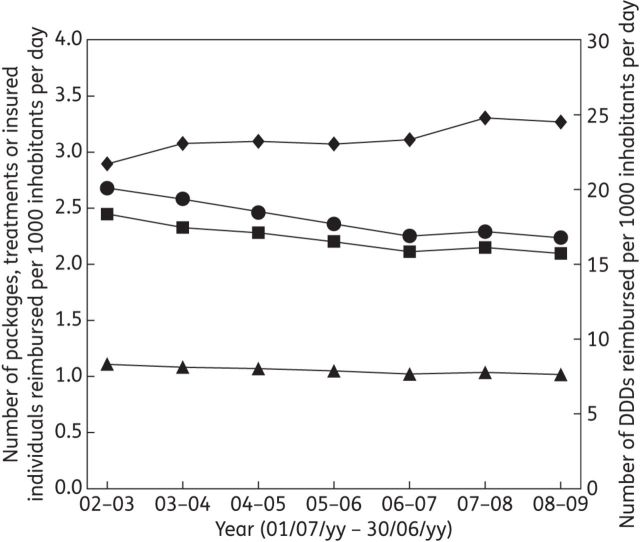
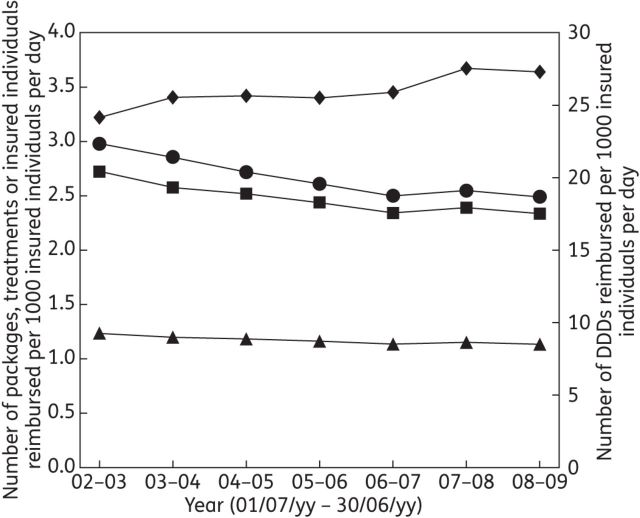
Detailed IMA data on outpatient antibiotic use were available for July–June years 2002–03 to 2008–09 (Tables S2, S3 and S4, available as Supplementary data at JAC Online). Expressed as DID, outpatient antibiotic use in Belgium increased between 2002–03 and 2008–09, whereas in PID, TID and I3D it decreased (Figure 1). The same was true for DIID (+13%) versus PIID (−16%), TIID (−14%) and I4D (−8%) (Figure 2).
Figure 1.
Outpatient antibiotic use in Belgium per July–June year expressed as the number of DDDs (diamonds), packages (circles), treatments (squares) and insured individuals (triangles) reimbursed per 1000 inhabitants per day.
Figure 2.
Outpatient antibiotic use in Belgium per July–June year expressed as the number of DDDs (diamonds), packages (circles), treatments (squares) and insured individuals (triangles) reimbursed per 1000 insured individuals per day.
Assessing these trends by major antibiotic subgroup, the same pattern could be observed only for penicillin use (Tables S2, S3 and S4, available as Supplementary data at JAC Online). These discrepancies can be explained by the increasing number of DDDs per package for amoxicillin (up 50%, from 7.11 in 2002–03 to 10.69 in 2008–09) and the combination of amoxicillin and clavulanic acid (co-amoxiclav; up 70%, 8.67 to 14.23). Together, these two antibiotic substances represented 54% of outpatient antibiotic use in DDDs (47% in packages, 48% in treatments and 71% in insured individuals) in 2008–09. For both antibiotic substances the number of units per package and the amount of active substance per unit increased over time, with e.g. a shift from 16 to 20 units per package and from 500 to 1000 mg per unit (Figure S1, available as Supplementary data at JAC Online). In addition, the number of antibiotic packages per treatment explains the small difference between the number of treatments and the number of packages, which decreased over time from 1.09 in 2002–03 to 1.07 in 2008–09. The number of antibiotic treatments per insured individual explains the difference between these two measures, which decreased over time from 2.20 to 2.06.
Furthermore, there were some distinct trends in the denominators (Table S4, available as Supplementary data at JAC Online), with the numbers of inhabitants, insured individuals and total patient contacts (GPs and specialists) all increasing over time. However, the number of patient contacts with GPs decreased, because GP home visits decreased more than GP office visits increased.
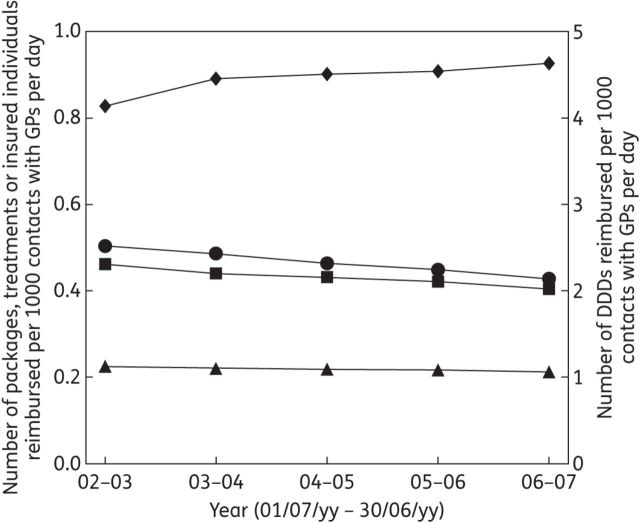
For all contacts, outpatient antibiotic use increased in terms of DCD, and decreased in terms of PCD, TCD and IICD. The same was true for contacts (consultations and home visits) with GPs only (Figure 3).
Figure 3.
Outpatient antibiotic use in Belgium prescribed by GPs per July–June year expressed as the number of DDDs (diamonds), packages (circles), treatments (squares) and insured individuals (triangles) reimbursed per 1000 contacts with GPs per day.
Tables S2 and S3, available as Supplementary data at JAC Online show that in the last year of observations (July 2008 to June 2009) amoxicillin and co-amoxiclav use was nearly equivalent when measured in terms of packages, treatments and insured individuals, but not in terms of DDDs. Nonetheless, between 2002–03 and 2008–09, the amoxicillin:co-amoxiclav use ratio increased according to all these measures (in DDDs from 0.58 to 0.74; in packages from 0.71 to 1.02; in treatments from 0.69 to 1.01; in insured individuals from 0.76 to 1.05). Tables S2 and S3, available as Supplementary data at JAC Online also show decreasing use of tetracyclines and cephalosporins and continued substantial use of macrolides (clarithromycin and azithromycin) as well as of quinolones (moxifloxacin).
Discussion
To our knowledge this is the first study describing outpatient antibiotic use over time by means of different numerators, including DDDs, packages, treatments and treated individuals, calculated from detailed national data on all systemic antibiotics dispensed and reimbursed in ambulatory care, and different denominators, including inhabitants, insured individuals and patient contacts. Discrepancies between trends in DDDs and packages in Belgium between 2002 and 2009 can be explained by increasing numbers of DDDs per package, which are mainly driven both by bigger pack size and increasing dose per unit of two penicillin antibiotics, amoxicillin and co-amoxiclav. Trends of outpatient antibiotic use in the number of packages are similar to those in the number of treatments and the number of insured individuals treated with antibiotics. These numbers are all decreasing both when taking into account the increasing number of inhabitants or insured individuals and when allowing for the decreasing number of contacts (with GPs). Amoxicillin use increased over time and surpassed co-amoxiclav use in packages, treatments and insured individuals, but not in DDDs.
Strengths and limitations
Using aggregated antibiotic use data, we were unable to differentiate appropriate from inappropriate use. The IMA data are based on the same data source as the Belgian data included in the ESAC database. However, whereas the number of inhabitants is the denominator in the internationally accepted DID, the number of insured individuals is likely to be a more valid denominator for reimbursement data.
Another relevant denominator is the number of patient contacts, which decreased over time in Belgium. Nevertheless, per 1000 contacts with GPs per day, outpatient antibiotic use prescribed by GPs also decreased in packages, treatments and insured individuals (−15%, −12% and −6%, respectively), but not in DDDs (+12%) between 2002 and 2007.
The number of packages might not be a good proxy for the number of treatments in e.g. the Netherlands or the UK, where only the exact number of items prescribed will be dispensed and pack size might not correspond well with one treatment. In countries dispensing complete packages, such as Belgium and most other European countries, a decrease in packages could also be regarded as a flawed indicator of decreased outpatient antibiotic use if it was explained by the prescription over time of fewer packages including more items per treatment. However, the decrease in treatments was fairly similar to that in packages. The number of packages per treatment decreased from 1.09 in 2002–03 to 1.07 in 2008–09. This means that decreases in packages of 2% or more correspond to decreases in treatments, that the number of packages has become a better estimate of the number of treatments with antibiotics over time and that, on average, antibiotic use in packages is overestimating the number of such treatments by 7% in 2008–09. Use in packages is also overestimating the number of insured individuals treated with antibiotics, with on average just over two treatments per insured individual treated with antibiotics per year. In addition, the exact number of treated individuals might be a very relevant measure, especially when relating antibiotic use to antimicrobial resistance.4 Nevertheless, all measures of antibiotic prescribing and consumption presented here represent related, but different, aspects of antibiotic use, i.e. the number of treated individuals, the number of treatments and the amount of active substance, that are relevant with respect to antimicrobial resistance.
One could argue that substantial amounts of active substances are still being consumed in Belgium. However, the increasing amount of active substance was used in less frequent treatments of fewer individuals, and did not have a detrimental influence on the selection of antimicrobial resistance. On the contrary, decrease in antibiotic use between 1999–2000 and 2006–07 from 3.6 to 2.3 PID coincided with a decrease in the proportion of pneumococci—one of the major bacterial pathogens of respiratory tract infections, e.g. pneumonia—resistant to penicillins, tetracyclines and macrolides between 2000 and 2007 from 18% to 10%, from 32% to 23% and from 36% to 25%, respectively.10 Whether fewer treatments with higher doses of amoxicillin and co-amoxiclav, as in Belgium, resulted in the observed trend of decreased antibiotic resistance remains hypothetical. Indeed, in the UK, which is considered a low-prescribing country,3,4 low doses of amoxicillin have been prescribed for decades,21 yet resistance of pneumococci remains low.22 The impact of pneumococcal vaccination on pneumococcal resistance in Belgium, where the 7-valent pneumococcal conjugate vaccine (PCV7) was introduced into the national schedule only in 2007 but had been used already in risk groups since 2004,23 has not been established either.
Comparison with existing literature
Trends of outpatient antibiotic use in Belgium have been assessed before. Bauraind et al.24 and ESAC used DDDs.4,15–19 We have used packages before.15,25 Davey et al.26 used both measures to assess trends in outpatient antibiotic use in the four administrations of the UK, and used Belgium as a control. They also showed discrepancies in the trend over time between DDDs and packages. In a study by Sabuncu et al.9 assessing the trend of outpatient antibiotic use in France, also using national reimbursement data, it is not clear whether the number of prescriptions equals the number of packages or the number of treatments as described in this paper, or both. They did not include other measures in their analysis. The target of 25% reduction over 5 years would not have been reached in DID, however, since outpatient antibiotic use in France decreased by only 11%, from 32.23 DID in 2002 to 28.63 DID in 2007.4 Except for Bauraind et al.,24 these studies used the number of inhabitants as the denominator to express antibiotic use, not the number of insured individuals; in addition, the number of consultations was not taken into account. Another study of the French campaign aiming to reduce inappropriate ambulatory antibiotic use assessed its impact on both prescription and consultation rates for respiratory tract infections.27 It worked with sample data, not population data, but did not show the uncertainty around the estimates and might have needed to control these estimates for clustering of patients within prescribers and for the longitudinal nature of the data. Intra-cluster correlation coefficients are estimated to be as high as 0.20.28 We worked with population data, but could not link our data on antibiotic use with the indication for which the antibiotics had been prescribed. We worked with July–June years, capturing winter peaks of influenza activity within the same 12 month period, but not formally corrected for influenza activity. It has, however, been shown that this would only slightly change the estimates of the trends.9 A decrease in the number of consultations for respiratory tract infections can explain a reduction in antibiotic use.27,29,30 We have no census data on the number of consultations for respiratory tract infections, except for the data on Belgian GP sentinel practices (http://influenza.wiv-isp.be). These do not show a clear decrease. Neither could we describe the trend of antibiotic use for respiratory tract infections, which Chahwakilian et al. did.27 Nevertheless, we know from a sample of Flemish GPs (www.intego.be) that the number of patients prescribed an antibiotic for respiratory infections decreased during the study period.31 However, taking the total number of contacts into account, outpatient antibiotic use decreased in Belgium despite decreasing numbers of contacts in ambulatory care. Since higher non-adherence to antibiotics prescribed in primary care has been shown in high-prescribing settings,14 the decrease in outpatient antibiotic use in Belgium might even represent a higher reduction in antibiotic prescribing by ambulatory care prescribers. As well as the decrease in antibiotic use coinciding with the public campaigns in Belgium, the proportion of amoxicillin use increased. This coincided with professional interventions, including guideline dissemination and individual prescribing feedback, recommending amoxicillin as the first-choice antibiotic for most respiratory infections.7 Although these changes coincided with decreasing antimicrobial resistance, there is still room to improve antibiotic use, i.e. decrease total and increase recommended outpatient antibiotic use.
Implications for policy and practice
For valid comparisons of outpatient antibiotic use over time within the same country or between countries, a combination of measures should be assessed, especially where there are differences in the number of DDDs per package and/or the number of packages per treatment. Such differences need to be explored because, due to differences in resistance rates and MICs for relevant bacterial pathogens, the treatment doses of antibiotics, in contrast to other drugs, might indeed not be stable over time or differ between countries. When such differences exist or are unknown, the number of packages appears to be a better proxy of antibiotic prescribing than the number of DDDs. The number of insured individuals is a more valid denominator for reimbursement data than the number of inhabitants. We suggest the use of July–June years to express annual antibiotic use data, hence including all influenza activity in winter in one 12 month period.
Efforts to assess the number of individuals exposed to antibiotics might allow better understanding of which measures are most indicative of changes in antimicrobial resistance trends. Combining these measures would make it possible to single out the impact of the number of DDDs per treatment and the number of treatments per individual as well. Moreover, as well as the number of DDDs, packages, treatments and insured individuals treated with antibiotics, the kind of antibiotic prescribed is an important factor related to antimicrobial resistance,1 and therefore of the quality of antibiotic use.32–34
Conclusions
In conclusion, the number of packages is a more appropriate measure than the number of DDDs when assessing outpatient antibiotic use over time in countries dispensing complete package, such as Belgium. By using different complementary measures, we observed less frequent treatments of fewer individuals with higher amounts of active substance (more DDDs) and higher proportions of recommended antibiotics (more amoxicillin) since the start of the national public antibiotic awareness campaigns. To assess interventions in other countries, including the EAAD, we recommend the use of a similar combination of measures or exercising caution when interpreting trends based only on DDDs.
Funding
This study was funded by the National Institute for Health and Disability Insurance (NIHDI) in Belgium via the Belgian Antibiotic Policy Coordination Committee (BAPCOC) and the Intermutualistic Agency (IMA), by a Methusalem research grant from the Flemish government and by the University of Antwerp.
Transparency declarations
None to declare.
Supplementary data
Acknowledgements
The ESAC project, launched in 2001, is currently continued as ESAC-Net by the ECDC.
References
- 1.Malhotra-Kumar S, Lammens C, Coenen S, et al. Impact of azithromycin and clarithromycin therapy on pharyngeal carriage of macrolide-resistant streptococci among healthy volunteers: a randomised, double-blind, placebo-controlled study. Lancet. 2007;369:482–90. doi: 10.1016/S0140-6736(07)60235-9. [DOI] [PubMed] [Google Scholar]
- 2.Costelloe C, Metcalfe C, Lovering A, et al. Effect of antibiotic prescribing in primary care on antimicrobial resistance in individual patients: systematic review and meta-analysis. BMJ. 2010;340:c2096. doi: 10.1136/bmj.c2096. [DOI] [PubMed] [Google Scholar]
- 3.Goossens H, Ferech M, Vander Stichele R, et al. Outpatient antibiotic use in Europe and association with resistance: a cross-national database study. Lancet. 2005;365:579–87. doi: 10.1016/S0140-6736(05)17907-0. [DOI] [PubMed] [Google Scholar]
- 4.Adriaenssens N, Coenen S, Versporten A, et al. European Surveillance of Antimicrobial Consumption (ESAC): outpatient antibiotic use in Europe (1997–2009) J Antimicrob Chemother. 2011;66(Suppl 6):vi3–12. doi: 10.1093/jac/dkr453. [DOI] [PubMed] [Google Scholar]
- 5.Goossens H, Guillemot D, Ferech M, et al. National campaigns to improve antibiotic use. Eur J Clin Pharmacol. 2006;62:373–9. doi: 10.1007/s00228-005-0094-7. [DOI] [PubMed] [Google Scholar]
- 6.Huttner B, Goossens H, Verheij T, et al. Characteristics and outcomes of public campaigns aimed at improving the use of antibiotics in outpatients in high-income countries. Lancet Inf Dis. 2010;10:17–31. doi: 10.1016/S1473-3099(09)70305-6. [DOI] [PubMed] [Google Scholar]
- 7.Coenen S, Costers M, De Corte S, et al. The first European Antibiotic Awareness Day after a decade of improving outpatient antibiotic use in Belgium. Acta Clin Belg. 2008;63:296–300. doi: 10.1179/acb.2008.059. [DOI] [PubMed] [Google Scholar]
- 8.Earnshaw S, Monnet DL, Duncan B, et al. European Antibiotic Awareness Day, 2008—the first Europe-wide public information campaign on prudent antibiotic use: methods and survey of activities in participating countries. Euro Surveill. 2009;14:pii=19280. doi: 10.2807/ese.14.30.19280-en. [DOI] [PubMed] [Google Scholar]
- 9.Sabuncu E, David J, Bernède-Bauduin C, et al. Significant reduction of antibiotic use in the community after a nationwide campaign in France, 2002–2007. PLoS Med. 2009;6:e1000084. doi: 10.1371/journal.pmed.1000084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goossens H, Coenen S, Costers M, et al. Achievements of the Belgian Antibiotic Policy Coordination Committee (BAPCOC) Euro Surveill. 2008;13:pii=19036. [PubMed] [Google Scholar]
- 11.Tegmark-Wisell K, Cars O. Förbättrad antibiotikaanvändning i fokus i regeringens patientsäkerhetssatsning. Läkartidningen. 2011;108:96. [PubMed] [Google Scholar]
- 12.Stora Regionala Skillnader i Antibiotikaförbrukningen Under 2010. Pressmeddelande Fran Smittskyddsinstitutet 2011–01–28. http://www.smittskyddsinstitutet.se/presstjanst/pressmeddelanden-och-pressinbjudningar/2011/stora-regionala-skillnader-i-antibiotikaforbrukningen-under-2010-/ (2 September 2013, date last accessed.
- 13.RIZIV. Ambulant Voorschrijfgedrag Antibiotica & Antihypertensiva: Onderzoeksrapport April 2005. http://www.riziv.fgov.be/care/nl/doctors/promotion-quality/antibiotiques-antihypertenseurs/pdf/rapport.pdf. (date last accessed, 2 September 2013)
- 14.Francis N, Gillespie D, Nuttall J, et al. Adherence to antibiotic prescriptions for acute cough: an international observational study. Br J Gen Pract. 2012;62:304–5. doi: 10.3399/bjgp12X649124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Versporten A, Coenen S, Adriaenssens N, et al. European Surveillance of Antimicrobial Consumption (ESAC): outpatient penicillin use in Europe (1997–2009) J Antimicrob Chemother. 2011;66(Suppl 6):vi13–23. doi: 10.1093/jac/dkr454. [DOI] [PubMed] [Google Scholar]
- 16.Versporten A, Coenen S, Adriaenssens N, et al. European Surveillance of Antimicrobial Consumption (ESAC): outpatient cephalosporin use in Europe (1997–2009) J Antimicrob Chemother. 2011;66(Suppl 6):vi25–35. doi: 10.1093/jac/dkr455. [DOI] [PubMed] [Google Scholar]
- 17.Adriaenssens N, Coenen S, Versporten A, et al. European Surveillance of Antimicrobial Consumption (ESAC): outpatient macrolide, lincosamide and streptogramin (MLS) use in Europe (1997–2009) J Antimicrob Chemother. 2011;66(Suppl 6):vi37–45. doi: 10.1093/jac/dkr456. [DOI] [PubMed] [Google Scholar]
- 18.Adriaenssens N, Coenen S, Versporten A, et al. European Surveillance of Antimicrobial Consumption (ESAC): outpatient quinolone use in Europe (1997–2009) J Antimicrob Chemother. 2011;66(Suppl 6):vi47–56. doi: 10.1093/jac/dkr457. [DOI] [PubMed] [Google Scholar]
- 19.Coenen S, Adriaenssens N, Versporten A, et al. European Surveillance of Antimicrobial Consumption (ESAC): outpatient use of tetracyclines, sulphonamides and trimethoprim, and other antibacterials in Europe (1997–2009) J Antimicrob Chemother. 2011;66(Suppl 6):vi57–70. doi: 10.1093/jac/dkr458. [DOI] [PubMed] [Google Scholar]
- 20.WHO Collaborating Centre for Drug Statistics Methodology. Anatomical Therapeutic Chemical (ATC) Classification System: Guidelines for ATC Classification and DDD Assignment 2010. Oslo: WHO, 2011: [Google Scholar]
- 21.Joint Formulary Committee. British National Formulary. 65th edn. London: BMJ Group and Pharmaceutical Press; 2013. [Google Scholar]
- 22.European Centre for Disease Prevention and Control. Antimicrobial Resistance Surveillance in Europe 2011. Annual Report of the European Antimicrobial Resistance Surveillance Network (EARS-Net) Stockholm: ECDC; 2012. [Google Scholar]
- 23.Hanquet G, Lernout T, Vergison A, et al. Impact of conjugate 7-valent vaccination in Belgium: addressing methodological challenges. Vaccine. 2011;29:2856–64. doi: 10.1016/j.vaccine.2011.02.016. [DOI] [PubMed] [Google Scholar]
- 24.Bauraind I, Lopez-Lozano J-M, Beyaert A, et al. Association between antibiotic sales and public campaigns for their appropriate use. JAMA. 2004;292:2468–70. doi: 10.1001/jama.292.20.2468-b. [DOI] [PubMed] [Google Scholar]
- 25.Campos J, Ferech M, Lazaro E, et al. Surveillance of outpatient antibiotic consumption in Spain according to sales data and reimbursement data. J Antimicrob Chemother. 2007;60:698–701. doi: 10.1093/jac/dkm248. [DOI] [PubMed] [Google Scholar]
- 26.Davey P, Ferech M, Ansari F, et al. Outpatient antibiotic use in the four administrations of the UK: cross-sectional and longitudinal analysis. J Antimicrob Chemother. 2008;62:1141–7. doi: 10.1093/jac/dkn386. [DOI] [PubMed] [Google Scholar]
- 27.Chahwakilian P, Huttner B, Schlemmer B, et al. Impact of the French campaign to reduce inappropriate ambulatory antibiotic use on the prescription and consultation rates for respiratory tract infections. J Antimicrob Chemother. 2011;66:2872–9. doi: 10.1093/jac/dkr387. [DOI] [PubMed] [Google Scholar]
- 28.Coenen S, Michiels B, Renard D, et al. Antibiotics for coughing in general practice: the effect of perceived patient demand. Br J Gen Pract. 2006;56:183–90. [PMC free article] [PubMed] [Google Scholar]
- 29.Fleming D, Ross A, Cross K, et al. The reducing incidence of respiratory tract infection and its relation to antibiotic prescribing. Br J Gen Pract. 2003;53:778–83. [PMC free article] [PubMed] [Google Scholar]
- 30.Neumark T, Brudin L, Mölstad S. Use of rapid diagnostic tests and choice of antibiotics in respiratory tract infections in primary healthcare—a 6-y follow-up study. Scand J Infect Dis. 2010;42:90–6. doi: 10.3109/00365540903352932. [DOI] [PubMed] [Google Scholar]
- 31.Adriaenssens N, Bartholomeeusen S, Ryckebosch P, et al. Quality of antibiotic prescription during office hours and out-of-hours in Flemish primary care, using European quality indicators. Eur J Gen Pract. 2013. doi:10.3109/13814788.2013.828200. [DOI] [PubMed]
- 32.Adriaenssens N, Coenen S, Tonkin-Crine S, et al. European Surveillance of Antimicrobial Consumption (ESAC): disease-specific quality indicators for outpatient antibiotic prescribing. BMJ Qual Saf. 2011;20:764–72. doi: 10.1136/bmjqs.2010.049049. [DOI] [PubMed] [Google Scholar]
- 33.Adriaenssens N, Coenen S, Versporten A, et al. European Surveillance of Antimicrobial Consumption (ESAC): quality appraisal of outpatient antibiotic use in Europe. J Antimicrob Chemother. 2011;66(Suppl 6):vi71–7. doi: 10.1093/jac/dkr459. [DOI] [PubMed] [Google Scholar]
- 34.Coenen S, Ferech M, Haaijer-Ruskamp FM, et al. European Surveillance of Antimicrobial Consumption (ESAC): quality indicators for outpatient antibiotic use in Europe. Qual Saf Health Care. 2007;16:440–5. doi: 10.1136/qshc.2006.021121. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.