Abstract
Objectives
Candida albicans is a common cause of nosocomial urinary tract infections (UTIs) and is responsible for increased morbidity and healthcare costs. Moreover, the US Centers for Medicare & Medicaid Services no longer reimburse for hospital-acquired catheter-associated UTIs. Thus, development of specific approaches for the prevention of Candida urinary infections is needed. Cranberry juice-derived proanthocyanidins (PACs) have efficacy in the prevention of bacterial UTIs, partially due to anti-adherence properties, but there are limited data on their use for the prevention and/or treatment of Candida UTIs. Therefore, we sought to systematically assess the in vitro effect of cranberry-derived PACs on C. albicans biofilm formation in artificial urine.
Methods
C. albicans biofilms in artificial urine were coincubated with cranberry PACs at serially increasing concentrations and biofilm metabolic activity was assessed using the XTT assay in static microplate and silicone disc models.
Results
Cranberry PAC concentrations of ≥16 mg/L significantly reduced biofilm formation in all C. albicans strains tested, with a paradoxical effect observed at high concentrations in two clinical isolates. Further, cranberry PACs were additive in combination with traditional antifungals. Cranberry PACs reduced C. albicans adherence to both polystyrene and silicone. Supplementation of the medium with iron reduced the efficacy of cranberry PACs against biofilms.
Conclusions
These findings indicate that cranberry PACs have excellent in vitro activity against C. albicans biofilm formation in artificial urine. We present preliminary evidence that cranberry PAC activity against C. albicans biofilm formation is due to anti-adherence properties and/or iron chelation.
Keywords: cranberry PACs, urinary tract infections, urinary catheters, Vaccinium macrocarpon Ait.
Introduction
Candida albicans is a major human fungal pathogen and one of the most common causes of nosocomial urinary tract infection.1 Candiduria is common in hospitalized patients and, although often asymptomatic and of unknown clinical significance, candiduria is still associated with increased morbidity and mortality.1,2 In rare cases, candiduria has been associated with the subsequent development of candidaemia, an infection associated with high rates of morbidity and mortality.1–4 Three major classes of antifungal drugs are currently available for use against Candida infections, but each class has notable limitations, particularly against candiduria.5 Azoles are the most commonly used antifungal for the treatment of C. albicans infections, but are fungistatic and poorly active against biofilms.6–8 Echinocandins are highly active against C. albicans, but generally do not achieve clinically useful concentrations in the urinary tract.9 Amphotericin B has significant toxicities and poor aqueous solubility.10 In part due to these limitations, the mortality, morbidity and cost of care due to candiduria and candidaemia remain high.1,4 Therefore, the search for alternative treatments and preventive approaches is warranted.11
A major component of C. albicans pathogenesis is the ability to form biofilms on both biotic and abiotic surfaces.8,12 Unlike their planktonic counterparts, fungal organisms embedded in biofilms undergo phenotypic changes that protect them from the local microenvironment and the host immune system. The formation of biofilms is also associated with increased drug resistance. Although the role of biofilm formation in candiduria is less well understood than on mucosal surfaces and in bloodstream infections (i.e. central venous catheters), C. albicans clinical isolates from patients presenting with candiduria form strong biofilms in artificial urine (AU) in vitro.6 Further, C. albicans has been shown to form biofilms in indwelling catheters in an in vivo murine model of catheter-associated candiduria13 and biofilms have been observed on infected urinary catheters in vivo.14 The initial stage of biofilm formation occurs when yeast cells adhere to the substrate material, a process mediated both by abiotic factors, such as surface hydrophobicity, and biotic factors, such as increased expression of adhesins and other cell-surface proteins.12 Therefore, the inhibition of adherence by C. albicans is a promising target for disrupting the initial stages of biofilm formation in C. albicans.
Cranberry juice has been touted as a safe, alternative therapy against bacterial urinary tract infections for centuries. Further, the preventative value of cranberry juice against Escherichia coli urinary tract infections has been demonstrated clinically15–17 and the anti-adherence quality of cranberry juice extract against E. coli has been studied extensively.18–22 Specifically, A-type proanthocyanidins (PACs) isolated from cranberries have been implicated in the anti-adherence properties of cranberry juice.18 The effect of cranberry PACs on E. coli adherence is not substrate specific; cranberry PACs decrease E. coli adherence to uroepithelial and vaginal epithelial cells,19 as well as to non-biological materials such as PVC and polytetrafluoroethylene.20 Ingestion of cranberry PACs has also been shown to decrease virulence due to E. coli in an in vivo Caenorhabditis elegans model.21 Interestingly, cranberry PACs have both non-biospecific activity against adherence, perhaps due to steric hindrance,20 and biospecific activity against adherence, including decreased expression of adhesion genes such as fliC.22 These studies highlight the potential use of cranberry PACs in the prevention of adherence in other species. A-type cranberry PACs have previously been reported to decrease C. albicans adherence to oral epithelial cells and to reduce biofilm formation and inflammatory responses in vitro.23
In order to determine the activity of cranberry PACs against C. albicans urinary biofilms, we systematically assayed the effect of serially increasing doses of cranberry PACs on biofilm formation and mature biofilms in AU on both polystyrene and silicone surfaces. To study the degree of strain-specific variation upon treatment with cranberry PACs, we next studied the effect of cranberry PACs on biofilm formation by nine clinical isolates, including four C. albicans strains isolated from patients with candiduria. In addition, we tested cranberry PAC treatment in combination with traditional antifungals for activity against C. albicans biofilm formation in a chequerboard assay of antimicrobial combinations. To gain further insight into the mechanism of activity, we assayed C. albicans adherence to both polystyrene and silicone surfaces in response to cranberry PACs. Finally, we tested whether iron chelation could account for cranberry PAC activity against C. albicans biofilms.
Methods
Isolation of cranberry-derived PACs
Cranberry PACs were isolated from cranberry fruit (Vaccinium macrocarpon Ait.) using solid-phase chromatography as previously described.18 Briefly, cranberry fruit was homogenized with 70% aqueous acetone, filtered and the pulp discarded. To remove acetone, the collected extract was concentrated under reduced pressure. The cranberry extract was suspended in water, applied to a pre-conditioned C-18 solid-phase chromatography column and washed with water to remove sugars, followed by acidified aqueous methanol to remove acids. The fats and waxes retained on the C-18 sorbent were discarded. The polyphenolic fraction containing anthocyanins, flavonol glycosides and PACs (confirmed using reverse-phase HPLC with diode array detection) was eluted with 100% methanol and dried under reduced pressure. This fraction was suspended in 50% EtOH and applied to a pre-conditioned Sephadex LH-20 column washed with 50% EtOH to remove low molecular weight anthocyanins and flavonol glycosides. PACs adsorbed to the LH-20 were eluted from the column with 70% aqueous acetone and monitored using diode array detection at 280 nm. The absence of absorption at 360 and 450 nm confirmed that anthocyanins and flavonol glycosides were removed. Acetone was removed under reduced pressure and the resulting purified PAC extract freeze-dried. The presence of A-type linkages and concentration of PACs present in the extract were confirmed using electrospray mass spectrometry, 13C-NMR, matrix-assisted laser desorption/ionization time-of-flight mass spectrometry and acid-catalysed degradation with phloroglucinol.18,24,25
Assays for biofilm activity
For initial studies of the activity of cranberry PACs against mature biofilms and against biofilm formation (i.e. biofilm prevention), C. albicans SC5314 was used as a reference isolate (gift from W. Fonzi, Georgetown University, Washington DC, USA).26 To characterize the degree of strain-specific variation, C. albicans ATCC 10231, ATCC 14053 and ATCC 24433 strains and two C. albicans fks1 mutant strains (strains 42379 and 53264) characterized by echinocandin resistance were also studied.27,28 Clinical urinary isolates of C. albicans were a gift from L. Massie (University of New Mexico and NM VA Healthcare System, Albuquerque, NM, USA). C. albicans biofilm formation in polystyrene 96-well microtitre plates and the XTT assay, used to determine the metabolic activity of the biofilms, were performed as described previously,29 with slight modifications. Briefly, each strain was grown in yeast extract/peptone/dextrose (‘YPD’) broth with 0.008% uridine overnight (30°C, 250 rpm). Cells were harvested via centrifugation, washed twice and resuspended in PBS. Cells were added to AU6 to a starting density of 1.0 × 106 cells/mL. An aliquot of 100 μL of the cell suspension was then added to designated wells of microtitre plates. Cell-free AU was added to wells designated as negative controls. The plates were incubated at 37°C for 24 h. The biofilms were then washed with PBS three times to remove non-adherent cells. Next, 100 μL of cranberry PACs serially diluted in AU to concentrations of 4–1024 mg/L was added to biofilm-containing wells. Drug-free AU was added to wells designated for positive and negative controls. The plates were then reincubated at 37°C for 24 h. After treatment with cranberry PACs, all biofilms were again washed three times with PBS and the XTT assay was used to determine the metabolic activity of the biofilms.29 The optical density (OD) at 490 nm was measured via spectrophotometry on a BioTek ELx808 microplate reader (BioTek Instruments, Inc., Winooski, VT, USA). The antifungal activity of each cranberry PAC treatment was expressed as a percentage relative to the metabolic activity of the untreated control. Each experiment was performed in triplicate or quadruplicate. The metabolic activities of the treatment groups were compared with controls using one- or two-way analyses of variance and Tukey's multiple-comparison post-test. Differences were considered significant at P < 0.05. Statistical analyses were performed and graphs were produced using GraphPad Prism 5.0 (GraphPad Software, San Diego, CA, USA) and Microsoft® Excel (Microsoft Corp., Redmond, WA, USA). The MIC was defined as the concentration of cranberry PAC or traditional antifungal agent needed to reduce the metabolic activity of the biofilms of the strain by 50% (MIC50) and 80% (MIC80).
To test biofilm formation in the presence of cranberry PACs, the protocol described above was followed, except that cells in AU were coincubated with cranberry PACs at concentrations of 4–1024 mg/L. The plates were then incubated at 37°C for 24 h to allow for biofilm formation. After 24 h, the medium was removed and biofilms were visualized via light microscopy before proceeding with the XTT assay.
Assays for biofilm activity on silicone discs
To model the effect of cranberry PACs on biofilms formed on silicone surfaces, medical grade silicone sheeting (Invotek International, Jacksonville, FL, USA) was washed in a mild detergent. Discs were cut to fit microtitre plate wells using a leather punch, autoclaved and transferred via sterile technique to microtitre plate wells. C. albicans SC5314 cells in AU were treated with cranberry PACs as described above for both biofilm formation and mature biofilms. The XTT assay was used to determine the metabolic activity of the biofilms. To test for synergistic activity between cranberry PACs and known antifungals against biofilm formation, we utilized a chequerboard assay.30 C. albicans SC5314 cells in AU (at a final concentration of 1 × 106 cells/mL) were coincubated with various concentrations of antifungal drugs and various concentrations of cranberry PACs. The fluconazole concentrations tested were 16–1024 mg/L, in 2-fold dilutions. The amphotericin B concentrations tested were 0.015625–1 mg/L, in 2-fold dilutions. The caspofungin concentrations tested were 0.125–16 mg/L, in 2-fold dilutions. The cranberry PAC concentrations tested were 1–512 mg/L, in 2-fold dilutions. Antifungal drug dilutions were prepared according to CLSI guidelines.31 The XTT assay was used to determine the metabolic activity of the biofilms and each combination was characterized as additive, synergistic or antagonistic, as described previously.30
Assessment of planktonic growth
The effect of cranberry PACs on C. albicans SC5314 growth in AU was also tested; cells from overnight cultures were diluted to an OD600 of 0.05 in AU, with cranberry PACs added to concentrations of 0, 2, 4, 16, 64, 256 or 1024 mg/L. To control for cranberry PAC absorbance at OD 600, AU containing 0, 2, 4, 16, 64, 256 or 1024 mg/L cranberry PACs was used as a negative control. An aliquot of 150 μL of cells was added to 96-well plates (four replicates per treatment). Then, cells were grown at 30°C using a BioTek Synergy H1M with double orbital shaking at fast speed and 2 mm frequency, with OD600 readings taken at 15 min intervals. OD600 readings were calculated as the OD600 readings of AU with cranberry PACs and cells minus the OD600 readings of AU with cranberry PACs alone.
Effects of PACs on planktonic adherence
We tested C. albicans SC5314 adherence to polystyrene as previously described, with some modifications.32 First, planktonic cells from overnight cultures were harvested via centrifugation, washed twice and resuspended in PBS. Cells were inoculated in PBS containing 0, 4, 16, 64, 256 or 1024 mg/L cranberry PACs to a concentration of 1 × 107 cells/mL. Next, 150 μL of each solution was added to a 96-well plate (eight replicates per treatment) and, as an unwashed control, 150 μL of each solution was added to 1.5 mL microcentrifuge tubes. Both the microcentrifuge tubes and the 96-well plate were incubated at 37°C for 2 h. Then, spent medium was aspirated from the 96-well plate and adherent cells were washed twice with PBS. Cells in microcentrifuge tubes were spun down at 13 000 rpm for 10 min and spent medium was aspirated. An aliquot of 100 μL of 300 mg/L XTT/0.2 mM menadione was added to the 96-well plate, as well as the microcentrifuge tubes, and both were incubated at 37°C. After 3 h, 75 μL of each treatment was transferred to a new 96-well plate and XTT formazan was measured at OD490. The percentage adherence was calculated as the mean of absorbance readings from washed wells over the mean of absorbance readings from unwashed microcentrifuge controls. To model the effect of cranberry PACs on adherence on silicone surfaces, the protocol described above was used to cut silicone discs to size and to transfer them via sterile technique to a 96-well plate, and the adherence assay was performed as described.
Effect of iron supplementation on PAC activity against biofilms
We also tested whether the supplementation of AU with 1 mM FeCl3 could compensate for the decrease in biofilm formation observed upon addition of cranberry PACs. C. albicans SC5314 cells in AU were coincubated with various concentrations of cranberry PACs, with or without the addition of 1 mM FeCl3. After incubation at 37°C for 24 h, the XTT assay was used to measure the metabolic activity of the biofilms. Statistical significance was assessed by comparing the metabolic activities of biofilms treated with cranberry PACs alone versus biofilms treated with cranberry PACs + 1 mM FeCl3.
Results
To test the ability of cranberry PACs to prevent C. albicans biofilm formation in an in vitro model of urinary biofilms, cranberry PACs were coincubated with C. albicans cells in AU. Cranberry PACs significantly decreased C. albicans SC5314 biofilm formation on polystyrene at concentrations as low as 8 mg/L (Figure 1a). Notably, at a concentration of 128 mg/L, which is comparable to the concentration of cranberry PACs previously reported in commercially available cranberry juice,21 C. albicans biofilm formation was decreased by 89.3%. The MIC50 of cranberry PACs was 16 mg/L and the MIC80 was 64 mg/L. Similarly, cranberry PACs significantly decreased C. albicans SC5314 biofilm formation on silicone (Figure 1b). The MIC50 of cranberry PACs against biofilm formation on silicone discs was 32 mg/L and the MIC80 was 512 mg/L. Light microscopy of biofilms on polystyrene coincubated with cranberry PACs revealed that cells were still forming hyphae even in the presence of high concentrations of cranberry PACs (Figure 2). Despite this, biofilms coincubated with cranberry PACs were less dense than controls. We also tested cranberry PAC activity against pre-formed, or mature, biofilms. Treatment of pre-formed biofilms with cranberry PAC concentrations from 4 to 128 mg/L had no statistically significant effect on biofilms on either polystyrene or silicone (data not shown). However, even high doses of cranberry PACs (256–1024 mg/L) only modestly decreased the metabolic activity of pre-formed biofilms (16.6%–23.2% decrease on polystyrene and 25.3%–49.1% decrease on silicone; data not shown). Finally, we tested the effect of cranberry PAC concentrations from 2 to 1024 mg/L on C. albicans SC5314 planktonic growth. Of note, concentrations of 8–128 mg/L cranberry PACs, which significantly reduce biofilm formation, did not have a statistically significant effect on growth (Table 1). Only very high concentrations of cranberry PACs (256–1024 mg/L) had a dose-dependent inhibitory effect on the doubling time of C. albicans cells (Table 1), thus suggesting a biofilm formation-specific inhibitory effect.
Figure 1.
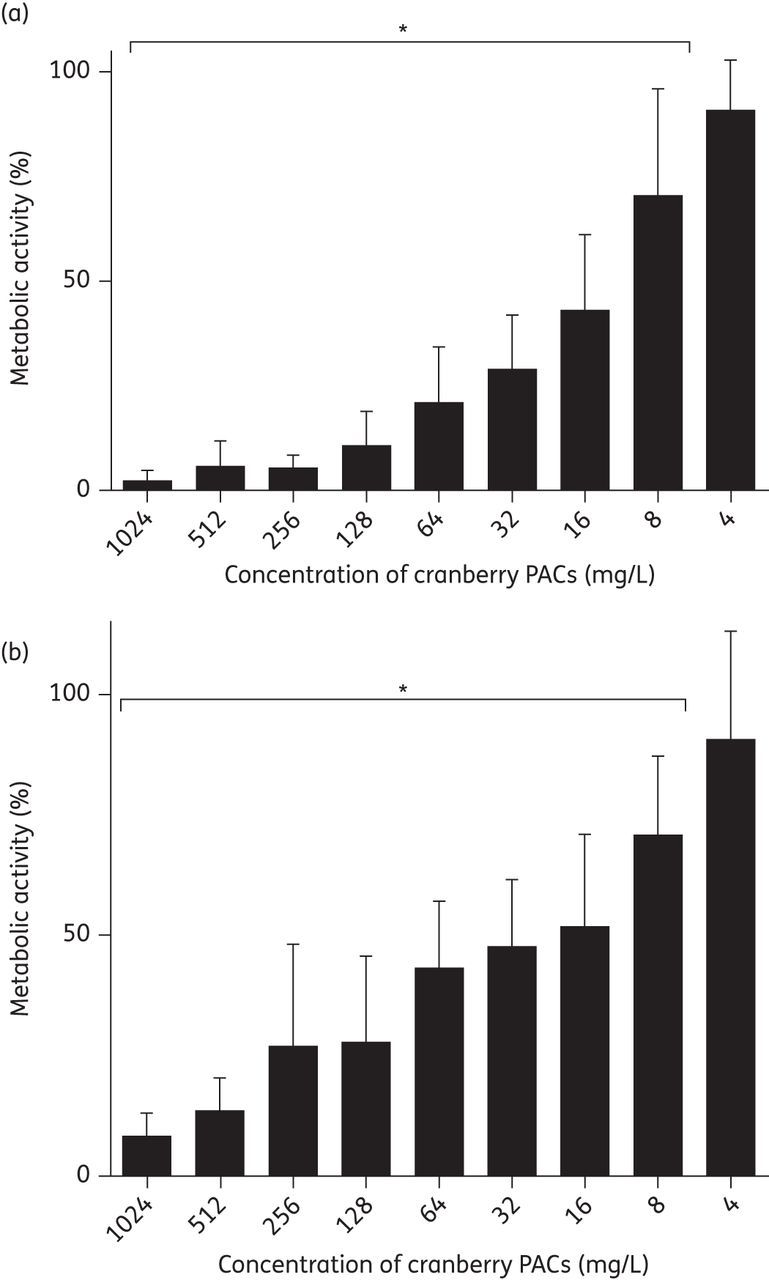
Cranberry PACs inhibit C. albicans SC5314 biofilm formation on polystyrene and silicone. (a) Cranberry PACs prevent C. albicans SC5314 biofilm formation on polystyrene. Biofilms in AU were coincubated with cranberry PACs at concentrations of 4–1024 mg/L for 24 h. After the incubation period, the metabolic activity of treated biofilms was assessed using the XTT assay. Average of three separate experiments. (b) Cranberry PACs prevent C. albicans SC5314 biofilm formation on silicone. Biofilms in AU were coincubated with cranberry PACs at concentrations of 4–1024 mg/L for 24 h. After the incubation period, the metabolic activity of treated biofilms was assessed using the XTT assay. Average of three independent experiments.
Figure 2.
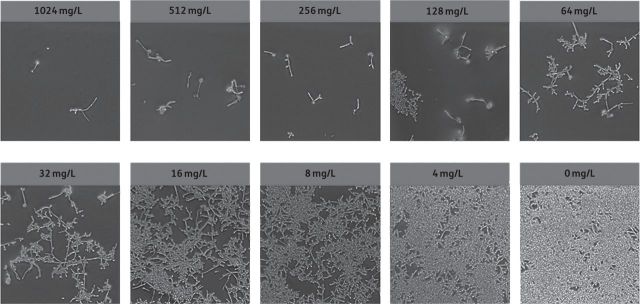
Light microscopy of C. albicans biofilms grown in AU in combination with various concentrations of cranberry PACs. Biofilms in AU were coincubated with cranberry PACs at concentrations of 4–1024 mg/L for 24 h before light microscopy images were taken. High concentrations of cranberry PACs decrease the density of biofilms, but do not inhibit filamentation within biofilms.
Table 1.
Doubling time of planktonic C. albicans SC5314 cells coincubated with various concentrations of cranberry PACs
| Concentration (mg/L) | Doubling time (h) | Significance |
|---|---|---|
| 0 | 2.28 ± 0.05 | NS |
| 2 | 2.23 ± 0.04 | NS |
| 4 | 2.13 ± 0.02 | NS |
| 8 | 2.14 ± 0.02 | NS |
| 16 | 2.13 ± 0.02 | NS |
| 32 | 2.18 ± 0.04 | NS |
| 64 | 2.38 ± 0.02 | NS |
| 128 | 2.39 ± 0.06 | NS |
| 256 | 2.88 ± 0.08 | *** |
| 512 | 4.47 ± 0.27 | *** |
| 1024 | 6.34 ± 0.23 | *** |
NS, not statistically significant.
***Statistical significance at P < 0.001.
The effect of cranberry PACs on C. albicans biofilm formation was highly strain dependent (Table 2). Concentrations of ≥16 mg/L demonstrated a significant reduction in metabolic activity for most isolates tested (data not shown). The exceptions were C. albicans urinary isolates 6 and 8, which demonstrated a paradoxical effect such that high doses (256–1024 mg/L) had no significant effect on metabolic activity despite a statistically significant reduction in metabolic activity at moderate doses (16–128 mg/L) (data not shown). A strain- and drug-dependent paradoxical effect has previously been noted upon treatment of various Candida species with echinocandins.33–35 Notably, the MIC80 for all four urinary isolates and for one of two caspofungin-resistant fks1 mutants, 42379, was appreciably elevated compared with other strains (>1024 mg/L).
Table 2.
Effect of cranberry PACs on C. albicans biofilm formation
| Strain | MIC50 (mg/L) | MIC80 (mg/L) |
|---|---|---|
| SC5314 | 16 | 128 |
| ATCC 10231 | 8 | 64 |
| ATCC 14053 | 128 | 512 |
| ATCC 24433 | 64 | 512 |
| 42379 (fks1 mutant) | 1024 | >1024 |
| 53264 (fks1 mutant) | 64 | 256 |
| C. albicans urinary isolate 3 | 16 | >1024 |
| C. albicans urinary isolate 5 | 32 | >1024 |
| C. albicans urinary isolate 6 | >1024 | >1024 |
| C. albicans urinary isolate 8 | >1024 | >1024 |
The combination of cranberry PACs with fluconazole, amphotericin B and caspofungin against biofilm formation had an additive, but not synergistic, effect (data not shown).
Cranberry PACs significantly reduced C. albicans adherence to both polystyrene and silicone in an in vitro model of planktonic adherence (Figure 3). Concentrations of 16–1024 mg/L cranberry PACs caused a statistically significant reduction of C. albicans adherence to polystyrene (Figure 3a). However, C. albicans SC5314 adherence to silicone is reduced compared with polystyrene and higher concentrations of cranberry PACs (256–1024 mg/L) were required to achieve a statistically significant effect on adherence to silicone (Figure 3b).
Figure 3.
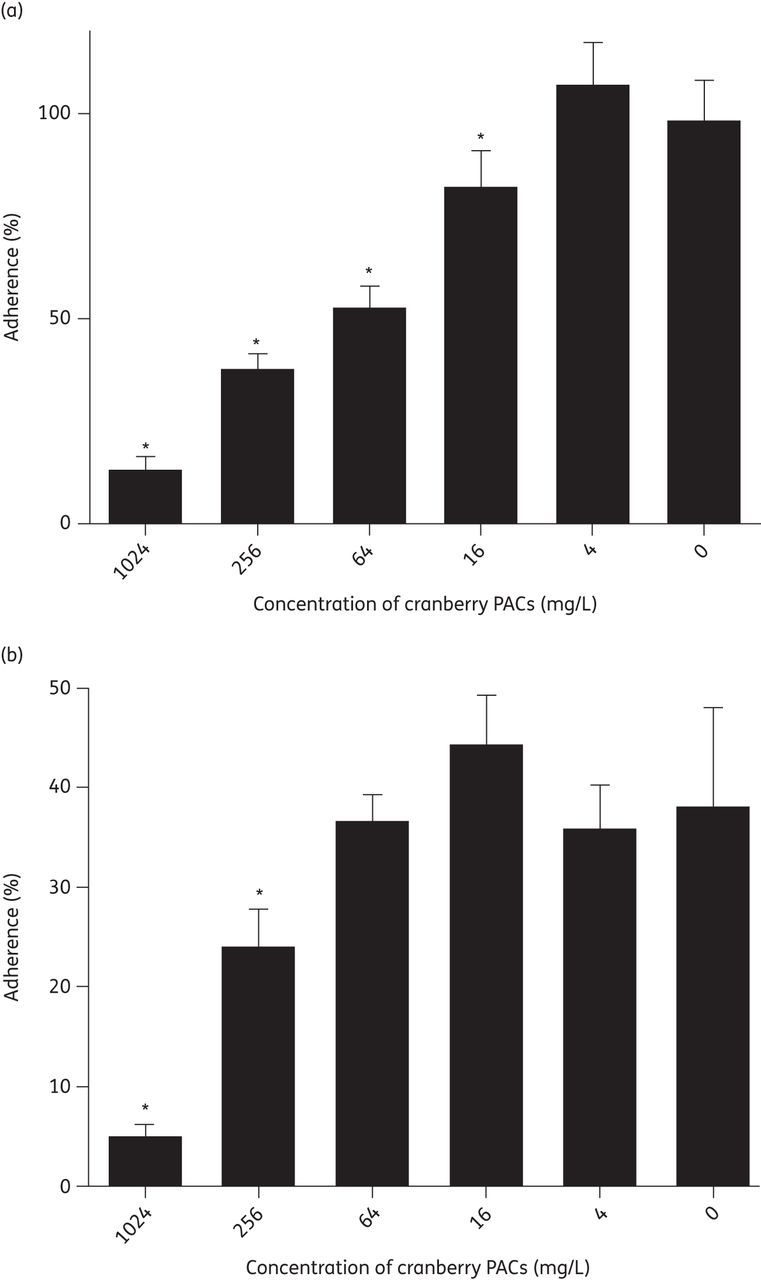
Cranberry PACs prevent C. albicans planktonic adherence. (a) Cranberry PACs prevent C. albicans adherence to polystyrene. Percentage adherence is calculated as the metabolic activity of adherent cells after 2 h of incubation at 37°C, divided by the metabolic activity of adherent plus non-adherent cells. Concentrations of 16–1024 mg/L cranberry PACs significantly decrease the percentage adherence to polystyrene. (b) Cranberry PACs prevent C. albicans adherence to silicone. C. albicans SC5314 adheres less well to silicone than to polystyrene. Concentrations of 256–1024 mg/L cranberry PACs significantly decrease the percentage adherence to silicone.
Cranberry PACs have previously been shown to chelate iron, thus removing available iron from the environment.22,36 Therefore, to test whether supplementing the medium with iron could compensate for the inhibitory effect of cranberry PACs on C. albicans biofilm formation, we coincubated C. albicans SC5314 with 2–1024 mg/L cranberry PACs in the presence and absence of 1 mM FeCl3 (Figure 4). The addition of iron to the medium significantly increased both the MIC50 and MIC80 of cranberry PACs from 16 and 128 mg/L, respectively, to 256 and 1024 mg/L, respectively. Iron addition to medium without cranberry PACs did not affect biofilm growth. Thus, iron supplementation partially rescued the inhibitory effect of cranberry PACs on C. albicans biofilms in our in vitro urinary candidiasis model.
Figure 4.
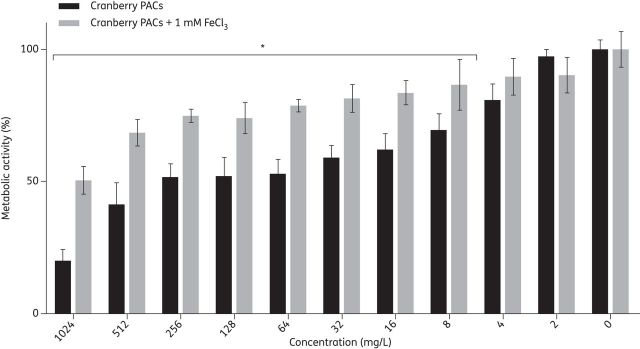
Addition of iron (III) chloride partially rescues biofilm formation in the presence of cranberry PACs. Biofilms in AU were coincubated with 2–1024 mg/L cranberry PACs, with or without the addition of 1 mM FeCl3. Biofilms in AU with or without 1 mM FeCl3 were used as controls. The metabolic activities of biofilms were assessed using the XTT assay.
Discussion
Candiduria is a frequent occurrence among hospitalized patients, particularly immunocompromised patients, the elderly and neonates. Of note, patients with indwelling urinary catheters are more likely to develop candiduria. Cranberry juice, cranberry extracts and cranberry-derived PACs are an appealing alternative strategy for the prevention of candiduria due to their low cost, low toxicity and high tolerability.16,37 Cranberry products have been the subject of numerous clinical trials involving varied patient populations for the prevention of bacterial urinary tract infections, as well as other conditions; these studies reported either no adverse events or minor adverse events such as headache or gastrointestinal upset at similar levels to placebo treatments.37–48 These clinical trials administered doses ranging from 46 to 960 mL of cranberry product per day, which contained 0.8–114 mg of PACs per day (where PAC values were not directly provided by study authors, we estimated PAC values based on previous analyses of cranberry products).21 These analyses indicate that doses of cranberry PACs as high as 114 mg/day are safe for consumption. However, further study on the concentration of PACs available in the urinary tract is needed to determine whether concentrations with activity are achievable in vivo.
Biofilm formation is a key pathogenesis-related trait and its prevention in vivo could decrease the incidence of candiduria. Furthermore, ideal drug treatments target pathogenesis without affecting growth, as growth inhibition typically induces strong selection for drug resistance. Our study demonstrated that PACs isolated from cranberries have strong activity against the formation of C. albicans biofilms in an in vitro model of urinary biofilms. Further, cranberry PACs retain their antibiofilm activity on silicone surfaces, suggesting that cranberry PAC treatment could be effective in preventing urinary catheter infections due to C. albicans. It should also be noted that there are few, if any, instances where antifungal prophylaxis to prevent candiduria and Candida urinary tract infections is justifiable. Echinocandins do not achieve clinically reliable urinary concentrations; amphotericin preparations can be highly nephrotoxic amongst other potentially serious toxicities. Azoles might be useful as prophylaxis, but the risk of selection of resistant yeast species would not appear to outweigh the potential benefits of prevention. Thus, cranberry PACs could represent a novel and safe option for the prevention of candiduria and Candida urinary tract infections.
Importantly, whereas high doses of cranberry PACs (256–1024 mg/L) in this study had a statistically significant effect on planktonic growth, doses with no effect on growth exhibited a significant reduction in biofilm formation. Moreover, even at the highest doses tested, cranberry PACs had minimal activity against pre-formed or ‘mature’ biofilms. Therefore, decreased growth cannot account for the effect of moderate doses of cranberry PACs on C. albicans biofilm formation, further supporting the potential of cranberry PACs as a prevention strategy through anti-adherence and biofilm-specific mechanisms. The antifungal effect of cranberry juice and cranberry extract on C. albicans has been studied previously. The majority of studies found no significant effect of cranberry and its derivatives on C. albicans growth,49–53 while a single study found decreased C. albicans growth after treatment with cranberry.54 These results are in accordance with our data. However, because these studies used various crude cranberry products rather than purified cranberry PACs, direct comparison between studies is difficult. Taken together, however, these data suggest that cranberry PACs prevent Candida urinary biofilm formation through mechanisms other than direct antifungal activity.
Cranberry PAC activity against E. coli is thought to be at least partially due to its anti-adherence properties. In this study, we demonstrated that cranberry PACs prevent C. albicans adherence to both polystyrene and silicone. Previous studies have demonstrated that the effect of cranberry PACs on adherence is both biospecific for E. coli and non-biospecific; it is unclear what mechanisms are primarily responsible for the anti-adherence properties of cranberry PACs against C. albicans cells. Further studies in our laboratory to address this question are in progress. Urine collected from patients treated with cranberry PAC doses as low as 36 mg/day displays E. coli anti-adherence activity.21 Notably, the concentration at which anti-adherence activity is first detectable in vitro is similar for C. albicans and E. coli (16 mg/L cranberry PACs for C. albicans in our study versus 10 mg/L cranberry PACs for E. coli).55 Further studies assessing local tissue concentrations of cranberry PACs in the urinary tract are required to determine whether clinically relevant urinary concentrations of cranberry PACs are achievable in vivo.
Cranberry PACs have been shown to form a chelate with iron, thereby removing available iron from the environment. We demonstrated that supplementation of medium with iron partially rescues the reduction in biofilm formation upon treatment with cranberry PACs. This result suggests that the effect of cranberry PACs on C. albicans biofilm formation may be partially explained by iron starvation due to the iron-chelating ability of cranberry PACs; further studies are required to test this hypothesis. We observed high tolerance for cranberry PACs in C. albicans strains isolated from patients presenting with candiduria compared with wild-type C. albicans. One intriguing possible explanation for this observation is that if cranberry PAC activity is due to iron chelation, these isolates may have undergone selection for the ability to tolerate the low-iron environment of the host and, therefore, are less susceptible to iron starvation than the laboratory strain SC5314. Alternatively, urinary isolates may undergo high levels of exposure to cranberry PACs and/or PACs derived from other foods and this selective pressure may explain their higher tolerance for cranberry PACs.
It is important to note that the effect of cranberry PACs on C. albicans clinical isolates may differ in vivo, as cranberry and cranberry PACs have previously been shown to suppress the host inflammatory response.23,56–59 For example, a previous study showed that treatment with cranberry PACs lowered C. albicans cell surface hydrophobicity and decreased the production of proinflammatory cytokines by oral epithelial cells infected with C. albicans.23 These results and the results of our study demonstrate that cranberry PACs have a complex, multifactorial effect on C. albicans, further underscoring the potential of cranberry PACs and cranberry products as a novel strategy for the prevention of Candida urinary infections. Our current efforts are now focused on determining the molecular mechanisms related to the antibiofilm activity of cranberry PACs and, next, to initiate in vivo studies of cranberry PAC activity against C. albicans.
Funding
This work was supported by grants from the Department of Veterans' Affairs (Merit Award to S. A. L.), from the Biomedical Research Institute of New Mexico (to S. A. L.) and from UNM IDIP T32 institutional training grant NIH 5 T32 AI007538-13 (to S. M. B.).
Transparency declarations
None to declare.
Author contributions
Conceived and designed the experiments: S. A. L., S. M. B. and H. S. R. Performed the experiments: H. S. R. Analysed the data: H. S. R. and S. M. B. Contributed materials/analytical tools: A. B. H. and S. A. L. Wrote the paper: H. S. R. and S. A. L.
Acknowledgements
We thank William Fonzi (Georgetown University, Washington DC, USA) for providing strain SC5314 and L. Massie (University of New Mexico and NM VA Healthcare System, Albuquerque, NM, USA) for providing the C. albicans urinary isolates used in this study.
References
- 1.Achkar JM, Fries BC. Candida infections of the genitourinary tract. Clin Microbiol Rev. 2010;23:253–73. doi: 10.1128/CMR.00076-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kauffman CA. Candiduria. Clin Infect Dis. 2005;41(Suppl 6):S371–6. doi: 10.1086/430918. [DOI] [PubMed] [Google Scholar]
- 3.Bougnoux M-E, Kac G, Aegerter P, et al. Candidemia and candiduria in critically ill patients admitted to intensive care units in France: incidence, molecular diversity, management and outcome. Intensive Care Med. 2008;34:292–9. doi: 10.1007/s00134-007-0865-y. [DOI] [PubMed] [Google Scholar]
- 4.Morgan J, Meltzer MI, Plikaytis BD, et al. Excess mortality, hospital stay, and cost due to candidemia: a case–control study using data from population-based candidemia surveillance. Infect Control Hosp Epidemiol. 2005;26:540–7. doi: 10.1086/502581. [DOI] [PubMed] [Google Scholar]
- 5.Cowen LE, Steinbach WJ. Stress, drugs, and evolution: the role of cellular signaling in fungal drug resistance. Eukaryotic Cell. 2008;7:747–64. doi: 10.1128/EC.00041-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jain N, Kohli R, Cook E, et al. Biofilm formation by and antifungal susceptibility of Candida isolates from urine. Appl Environ Microbiol. 2007;73:1697–703. doi: 10.1128/AEM.02439-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lupetti A, Danesi R, Campa M, et al. Molecular basis of resistance to azole antifungals. Trends Mol Med. 2002;8:76–81. doi: 10.1016/s1471-4914(02)02280-3. [DOI] [PubMed] [Google Scholar]
- 8.Douglas LJ. Candida biofilms and their role in infection. Trends Microbiol. 2003;11:30–6. doi: 10.1016/s0966-842x(02)00002-1. [DOI] [PubMed] [Google Scholar]
- 9.Malani AN, Kauffman CA. Candida urinary tract infections: treatment options. Expert Rev Anti Infect Ther. 2007;5:277–84. doi: 10.1586/14787210.5.2.277. [DOI] [PubMed] [Google Scholar]
- 10.Klepser M. The value of amphotericin B in the treatment of invasive fungal infections. J Crit Care. 2011;26:225.e1–10. doi: 10.1016/j.jcrc.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 11.Butts A, Krysan DJ. Antifungal drug discovery: something old and something new. PLoS Pathog. 2012;8:e1002870. doi: 10.1371/journal.ppat.1002870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramage G, Saville SP, Thomas DP, et al. Candida biofilms: an update. Eukaryotic Cell. 2005;4:633–8. doi: 10.1128/EC.4.4.633-638.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang X, Fries BC. A murine model for catheter-associated candiduria. J Med Microbiol. 2011;60:1523–9. doi: 10.1099/jmm.0.026294-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maki DG, Tambyah PA. Engineering out the risk for infection with urinary catheters. Emerg Infect Dis. 2001;7:342–7. doi: 10.3201/eid0702.010240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang C-H, Fang C-C, Chen N-C, et al. Cranberry-containing products for prevention of urinary tract infections in susceptible populations: a systematic review and meta-analysis of randomized controlled trials. Arch Intern Med. 2012;172:988–96. doi: 10.1001/archinternmed.2012.3004. [DOI] [PubMed] [Google Scholar]
- 16.Tempesta M, Barrett M. Cranberry products prevent urinary tract infections in women: clinical evidence. In: Cooper R, Kronenberg F, editors. Botanical Medicine: From Bench to Beside. New Rochelle, NY: Mary Ann Liebert; 2009. pp. 203–11. [Google Scholar]
- 17.Raz R, Chazan B, Dan M. Cranberry juice and urinary tract infection. Clin Infect Dis. 2004;38:1413–9. doi: 10.1086/386328. [DOI] [PubMed] [Google Scholar]
- 18.Howell AB, Reed JD, Krueger CG, et al. A-type cranberry proanthocyanidins and uropathogenic bacterial anti-adhesion activity. Phytochemistry. 2005;66:2281–91. doi: 10.1016/j.phytochem.2005.05.022. [DOI] [PubMed] [Google Scholar]
- 19.Gupta K, Chou MY, Howell AB, et al. Cranberry products inhibit adherence of P-fimbriated Escherichia coli to primary cultured bladder and vaginal epithelial cells. J Urol. 2007;177:2357–60. doi: 10.1016/j.juro.2007.01.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eydelnant IA, Tufenkji N. Cranberry derived proanthocyanidins reduce bacterial adhesion to selected biomaterials. Langmuir. 2008;24:10273–81. doi: 10.1021/la801525d. [DOI] [PubMed] [Google Scholar]
- 21.Howell AB, Botto H, Combescure C, et al. Dosage effect on uropathogenic Escherichia coli anti-adhesion activity in urine following consumption of cranberry powder standardized for proanthocyanidin content: a multicentric randomized double blind study. BMC Infect Dis. 2010;10:94. doi: 10.1186/1471-2334-10-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hidalgo G, Chan M, Tufenkji N. Inhibition of Escherichia coli CFT073 fliC expression and motility by cranberry materials. Appl Environ Microbiol. 2011;77:6852–7. doi: 10.1128/AEM.05561-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feldman M, Tanabe S, Howell AB, et al. Cranberry proanthocyanidins inhibit the adherence properties of Candida albicans and cytokine secretion by oral epithelial cells. BMC Complement Altern Med. 2012;12:6. doi: 10.1186/1472-6882-12-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Foo LY, Lu Y, Howell AB, et al. A-type proanthocyanidin trimers from cranberry that inhibit adherence of uropathogenic P-fimbriated Escherichia coli. J Nat Prod. 2000;63:1225–8. doi: 10.1021/np000128u. [DOI] [PubMed] [Google Scholar]
- 25.Foo LY, Lu Y, Howell AB, et al. The structure of cranberry proanthocyanidins which inhibit adherence of uropathogenic P-fimbriated Escherichia coli in vitro. Phytochemistry. 2000;54:173–81. doi: 10.1016/s0031-9422(99)00573-7. [DOI] [PubMed] [Google Scholar]
- 26.Fonzi WA, Irwin MY. Isogenic strain construction and gene mapping in Candida albicans. Genetics. 1993;134:717–28. doi: 10.1093/genetics/134.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.LaFleur MD, Kumamoto CA, Lewis K. Candida albicans biofilms produce antifungal-tolerant persister cells. Antimicrob Agents Chemother. 2006;50:3839–46. doi: 10.1128/AAC.00684-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ramage G, Mowat E, Jones B, et al. Our current understanding of fungal biofilms. Crit Rev Microbiol. 2009;35:340–55. doi: 10.3109/10408410903241436. [DOI] [PubMed] [Google Scholar]
- 29.Ramage G, López-Ribot JL. Techniques for antifungal susceptibility testing of Candida albicans biofilms. Methods Mol Med. 2005;118:71–9. doi: 10.1385/1-59259-943-5:071. [DOI] [PubMed] [Google Scholar]
- 30.Pillai SK, Moellering RC, Eliopoulous GM. Antimicrobial combinations. In: Lorian V, editor. Antibiotics in Laboratory Medicine. 5th edn. Baltimore, MD: Williams & Wilkins; 2005. pp. 365–440. [Google Scholar]
- 31.Clinical and Laboratory Standards Institute. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts: Third Informational Supplement M27-S3. Wayne, PA, USA: CLSI; 2008. [Google Scholar]
- 32.Imbert C, Rodier MH, Daniault G, et al. Influence of sub-inhibitory concentrations of conventional antifungals on metabolism of Candida albicans and on its adherence to polystyrene and extracellular matrix proteins. Med Mycol. 2002;40:123–9. doi: 10.1080/mmy.40.2.123.129. [DOI] [PubMed] [Google Scholar]
- 33.Chamilos G, Lewis RE, Albert N, et al. Paradoxical effect of echinocandins across Candida species in vitro: evidence for echinocandin-specific and Candida species-related differences. Antimicrob Agents Chemother. 2007;51:2257–9. doi: 10.1128/AAC.00095-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Melo AS, Colombo AL, Arthington-Skaggs BA. Paradoxical growth effect of caspofungin observed on biofilms and planktonic cells of five different Candida species. Antimicrob Agents Chemother. 2007;51:3081–8. doi: 10.1128/AAC.00676-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ku TSN, Bernardo SM, Lee SA. In vitro assessment of the antifungal and paradoxical activity of different echinocandins against Candida tropicalis biofilms. J Med Microbiol. 2011;60:1708–10. doi: 10.1099/jmm.0.032235-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin B, Johnson BJ, Rubin RA, et al. Iron chelation by cranberry juice and its impact on Escherichia coli growth. Biofactors. 2010;37:121–30. doi: 10.1002/biof.110. [DOI] [PubMed] [Google Scholar]
- 37.Pérez-López FR, Haya J, Chedraui P. Vaccinium macrocarpon: an interesting option for women with recurrent urinary tract infections and other health benefits. J Obstet Gynaecol Res. 2009;35:630–9. doi: 10.1111/j.1447-0756.2009.01026.x. [DOI] [PubMed] [Google Scholar]
- 38.Afshar K, Stothers L, Scott H, et al. Cranberry juice for the prevention of pediatric urinary tract infection: a randomized controlled trial. J Urol. 2012;188:1584–7. doi: 10.1016/j.juro.2012.02.031. [DOI] [PubMed] [Google Scholar]
- 39.Barbosa-Cesnik C, Brown MB, Buxton M, et al. Cranberry juice fails to prevent recurrent urinary tract infection: results from a randomized placebo-controlled trial. Clin Infect Dis. 2011;52:23–30. doi: 10.1093/cid/ciq073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Campbell G, Pickles T, D'yachkova Y. A randomised trial of cranberry versus apple juice in the management of urinary symptoms during external beam radiation therapy for prostate cancer. Clin Oncol (R Coll Radiol) 2003;15:322–8. doi: 10.1016/s0936-6555(03)00161-4. [DOI] [PubMed] [Google Scholar]
- 41.Cowan CC, Hutchison C, Cole T, et al. A randomised double-blind placebo-controlled trial to determine the effect of cranberry juice on decreasing the incidence of urinary symptoms and urinary tract infections in patients undergoing radiotherapy for cancer of the bladder or cervix. Clin Oncol (R Coll Radiol) 2012;24:e31–8. doi: 10.1016/j.clon.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 42.Ferrara P, Romaniello L, Vitelli O, et al. Cranberry juice for the prevention of recurrent urinary tract infections: a randomized controlled trial in children. Scand J Urol Nephrol. 2009;43:369–72. doi: 10.3109/00365590902936698. [DOI] [PubMed] [Google Scholar]
- 43.Kontiokari T, Sundqvist K, Nuutinen M, et al. Randomised trial of cranberry-lingonberry juice and Lactobacillus GG drink for the prevention of urinary tract infections in women. BMJ. 2001;322:1571. doi: 10.1136/bmj.322.7302.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kontiokari T, Salo J, Eerola E, et al. Cranberry juice and bacterial colonization in children—a placebo-controlled randomized trial. Clin Nutr. 2005;24:1065–72. doi: 10.1016/j.clnu.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 45.McMurdo MET, Bissett LY, Price RJG, et al. Does ingestion of cranberry juice reduce symptomatic urinary tract infections in older people in hospital? A double-blind, placebo-controlled trial. Age Ageing. 2005;34:256–61. doi: 10.1093/ageing/afi101. [DOI] [PubMed] [Google Scholar]
- 46.Stapleton AE, Dziura J, Hooton TM, et al. Recurrent urinary tract infection and urinary Escherichia coli in women ingesting cranberry juice daily: a randomized controlled trial. Mayo Clin Proc. 2012;87:143–50. doi: 10.1016/j.mayocp.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Takahashi S, Hamasuna R, Yasuda M, et al. A randomized clinical trial to evaluate the preventive effect of cranberry juice (UR65) for patients with recurrent urinary tract infection. J Infect Chemother. 2013;19:112–7. doi: 10.1007/s10156-012-0467-7. [DOI] [PubMed] [Google Scholar]
- 48.Zhang L, Ma J, Pan K, et al. Efficacy of cranberry juice on Helicobacter pylori infection: a double-blind, randomized placebo-controlled trial. Helicobacter. 2005;10:139–45. doi: 10.1111/j.1523-5378.2005.00301.x. [DOI] [PubMed] [Google Scholar]
- 49.Patel KD, Scarano FJ, Kondo M, et al. Proanthocyanidin-rich extracts from cranberry fruit (Vaccinium macrocarpon Ait.) selectively inhibit the growth of human pathogenic fungi Candida spp. and Cryptococcus neoformans. J Agric Food Chem. 2011;59:12864–73. doi: 10.1021/jf2035466. [DOI] [PubMed] [Google Scholar]
- 50.Lee YL, Najm WI, Owens J, et al. Anti-microbial activity of urine after ingestion of cranberry: a pilot study. Evid Based Complement Alternat Med. 2010;7:227–32. doi: 10.1093/ecam/nem183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nohynek LJ, Alakomi H-L, Kähkönen MP, et al. Berry phenolics: antimicrobial properties and mechanisms of action against severe human pathogens. Nutr Cancer. 2006;54:18–32. doi: 10.1207/s15327914nc5401_4. [DOI] [PubMed] [Google Scholar]
- 52.Cavanagh HMA, Hipwell M, Wilkinson JM. Antibacterial activity of berry fruits used for culinary purposes. J Med Food. 2003;6:57–61. doi: 10.1089/109662003765184750. [DOI] [PubMed] [Google Scholar]
- 53.Swartz JH, Medrek TF. Antifungal properties of cranberry juice. Appl Microbiol. 1968;16:1524–7. doi: 10.1128/am.16.10.1524-1527.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee YL, Owens J, Thrupp L, et al. The antifungal activity of urine after ingestion of cranberry products. J Altern Complement Med. 2009;15:957–8. doi: 10.1089/acm.2009.0148. [DOI] [PubMed] [Google Scholar]
- 55.Howell AB, Vorsa N, Der Marderosian A, et al. Inhibition of the adherence of P-fimbriated Escherichia coli to uroepithelial-cell surfaces by proanthocyanidin extracts from cranberries. N Engl J Med. 1998;339:1085–6. doi: 10.1056/NEJM199810083391516. [DOI] [PubMed] [Google Scholar]
- 56.Huang Y, Nikolic D, Pendland S, Doyle BJ, et al. Effects of cranberry extracts and ursolic acid derivatives on P-fimbriated Escherichia coli, COX-2 activity, pro-inflammatory cytokine release and the NF-κβ transcriptional response in vitro. Pharm Biol. 2009;47:18–25. doi: 10.1080/13880200802397996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.La VD, Howell AB, Grenier D. Cranberry proanthocyanidins inhibit MMP production and activity. J Dent Res. 2009;88:627–32. doi: 10.1177/0022034509339487. [DOI] [PubMed] [Google Scholar]
- 58.La VD, Howell AB, Grenier D. Anti-Porphyromonas gingivalis and anti-inflammatory activities of A-type cranberry proanthocyanidins. Antimicrob Agents Chemother. 2010;54:1778–84. doi: 10.1128/AAC.01432-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Feldman M, Grenier D. Cranberry proanthocyanidins act in synergy with licochalcone A to reduce Porphyromonas gingivalis growth and virulence properties, and to suppress cytokine secretion by macrophages. J Appl Microbiol. 2012;113:438–47. doi: 10.1111/j.1365-2672.2012.05329.x. [DOI] [PubMed] [Google Scholar]


