Background: CD44 mediates vascular barrier integrity via regulation of VE-cadherin and CD31 expression.
Results: CD44 regulates cell proliferation and specific caspase-mediated apoptosis via modulation of CD31 and VE-cadherin expression through the Hippo pathway.
Conclusion: CD44 modulates proliferation and apoptosis of microvascular endothelial cells.
Significance: CD44 regulates endothelial cell survival by modulating specific cell adhesion molecules via the Hippo pathway.
Keywords: Apoptosis, Caspase, Cd44, Cell Proliferation, Endothelial Cell, Survivin, Vascular Biology, Western Blotting, Cd31
Abstract
CD44 has been implicated in a diverse array of cell behaviors and in a diverse range of signaling pathway activations under physiological and pathophysiological conditions. We have documented a role for CD44 in mediating vascular barrier integrity via regulation of PECAM-1 (CD31) expression. We now report our findings on the roles of CD44 in modulating proliferation and apoptosis of microvascular endothelial cells via its modulation of CD31 and VE-cadherin expression and the Hippo pathway. In this report, we demonstrate persistent increased proliferation and reduced activations of both effector and initiator caspases in high cell density, postconfluent CD44 knock-out (CD44KO), and CD31KO cultures. We found that reconstitution with murine CD44 or CD31 restored the proliferative and caspase activation rates to WT levels. Moreover, we have confirmed that the CD31 ecto-domain plays a key role in specific caspase cascades as well as cell adhesion-mediated cell growth and found that CD31 deficiency results in a reduction in VE-cadherin expression. Last, we have shown that both CD44KO and CD31KO endothelial cells exhibit a reduced VE-cadherin expression correlating with increased survivin expression and YAP nuclear localization, consistent with inactivation of the Hippo pathway, resulting in increased proliferation and decreased apoptosis. These findings support the concept that CD44 mediates several of its effects on endothelia through modulation of adhesion protein expression, which, in addition to its known modulation of junctional integrity, matrix metalloproteinase levels and activation, interactions with cortical membrane proteins, and selected signaling pathways, plays a key role as a critical regulator of vascular function.
Introduction
CD44 is a widely expressed alternatively spliced, post-translationally modified transmembrane glycoprotein that has been implicated in a diverse array of cell behaviors, including cell-cell and cell matrix interactions, migration, differentiation, and survival (1–5). CD44 has been shown to interact with a variety of extracellular and intracellular ligands, including hyaluronan as well as translocation to the nucleus, resulting in a diverse range of signaling pathway activations under physiological and pathophysiological conditions (6–16). Its known roles in the modulation of vascular endothelial cells include maintaining a functional vascular barrier (17, 18) and modulating angiogenesis (19).
Recently, we (20) and others (21) have documented roles for CD44 in the regulation of Th17 and Treg lymphocytes in experimental autoimmune encephalomyelitis, and we have noted that CD44 plays a significant role in the maintenance of central nervous system (CNS) microvascular barrier integrity (22). We have documented a role for CD44 in mediating its modulation of vascular barrier integrity via regulation of PECAM-1 (platelet endothelial cell adhesion molecule) (CD31) expression, specifically by CD31 modulation of endothelial cell junctional integrity (22).
In light of our recent findings, we now report our findings on the roles of CD44 in modulating both proliferation and apoptosis of microvascular endothelial cells via its modulation of CD31 and VE-cadherin expression via Hippo pathway activation. In this report, we demonstrate persistent increased proliferation and reduced activations of both effector and initiator caspases in high cell density, post-confluent CD44 knock-out (CD44KO),2 and CD31KO cultures and that reconstitution with murine CD44 or CD31 restored the proliferative and caspase activation rates to WT levels under high cell density conditions. In addition, we have confirmed that the CD31 ecto-domain plays a key role in specific caspase cascades (23) as well as cell adhesion-mediated cell growth and found that CD31 deficiency results in a reduction in VE-cadherin expression. We also show that both CD44KO and CD31KO endothelial cells exhibit a reduced VE-cadherin expression correlating with increased survivin expression and increased YAP nuclear localization, consistent with inactivation of the Hippo pathway, resulting in increased PCNA expression and caspase inhibition. These findings support the concept that CD44 mediates several of its effects in endothelia through modulation of cell junctional molecule expression (e.g. CD31 and VE-cadherin), which, in addition to its known modulation of junctional integrity, matrix metalloproteinase levels and activation, interactions with cortical membrane proteins, and selected signaling pathways, modulates proliferation and apoptosis (22–29), further defining the integrated roles of CD44, VE-cadherin, and CD31 as critical regulators of vascular function.
EXPERIMENTAL PROCEDURES
Endothelial Cell Culture
Brain endothelial cells (BEC) were isolated from cerebral microvessels of C57BL/6 wild type (WT-BEC) and CD44-knock-out (CD44KO-BEC) 6-week-old mice (B6.129(Cg)-Cd44tmlHbg/J) (Jackson Laboratory, Bar Harbor, ME) back-crossed to a C57BL/6 background as described previously (22). CD31-knock-out microvascular endothelial cells were isolated from brain (CD31KO-BEC) and lung (CD31KO-LEC) microvasculature from 6-week-old CD31KO mice as described elsewhere (30). For rescue experiments, we used murine CD44- or CD31-reconstituted endothelial cells (CD44KO-BEC-mCD44, CD44KO-BEC-mCD31, and CD31KO-LEC-mCD31) as well as empty lentiviral vector pLVX-IRES-Puro (Clontech) constructs (WT-BEC-VO and CD44KO-BEC-VO) (22, 30). Endothelial cells were cultured on 1.5% gelatin-coated plates in endothelial cell medium (Dulbecco's modified Eagle's medium (DMEM) with high glucose (Invitrogen) containing 10% FBS, 2 mm l-glutamine, 0.1 mm nonessential amino acids, 1 mm sodium pyruvate, 10 mm HEPES (pH 7.4), 10−5 m β-mercaptoethanol, 100 units/ml penicillin, and 100 μg/ml streptomycin) in 8% CO2 at 37 °C. Cells were used between passages 22 and 24. Endothelial cells were cultured under normoxic (20% O2) conditions at high cell density (over 90% confluence).
Cell Proliferation Analysis
To study the role of CD44 and CD31 in cell proliferation, we demonstrated growth curves of WT-BEC, CD44KO-BEC, WT-BEC-VO, CD44KO-BEC-VO, CD44KO-BEC-mCD44, CD44KO-BEC-mCD31, CD31KO-BEC, CD31KO-LEC, and CD31KO-LEC-mCD31. Each cell type was plated at 3000 cells/well in 96-well plates (n = 4 each). At 24, 48, 72, 96, 120, 144, and 168 h after plating, the wells were washed with PBS (pH 7.4). After freezing-thawing, wells were treated with 200 μl of the dye/cell lysis buffer using the CyQUANT cell proliferation assay kit (Invitrogen). After incubation at room temperature for 5 min, the sample fluorescence was measured using the Wallac 1420 fluorescence microplate reader (PerkinElmer Life Sciences) with filters for 485 nm. For proliferation rate analysis, initial and secondary proliferation rates were determined as follows: initial proliferation rates for WT-BEC, CD44KO-BEC-mCD44, CD44KO-BEC-mCD31, and CD31KO-LEC-mCD31 = (average cell number at 72 h − 48 h)/24; initial proliferation rates for CD44KO-BEC, CD31KO-BEC, and CD31KO-LEC = (average cell number at 120 h − 72 h)/48; secondary proliferation rates for all endothelial cells = (average cell number at 168 h − 120 h)/48. Cells were used at passage 23.
Antibodies
Antibodies against mouse CD31 ecto-domain (affinity-purified SL-4) were raised in rabbits and purified as described elsewhere (31). Rabbit polyclonal antibodies against mouse caspase-3 (catalog no. 9662), cleaved caspase-3 (Asp-175) (catalog no. 9661), caspase-6 (catalog no. 9762), caspase-7 (catalog no. 9492), caspase-9 (catalog no. 9504), caspase-10 (catalog no. 9752), and caspase-12 (catalog no. 2202); rabbit monoclonal antibodies against cleaved caspase-8 (Asp-387) (D5B2; catalog no. 8592), Apaf-1 (D5C3; catalog no. 8969), cytochrome c (D18C7; catalog no. 11940), survivin (71G4B7; catalog no. 2808), YAP (D24E4) (catalog no. 8418), and phospho-YAP (Ser-127) (D9W2I; catalog no. 13008); and a mouse monoclonal antibody against proliferating cell nuclear antigen (PCNA) (PC10; catalog no. 2586) were purchased from Cell Signaling Technology, Inc. (Danvers, MA). Rat monoclonal antibody against VE-cadherin (11D4.1; catalog no. 550548) was purchased from BD Biosciences (San Jose, CA). A mouse monoclonal antibody against mouse β-actin (AC-15; ab6276) and rabbit polyclonal antibodies against Ki-67 (ab15580) and VE-cadherin (ab33168) were purchased from Abcam (Cambridge, MA). Rabbit polyclonal antibodies against Histone H1 (FL-219) and secondary antibodies raised in donkeys against rabbit IgG (sc-2313) and mouse IgG (sc-2318), which were conjugated to horseradish peroxidase (HRP), were purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX). Every caspase antibody was confirmed to detect full-length caspase as well as the cleaved (active) form.
Preparation of Cell Lysates
Cells were rinsed twice with cold PBS (pH 7.4) containing 1 mm sodium orthovanadate (Na3VO4) and then lysed with radioimmune precipitation assay buffer (EMD Millipore, Billerica, MA) supplemented with 1% SDS, protease inhibitor (Roche Applied Science), and phosphatase inhibitor mixtures (EMD Millipore), and 1 mm PMSF. Cell lysate samples were placed on ice for 30 min, vortexed thoroughly, and then centrifuged at 14,000 rpm for 15 min to remove insoluble materials. After the total protein was determined using the PierceTM BCA protein assay kit (Thermo Fisher Scientific Inc.), an aliquot of 12 μg of protein samples was resuspended in SDS-sample buffer and boiled for 5 min.
Nuclear and Cytoplasmic Fractionation
Confluent 60-mm dishes of WT-BEC, CD44KO-BEC, and CD31KO-BEC cells were harvested with 0.05% trypsin-EDTA and centrifuged. Cells were washed twice with cold PBS and pelleted by centrifugation. Nuclear and cytoplasmic fractionation was performed using NE-PER nuclear and cytoplasmic extraction reagents (catalog no. 78833) (Thermo Scientific Inc.) according to the instructions. Protease and phosphatase inhibitors as mentioned above were added to cytoplasmic extraction reagent I and nuclear extraction reagent. Protein concentrations of nuclear and cytoplasmic fractions were determined by the BCA protein assay kit, and an aliquot of 10 μg of protein was resuspended in SDS-sample buffer and boiled for 5 min.
Western Blot
Samples suspended in sample buffer were subjected to 10–15% SDS-PAGE under reducing conditions, and the gels were transferred to polyvinylidene difluoride (PVDF) membranes (EMD Millipore). The membranes were incubated with 1% bovine serum albumin (BSA) in 50 mm Tris-buffered saline (pH 7.4) containing 0.1% Tween 20 (TTBS) for 1 h at room temperature to block nonspecific protein binding followed by overnight incubation of the membranes in TTBS containing primary antibody diluted at 1:200 (anti-histone H1), 1:1000 (anti-caspase-3, -6, -7, -8, -9, -10, and -12, Apaf-1, cytochrome c, VE-cadherin (ab33168), survivin, YAP, and phospho-YAP), 1:2000 (anti-PCNA), 1:2500 (anti-CD31), and 1:5000 (anti-β-actin). After washing with TTBS, the membranes were reacted with HRP-conjugated secondary antibodies diluted at 1:10,000 in TTBS for 1 h at room temperature. Target protein bands were detected using Western Lightning enhanced chemiluminescence substrate (PerkinElmer Life Sciences) according to the manufacturer's instructions. Quantitation was performed on scanned densitometric images using Quantity One software (Bio-Rad) in triplicate experiments.
Neutralizing CD31
To study a role of the CD31 ecto-domain in endothelial cell morphology and caspase signaling, WT-BEC cells were plated at 1.0 × 105 cells into 6-well plates and incubated for 72 h at 37 °C. Cells were treated with 1 or 5 μg/ml anti-CD31 antibodies (affinity-purified SL-4), which recognized the ecto-domain of CD31, and phase-contrast microscopic photos were taken in every 24 h (24, 48, and 72 h) after plating using an Olympus inverted research microscope IX-70 (Center Valley, PA) equipped with a MicroFireTM camera and PictureFrameTM version 1.0 software for Macintosh (OPTRONICS, Goleta, CA) and Photoshop CS6 software (Adobe, San Jose, CA) on a Macintosh computer. After incubation for 72 h, cells were lysed, and active caspase-3 and -8 expression was determined by Western blot as mentioned above. For control studies, equivalent concentrations of IgG isolated from prebleed serum were treated instead of anti-CD31 antibodies.
Immunofluorescence
Confluent cultures of WT-BEC-VO, CD44KO-BEC-VO, C44KO-BEC-mCD44, CD44KO-BEC-mCD31, CD31KO-BEC, CD31KO-LEC, and CD31KO-LEC-mCD31 cells, grown on 6-well plates, were fixed with 4% paraformaldehyde in 50 mm HEPES buffer (pH 7.3) for 20 min, permeabilized for 20 min with 0.2% Triton X-100 in PBS (pH 7.4). To prevent nonspecific protein binding, cells were incubated with 5% BSA in PBS containing 0.05% Triton X-100 (T-PBS) for 1 h at room temperature. The cells were then incubated with the primary antibodies (VE-cadherin (11D4.1), diluted at 1:50 in T-PBS; CD31 (SL-4) and YAP (D24E4), 1:200; cleaved caspase-3, 1:300; cleaved caspase-8 (D5B2) and survivin (71G4B7), 1:400; Ki-67, 1:500; and phospho-YAP (D9W2I), 1:2000) overnight at 4 °C and further with secondary antibodies (Alexa Fluor 488- or 594-conjugated goat anti-rabbit IgG (H+L) or anti-rat IgG (H+L) (Invitrogen) (diluted at 1:100) and Alexa Fluor 594 phalloidin (A12381) (Invitrogen) (diluted at 1:40)) in T-PBS for 1 h at room temperature. Finally, cells were counterstained with DAPI, diluted at 1:5000 in T-PBS for 10 min at room temperature. Digital fluorescence images were captured on an Olympus IX71 inverted microscope equipped with a MicroFireTM camera and PictureFrameTM version 1.0 software for Macintosh (OPTRONICS) and Photoshop CS6 software (Adobe) on a Macintosh computer.
Statistical Analysis
All data are means ± S.D. for a series of experiments. Statistical analysis was performed by unpaired Student's t test using GraphPad Prism version 6 for Windows (GraphPad Software Inc., La Jolla, CA). p values less than 0.05 were considered significant. Each significant p value is mentioned in the figure legends.
RESULTS
In light of the known roles of CD44 in a wide variety of cellular functions and behaviors (1–3, 6–8, 11, 12, 17, 32) and our documentation of its role as a modulator of CD31 and VE-cadherin expression (22) (Fig. 4, second panel), we embarked on a series of studies to elucidate the role of its effects on proliferation and apoptosis via its effects on CD31 and VE-cadherin expression.
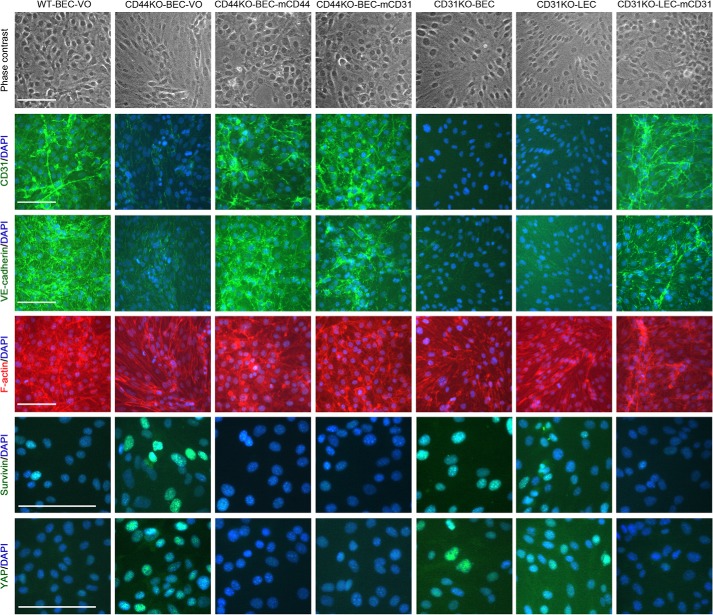
FIGURE 4.
Immunofluorescence evidence of CD44 and CD31 playing key roles in endothelial cell proliferation by modulating Hippo pathway activation. Shown are phase (top row) and merged DAPI and CD31, DAPI and VE-cadherin, DAPI and F-actin, DAPI and survivin, and DAPI and YAP immunofluorescence micrographs in descending order of WT-BEC-VO, CD44KO-BEC-VO, CD44KO-BEC-mCD44, CD44KO-BEC-mCD31, CD31KO-BEC, CD31KO-LEC, and CD31KO-LEC-mCD31 from left to right. Deficiency of CD44 and CD31 imparts a more variable, spindle shape to the cells as well as altered F-actin labeling profiles that were abrogated by reconstitution with mCD44 or mCD31. Additionally, loss of VE-cadherin and CD31 labeling and increases in survivin and YAP labeling were found in the CD44KO and CD31KO cells that were abrogated by reconstitution with mCD44 or mCD31. Scale bar, 100 μm.
CD44 Plays a Key Role in Regulating Endothelial Cell Proliferation via Modulation of CD31 and VE-cadherin
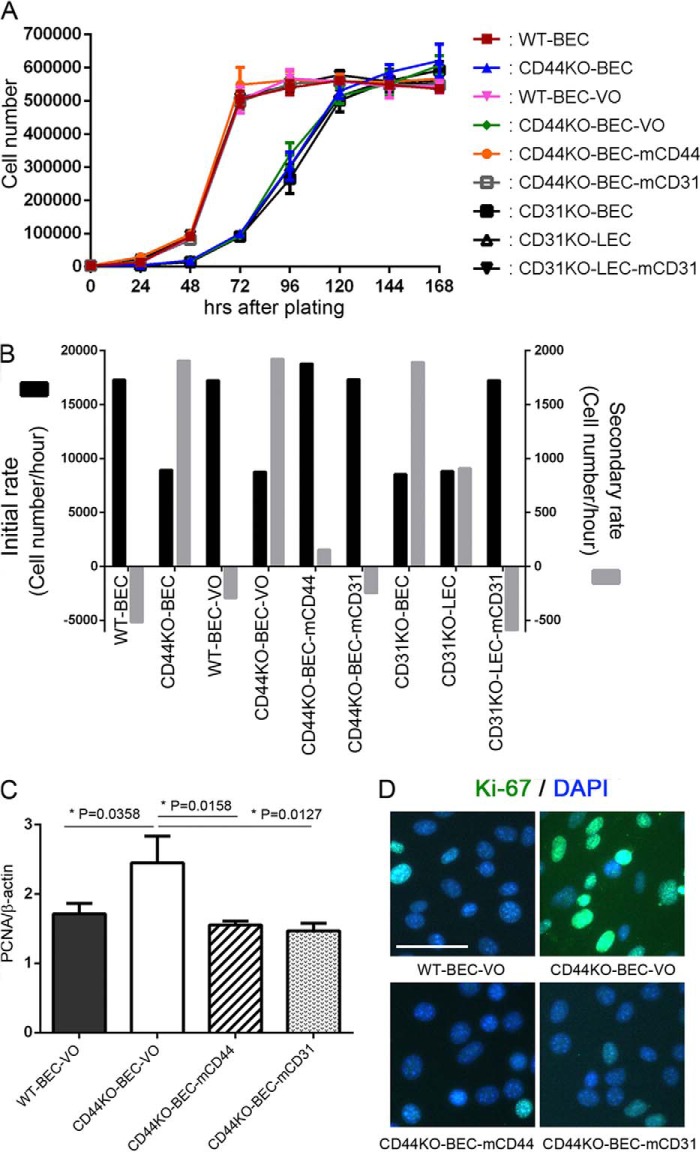
Previous studies by our laboratory (27, 29) and others (33) have demonstrated that CD31 affects proliferation in a variety of cells. Here we show that the absence of CD44 significantly decreased the initial proliferation rate (measured from 48 to 72 h in WT and mCD44- and mCD31-reconstituted cells and from 72 to 120 h in CD44KO cells) of brain microvascular endothelial cells (Fig. 1, A and B). In contrast, the absence of CD44 increased the secondary proliferation rate (measured from 120 to 168 h for all cell samples) (Fig. 1, A and B). Further, reconstitution of the CD44KO with full-length recombinant mCD44 rescued the proliferative phenotype back to the WT phenotype (Fig. 1, A and B). We recently observed that CD44KO also exhibited a reduction in CD31 protein and mRNA compared with WT and WT-VO cells, whose levels were restored upon reconstitution with mCD44 and mCD31 (22), and here we confirm this observation (Fig. 4, second panel). Examination of proliferation rates in CD44KO reconstituted with mCD31 also restored the initial and secondary proliferation rates to WT levels (Fig. 1, A and B). Fig. 1C illustrates similar confirmatory findings using PCNA analyses showing lower proliferative rates in WT-BEC-VO, CD44KO-BEC-mCD44, and CD44KO-BEC-mCD31 compared with the CD44KO-BEC-VO cultures. Additionally, Ki-67 labeling of WT-BEC-VO, CD44KO-BEC-VO, CD44KO-BEC-mCD44, and CD44KO-BEC-mCD31 cultures yielded similar results, with the CD44KO-BEC-VO cells exhibiting robust nuclear labeling compared with WT and CD44- and CD31-reconstituted cultures (Fig. 1D). Significantly high expression of Ki-67 in CD44KO-BEC-VO cells compared with WT-BEC-VO, CD44KO-BEC-mCD44, and CD44KO-BEC-mCD31 cells was confirmed by Western blotting (data not shown).
FIGURE 1.
CD44 and CD31 play a key role in endothelial cell proliferation. A, growth curves of WT (red squares), CD44KO-BEC (blue triangles), WT-BEC-VO (pink triangles), CD44KO-BEC-VO (green diamonds), CD44KO-BEC-mCD44 (orange circles), CD44KO-BEC-mCD31 (black open squares), CD31KO-BEC (black closed squares), CD31KO-LEC (black open triangles), and CD31KO-LEC-mCD31 (black closed triangles) cells for 168 h. All data are means ± S.D. from triplicate experiments. B, initial (black columns) and secondary (gray columns) proliferation rate analyses of CD44KO-BEC, CD44KO-BEC-VO, CD31KO-BEC, and CD31KO-LEC cells exhibited a lower initial rate (72–120 h) and higher secondary rate (120–168 h) as compared with the other cells. All data are means ± S.D. from triplicate experiments. C, PCNA analysis of WT-BEC-VO, CD44KO-BEC-VO, CD44KO-BEC-mCD44, and CD44KO-BEC-mCD31 cells illustrating the increased expression of PCNA in CD44KO-BEC-VO cells compared with the WT and reconstituted cells consistent with the increased secondary proliferation rate exhibited by the KO cells. Data represent the mean PCNA versus β-actin ratios of n = 3 for each cell type and are expressed as means ± S.D. (error bars). *, p < 0.05; **, p < 0.01. D, representative merged DAPI and Ki-67 immunofluorescence images of WT-BEC-VO, CD44KO-BEC-VO, CD44KO-BEC-mCD44, and CD44KO-BEC-mCD31 cells, illustrating robust Ki-67 labeling in CD44KO-BEC-VO (top right panel) compared with the diminished labeling in WT-BEC-VO and both mCD44- and mCD31-reconstituted cultures (top left and both bottom panels). Scale bar, 50 μm.
In order to better elucidate the effect of the absence of CD31 on endothelial cell proliferation rates, we employed lung microvascular endothelial cells devoid of CD31 (CD31KO-LEC) and reconstituted with mCD31 (CD31KO-LEC-mCD31). Fig. 1, A and B, illustrates the similar profile of WT and CD31KO-LEC-mCD31 cells, which exhibit a greater initial proliferation rate compared with CD31KO-BEC and CD31KO-LEC cells devoid of CD31 expression. We also observed the complete loss of CD31 expression in both CD31KO-BEC and CD31KO-LEC compared with WT-BEC and CD31KO-LEC-mCD31 cells by Western blotting (data not shown) as well as immunofluorescence (Fig. 4, second panel). The differences in initial and secondary proliferation rates of CD31KO-LEC and CD31-LEC-mCD31 cells are similar to the profiles observed for CD44KO-BEC and CD44KO-BEC-mCD44 (or CD44KO-BEC-mCD31) cells in Fig. 1, A and B.
Absence of CD44 Affects Selective Caspase Activation
Previous studies have indicated that CD44 plays a role in apoptosis (34–40). To investigate the effect of loss of CD44 on endothelial cells, we examined the levels and activation profiles of selected effector and initiator caspases.
Initiator Caspases
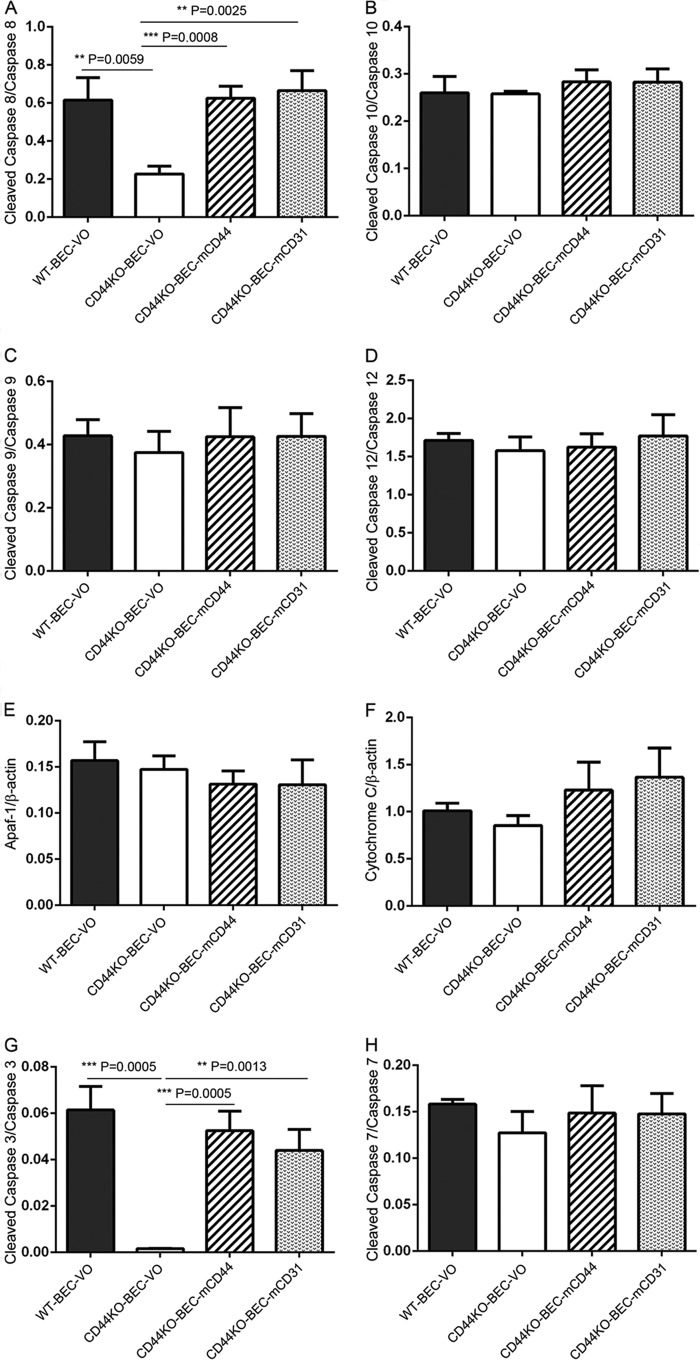
Although we observed that total caspase expression levels were similar in the different cell lysates studied (data not shown), we found that the initiator caspase-8 activation was decreased in CD44KO-BEC-VO cells compared with WT-BEC-VO cells, and its activities were rescued with recombinant mCD44 and mCD31 (Fig. 2A). In contrast, caspase-10 activation was not reduced in CD44KO-BEC-VO, and there was no effect of reconstitution of CD44KO cells with mCD44 or mCD31 (Fig. 2B).
FIGURE 2.
Initiator and effector caspases are down-regulated in CD44KO cells. A–H, Western blot analyses revealed decreased cleaved caspase-8 (A) and caspase-3 (G) activation in CD44KO-VO cells and rescue in the presence of mouse CD44 (CD44KO-mCD44) and CD31 (CD44KO-mCD31). No changes in activation or expression were observed in caspase-10, -9, -12, and -7 or in the expression levels of Apaf-1 or cytochrome c (B–F and H). All data represent the mean cleaved caspase versus full-length caspase ratios of n = 3 for each cell type and are expressed as means ± S.D. (error bars). *, p < 0.05; **, p < 0.01; ***, p < 0.001.
Caspases Associated with Endoplasmic Reticulum (ER) Stress
Caspase-9 and -12, known to be activated following ER stress (41), were also investigated. Activation of both caspase-9 and -12 was not affected by CD44 deficiency (Fig. 3, C and D). Full-length caspase-9 and -12 expression was not affected in the different cells studied (data not shown).
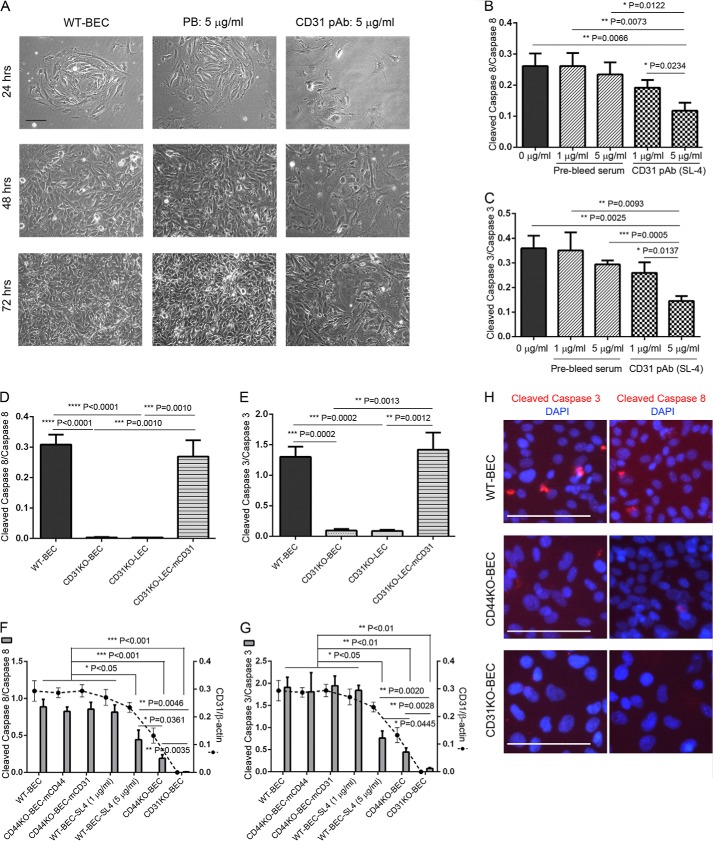
FIGURE 3.
CD31 extracellular domain plays an important role in endothelial cell morphology and proliferation as well as caspase-3 and -8 activation. A, WT brain endothelial cells were incubated without (left column) and with 5 μg/ml prebleed (PB) rabbit serum (middle column) and anti-CD31 ectodomain rabbit polyclonal antibody (SL-4) (right column) for 24 h (upper row), 48 h (second row from bottom), and 72 h (bottom row), resulting in distinct morphological changes following treatment with SL-4. All experiments were performed in triplicate. Scale bar, 100 μm. B and C, Western blot and densitometric analyses of cleaved (active) caspase-3 (B) and -8 (C) expression in WT cells that were treated without (0 μg/ml) or with 1 and 5 μg/ml prebleed rabbit serum or SL-4 antibody at 72 h. Attenuated activation of caspase-3 (B) and -8 (C) was observed in WT cells treated with 5 μg/ml SL-4 antibody. Data represent the mean cleaved caspase versus full-length caspase ratios of n = 3 for each treatment and are expressed as means ± S.D. *, p < 0.05; **, p < 0.01; ***, p < 0.001. D and E, Western blot and densitometric analyses revealed robust decreased expression of caspase-3 and -8 in CD31KO-BEC and CD31KO-LEC endothelial cells, which was completely rescued in the presence of mouse CD31 (CD31KO-LEC-mCD31). Data represent the mean cleaved caspase versus full-length caspase ratios of n = 3 for each treatment and are expressed as means ± S.D. *, p < 0.05; **, p < 0.01; ***, p < 0.001. F and G, moreover, activation of caspase-3 (F) and -8 (G) was correlated with CD31 expression levels in endothelial cells (WT, CD44KO-BEC, and CD31KO-BEC) and different levels of CD31 sequestration (WT-BEC-SL4 (1 μg/ml) and WT-BEC-SL4 (5 μg/ml)) as illustrated by Western blot and densitometric analyses of cleaved (active) caspase-3 (F) and -8 (G) expression. Data represent the mean cleaved caspase versus full-length caspase and CD31 versus β-actin ratios of n = 3 for each cell type and are expressed as means ± S.D. (error bars). *, p < 0.05; **, p < 0.01; ***, p < 0.001; ****, p < 0.0001. H, immunofluorescence micrographs of WT-BEC (top), CD44KO-BEC (middle), and CD31KO-BEC (bottom) labeled with DAPI and either anti-cleaved caspase-3 (left) or cleaved caspase-8 (right), illustrating decreased caspase activation in the KO cells. Scale bar, 100 μm.
Mitochondrially Associated Caspases
To determine whether mitochondrially mediated caspase activation may be responsible for caspase-9 activation in CD44KO cells, Apaf-1 and cytochrome c levels were assessed. Neither Apaf-1 nor cytochrome c levels were changed in CD44KO-BEC-VO when examined (Fig. 2, E and F).
Effector Caspases
As observed in Fig. 2, we found that CD44KO-BEC-VO exhibited decreased effector caspase-3 but not caspase-7 activation compared with WT-BEC-VO cells (Fig. 2, G and H). We also examined caspase-6, the other effector caspase; however, there was no active caspase-6 expression observed in WT-BEC-VO and CD44KO-BEC-VO cells as well as CD44KO-BEC-mCD44 and CD44KO-BEC-mCD31 cells (data not shown). Full-length caspase-3, -6, and -7 expression was not changed in the different cells studied (data not shown). Correspondingly, decreased initiator caspase-8 and effector caspase-3 and unaltered initiator caspase-10, -9, and -12 and effector caspase-7 and -6 were observed in CD44KO-BEC compared with WT-BEC (data not shown).
CD44-mediated Effects on Caspase Activation via Its Modulation of CD31 Expression
Having recently determined that CD44 presence or absence regulates CD31 expression levels in BEC and reconstitution of CD44KO with mCD31 rescues BEC junctional integrity and VE-cadherin and MMP-2 expression levels to WT levels (22), we hypothesized that the level of CD31 expression may also play a critical role in mediating the changes in caspase activation observed in CD44KO cells. To support this hypothesis, we utilized affinity-purified polyclonal antibodies (SL-4) directed at the ecto-domain of CD31 (31) to affect CD31 mediated cell-cell interactions and examined WT-BEC morphologically (Fig. 3A) and for activation of caspase-3 and -8 (Fig. 3, B and C). Incubation of WT-BEC with anti-CD31 antibodies resulted in decreased cell-cell contact at early time points (24 h) compared with incubation with equivalent concentrations of immunoglobulin (Ig) isolated from preimmunized sera (Fig. 3A, top). At later time points (48 and 72 h), the cultures containing anti-CD31 antibodies exhibited a looser morphology with fewer cell-cell contacts (Fig. 3A, middle and bottom). Determination of activation levels of caspase-3 and -8 at 72 h (Fig. 3A, bottom) revealed decreases in both caspases in cultures treated with anti-CD31 antibodies in a dose-dependent manner. In contrast, control cultures (0 μg/ml) and the cultures treated with preimmune Ig exhibited no decreases in these caspases (Fig. 3, B and C). Full-length caspase-3 and -8 expression was not affected in the absence or presence of anti-CD31 antibodies as well as preimmune Ig (data not shown). To further explore the importance of CD31 as a modulator of caspase activation, we utilized CD31KO-BEC and CD31KO-LEC derived from the pulmonary microvasculature and CD31KO-LEC-transfected and stably expressing full-length murine CD31 (CD31KO-LEC-mCD31) (data not shown). As illustrated in Fig. 3, D and E, the CD31KO-LEC exhibited decreased activation of caspase-3 and -8 as well as CD31KO brain endothelial cells. As observed in the CD44KO-BEC-VO cultures (Fig. 2, A and G), activation levels of caspase-3 and -8 returned to WT levels upon rescue with reconstitution with recombinant mCD31 (Fig. 3, D and E). Interestingly, the levels of caspase-3 and -8 activation correlated with the expression levels of CD31 (Fig. 3, F and G). Full-length caspase-3 and -8 expression was not affected in the absence or presence of CD31 (data not shown). Moreover, robust decreased cleaved caspase-3 (Fig. 3H, left column) and caspase-8 (Fig. 3H, right column) immunolocalization was observed in CD44KO-BEC (Fig. 3H, middle row) and CD31KO-BEC (Fig. 3H, third row) cells as compared with WT-BEC (Fig. 3H, top row) by immunofluorescence staining.
CD44-mediated Hippo Pathway Regulates Endothelial Cell Survival
The increased proliferation rate and decreased caspase activation observed in CD44 and CD31KO cells suggest the possibility that these effects may be due in part to modulation of the Hippo pathway because CD44 has been associated with modulation of Merlin (16), a regulator of the Hippo pathway and recognized cell density (contact inhibition) (16, 42–46). Additionally, CD44 deficiency's reduction in CD31 and VE-cadherin has been associated with a reduction in adherens and tight junction assembly (22), leading to increased survivin expression, Ajuba inhibition of LATS, and translocation of YAP to the nucleus, leading to increased proliferation and reduced caspase activation (42, 43, 45, 47–59).
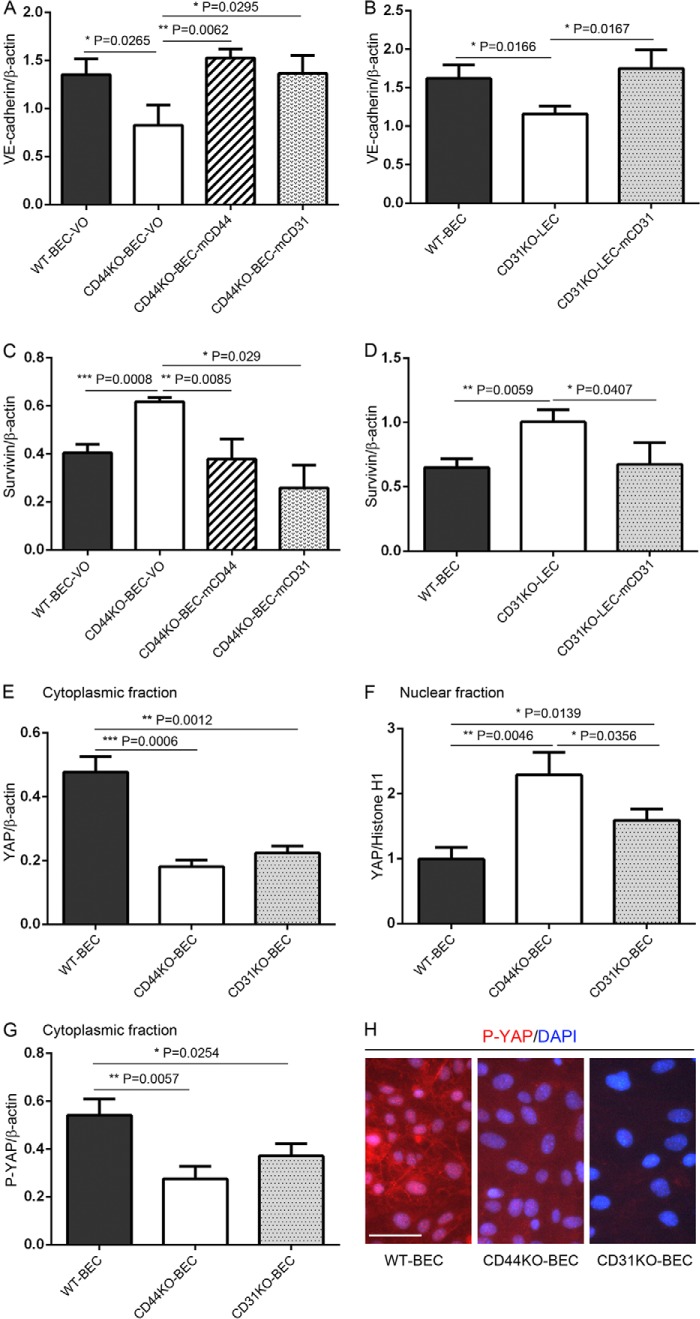
To address this possibility, we performed immunofluorescence and Western blotting analyses of WT-BEC-VO, CD44KO-BEC-VO, CD44KO-BEC-mCD44, CD44KO-BEC-mCD31, CD31KO-BEC, CD31KO-LEC, and CD31KO-LEC-mCD31 cells. As illustrated in Fig. 4, these cultures were examined using phase-contrast microscopy and immunofluorescence. WT-BEC-VO, CD44KO-BEC-mCD44, CD44KO-BEC-mCD31, and CD31KO-LEC-mCD31 cells exhibited a more uniform, cobblestone morphology, whereas CD44KO-BEC-VO and CD31KO (CD31KO-BEC and CD31KO-LEC) cells displayed a more variable, spindle morphology with occasional groups of cells present above the monolayer (Fig. 4, top panel). Labeling with anti-CD31 (Fig. 4, second panel) and anti-VE-cadherin (Fig. 4, third panel) revealed a significant loss of CD31 and VE-cadherin staining in CD44 and CD31KO cells. F-actin labeling (Fig. 4, fourth panel) revealed staining patterns consistent with the phase-contrast morphology. Labeling with anti-survivin (Fig. 4, fifth panel) and anti-YAP (Fig. 4, sixth panel) revealed increased nuclear staining in the CD44 and CD31KO cells. These data illustrate a reciprocal relationship of survivin and YAP nuclear staining with CD31 and CD44 expression in WT, CD44- and CD31-reconstituted, and CD44 and CD31KO cells (Fig. 4, fifth and sixth panels).
These immunofluorescence data were confirmed by Western blotting. Specifically, CD44KO-BEC-VO and CD31KO-LEC both exhibited reduced VE-cadherin expression, whereas CD44KO-BEC reconstituted with either mCD44 or mCD31 and CD31KO-LEC reconstituted with mCD31 exhibited WT level expression (Fig. 5, A and B). Fig. 5, C and D, illustrates increased survivin expression in CD44KO-BEC-VO and CD31KO-LEC cells, whereas CD44KO-BEC reconstituted with either mCD44 or mCD31 and CD31KO-LEC reconstituted with mCD31 exhibited reduced WT level expression. In Fig. 5, E and F, Western blotting illustrates decreased cytoplasmic and increased nuclear YAP expression in CD44KO-BEC and CD31KO-BEC cells and increased cytoplasmic and decreased nuclear YAP expression in WT-BEC. Western blotting of WT-BEC, CD44KO-BEC, and CD31KO-BEC cultures revealed increased phospho-YAP (Ser-127) cytoplasmic levels in WT-BEC compared with CD44KO-BEC and CD31KO-BEC cultures (Fig. 5G), which was confirmed in phospho-YAP (Ser-127) immunofluorescence analysis of WT-BEC, CD44KO-BEC, and CD31KO-BEC cultures (Fig. 5H).
FIGURE 5.
Western blotting evidence of CD44 and CD31 playing key roles in endothelial cell proliferation by modulating Hippo pathway activation. Shown are Western blotting and densitometric analyses of lysates of WT-BEC-VO, CD44KO-BEC-VO, CD44KO-BEC-mCD44, CD44KO-BEC-mCD31 (A and C); WT-BEC, CD31KO-LEC, and CD31KO-LEC-mCD31 (B and D); cytoplasmic (E) and nuclear (F) fractions of WT-BEC, CD44KO-BEC, and CD31KO-BEC; cytoplasmic fractions of WT-BEC, CD44KO-BEC, and CD31KO-BEC (G); and quantitation of expression of VE-cadherin (A and B), survivin (C and D), YAP (E and F), and phospho-YAP (P-YAP) (G). Data represent the mean VE-cadherin versus β-actin (A and B); survivin versus β-actin (C and D); YAP versus β-actin (E); YAP versus histone H1 (F); and phospho-YAP versus β-actin (G) (ratios of n = 3 for each cell type; expressed as means ± S.D (error bars).; *, p < 0.05; **, p < 0.01; ***, p < 0.001). H, representative merged DAPI and phospho-YAP immunofluorescence images of WT-BEC, CD44KO-BEC, and CD31KO-BEC, illustrating robust phospho-YAP labeling in WT-BEC (left) and diminished phospho-YAP labeling in both KO cultures (middle and right). Scale bar, 50 μm.
DISCUSSION
In physiological and pathophysiological conditions, CD44 plays a crucial role in cell proliferation and apoptosis via extracellular and intracellular signaling (16, 32, 34–37, 39, 40, 60) as well as cell adhesion and migration (4, 5, 8, 9, 13, 19). We recently have reported a novel role for CD44 in vascular endothelial cell integrity and permeability, namely its modulation of CD31 expression in vascular endothelial cells (20, 22). These results suggested that loss of CD44 function would affect cell adhesion-mediated proliferation and apoptosis in part by affecting cell-cell contact. Regarding the role of CD44 in endothelial cell proliferation, it has been reported that anti-CD44 antibodies affected the proliferation of human umbilical vein endothelial cells (19). However, a critical role of CD44 in endothelial cell proliferation has been controversial because it has been reported that there is no significant difference between WT CD44 and CD44KO lung endothelial cells in proliferation in in vivo studies (61). Here we show novel CD44 regulatory control in vascular endothelial cell proliferation and survival mediated by CD44 modulation of CD31 and VE-cadherin expression via the Hippo pathway.
CD44 Plays a Key Role in Regulating Endothelial Cell Proliferation via Modulation of CD31
CD44 is a known modulator of cell adhesion, motility, proliferation, and survival mediated by ligand binding, which initiates a cascade of events encompassing adherence to extracellular matrix, binding to growth factors and matrix-degrading enzymes, complex formation with other transmembrane molecules, and association with cytoskeletal elements and signal-transducing molecules (4, 5, 8, 16, 62). Previous in vivo studies have shown that endothelial cell proliferation was observed not to affect endothelial cell proliferation and survival in CD44 null mice (61, 63). Using an in vitro approach, which allows for the examination of a single cell type under defined conditions without the potential of heterotypic cell-cell contacts or heterotypic cell-derived solid phase or soluble factors, we demonstrated decreased initial and increased secondary proliferation rates in CD44KO brain endothelial cells. In addition to the rescue of the proliferative phenotype by reconstitution with mCD44, we found that reconstitution with mCD31 also rescued the proliferative phenotype. In light of the decreased VE-cadherin and CD31 expression noted in the CD44KO cells (22) causing decreased intimate cell-cell contacts observed in cultures of CD44KO cells, we postulate that this results in a perturbation of juxtacrine and paracrine influences, accounting for the lower initial proliferation rate noted. Interestingly, the loss of intimate cell-cell contact in high density cultures, again caused by decreased VE-cadherin and CD31 expression in the CD44KO cells, correlated with decreased contact inhibition of the secondary proliferation rate. These alterations in proliferation rates are consistent with dynamic changes in cell-cell contacts, which in turn affect contact inhibition of proliferation (64). Additionally, it is possible that interactions between CD44 and hyaluronan play a role in promoting endothelial cell contact inhibition at high cell density culture because CD44 expression was recently correlated with hyaluronan deposition in the intercellular space of endothelial cells (65).
Having recently determined that CD44KO mice and endothelial cells derived from them exhibit reduced expression levels of CD31 and behave in many ways similar to CD31KO mice and endothelial cells (20, 22), we investigated whether our findings of altered proliferation rates manifested by CD44KO endothelial cells were due to changes in CD31 expression levels mediated by the presence or absence of CD44 expression. Indeed, the initial and secondary proliferation rates expressed by CD31KO-BEC mirrored the proliferation rates noted for CD44KO-BEC. Reconstitution with either CD44 or, interestingly, with CD31 resulted in a return to WT proliferation rates. Similar to the effects of reduced expression levels of VE-cadherin, these findings are consistent with the modulation by CD44 of proliferation via regulation of CD31 expression and the subsequent changes in cell-cell interactions (22) mediated by CD31 modulation of MMP expression (66), GSK-3β activation state (27), β-catenin phosphorylation and localization (25), and STAT family member phosphorylation (67–69). Because CD44 is known to be capable of either positively or negatively modulating cell proliferation via regulation of Akt activation (32, 70), we examined the effects of CD44 and CD31 deficiency on the expression and activation of PI3K and Akt. Although total PI3K and Akt expression was similar in WT-BEC, CD44KO-BEC, and CD31KO-BEC (data not shown), we found that both CD44- and CD31-deficient BEC exhibited significantly decreased PI3K and Akt activation (data not shown), which correlated with decreased initial proliferative rates noted in both KO cell cultures at 72 h (Fig. 1, A and B). Currently, we do not have any data relating to PI3K and Akt activation at later time points (120–168 h) (Fig. 1, A and B).
We postulate that these changes mediate alterations in cell-cell contact noted during culture, reducing the proximity of cells to each other and reducing juxtacrine and paracrine actions, which would facilitate a decreased initial proliferation rate. The increased later secondary proliferation rate in CD44KO-BEC can be explained by the loss of contact inhibition of proliferation due to decreased CD31- and VE-cadherin-mediated cell-cell interactions (64) and possibly to increased MMP-2 expression (22, 66) and enhanced β-catenin targeting to the proteosome (27), both of which have been observed in CD31KO-BEC. In addition to the cell growth curves, the results were confirmed using PCNA Western blot analyses as well as Ki-67 immunostaining.
Absence of CD44 Affects Selective Caspase Activation
CD44 is known to play a significant role in modulating apoptosis in lymphocyte populations (38, 40). In light of this, we assessed the role of CD44 as a modulator of endothelial cell apoptosis using CD44KO and CD31KO endothelial cells and KO cells reconstituted with mCD44 and mCD31.
It is widely accepted that so-called effector caspases (caspase-3, -6, and -7) play a direct role in cell apoptosis (71). Our results confirmed that active caspase-3 plays a key role in CD44-mediated apoptosis. On the other hand, CD44-mediated caspase-7 and caspase-6 activation was not observed. Interestingly, down-regulated active caspase-3 in CD44KO cells was rescued in the presence of mouse CD31, indicating the possibility of initiator caspase activation being mediated by a downstream signal of CD31. Effector caspase activation is initiated by initiator caspases (71, 72), which are divided into three groups: (i) death receptor-mediated caspase-8 and -10 (72), (ii) ER stress-specific caspase-9 and -12 (41), and (iii) mitochondrially mediated caspase-9 activation via cytochrome c and Apaf-1 (71, 73). (i) Active caspase-8 expression was suppressed in CD44KO cells as well as active caspase-3, and both were rescued by the expression of mouse CD44 and CD31 in CD44KO cells. These results confirmed that CD31 expression and active caspase-8 are related to each other, as we suggested in 2001 (23). Thus, the attenuated activation of caspase-8 in the absence of CD44 is related to CD31 expression. (ii) ER stress-specific active caspase-9 and -12 were noted to be unaffected by the absence of CD44. To address these findings further, we examined the ER stress-mediated protein response using PERK (protein kinase-like endoplasmic reticulum kinase) as a surrogate for this process, and total PERK levels and PERK phosphorylation were unchanged (data not shown). (iii) Active caspase-9 is involved not only in ER stress but also in mitochondrially mediated apoptosis initiation (71). Our results regarding cytochrome c and Apaf-1 expression suggest that mitochondrially mediated apoptosis initiation may not be altered in the absence of CD44 in endothelial cells. A potential mechanism explaining the effects of CD44 expression levels on endothelial cell apoptosis involves the effects of CD44 on VE-cadherin expression levels. In general, it has been found that cadherin expression negatively regulates survivin synthesis (57, 74), and Iulrano et al. (55) have shown that VE-cadherin expression negatively regulates expression of survivin and induces contact inhibition in endothelia. Further, survivin has been shown to be a direct inhibitor of caspase-3 and -7 (56). Because survivin is known to bind to and inactivate effector caspases (57, 74), the decrease in VE-cadherin in CD44KO- and CD31KO-BEC is consistent with our observation of increases in survivin increasing effector caspase inhibition.
Regarding the role of CD31 in modulating apoptosis, using antibodies directed against the ecto-domain of CD31, we have determined that this domain plays a key role as a regulator of initiator and effector caspases. Although the specific mechanism(s) underlying this process are currently ill defined, possibilities include the role of CD31 in affecting adherens junctions, including its role in regulating β-catenin tyrosine phosphorylation state, which affects its ability to interact with VE-cadherin (24, 27, 68, 75), as well as its role in modulating the inhibition of LATS kinase, a component of the Hippo pathway, by binding to Ajuba, a LATS inhibitor (43); its role in regulating STAT phosphorylation, which can effect cytokine expression (69); its role in regulating the expression levels of matrix metalloproteinases, enzymes known to affect junctional integrity (66); and the potential role of CD31 expression levels to affect VE-cadherin expression (76).
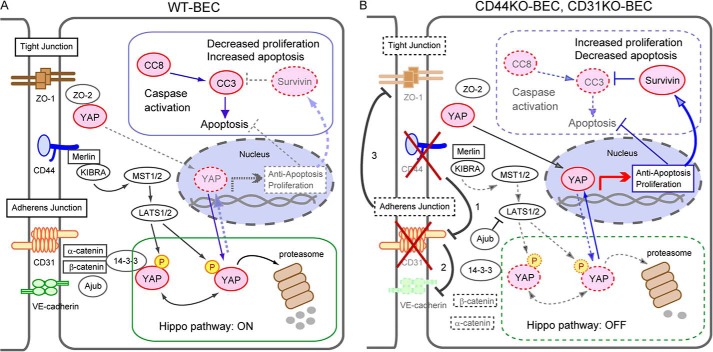
Additionally, CD31-mediated decreased VE-cadherin expression affects not only adherens junction formation and maintenance but also tight junction formation (22). Reduced tight junction formation would then result in loss of YAP-ZO-2 binding (43), resulting in increased YAP nuclear translocation, ultimately leading to increased induction of proliferation and loss of caspase activation in addition to increased survivin expression, as illustrated in Fig. 6.
FIGURE 6.
Working model for the involvement of CD44 and CD31 in the modulation of proliferation and active caspase cascades in endothelial cells. A, in WT-BEC, in the presence of CD44, there is appropriate adherens junction formation that facilitates Merlin interactions with KIBRA and effectively sequesters Ajuba, a LIM family member that is an inhibitor of the Hippo pathway, activating the core Hippo pathway and allowing sequestration of phosphorylated YAP in the cytoplasm and allowing sequestration of phosphorylated YAP in the cytoplasm and ultimately proteosomal degradation. Tight junction formation also participates in sequestration of phosphorylated YAP in the cytoplasm via ZO-2. Thus, the presence of CD44 facilitates activation of the Hippo pathway, resulting in a suppression of proliferation and increased apoptosis. B, in contrast, in CD44KO- and CD31KO-BEC, CD31 expression is reduced (1), which leads to reduction in VE-cadherin (2). This in turn, facilitates Ajuba inhibition of LATS1/2. Additionally, the loss of adherens junctions causes a reduction in tight junction formation (3), resulting in a loss of ZO-2-YAP sequestration. CD44 deficiency also diminishes Merlin activation of the Hippo pathway. Thus, CD44 deficiency inhibits the Hippo pathway, allowing greater YAP translocation to the nucleus, eliciting increased survivin expression, decreased apoptosis, and increased proliferation. Our data and previously published material (16, 43, 45, 46, 58, 59) are consistent with this model.
In summary, CD44 is a complex multifunctional molecule that can differentially regulate cell proliferative and apoptotic processes, depending, in part, on its ability to modulate the expression levels of CD31 and VE-cadherin (Fig. 6). Here we have identified several CD31- and VE-cadherin-dependent mechanisms that affect proliferation and apoptosis. Mechanistic understandings of how CD44 alters each process via its modulation of CD31 and VE-cadherin are still ill defined. However, our data from CD44-deficient endothelial cells illustrate a role for CD44 in modulating proliferation and apoptosis through several mechanisms that involve CD31 and VE-cadherin (Fig. 6) that may be applied to a variety of normal and disease states.
Acknowledgment
We thank Dr. Kelly Flynn for helpful discussions.
This work was supported, in whole or in part, by National Institutes of Health Grant PO1-NS00344738 (to J. A. M.). This work was also supported by a grant-in-aid from the Japan Society for the Promotion of Science for Fellows and by the Uehara Memorial Foundation (to M. T.).
- CD44KO and CD31KO
- CD44 knockout and CD31 knockout, respectively
- mCD44 and mCD31
- mouse CD44 and CD31, respectively
- BEC
- brain endothelial cell(s)
- LEC
- lung endothelial cell(s)
- VO
- vector-only
- PCNA
- proliferating cell nuclear antigen
- ER
- endoplasmic reticulum.
REFERENCES
- 1. Hiraga T., Ito S., Nakamura H. (2013) Cancer stem-like cell marker CD44 promotes bone metastases by enhancing tumorigenicity, cell motility, and hyaluronan production. Cancer Res. 73, 4112–4122 [DOI] [PubMed] [Google Scholar]
- 2. Tsuneki M., Yamazaki M., Maruyama S., Cheng J., Saku T. (2013) Podoplanin-mediated cell adhesion through extracellular matrix in oral squamous cell carcinoma. Lab. Invest. 93, 921–932 [DOI] [PubMed] [Google Scholar]
- 3. Kirschner N., Haftek M., Niessen C. M., Behne M. J., Furuse M., Moll I., Brandner J. M. (2011) CD44 regulates tight-junction assembly and barrier function. J. Invest. Dermatol. 131, 932–943 [DOI] [PubMed] [Google Scholar]
- 4. Cichy J., Puré E. (2003) The liberation of CD44. J. Cell Biol. 161, 839–843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ladeda V., Aguirre Ghiso J. A., Bal de Kier Joffé E. (1998) Function and expression of CD44 during spreading, migration, and invasion of murine carcinoma cells. Exp. Cell Res. 242, 515–527 [DOI] [PubMed] [Google Scholar]
- 6. Mellor L., Knudson C. B., Hida D., Askew E. B., Knudson W. (2013) Intracellular domain fragment of CD44 alters CD44 function in chondrocytes. J. Biol. Chem. 288, 25838–25850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nagano O., Saya H. (2004) Mechanism and biological significance of CD44 cleavage. Cancer Sci. 95, 930–935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chetty C., Vanamala S. K., Gondi C. S., Dinh D. H., Gujrati M., Rao J. S. (2012) MMP-9 induces CD44 cleavage and CD44 mediated cell migration in glioblastoma xenograft cells. Cell. Signal. 24, 549–559 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 9. Seiki M. (2002) The cell surface. The stage for matrix metalloproteinase regulation of migration. Curr. Opin. Cell Biol. 14, 624–632 [DOI] [PubMed] [Google Scholar]
- 10. Twarock S., Tammi M. I., Savani R. C., Fischer J. W. (2010) Hyaluronan stabilizes focal adhesions, filopodia, and the proliferative phenotype in esophageal squamous carcinoma cells. J. Biol. Chem. 285, 23276–23284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Suwan K., Choocheep K., Hatano S., Kongtawelert P., Kimata K., Watanabe H. (2009) Versican/PG-M assembles hyaluronan into extracellular matrix and inhibits CD44-mediated signaling toward premature senescence in embryonic fibroblasts. J. Biol. Chem. 284, 8596–8604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bourguignon L. Y., Peyrollier K., Xia W., Gilad E. (2008) Hyaluronan-CD44 interaction activates stem cell marker Nanog, Stat-3-mediated MDR1 gene expression, and ankyrin-regulated multidrug efflux in breast and ovarian tumor cells. J. Biol. Chem. 283, 17635–17651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hertweck M. K., Erdfelder F., Kreuzer K. A. (2011) CD44 in hematological neoplasias. Ann. Hematol. 90, 493–508 [DOI] [PubMed] [Google Scholar]
- 14. Perez A., Neskey D. M., Wen J., Pereira L., Reategui E. P., Goodwin W. J., Carraway K. L., Franzmann E. J. (2013) CD44 interacts with EGFR and promotes head and neck squamous cell carcinoma initiation and progression. Oral Oncol. 49, 306–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bourguignon L. Y., Xia W., Wong G. (2009) Hyaluronan-mediated CD44 interaction with p300 and SIRT1 regulates β-catenin signaling and NFκB-specific transcription activity leading to MDR1 and Bcl-xL gene expression and chemoresistance in breast tumor cells. J. Biol. Chem. 284, 2657–2671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Stamenkovic I., Yu Q. (2010) Merlin, a “magic” linker between extracellular cues and intracellular signaling pathways that regulate cell motility, proliferation, and survival. Curr. Protein Pept. Sci. 11, 471–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Singleton P. A., Salgia R., Moreno-Vinasco L., Moitra J., Sammani S., Mirzapoiazova T., Garcia J. G. (2007) CD44 regulates hepatocyte growth factor-mediated vascular integrity. Role of c-Met, Tiam1/Rac1, dynamin 2, and cortactin. J. Biol. Chem. 282, 30643–30657 [DOI] [PubMed] [Google Scholar]
- 18. Singleton P. A., Dudek S. M., Ma S. F., Garcia J. G. (2006) Transactivation of sphingosine 1-phosphate receptors is essential for vascular barrier regulation. Novel role for hyaluronan and CD44 receptor family. J. Biol. Chem. 281, 34381–34393 [DOI] [PubMed] [Google Scholar]
- 19. Savani R. C., Cao G., Pooler P. M., Zaman A., Zhou Z., DeLisser H. M. (2001) Differential involvement of the hyaluronan (HA) receptors CD44 and receptor for HA-mediated motility in endothelial cell function and angiogenesis. J. Biol. Chem. 276, 36770–36778 [DOI] [PubMed] [Google Scholar]
- 20. Flynn K. M., Michaud M., Madri J. A. (2013) CD44 deficiency contributes to enhanced experimental autoimmune encephalomyelitis. A role in immune cells and vascular cells of the blood-brain barrier. Am. J. Pathol. 182, 1322–1336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Guan H., Nagarkatti P. S., Nagarkatti M. (2011) CD44 Reciprocally regulates the differentiation of encephalitogenic Th1/Th17 and Th2/regulatory T cells through epigenetic modulation involving DNA methylation of cytokine gene promoters, thereby controlling the development of experimental autoimmune encephalomyelitis. J. Immunol. 186, 6955–6964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Flynn K. M., Michaud M., Canosa S., Madri J. A. (2013) CD44 regulates vascular endothelial barrier integrity via a PECAM-1 dependent mechanism. Angiogenesis 16, 689–705 [DOI] [PubMed] [Google Scholar]
- 23. Ilan N., Mohsenin A., Cheung L., Madri J. A. (2001) PECAM-1 shedding during apoptosis generates a membrane-anchored truncated molecule with unique signaling characteristics. FASEB J. 15, 362–372 [DOI] [PubMed] [Google Scholar]
- 24. Ilan N., Mahooti S., Rimm D. L., Madri J. A. (1999) PECAM-1 (CD31) functions as a reservoir for and a modulator of tyrosine-phosphorylated β-catenin. J. Cell Sci. 112, 3005–3014 [DOI] [PubMed] [Google Scholar]
- 25. Biswas P., Zhang J., Schoenfeld J. D., Schoenfeld D., Gratzinger D., Canosa S., Madri J. A. (2005) Identification of the regions of PECAM-1 involved in β- and γ-catenin associations. Biochem. Biophys. Res. Commun. 329, 1225–1233 [DOI] [PubMed] [Google Scholar]
- 26. Zhang J. J., Kelm R. J., Biswas P., Kashgarian M., Madri J. A. (2007) PECAM-1 modulates thrombin-induced tissue factor expression on endothelial cells. J. Cell Physiol. 210, 527–537 [DOI] [PubMed] [Google Scholar]
- 27. Biswas P., Canosa S., Schoenfeld D., Schoenfeld J., Li P., Cheas L. C., Zhang J., Cordova A., Sumpio B., Madri J. A. (2006) PECAM-1 affects GSK-3β-mediated β-catenin phosphorylation and degradation. Am. J. Pathol. 169, 314–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gratzinger D., Canosa S., Engelhardt B., Madri J. A. (2003) Platelet endothelial cell adhesion molecule-1 modulates endothelial cell motility through the small G-protein Rho. FASEB J. 17, 1458–1469 [DOI] [PubMed] [Google Scholar]
- 29. Biswas P., Canosa S., Schoenfeld J., Schoenfeld D., Tucker A., Madri J. A. (2003) PECAM-1 promotes β-catenin accumulation and stimulates endothelial cell proliferation. Biochem. Biophys. Res. Commun. 303, 212–218 [DOI] [PubMed] [Google Scholar]
- 30. Graesser D., Solowiej A., Bruckner M., Osterweil E., Juedes A., Davis S., Ruddle N. H., Engelhardt B., Madri J. A. (2002) Altered vascular permeability and early onset of experimental autoimmune encephalomyelitis in PECAM-1-deficient mice. J. Clin. Invest. 109, 383–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pinter E., Barreuther M., Lu T., Imhof B. A., Madri J. A. (1997) Platelet-endothelial cell adhesion molecule-1 (PECAM-1/CD31) tyrosine phosphorylation state changes during vasculogenesis in the murine conceptus. Am. J. Pathol. 150, 1523–1530 [PMC free article] [PubMed] [Google Scholar]
- 32. Jijiwa M., Demir H., Gupta S., Leung C., Joshi K., Orozco N., Huang T., Yildiz V. O., Shibahara I., de Jesus J. A., Yong W. H., Mischel P. S., Fernandez S., Kornblum H. I., Nakano I. (2011) CD44v6 regulates growth of brain tumor stem cells partially through the AKT-mediated pathway. PLoS One 6, e24217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Feng Y., Zou Z., Gao L., Zhang X., Wang T., Sun H., Liu Y., Chen X. (2012) Umbilical cord blood-derived stromal cells regulate megakaryocytic proliferation and migration through SDF-1/PECAM-1 pathway. Cell Biochem. Biophys. 64, 5–15 [DOI] [PubMed] [Google Scholar]
- 34. Fedorchenko O., Stiefelhagen M., Peer-Zada A. A., Barthel R., Mayer P., Eckei L., Breuer A., Crispatzu G., Rosen N., Landwehr T., Lilienthal N., Möllmann M., Montesinos-Rongen M., Heukamp L., Dürig J., Hallek M., Fingerle-Rowson G., Herling M. (2013) CD44 regulates the apoptotic response and promotes disease development in chronic lymphocytic leukemia. Blood 121, 4126–4136 [DOI] [PubMed] [Google Scholar]
- 35. Rouschop K. M., Claessen N., Pals S. T., Weening J. J., Florquin S. (2006) CD44 disruption prevents degeneration of the capillary network in obstructive nephropathy via reduction of TGF-β1-induced apoptosis. J. Am. Soc. Nephrol. 17, 746–753 [DOI] [PubMed] [Google Scholar]
- 36. Hauptschein R. S., Sloan K. E., Torella C., Moezzifard R., Giel-Moloney M., Zehetmeier C., Unger C., Ilag L. L., Jay D. G. (2005) Functional proteomic screen identifies a modulating role for CD44 in death receptor-mediated apoptosis. Cancer Res. 65, 1887–1896 [DOI] [PubMed] [Google Scholar]
- 37. Qian H., Xia L., Ling P., Waxman S., Jing Y. (2012) CD44 ligation with A3D8 antibody induces apoptosis in acute myeloid leukemia cells through binding to CD44s and clustering lipid rafts. Cancer Biol. Ther. 13, 1276–1283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ghazi-Visser L., Laman J. D., Nagel S., van Meurs M., van Riel D., Tzankov A., Frank S., Adams H., Wolk K., Terracciano L., Melief M. J., Sabat R., Günthert U. (2013) CD44 variant isoforms control experimental autoimmune encephalomyelitis by affecting the lifespan of the pathogenic T cells. FASEB J. 27, 3683–3701 [DOI] [PubMed] [Google Scholar]
- 39. McKallip R. J., Fisher M., Gunthert U., Szakal A. K., Nagarkatti P. S., Nagarkatti M. (2005) Role of CD44 and its v7 isoform in staphylococcal enterotoxin B-induced toxic shock. CD44 deficiency on hepatic mononuclear cells leads to reduced activation-induced apoptosis that results in increased liver damage. Infect. Immun. 73, 50–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. McKallip R. J., Do Y., Fisher M. T., Robertson J. L., Nagarkatti P. S., Nagarkatti M. (2002) Role of CD44 in activation-induced cell death. CD44-deficient mice exhibit enhanced T cell response to conventional and superantigens. Int. Immunol. 14, 1015–1026 [DOI] [PubMed] [Google Scholar]
- 41. Morishima N., Nakanishi K., Takenouchi H., Shibata T., Yasuhiko Y. (2002) An endoplasmic reticulum stress-specific caspase cascade in apoptosis. Cytochrome c-independent activation of caspase-9 by caspase-12. J. Biol. Chem. 277, 34287–34294 [DOI] [PubMed] [Google Scholar]
- 42. Bao Y., Hata Y., Ikeda M., Withanage K. (2011) Mammalian Hippo pathway. From development to cancer and beyond. J. Biochem. 149, 361–379 [DOI] [PubMed] [Google Scholar]
- 43. Badouel C., McNeill H. (2011) SnapShot. The hippo signaling pathway. Cell 145, 484–484.e1 [DOI] [PubMed] [Google Scholar]
- 44. Xu Y., Stamenkovic I., Yu Q. (2010) CD44 attenuates activation of the hippo signaling pathway and is a prime therapeutic target for glioblastoma. Cancer Res. 70, 2455–2464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Halder G., Johnson R. L. (2011) Hippo signaling. Growth control and beyond. Development 138, 9–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Li W., Cooper J., Karajannis M. A., Giancotti F. G. (2012) Merlin. A tumour suppressor with functions at the cell cortex and in the nucleus. EMBO Rep. 13, 204–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bai H., Zhang N., Xu Y., Chen Q., Khan M., Potter J. J., Nayar S. K., Cornish T., Alpini G., Bronk S., Pan D., Anders R. A. (2012) Yes-associated protein regulates the hepatic response after bile duct ligation. Hepatology 56, 1097–1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Huang J. M., Nagatomo I., Suzuki E., Mizuno T., Kumagai T., Berezov A., Zhang H., Karlan B., Greene M. I., Wang Q. (2013) YAP modifies cancer cell sensitivity to EGFR and survivin inhibitors and is negatively regulated by the non-receptor type protein tyrosine phosphatase 14. Oncogene 32, 2220–2229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Fan R., Kim N. G., Gumbiner B. M. (2013) Regulation of Hippo pathway by mitogenic growth factors via phosphoinositide 3-kinase and phosphoinositide-dependent kinase-1. Proc. Natl. Acad. Sci. U.S.A. 110, 2569–2574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zhao B., Wei X., Li W., Udan R. S., Yang Q., Kim J., Xie J., Ikenoue T., Yu J., Li L., Zheng P., Ye K., Chinnaiyan A., Halder G., Lai Z. C., Guan K. L. (2007) Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 21, 2747–2761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Chan S. W., Lim C. J., Chen L., Chong Y. F., Huang C., Song H., Hong W. (2011) The Hippo pathway in biological control and cancer development. J. Cell Physiol. 226, 928–939 [DOI] [PubMed] [Google Scholar]
- 52. Ramos A., Camargo F. D. (2012) The Hippo signaling pathway and stem cell biology. Trends Cell Biol. 22, 339–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Campbell K. N., Wong J. S., Gupta R., Asanuma K., Sudol M., He J. C., Mundel P. (2013) Yes-associated protein (YAP) promotes cell survival by inhibiting proapoptotic dendrin signaling. J. Biol. Chem. 288, 17057–17062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hong W., Guan K. L. (2012) The YAP and TAZ transcription co-activators. Key downstream effectors of the mammalian Hippo pathway. Semin. Cell Dev. Biol. 23, 785–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Iurlaro M., Demontis F., Corada M., Zanetta L., Drake C., Gariboldi M., Peiro S., Cano A., Navarro P., Cattelino A., Tognin S., Marchisio P. C., Dejana E. (2004) VE-cadherin expression and clustering maintain low levels of survivin in endothelial cells. Am. J. Pathol. 165, 181–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Shin S., Sung B. J., Cho Y. S., Kim H. J., Ha N. C., Hwang J. I., Chung C. W., Jung Y. K., Oh B. H. (2001) An anti-apoptotic protein human survivin is a direct inhibitor of caspase-3 and -7. Biochemistry 40, 1117–1123 [DOI] [PubMed] [Google Scholar]
- 57. Tamm I., Wang Y., Sausville E., Scudiero D. A., Vigna N., Oltersdorf T., Reed J. C. (1998) IAP-family protein survivin inhibits caspase activity and apoptosis induced by Fas (CD95), Bax, caspases, and anticancer drugs. Cancer Res. 58, 5315–5320 [PubMed] [Google Scholar]
- 58. Sun G., Irvine K. D. (2013) Ajuba family proteins link JNK to Hippo signaling. Sci. Signal. 6, ra81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Das Thakur M., Feng Y., Jagannathan R., Seppa M. J., Skeath J. B., Longmore G. D. (2010) Ajuba LIM proteins are negative regulators of the Hippo signaling pathway. Curr. Biol. 20, 657–662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Trochon V., Mabilat C., Bertrand P., Legrand Y., Smadja-Joffe F., Soria C., Delpech B., Lu H. (1996) Evidence of involvement of CD44 in endothelial cell proliferation, migration and angiogenesis in vitro. Int. J. Cancer 66, 664–668 [DOI] [PubMed] [Google Scholar]
- 61. Cao G., Savani R. C., Fehrenbach M., Lyons C., Zhang L., Coukos G., Delisser H. M. (2006) Involvement of endothelial CD44 during in vivo angiogenesis. Am. J. Pathol. 169, 325–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Marhaba R., Zöller M. (2004) CD44 in cancer progression. Adhesion, migration and growth regulation. J. Mol. Histol. 35, 211–231 [DOI] [PubMed] [Google Scholar]
- 63. Cao G., Fehrenbach M. L., Williams J. T., Finklestein J. M., Zhu J. X., Delisser H. M. (2009) Angiogenesis in platelet endothelial cell adhesion molecule-1-null mice. Am. J. Pathol. 175, 903–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. McClatchey A. I., Yap A. S. (2012) Contact inhibition (of proliferation) redux. Curr. Opin. Cell Biol. 24, 685–694 [DOI] [PubMed] [Google Scholar]
- 65. Winkler C. W., Foster S. C., Matsumoto S. G., Preston M. A., Xing R., Bebo B. F., Banine F., Berny-Lang M. A., Itakura A., McCarty O. J., Sherman L. S. (2012) Hyaluronan anchored to activated CD44 on central nervous system vascular endothelial cells promotes lymphocyte extravasation in experimental autoimmune encephalomyelitis. J. Biol. Chem. 287, 33237–33251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Enciso J. M., Gratzinger D., Camenisch T. D., Canosa S., Pinter E., Madri J. A. (2003) Elevated glucose inhibits VEGF-A-mediated endocardial cushion formation. Modulation by PECAM-1 and MMP-2. J. Cell Biol. 160, 605–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ilan N., Cheung L., Miller S., Mohsenin A., Tucker A., Madri J. A. (2001) Pecam-1 is a modulator of stat family member phosphorylation and localization. Lessons from a transgenic mouse. Dev. Biol. 232, 219–232 [DOI] [PubMed] [Google Scholar]
- 68. Ilan N., Madri J. A. (2003) PECAM-1. Old friend, new partners. Curr. Opin. Cell Biol. 15, 515–524 [DOI] [PubMed] [Google Scholar]
- 69. Carrithers M., Tandon S., Canosa S., Michaud M., Graesser D., Madri J. A. (2005) Enhanced susceptibility to endotoxic shock and impaired STAT3 signaling in CD31-deficient mice. Am. J. Pathol. 166, 185–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Zhang L. S., Ma H. W., Greyner H. J., Zuo W., Mummert M. E. (2010) Inhibition of cell proliferation by CD44. Akt is inactivated and EGR-1 is down-regulated. Cell Prolif. 43, 385–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Riedl S. J., Shi Y. (2004) Molecular mechanisms of caspase regulation during apoptosis. Nat. Rev. Mol. Cell Biol. 5, 897–907 [DOI] [PubMed] [Google Scholar]
- 72. Boatright K. M., Salvesen G. S. (2003) Mechanisms of caspase activation. Curr. Opin. Cell Biol. 15, 725–731 [DOI] [PubMed] [Google Scholar]
- 73. Allan L. A., Clarke P. R. (2009) Apoptosis and autophagy. Regulation of caspase-9 by phosphorylation. FEBS J. 276, 6063–6073 [DOI] [PubMed] [Google Scholar]
- 74. Sah N. K., Khan Z., Khan G. J., Bisen P. S. (2006) Structural, functional and therapeutic biology of survivin. Cancer Lett. 244, 164–171 [DOI] [PubMed] [Google Scholar]
- 75. Ilan N., Cheung L., Pinter E., Madri J. A. (2000) Platelet-endothelial cell adhesion molecule-1 (CD31), a scaffolding molecule for selected catenin family members whose binding is mediated by different tyrosine and serine/threonine phosphorylation. J. Biol. Chem. 275, 21435–21443 [DOI] [PubMed] [Google Scholar]
- 76. Wu J., Sheibani N. (2003) Modulation of VE-cadherin and PECAM-1 mediated cell-cell adhesions by mitogen-activated protein kinases. J. Cell Biochem. 90, 121–137 [DOI] [PubMed] [Google Scholar]