Background: Contribution of serglycin in myeloma pathobiology remains undefined.
Results: Serglycin promotes myeloma cell adhesion to bone marrow components and serglycin knockdown in myeloma cells dramatically diminish tumor growth.
Conclusion: Serglycin plays a crucial role in myeloma pathogenesis.
Significance: Targeting serglycin may provide a novel therapeutic approach for multiple myeloma.
Keywords: Cd44, Chondroitin Sulfate, Glycosaminoglycan, Multiple Myeloma, Proteoglycan
Abstract
Recently, it was discovered that serglycin, a hematopoietic cell proteoglycan, is the major proteoglycan expressed and constitutively secreted by multiple myeloma (MM) cells. High levels of serglycin are present in the bone marrow aspirates of at least 30% of newly diagnosed MM patients. However, its contribution to the pathophysiology of MM is unknown. Here, we show that serglycin knockdown (by ∼85% compared with normal levels), using lentiviral shRNA, dramatically attenuated MM tumor growth in mice with severe combined immunodeficiency. Tumors formed from cells deficient in serglycin exhibited diminished levels of hepatocyte growth factor expression and impaired development of blood vessels, indicating that serglycin may affect tumor angiogenesis. Furthermore, knockdown of serglycin significantly decreased MM cell adhesion to bone marrow stromal cells and collagen I. Even though serglycin proteoglycan does not have a transmembrane domain, flow cytometry showed that serglycin is present on the MM cell surface, and attachment to the cell surface is, at least in part, dependent on its chondroitin sulfate side chains. Co-precipitation of serglycin from conditioned medium of MM cells using a CD44-Fc chimera suggests that CD44 is the cell surface-binding partner for serglycin, which therefore may serve as a major ligand for CD44 at various stages during myeloma progression. Finally, we demonstrate that serglycin mRNA expression in MM cells is up-regulated by activin, a predominant cytokine among those increased in MM patients with osteolytic lesions. These studies provide direct evidence for a critical role for serglycin in MM pathogenesis and show that targeting serglycin may provide a novel therapeutic approach for MM.
Introduction
Multiple myeloma (MM)2 is a clonal neoplasm of terminally differentiated B-cells that remains largely incurable, and identification of novel therapeutic targets is urgently needed (1). However, recent developments in the treatment of MM using new classes of therapeutic agents, including the proteasome inhibitor bortezomib, thalidomide, and lenalidomide, have significantly improved patient outcomes (2). The success of these therapies is, at least in part, due to the fact that they are able to counteract certain aspects of MM-bone marrow (BM) interactions. This underscores the importance of comprehensively characterizing the role of the local microenvironment in the pathophysiology of MM (2). The BM microenvironment plays a critical role in MM by supporting tumor growth, bone damage, and drug resistance. Tumor cells in this disease are surrounded within the BM microenvironment by an array of adhesive interactions between the BM cellular residents/extracellular matrix (ECM) proteins and a variety of adhesion molecules on the surface of myeloma cells. Identification of these adhesion molecules is essential for understanding MM biology and elucidating novel therapeutic targets for this disease.
Proteoglycans (PGs), which serve as receptors for extracellular ligands and growth factors, are known to promote the initiation and progression of many cancers. Studies over the last decade have shown that the heparan sulfate (HS) PG syndecan-1 (CD138), expressed by MM cells, plays a major role in the progression of this disease (2, 3). However, a recent study has shown that the chondroitin sulfate PG (CSPG) serglycin is the major PG constitutively secreted by MM cells and that serglycin levels are elevated in the BM biopsies and BM aspirates of at least 30% of MM patients compared with healthy controls (4). Despite this, the role of serglycin in myeloma pathobiology remains undefined.
Serglycin is a major PG found in hematopoietic cells and endothelial cells (5, 6) and contains a peptide core of 17.6 kDa. The core protein of serglycin is rich in serine-glycine repeats and has eight glycosaminoglycan (GAG) side chains (7). The GAG chains can be either heparin or chondroitin sulfate (CS) depending on the cell type in which serglycin is expressed. Using serglycin knock-out mice, Pejler et al. have shown that it is involved in the generation of storage granules and the retention of proteases, growth factors, chemokines, and granzyme B in hematopoietic cells (8, 9). Serglycin does not contain a transmembrane domain and is commonly regarded as an intracellular PG. However, serglycin can also be secreted by cells and can therefore be incorporated into the ECM or associate with the surfaces of target cells (7). For example, in monocytes or macrophages, serglycin is a secretory product and is stored in secretory vesicles. Elimination of serglycin from monocytes affects secretory vesicle formation and thereby the secretion of several binding partners of serglycin (10). In mast cells and platelets, serglycin packaged in storage granules or secretory vesicles is secreted upon activation (5), whereas in endothelial cells or hematopoietic tumor cells, serglycin is constitutively secreted along with granule proteins (11, 12). Serglycin interacts with a wide variety of proteins, including ECM components (fibronectin and collagen) growth factors/cytokines/chemokines (platelet factor 4, MIP-1α, and BMP-like protein), the membrane receptor CD44, and lysozymes. Even after secretion, serglycin plays a role in modulating the activities of its binding partners through the protection, transport, and activation of as well as interactions with substrates or target cells (7).
Several aspects of the biology of serglycin have not previously been studied in detail, either in normal or pathological conditions. However, some studies have shown the involvement of serglycin in progression of certain types of cancer. For example, Li et al. (13) showed that serglycin can promote the metastasis of nasopharyngeal carcinoma by inducing epithelial-mesenchymal transition. Serglycin is associated with tumorigenesis in acute myeloid leukemia (AML) and is a selective marker for distinguishing AML from Philadelphia chromosome-negative chronic myeloproliferative disorders (14). The expression of serglycin in leukemic cells is also regulated by epigenetic modifications at its promoter region and is often hypomethylated and therefore transcribed more efficiently in these cells (15). In this study we therefore investigated further the role of serglycin in multiple myeloma.
EXPERIMENTAL PROCEDURES
Cell Culture
Human MM cell lines analyzed in this study (CAG, U266, OPM-1, delta47, OCIMy5, KMS-11, and RPMI 8226) were cultured in RPMI 1640 (Cellgro, Mediatech, VA) and supplemented with 10% heat-inactivated fetal bovine serum, 100 units/ml penicillin, 100 μg/ml streptomycin, and 2 mm l-glutamine. Human stromal cell line HS-5 was purchased from the ATCC and maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% heat-inactivated FBS, 100 units/ml penicillin, 100 μg/ml streptomycin, and 2 mm l-glutamine.
Immunoblotting and Flow Cytometric Analysis
Total cell lysates or conditioned medium from human MM cell lines were subjected to 4–20% SDS-PAGE under reducing conditions and transferred onto nylon transfer membrane (Whatman), as described previously (16). Serglycin was detected using an anti-human serglycin antibody obtained from Sigma (product no. HPA000759). In some experiments, conditioned medium was treated with chondroitinase ABC (Chase ABC) enzyme (Seikagaku) overnight at 37 °C, prior to SDS-PAGE. To determine whether serglycin secreted by MM cells binds to CD44, conditioned medium from various human MM cells were incubated with recombinant human CD44 Fc chimera (R&D) at 37 °C for 8 h. CD44-IgG was then precipitated using protein-G Sepharose beads (GE Healthcare) and subjected to SDS-PAGE for serglycin. To determine whether serglycin was present in the nucleus of MM cells, nuclear and non-nuclear fractions from CAG cells were isolated, as reported previously (16), and processed for Western blotting. The nuclear marker lamin was detected using an antibody from Abcam and the non-nuclear marker actin using an antibody from Sigma. Serglycin expression was further monitored by flow cytometric analyses using anti-human serglycin antibody (Sigma) and phycoerythrin-conjugated anti-rabbit IgG as a secondary (R&D).
Immunohistochemistry and Immunocytochemistry
Bone marrow tissue microarrays of MM patients and normal controls, obtained from US Biomax, Inc., were stained for serglycin as described previously (17). In brief, sections were deparaffinized with xylene and then rehydrated through graded concentrations of ethanol and distilled water. Epitope retrieval was performed by steaming the slides for 20 min in citrate buffer solution (pH 6.0). Slides were washed and incubated with 2.5% H2O2 for 30 min to quench endogenous peroxide activities and were then blocked with normal goat serum for 20 min at room temperature. Following this, the slides were stained for 1 h with rabbit anti-human serglycin antibody (Sigma) at room temperature. The sections were washed with phosphate-buffered saline and stained with biotinylated goat anti-rabbit IgG (Vector Laboratories, Burlingame, CA) for 30 min at room temperature followed by Vectastain ABC reagent (Vector Laboratories) for another 30 min at room temperature. Detection was accomplished using a 3,3′-diaminobenzidine substrate kit (Vector Laboratories). Photographs were taken using an Olympus BX60 System Microscope.
Sections from formalin-fixed tumor tissues formed from control or serglycin knockdown cells were stained for mouse CD34 (Hycult), human HGF (R&D), and human serglycin, as described above, except that the blocking solution used was 1% BSA/PBS and the primary antibody incubation was overnight at 4 °C. For cell staining, cytospins of MM cells were fixed in 4% paraformaldehyde for 10 min, permeabilized with 2% Triton X-100 in PBS for 5 min, blocked for 1 h at room temperature using 1% BSA/PBS, and incubated overnight at 4 °C with rabbit anti-human serglycin Ab (Sigma) (1:250 in 1% BSA/PBS). After washing, samples were incubated for 1 h at room temperature in 1% BSA/PBS containing Alexa 488 goat anti-rabbit IgG, cover slipped and sealed using Vectashield mounting media. Images were captured using a Nikon A1 confocal microscope.
Knockdown of Serglycin by shRNA
Serglycin knockdown was performed using MISSION lentiviral transduction particles from Sigma, as reported previously (18). Lentiviral transduction particles are produced from a lentiviral plasmid vector containing the shRNA sequences (shRNA #1) for the human serglycin gene: CCGGCCTCAGTTCAAGGTTATCCTACTCGAGTAGGATAACCTTGAACTGAGGTTTTT. The non-target shRNA control transduction particles containing the sequence CCGGCAACAAGATGAAGAGCACCAACTCGAGTTGGTGCTCTTCATCTTGTTGTTTTT do not target any human genes but do activate the RNAi pathway. In brief, 25 μl of lentiviral particles were added to 105 CAG cells and incubated for 18–20 h at 37 °C in a humidified incubator. The next day, the medium containing the lentiviral particles was removed, and fresh complete RPMI medium was added to each well. The cells were then selected using puromycin (5 μg/ml) and assessed for serglycin knockdown by real-time PCR and Western blotting.
Human Plasmacytoma Xenograft Model
All animal studies were approved by the University of Alabama Institutional Animal Care and Use Committee. The xenograft tumor model was performed as previously described (19). CB17 severe combined immunodeficiency (SCID) mice (Charles River Breeding Laboratories, Portage, MI) were subcutaneously inoculated with control or serglycin-knockdown CAG cells (1 × 106) in 100 μl of PBS. The serum was collected once every 3 weeks, and the animals were euthanized 6 weeks after tumor cell injection. Human immunoglobulin kappa light chain levels were measured in murine sera to assess whole animal tumor burden. Sera collected during animal studies were stored at −80 °C and analyzed by ELISA (Bethyl Laboratories, Montgomery, TX) in duplicate, as described previously (19).
HGF ELISA
Supernatants harvested from 2-day cultures of control of serglycin-knockdown cells were tested for HGF secretion by ELISA (R&D Systems).
Adhesion Assay
MM cells were labeled with calcein-AM (Invitrogen), washed, and resuspended in culture medium. Cells were added to human BMSC HS-5 coated 96-well plates at 37 °C for 2 h; unbound cells were then washed out, and adherence was assessed using a fluorescence plate reader (Synergy H1 hybrid reader, Biotek). In some experiments, MM cells were pretreated with Chase ABC (to remove CS chains) prior to being placed in HS-5 coated wells. To determine the involvement of CD44 in MM-BMSC interactions, adhesion assays were also performed in the presence of either an anti-CD44 ab (hermes-1) (Thermo Scientific) or hyaluronan oligosaccharides (o-HA) (100 μg/ml). Hyaluronan oligosaccharides used in this study were a mixed fraction, with the average molecular weight of 2.5 × 103, composed of 2–10 disaccharide units that were fractionated from testicular hyaluronidase (Sigma, type 1-S) digests of hyaluronan polymer (Sigma, sodium salt), as described previously (20). For adhesion to collagen I, wells coated with collagen I were blocked with 1% BSA in serum-free culture medium for 1 h at room temperature. After three washes, 5 × 105 cells were added to the wells and incubated for 2 h in a serum-free medium at 37 °C in 5% CO2. Following incubation, the wells were washed to remove non-adherent cells and the adherent cells were then fixed using cold methanol for 30 min and stained with 0.25% crystal violet for 15 min. Cells were then lysed with 200 μl of 10% acetic acid and the absorbance was measured at 590 nm.
Endothelial Invasion Assay
The invasiveness of endothelial cells was analyzed using Biocoat Matrigel invasion chambers (BD Biosciences), as previously reported (21). Endothelial (HUVEC) cells (2 × 104) suspended in 500 μl of endothelial cell basal medium containing 1% fetal bovine serum were seeded in the upper compartments of the chambers. A total of 750 μl of medium that had been conditioned by control or serglycin-knockdown CAG cells was added to the lower well of the chamber. In some experiments, conditioned medium from control cells was treated with Chase ABC prior to being added to the lower well. Once cells were added to the upper chamber, they were incubated at 37 °C and allowed to invade through the matrigel barrier for 12 h. After incubation, the non-invading cells were removed from the upper surface of the membrane using a cotton swab and invading cells on the underside of the filter were enumerated using an inverted microscope, after fixing and staining with Diff-Quick solution (IMEB Inc). Each assay was performed in triplicate. Data are expressed as the percentage of cell invasion with the invasion stimulated by medium from control cells set at 100%.
Cell Viability Assay
The survival of control or serglycin-knockdown cells was determined by MTT (-[4,5-dimethylthiazol-2-yl]-2,5diphenyl-tetrazolium bromide) assays. Cells (5 × 103 per well in 100 μl of medium) were seeded in a 96-well plate. After 24, 48, and 72 h, the cells were incubated with 50 μl of MTT (1 mg/ml) and left in the dark at 37 °C for an additional 4 h. Formazan crystals were dissolved in 200 μl of DMSO, and absorbance was measured at 540 nm in a microplate reader.
Real-time PCR
Approximately 1 × 106 cells were either untreated or treated with recombinant activin (500 ng/ml) (R&D) for 12 h. RNA was extracted (RNeasy Mini Kit, Qiagen), and cDNA was synthesized (Clontech). Real-time PCR was conducted using the following primers; serglycin (F) CTAAGTTGGTCATGATGCAGAA; serglycin (R) TCCGCGTAGGATAACCTTGAAC; C4ST1(F)-TCCCTTTGGTGTGGACATCT; C4ST1(R)-CACGTGTCTGTCACCTGGTC and SYBR Green Supermix (Bio-Rad). Expression was determined relative to 28 S rRNA.
Statistical Analyses
All results are representative of at least three independent experiments. Except where noted, comparisons between two groups were analyzed using the Student's t test, and a p value of <0.05 was considered to be statistically significant. Data are presented as mean ± S.D.
RESULTS
Multiple Myeloma Cells Express the Proteoglycan Serglycin
A previous study reported that the proteoglycan serglycin is constitutively secreted by myeloma cells and is present at higher levels in the bone marrow aspirates of myeloma patients, compared with healthy controls (4). Consistent with this, we found that bone marrow biopsies from MM patients exhibited robust serglycin immunoreactivity (Fig. 1A, upper panel). Strong reactivity with anti-serglycin antibodies occurred in the cytoplasm and nucleus of plasma cells (Fig. 1A). Normal bone marrow biopsies showed serglycin immunoreactivity in hematopoietic cells that are present in between the fat cells (white spaces) (Fig. 1A, lower panel). To demonstrate further that the serglycin proteoglycan is expressed by myeloma cells, protein extracts from different human myeloma cell lines were analyzed by Western blotting. Fig. 1B (left panel) shows that all of the myeloma cell lines expressed serglycin. However, serglycin was not detected in normal human mammary epithelial cells (MCF-10A) or normal human kidney proximal tubular cells (HK-2), compared with CAG myeloma cells (Fig. 1B, right panel). Serglycin ran as a broad smear on the Western blot, ranging from 300 to 140 kDa; this is typical of proteoglycans and is due to variations in the number and length of glycosaminoglycan chains attached to the core protein of serglycin. Serglycin expression was also confirmed by real-time PCR, and mRNA was detected in all six myeloma cell lines tested (data not shown). Further confocal microscopy analysis of human MM cells using a serglycin antibody demonstrated that this PG is present in the cytoplasm and in the nucleus (Fig. 1C). To confirm the nuclear localization of serglycin, cells were extracted and nuclear and non-nuclear fractions were isolated (although most studies refer to these as nuclear and cytoplasmic fractions, we use the terms nuclear and non-nuclear because the non-nuclear fraction included not only cytoplasmic molecules but also cell surface molecules). Western blots detected abundant serglycin within the nuclear and non-nuclear fractions of the CAG myeloma cell line (Fig. 1D). Staining of the blot for lamin (a nuclear marker) and actin (a non-nuclear marker) confirmed the fidelity of the nuclear and non-nuclear fractions, respectively.
FIGURE 1.
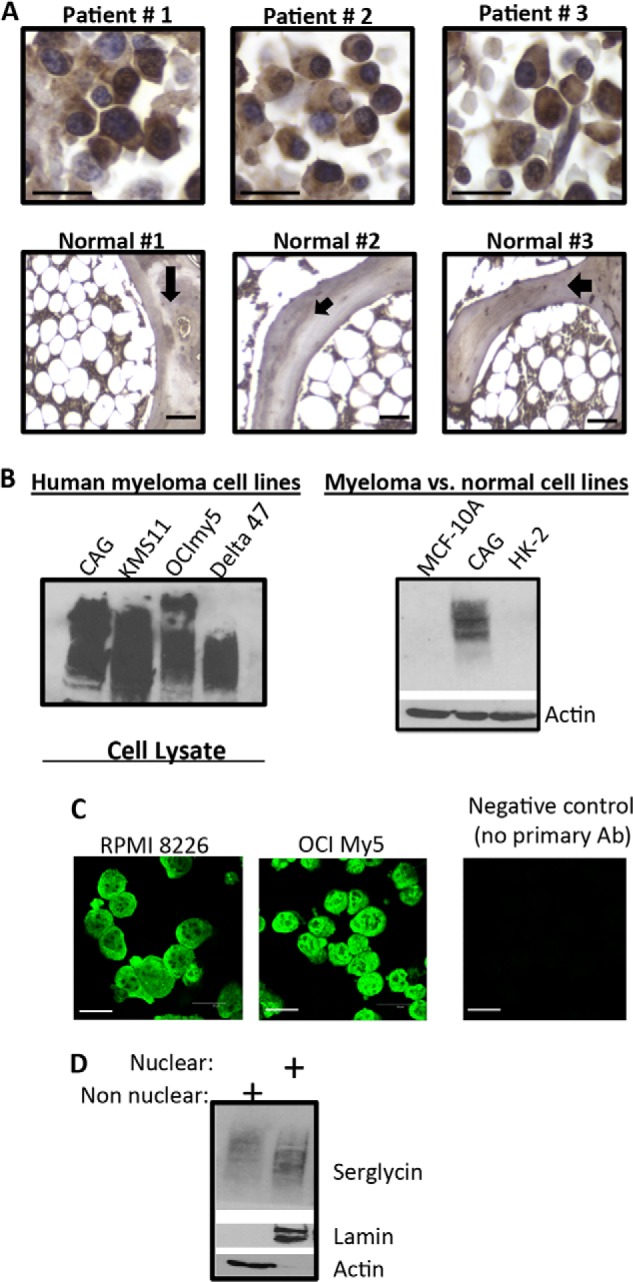
Serglycin is expressed by human myeloma cells. A, immunohistochemistry demonstrating serglycin expression on bone marrow biopsy sections from myeloma patients (nos.1–3) (upper panel). Scale bars, 200 μm. Lower panel represents immunohistochemistry for serglycin in normal human bone marrow biopsies (nos. 1–3). A normal bone marrow biopsy section consists of a meshwork of bone trabeculae (black arrow) containing fat cells (white spaces) and hematopoietic precursor cells in between the fat cells. Scale bars, 200 μm. B, total protein was extracted from multiple myeloma cell lines and analyzed for serglycin expression (rabbit anti-human serglycin Ab) by Western blotting (left panel). The right panel represents Western blot for serglycin in CAG myeloma cells compared with normal human mammary epithelial cells (MCF-10A) or normal human kidney proximal tubular cells (HK-2). Actin is used as the loading control. C, confocal analysis of serglycin in the respective cell lines. RPMI-8226 and OCImy5 human myeloma cell lines were permeabilized and incubated overnight with rabbit anti-human serglycin antibody (1: 250 dilution) and, after extensive washing, were incubated with Alexa-488 secondary antibody. Staining was visualized using confocal microscopy. Scale bars, 30 μm. D, nuclear and non-nuclear fractions were isolated from CAG human myeloma cell line and separated on SDS-PAGE. Western blots were probed with antibody to human serglycin, lamin, or actin.
Serglycin Is Secreted and Binds to the Surface of Myeloma Cells
Even though serglycin is commonly considered to be an intracellular PG, it can also be secreted by cells (22). A recent study showed that serglycin is a major PG in human endothelial cells and may regulate secretion of the chemokine GROα (23). To determine whether serglycin is secreted by myeloma cells, conditioned media from several myeloma cell lines were processed for Western blot analysis. As shown in Fig. 2A, serglycin PG was detected in the medium of all six human myeloma cell lines tested, consistent with a previous report showing that serglycin represents the predominant PG constitutively secreted by myeloma cells at high levels (4). Furthermore, treatment of serglycin with the CS/dermatan sulfate-degrading enzyme, Chase ABC, completely removed the GAG chains attached to the core protein of serglycin (Fig. 2B). This result suggests strongly that serglycin expressed by MM cells has only CS/dermatan sulfate GAG chains attached to it, most likely only CS since a previous study reported that CS chains, consisting almost entirely of 4-O-sulfated disaccharides, are the only GAG chains attached to myeloma-derived serglycin (4). Serglycin lacks a transmembrane domain, and is therefore not expressed as an integral proteoglycan on the cell surface; however, serglycin can bind to cell surface receptors via its GAG chains (24, 25). To determine whether serglycin is present on the surface of myeloma cells, flow cytometry analysis was carried out. As shown in Fig. 2C, all of the five human MM cell lines tested (CAG, RPMI 8226, KMS 11, OCIMy5, and OPM1) had serglycin present on their surface. However, the signal intensity varied somewhat between cell lines. Furthermore, to determine whether the presence of serglycin on the surface of myeloma cells is dependent on its CS GAG chains, myeloma cells were treated with the CS-degrading enzyme, Chase ABC. Pretreatment of cells with Chase ABC partly reduced the levels of serglycin on the MM cell surface (Fig. 2D), suggesting that the cell surface-binding ability of serglycin is dependent on its CS chains. The incomplete removal of serglycin may be due to the incomplete digestion of Chase ABC or due to the involvement of the core protein of serglycin in cell surface binding; however, this possibility needs to be investigated further. Together, the results presented in Figs. 1 and 2 shows that myeloma cells highly express and secrete the CSPG serglycin and that CS GAG chains (up to eight chains are attached to the core protein of serglycin) are a component of the myeloma glycocalyx.
FIGURE 2.
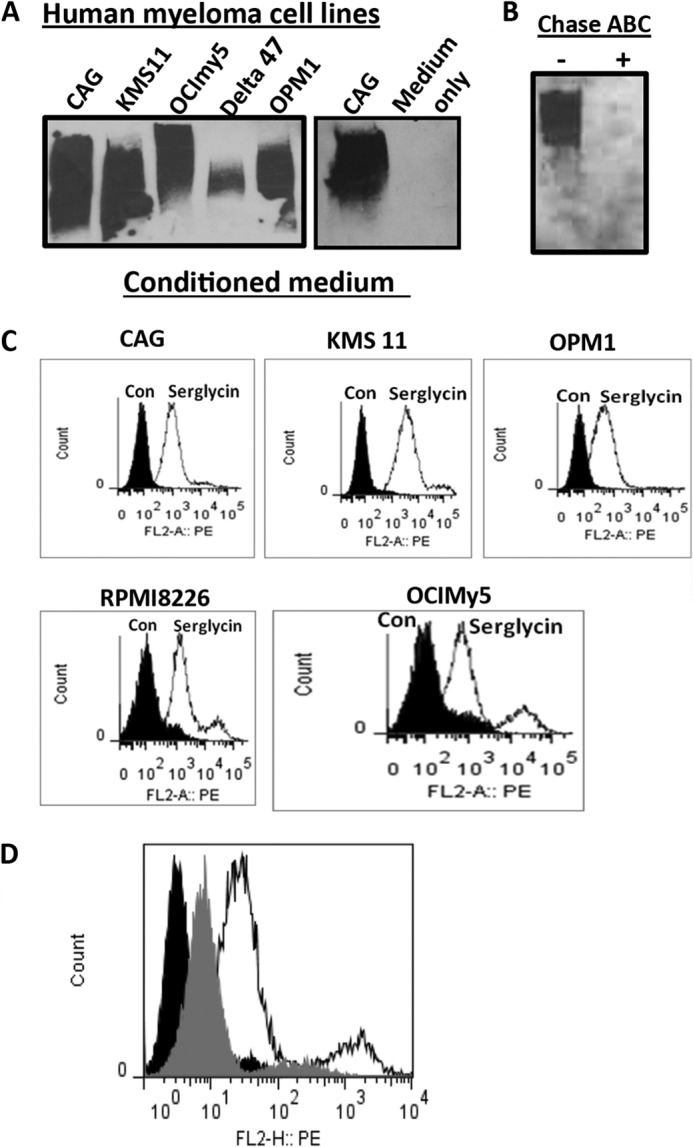
Serglycin is secreted by myeloma cells and is present on the cell surface. A, Western blot analysis for the presence of serglycin in the conditioned medium from five different human myeloma cell lines. Culture medium without cells is used as the control. B, serglycin expressed by myeloma cell lines has side-chains of CS glycosaminoglycan. Conditioned medium of KMS11 human myeloma cell line was either treated or not treated with the CS-degrading enzyme chondroitinase ABC (Chase ABC) and then analyzed for serglycin by Western blotting. C, flow cytometric analysis demonstrating serglycin expression on the surface of myeloma cell lines (CAG, KMS11, RPMI8226, OPM1, and OCImy5). Solid curves represent isotype control, and open curves indicate serglycin staining. D, serglycin binding to myeloma cell surface is dependent on their CS chains. OCImy5 human myeloma cell line was either treated or not treated with Chase ABC enzyme and then analyzed for cell surface serglycin by flow cytometry. Black curves represent isotype control, gray curves indicate serglycin level after Chase ABC digestion, and open curves represent serglycin level without ABC digestion.
Serglycin Promotes Myeloma Cell Adhesion to Bone Marrow Stromal Cells (BMSCs) and Collagen I
Because of the fact that the CS-type serglycin that is widely expressed by hematopoietic cells has the ability to bind to CD44 receptors through its CS chains, and also because serglycin has been shown to promote cell-cell interaction by bridging CD44 on neighboring cells (25, 26), we sought to determine whether serglycin secreted by myeloma cells has the ability to bind to CD44. This was examined by co-precipitation analysis of serglycin using recombinant soluble CD44 (CD44-Ig) with culture supernatants from various human myeloma cell lines. As shown in Fig. 3A, serglycin was precipitated with CD44-IgG from the conditioned medium from all seven human myeloma cell lines. Serglycin was not precipitated using a control human IgG (Fig. 3A). These results suggest that the CS serglycin secreted by myeloma cells represents a major CD44-binding macromolecule in these cell lines.
FIGURE 3.
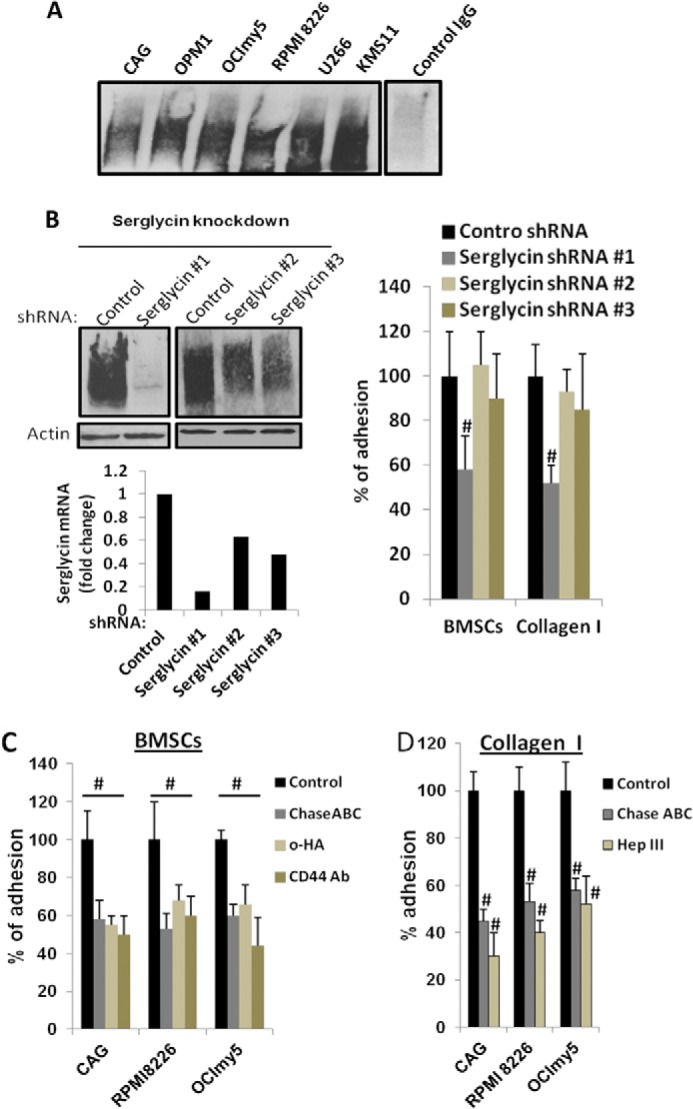
Serglycin promotes myeloma cell adhesion to bone marrow stromal cells and collagen I. A, serglycin secreted by myeloma cells binds to CD44. Recombinant soluble CD44 (CD44-IgG1) was added to the culture supernatants from 6 different human myeloma cell lines. After 8 h of incubation at 37 °C, CD44-IgG was precipitated using protein G-Sepharose beads and subjected to Western blotting for serglycin. Serglycin was not precipitated using control human IgG. B, CAG cells were infected with lentiviral vectors coding for control or serglycin shRNA. Left upper panel: Western blotting of cell extracts of stably infected cells demonstrates effective knockdown of serglycin expression using serglycin shRNA# 1 and partial knockdowns using serglycin shRNAs #2 and #3. Left lower panel: mRNA expression of serglycin assessed by real time PCR. Right panel: myeloma cells after serglycin knockdown adhere less to bone marrow stromal cell (BMSC) line HS-5 and collagen I. Control or serglycin-knockdown CAG cells were seeded to plates that were pre-coated with either BMSC HS-5 or collagen I. After 2 h, non-adherent cells were removed, and percentage of adherent cells were determined as described under “Experimental Procedures.” Values were normalized to control, which is arbitrarily set at 100% (mean ± S.D. of triplicates from three independent experiments). #, p < 0.05 compared with control. CAG cells with partial knockdowns for serglycin adhered to a similar extent to control cells. C, adhesion of myeloma cell lines (CAG, RPMI 8226, and OCImy5) to BMSCs was reduced significantly in the presence of CD44 antagonists. Calcein-labeled myeloma cell lines were added to BMSC HS-5 coated 96-well plates in the presence or absence of CD44 antagonists: small hyaluronan oligosaccharides (o-HA) (100 μg/ml) or adhesion blocking CD44 antibody (hermes-1). To determine the role of CS chains in cell adhesion, myeloma cells were also pretreated with Chase ABC enzyme prior to the addition to HS-5 coated wells. After 2 h, unbound cells were removed and adherence of myeloma cells was determined using a fluorescence plate reader. Data are expressed as percentage control from three different experiments. #, p < 0.05 compared with control. D, myeloma cells (CAG, RPMI 8226, and OCImy5) pre-treated or untreated either with Chase ABC enzyme or Hep III enzyme, were allowed to adhere to collagen I-coated plates for 2 h. Unbound cells were then removed, and adherent cells were then fixed and stained with 0.25% crystal violet. Cells were then lysed and absorbance read at 590 nm. Results are expressed as mean ± S.D. of triplicates from three separate experiments. #, p < 0.05 compared with control.
Next, we sought to investigate whether serglycin promotes the adhesion of myeloma cells to bone marrow stromal cells and collagen type I (CS chains can bind to collagen I). To examine the effect of serglycin in myeloma cell adhesion, we first attempted to completely knockdown serglycin expression in CAG human myeloma cell lines using lentiviral serglycin shRNAs. Five lentiviral serglycin shRNAs were generated targeting different regions in the serglycin mRNA. Only one of the five shRNAs (shRNA#1) yielded a dramatic knockdown (∼85%) of serglycin, as confirmed by Western blotting and real-time PCR (Fig. 3B). Two of the other shRNA sequences (shRNA #2 and shRNA #3) yielded a 40–50% knockdown (Fig. 3B). Incomplete knockdown of serglycin was probably due to its high expression. We then examined the effect of serglycin knockdown on CAG cell adhesion to the human bone marrow cell line HS-5. As shown in Fig. 3B (right panel), serglycin knockdown (∼85%) (shRNA#1) induced significant inhibition of adhesion of MM cells to BMSCs, demonstrating a role for MM-expressed serglycin in the adhesion of MM cells to stromal cells. Cells that had 40–50% knockdown levels of serglycin (shRNA #2 and #3) adhered to a similar extent to control cells (Fig. 3B, right panel)). To further confirm the role of serglycin in mediating adhesion, MM cells were treated with Chase ABC. This enzyme degrades CS chains, thereby releasing serglycin from the cell surface (Fig. 2D). As shown in Fig. 3C, pre-treatment with Chase ABC decreased the adhesion of human MM cell lines (CAG, RPMI8226, and OCIMy5) to BMSC HS-5 to the same level as that caused by serglycin knockdown by shRNA #1 (Fig. 3B). These results clearly demonstrate the role of serglycin in mediating myeloma cell adhesion and further confirm that the decreased adhesion shown by serglycin knockdown cells is not an artifact of knockdown. To determine if CD44 is involved in this adhesion mechanism, the adhesion of MM cells to BMSCs was examined in the presence of antagonists of CD44-ligand interaction, either hyaluronan oligosaccharides (o-HA) (20, 27) or anti-CD44 monoclonal antibodies (hermes-1). As shown in Fig. 3C, addition of o-HA or anti-CD44 Ab significantly decreased the adhesion of MM cells to HS-5 cells. Addition of o-HA or anti-CD44 Ab to serglycin-knockdown CAG cells had no further effect on cell adhesion (data not shown). Taken together, our findings suggest that serglycin secreted by myeloma cells is a partial mediator of cell adhesion, and this adhesion is likely due to its CD44 binding ability.
We also investigated whether serglycin expressed by MM cells promotes its adhesion to type I collagen, the major collagen type present within bone. Tissue culture plates were coated with collagen type I and control or serglycin-knockdown CAG cells were added. Fig. 3B (right panel) demonstrates that serglycin knockdown (∼85%) significantly decreased the binding of CAG cells (by ∼50%) to collagen I, compared with control cells. However, cells that had 40–50% knockdown levels of serglycin adhered to a similar extent to control cells (Fig. 3B, right panel)). Furthermore, pretreatment of MM cells (CAG, RPMI 8226, and OCIMy5) with Chase ABC, prior to the addition of cells to collagen I coated plates, inhibited collagen binding to a similar extent to cells with ∼85% serglycin knockdown (Fig. 3D). As expected, degradation of HS chains using heparinase III (Hep III) also exhibited a significant reduction in the adhesion of myeloma cells to collagen I (Fig. 3D). Together, these data reveal that the CSPG serglycin expressed by MM cells, in addition to HSPG syndecan-1 (28), helps to mediate MM cell binding to collagen type I.
Serglycin Knockdown Dramatically Diminishes Myeloma Tumor Growth in Vivo
Serglycin-knockdown cells were further analyzed to see whether serglycin alters the growth of myeloma cells in vitro or in vivo. As shown in Fig. 4A, the in vitro growth of serglycin-knockdown cells was not significantly different to that of control cells when measured by MTT assay. Further, no cleaved caspase-3 or nuclear DNA fragmentation (assessed by DAPI staining) were observed in control or serglycin knockdown cells, suggesting that apoptosis is also not altered by serglycin knockdown (data not shown).
FIGURE 4.
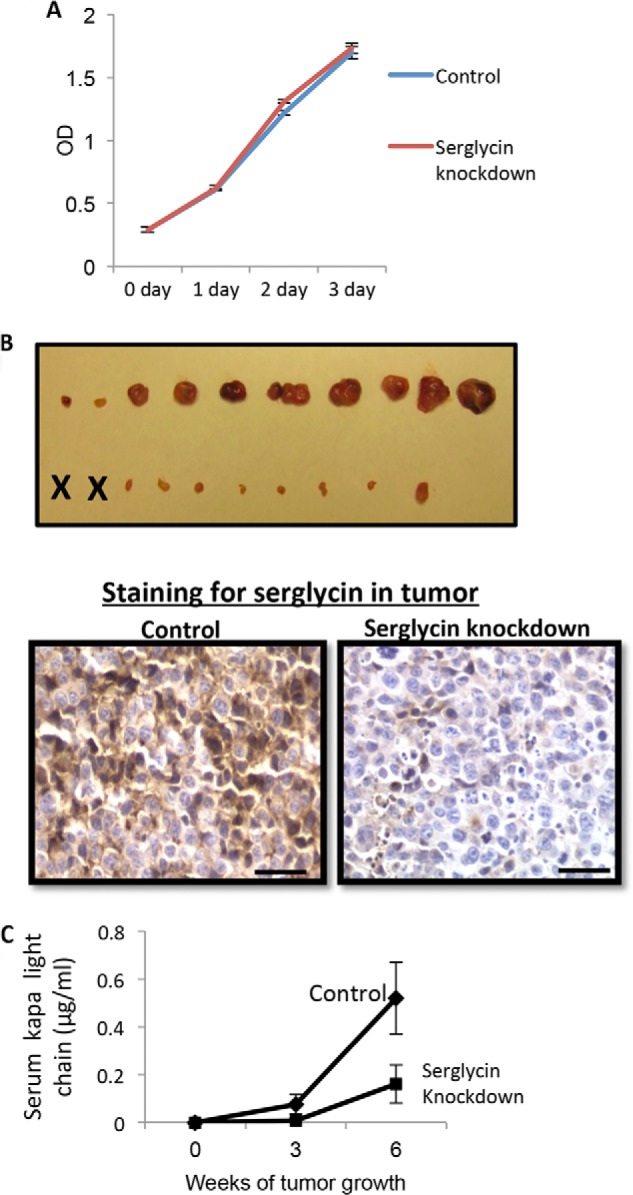
Knockdown of serglycin expression diminishes subcutaneous growth of myeloma cells. A, knockdown of serglycin expression does not alter the in vitro growth of myeloma cells. Control or serglycin-knockdown CAG cells were seeded at equal density, and the growth in cell numbers was assessed by MTT assay at 0, 1, 2, and 3 days. Points represents mean of triplicate samples ±S.D. from each of two separate experiments. B, 6 weeks after subcutaneous injection of control and serglycin-knockdown CAG cells into flanks of male SCID mice, the tumors were removed at necropsy and photographed (upper panel). “X” represents animals with no detectable tumor. Lower panel represents the immunohistochemistry of sections from xenograft tumors formed from control or serglycin-knockdown cells stained for human serglycin (brown) and counterstained with hematoxylin (blue). Scale bars, 200 μm. C, levels of human kappa light chain in the serum were measured by ELISA. Data include all animals that were injected with tumor cells and represents mean ± S.D. of duplicate from a single ELISA.
Because high levels of serglycin are present in the extracellular milieu of myeloma patients, we also tested whether serglycin knockdown blocked multiple myeloma cell growth in vivo. CB17 SCID mice were injected subcutaneously with serglycin-knockdown (∼85%) versus control CAG cells to compare the development of tumors. After 6 weeks, 8 of the 10 mice injected with control CAG cells had developed large tumors (Fig. 4B). In contrast, serglycin-knockdown cells developed much smaller tumors than controls and 2 of the 10 mice had not developed tumors after 6 weeks. Tumor onset in mice receiving serglycin-knockdown CAG cells occurred at an average of 35 days, compared with 14 days in mice injected with control CAG cells. Further, immunostaining for serglycin in tumors formed from control and serglycin knockdown cells showed that a sustained knockdown of serglycin is achieved in tumors formed by the serglycin knockdown cells (Fig. 4B). To quantitatively assess the rate of tumor growth, blood was collected every 3 weeks and levels of human kappa light chain were analyzed. Fig. 4C shows that the mean kappa levels in mice injected with serglycin-knockdown cells were significantly lower (0.16 μg/ml) compared with those of mice injected with control cells (0.52 μg/ml) at 6 weeks. Although 8 of the 10 animals injected with control cells had detectable levels of kappa in their serum after 3 weeks, only 1 of the 10 injected with serglycin-knockdown cells had detectable kappa chains. Together, these data indicate that serglycin knockdown in myeloma cells inhibits myeloma growth and tumor burden.
We also compared the growth in vivo of control cells with two CAG cell preparations that exhibited partial knockdown for serglycin, specifically 40 and 50% of serglycin expression compared with control. The cells with partial knockdown of serglycin grew in a similar way to control cells in vivo (data not shown). These data indicate that greater than 50% knockdown of serglycin is necessary to affect tumor growth in vivo.
Tumors Formed from Serglycin-Knockdown Cells Exhibit Poorly Developed Vasculature and Decreased Levels of HGF
While there are no differences in growth between serglycin-knockdown and control cells in vitro (Fig. 4A), the fact that there are major differences between their growth in vivo (Fig. 4B) suggests that the growth promoting effects of serglycin in vivo may be related to its effects on the tumor microenvironment. Because vascularization is required for tumors to continue to grow, we assessed the microvessel density in tumors formed from control and serglycin-knockdown cells. Poorly developed vasculature, as assessed by immunohistochemistry using anti-CD34 mAb (which stains endothelial cells), were observed in serglycin-knockdown tumors as compared with control tumors (Fig. 5A). Next we examined the effect of serglycin on myeloma-mediated endothelial cell invasion, a critical early step in the angiogenesis process. Conditioned media from control and serglycin-knockdown cells (shRNA #1) were harvested and incubated in lower wells with endothelial cells that had been plated on the upper surface of matrigel-coated membranes in a Boyden-chamber type assay. The number of invading cells present on the underside of the filter was counted 12 h later. Endothelial cell invasion was lower for cells that were incubated in medium from serglycin-knockdown cells compared with cells incubated with control cell medium (Fig. 5B). Because GAG chains can bind and aid in establishing gradients of pro-angiogenic growth factors to promote proper endothelial branching, we examined the role of CS chains of serglycin in endothelial invasion. Pretreatment of media from control cells with Chase ABC decreased the invasion stimulatory activity of the medium (Fig. 5B). This suggests that serglycin stimulates the invasion of endothelial cells via its CS chains.
FIGURE 5.
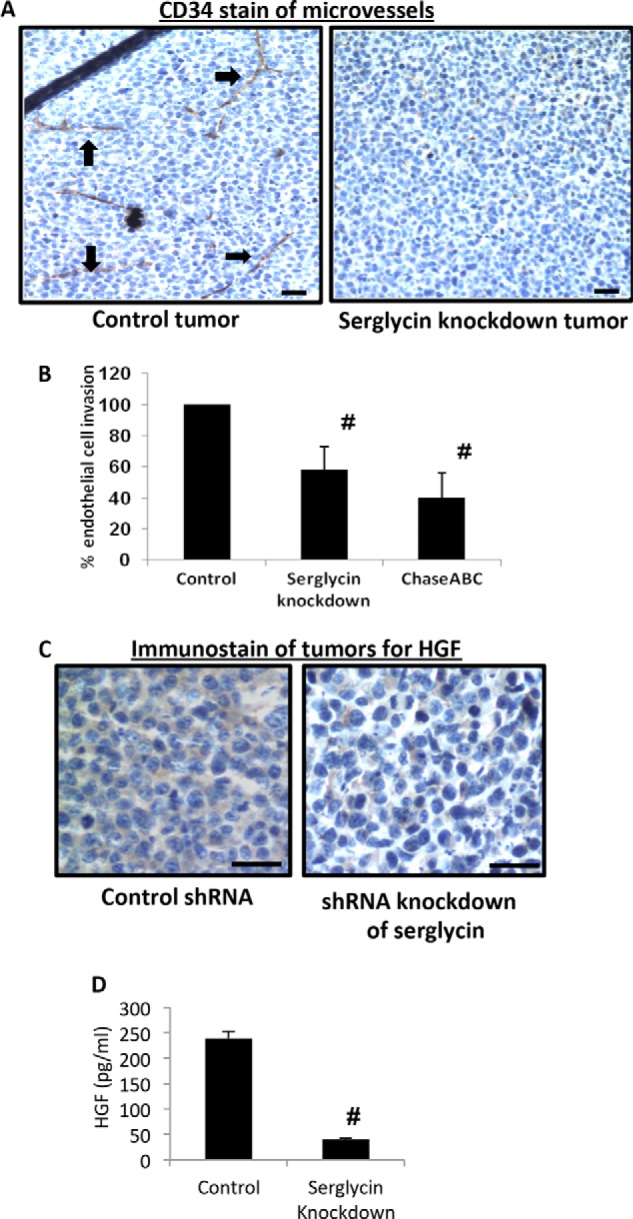
Tumors formed by serglycin-knockdown cells have a poorly developed vasculature and decreased levels of human HGF. A, immunohistochemistry of tumors formed by control (left panel) or serglycin-knockdown (right panel) cells. Tumor tissue was stained with antibody to CD34 (brown) and counterstained with hematoxylin (blue). Microvessels are indicated with black arrows. Scale bars, 200 μm. B, conditioned medium from serglycin-knockdown cells inhibits endothelial invasion. Endothelial cells were placed in the upper chamber of Matrigel invasion chambers and conditioned medium from control or serglycin-knockdown cells or from control cells pretreated with Chase ABC was placed in the lower chamber. After overnight incubation, endothelial cells that invaded through the Matrigel were fixed, stained, and counted. Data are mean ± S.D. of three independent experiments. #, p < 0.05 compared with control. C, immunohistochemistry of sections from xenograft tumors formed from control (left panel) or serglycin-knockdown (right panel) cells stained for human HGF (brown) and counterstained with hematoxylin (blue). Scale bars, 200 μm. D, medium conditioned by myeloma cells for 48 h was collected, and levels of human HGF quantified by ELISA. Data are from mean ± S.D. from three separate experiments. #, p < 0.05 compared with control.
Vascular endothelial growth factor (VEGF) and hepatocyte growth factor (HGF) are multifunctional cytokines that potently stimulate angiogenesis, including tumor neovascularization (29, 30). Therefore, we hypothesized that knocking down serglycin may alter the levels of pro-angiogenic cytokines in tumors. Even though no difference in VEGF expression was observed (data not shown), a striking difference in the HGF level was seen. Tumors formed from serglycin-knockdown cells showed much less detectable HGF compared with controls (Fig. 5C). Quantification of HGF levels in vitro revealed that serglycin-knockdown cells secreted significantly less HGF than control cells (Fig. 5D). HGF levels are highly elevated in myeloma patients, correlate with high microvessel density and are a significant predictor of mortality (31–33). Taken together, these results indicate that decreased endothelial cell invasion and decreased levels of HGF caused by serglycin knockdown may lead to impaired vascularization of tumors, resulting in poor tumor growth.
Activin Up-regulates the mRNA Levels of Serglycin and C4ST1
Activin, a TGF-β superfamily member cytokine, is a predominant cytokine that is up-regulated in myeloma patients with osteolytic lesions (34). Moreover, activin is known to induce C4ST1 expression, which is responsible for the 4-O-sulfation of N-acetylgalactosamine in CS chains (35). Because array analysis of genes involved in GAG synthesis and modification has shown that the C4ST1 gene is highly up-regulated in the plasma cells of MM patients compared with bone marrow plasma cells from healthy donors (36), and because the CS chains of myeloma-derived serglycin are almost completely composed of 4-O-sulfated disaccharides (4), we sought to investigate whether activin up-regulates C4ST1 expression in myeloma cells. CAG and 8226 human myeloma cells were treated with recombinant activin overnight and C4ST1 expression was analyzed. As shown in Fig. 6A, exposure of the myeloma cells to activin significantly enhanced the expression of C4ST1. Strikingly, high levels of up-regulation were also observed for serglycin mRNA, which was almost 3-fold higher in activin-treated cells compared with untreated controls (Fig. 6B). Further, we found that the ability of activin to induce serglycin expression in myeloma cells was much higher than another osteolytic cytokine TNF-α, which caused only a 2-fold increase in serglycin mRNA levels (data not shown). These results reveal a novel function for activin in myeloma progression and a molecular mechanism for the regulation of C4ST1 and serglycin expression in multiple myeloma. Whether or not the expression of C4ST1 and serglycin is associated with osteolytic disease in MM patients requires further investigation.
FIGURE 6.
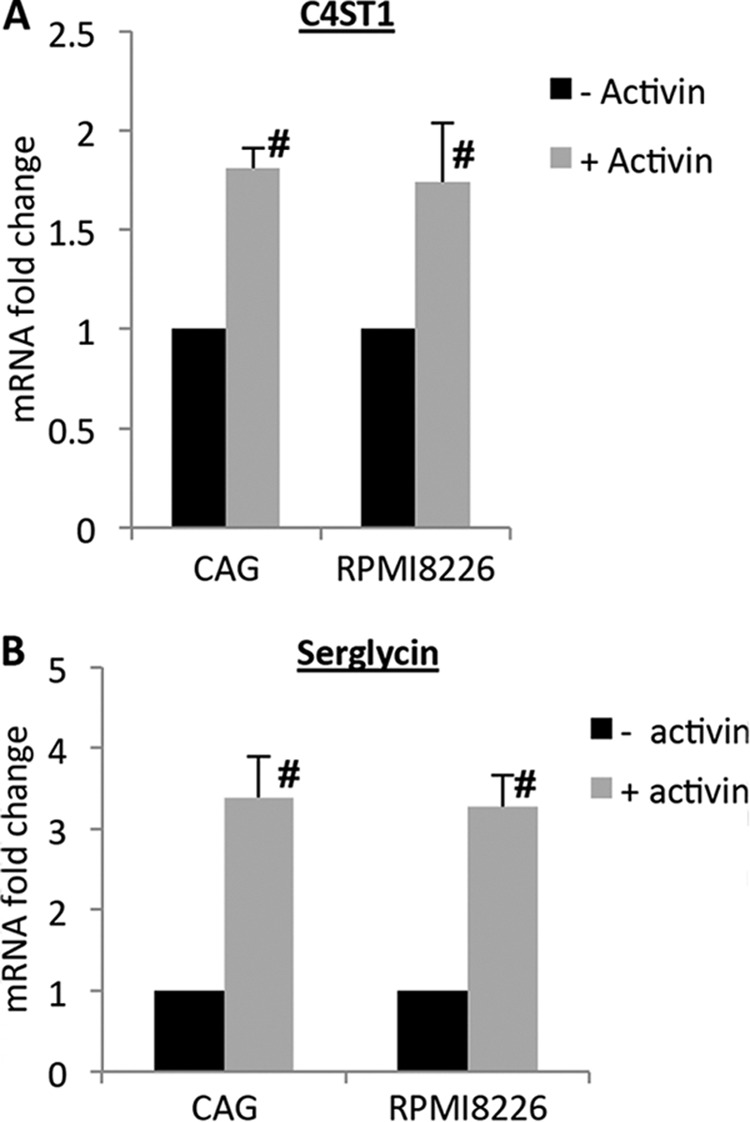
Activin enhances C4ST1 and serglycin expression in myeloma cells. CAG or RPMI 8226 myeloma cells were treated with recombinant activin (500 ng/ml) overnight and the expression of C4ST1 (A) and serglycin (B) was assessed by real time PCR and normalized to 28 S rRNA levels. Data are from three separate experiments ± S.D. #, p < 0.05 compared with activin-untreated cells.
DISCUSSION
The constitutive secretion of serglycin by myeloma cells and its up-regulation in the bone marrow biopsies and BM aspirates of myeloma patients (4) suggests that it may play a role in MM pathophysiology. However, until now, there has been no direct evidence that implicates serglycin in MM pathogenesis. Our present study demonstrates that serglycin knockdown in myeloma cells leads to poor tumor growth, low levels of HGF and impaired angiogenesis in myeloma tumors in vivo. The fact that serglycin knockdown has such a dramatic effect on these parameters strongly points to the importance of serglycin in myeloma progression in vivo. We also showed, for the first time, that serglycin secreted by myeloma cells has the ability to bind to CD44 receptors thereby mediating adhesion to bone marrow stromal cells, and that activin, a member of the TGF-β family, promotes the up-regulation of serglycin mRNA in myeloma cells. The latter findings point to possible mechanisms underlying the regulation and activity of serglycin in MM.
To the best of our knowledge, this is the first study showing a critical role for serglycin (an under-studied and important proteoglycan expressed in myeloma cells) in regulating the growth of myeloma tumors. But why does knocking down serglycin have such a dramatic impact on myeloma tumor growth and progression when the in vitro growth of serglycin-knockdown MM cells is not significantly different from that of control cells? Our hypothesis is that serglycin acts as a master regulator of multiple activities within the tumor microenvironment. Our results indicate that one of the critical activities of serglycin (which has up to eight GAG chains) in the myeloma tumor microenvironment is likely to be promotion of myeloma cell adhesion to bone marrow components, including bone marrow stromal cells (BMSCs) and collagen type I. Bone marrow provides a conducive environment for myeloma cells to grow, and the adhesive interaction of myeloma cells with bone marrow components is now recognized as a key factor in myeloma pathobiology. But how does serglycin promote adhesion, even though it is not an integral cell surface proteoglycan? The findings that serglycin is present on the surface of myeloma cells and that treatment with the CS-degrading enzyme, Chase ABC, partially removes cell-bound serglycin suggest that serglycin binds to the MM cell surface via its CS chains. The incomplete removal of cell surface serglycin after Chase ABC treatment also suggests the possible involvement of the core protein of serglycin in binding to yet unidentified cell surface binding partners. Further, our results show that CD44 is most likely to be the cell surface binding partner for serglycin CS chains. A previous study has shown that serglycin secreted by hematopoietic cells binds to CD44 receptors and promotes heterophilic or homophilic cell-cell interactions, by bridging the CD44 receptors of neighboring cells (25, 26). It is therefore possible that serglycin could promote the adhesion of myeloma cells through a similar mechanism. However, serglycin is only a partial mediator of cell adhesion, presumably since it is only one among several other adhesion molecules expressed by MM cells.
Yet another critical role of serglycin in regulating myeloma growth is the promotion of angiogenesis. Our finding that serglycin-knockdown cells fail to stimulate endothelial invasion in vitro and vasculature development in vivo supports this hypothesis. Proteoglycans present in the extracellular milieu aid in establishing gradients of pro-angiogenic growth factors and cytokines required for proper endothelial branching. For example, we previously demonstrated that syndecan-1, released from myeloma cells as a complex with bound VEGF, infiltrates and binds to the extracellular matrix where it promotes endothelial invasion and angiogenesis (21). It is possible that serglycin extends its range of function beyond that of cell-cell contacts within the tumor microenvironment. This capability could enhance tumor growth by “jump-starting” angiogenesis at sites where serglycin may not be at levels sufficient to initiate angiogenesis. For example, serglycin with bound angiogenic factors released from myeloma cells may also enter the circulation and nurture angiogenesis at sites distal to tumors, perhaps helping to establish a pre-metastatic niche. Although our data suggest that the majority of serglycin-associated angiogenic activity can be traced to HGF, it is possible that serglycin may interact with other myeloma-derived angiogenic factors, such as VEGF and MMP-9, and contribute to angiogenesis. It has been demonstrated that serglycin can covalently link to MMP-9 and this association is suggested to be important for the transport, targeting, and regulation of MMP-9 (37, 38).
Although serglycin has long been considered as a PG that is packaged into secretory granules and vesicles of hematopoietic cells and endothelial cells, including hematopoietic tumor cells, it is also constitutively secreted by cells (11, 12, 22, 39). While, within the cell, serglycin is involved in packaging and retention of various proteases, growth factors, and chemokines in storage granules and secretory vesicles, its secretion can aid in the release of its binding partners as complexes. This includes the release of proteases by mast cells (40, 41), GROα/CXCL1 by human endothelial cells (23), TNF-α by macrophages (42), and MMP-9 and several other proteins by monocytes (10, 43). Therefore, the presence or absence of serglycin can influence the balance of chemokines, growth factors, or cytokines within the tumor microenvironment. In addition, serglycin can further modulate the activities of partner molecules in the microenvironment through the protection, transport, activation of, and interactions with, substrates or target cells (7, 44). Therefore, more studies are needed to; (i) identify all the molecules whose secretion in MM is regulated by serglycin; (ii) determine if these molecules are secreted in complex with serglycin; and (iii) determine if serglycin regulates the function of their binding partners within the myeloma microenvironment.
Our confocal microscopic studies revealed that serglycin is distributed throughout the cytoplasm and in the nucleus of MM cells. Even though we have not characterized the intracellular distribution of serglycin in detail, it is likely that serglycin is contained within the cytoplasmic storage granules/secretory vesicles of MM cells (11) and involved in promoting the storage and regulating the activities of a number of proteases within these cells (8, 9, 45). The presence of serglycin in the nucleus of MM cells suggests that this PG may also be involved in regulating gene expression. We previously demonstrated that the HSPG syndecan-1 (CD138) are present within the nucleus of myeloma cells and can regulate the acetylation status of nuclear histones and thereby gene expression (16, 46). Further studies are required to understand the role of serglycin in the nucleus of myeloma cells.
Many of the functions of serglycin are mediated through its negatively charged GAG chains. However, based on the cell type, the GAG chains attached to serglycin can be either chondroitin sulfate or heparin. Our findings suggest that the serglycin expressed by MM cells is decorated by CS chains. This is consistent with a previous study showing that CS is the only type of GAG chain attached to myeloma-derived serglycin and that CS chains of serglycin are almost completely composed of 4-0-sulfate-substituted disaccharides (4). Using gene array analysis it has been previously shown that the CHST11 (C4ST1) gene, which is responsible for 4-0-sulfation of CS chains, is highly up-regulated in myeloma patients compared with that of healthy donors (36). Inhibiting C4ST1 expression could therefore shut down the sulfation of CS chains, and thereby the functions of serglycin. Our finding that activin, a TGF-β family member, can up-regulate the mRNA levels of both C4ST1 and serglycin in MM cells suggests that inhibiting activin could be a better approach for targeting serglycin functions in MM. Because activin levels are higher in myeloma patients with osteolytic disease (34) and because serglycin derived from MM cells is a potent inhibitor of bone mineralization (4), it will be interesting to see if there is any correlation between serglycin levels and osteolytic damage in MM.
For the first time, the data presented here suggest that serglycin, a major and an understudied PG expressed by myeloma cells, plays a critical role in promoting adhesion, angiogenesis and growth in MM. However, further studies are urgently required to completely understand the different roles played by serglycin in MM pathogenesis, which will hopefully provide us with new therapeutic techniques to tackle this fatal disease.
Acknowledgments
We thank Dr. Ralph D. Sanderson (UAB) for his excellent support and discussions throughout this work. We also thank Dr. Erming Tian (University of Arkansas at Little Rock) for providing OPM-1, Delta 47, OCIMy5, and KMS-11 cell lines. We acknowledge Enid Keyser from the University of Alabama at Birmingham for assistance with flow analysis and the RDCC-CFCC core (supported by National Institutes of Health Grant P30 AR48311 and P30 AI027767). We also thank Ed Phillips (University of Alabama at Birmingham, High Resolution Imaging Facility) for assistance with fluorescence microscopy. Histology services were performed by the University of Alabama at Birmingham Center for Metabolic Bone Diseases Histomorphometry and Molecular Analysis Core Laboratory (supported by National Institutes of Health Grant P30 AR46031).
This work was supported by the New Faculty Development Award from the Young Supporters Board of the UAB Comprehensive Cancer Center (to A. P.).
- MM
- multiple myeloma
- PG
- proteoglycan
- CS
- chondroitin sulfate
- C4ST1
- chondroitin 4-0-sulfotransferase-1
- Chase ABC
- chondroitinase ABC
- GAG
- glycosaminoglycan
- MTT
- [4,5-dimethylthiazol-2-yl]-2,5diphenyl-tetrazolium bromide.
REFERENCES
- 1. Neri P., Bahlis N. J. (2012) Targeting of adhesion molecules as a therapeutic strategy in multiple myeloma. Curr. Cancer Drug Targets 12, 776–796 [DOI] [PubMed] [Google Scholar]
- 2. Mitsiades C. S., Mitsiades N. S., Richardson P. G., Munshi N. C., Anderson K. C. (2007) Multiple myeloma: a prototypic disease model for the characterization and therapeutic targeting of interactions between tumor cells and their local microenvironment. J. Cell Biochem. 101, 950–968 [DOI] [PubMed] [Google Scholar]
- 3. Sanderson R. D., Yang Y. (2008) Syndecan-1: a dynamic regulator of the myeloma microenvironment. Clin. Exp. Metastasis 25, 149–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Theocharis A. D., Seidel C., Borset M., Dobra K., Baykov V., Labropoulou V., Kanakis I., Dalas E., Karamanos N. K., Sundan A., Hjerpe A. (2006) Serglycin constitutively secreted by myeloma plasma cells is a potent inhibitor of bone mineralization in vitro. J. Biol. Chem. 281, 35116–35128 [DOI] [PubMed] [Google Scholar]
- 5. Kolset S. O., Gallagher J. T. (1990) Proteoglycans in haemopoietic cells. Biochim. Biophys. Acta 1032, 191–211 [DOI] [PubMed] [Google Scholar]
- 6. Kulseth M. A., Kolset S. O., Ranheim T. (1999) Stimulation of serglycin and CD44 mRNA expression in endothelial cells exposed to TNF-α and IL-1α. Biochim. Biophys. Acta 1428, 225–232 [DOI] [PubMed] [Google Scholar]
- 7. Kolset S. O., Tveit H. (2008) Serglycin–structure and biology. Cell Mol. Life Sci. 65, 1073–1085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Abrink M., Grujic M., Pejler G. (2004) Serglycin is essential for maturation of mast cell secretory granule. J. Biol. Chem. 279, 40897–40905 [DOI] [PubMed] [Google Scholar]
- 9. Grujic M., Braga T., Lukinius A., Eloranta M. L., Knight S. D., Pejler G., Abrink M. (2005) Serglycin-deficient cytotoxic T lymphocytes display defective secretory granule maturation and granzyme B storage. J. Biol. Chem. 280, 33411–33418 [DOI] [PubMed] [Google Scholar]
- 10. Kolset S. O., Zernichow L. (2008) Serglycin and secretion in human monocytes. Glycoconj. J. 25, 305–311 [DOI] [PubMed] [Google Scholar]
- 11. Schick B. P., Gradowski J. F., San Antonio J. D. (2001) Synthesis, secretion, and subcellular localization of serglycin proteoglycan in human endothelial cells. Blood 97, 449–458 [DOI] [PubMed] [Google Scholar]
- 12. Schick B. P., Jacoby J. A. (1995) Serglycin and betaglycan proteoglycans are expressed in the megakaryocytic cell line CHRF 288–11 and normal human megakaryocytes. J. Cell Physiol. 165, 96–106 [DOI] [PubMed] [Google Scholar]
- 13. Li X. J., Ong C. K., Cao Y., Xiang Y. Q., Shao J. Y., Ooi A., Peng L. X., Lu W. H., Zhang Z., Petillo D., Qin L., Bao Y. N., Zheng F. J., Chia C. S., Iyer N. G., Kang T. B., Zeng Y. X., Soo K. C., Trent J. M., Teh B. T., Qian C. N. (2011) Serglycin is a theranostic target in nasopharyngeal carcinoma that promotes metastasis. Cancer Res. 71, 3162–3172 [DOI] [PubMed] [Google Scholar]
- 14. Niemann C. U., Kjeldsen L., Ralfkiaer E., Jensen M. K., Borregaard N. (2007) Serglycin proteoglycan in hematologic malignancies: a marker of acute myeloid leukemia. Leukemia 21, 2406–2410 [DOI] [PubMed] [Google Scholar]
- 15. Humphries D. E., Nicodemus C. F., Schiller V., Stevens R. L. (1992) The human serglycin gene. Nucleotide sequence and methylation pattern in human promyelocytic leukemia HL-60 cells and T-lymphoblast Molt-4 cells. J. Biol. Chem. 267, 13558–13563 [PubMed] [Google Scholar]
- 16. Purushothaman A., Hurst D. R., Pisano C., Mizumoto S., Sugahara K., Sanderson R. D. (2011) Heparanase-mediated loss of nuclear syndecan-1 enhances histone acetyltransferase (HAT) activity to promote expression of genes that drive an aggressive tumor phenotype. J. Biol. Chem. 286, 30377–30383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Purushothaman A., Chen L., Yang Y., Sanderson R. D. (2008) Heparanase stimulation of protease expression implicates it as a master regulator of the aggressive tumor phenotype in myeloma. J. Biol. Chem. 283, 32628–32636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Purushothaman A., Babitz S. K., Sanderson R. D. (2012) Heparanase enhances the insulin receptor signaling pathway to activate extracellular signal-regulated kinase in multiple myeloma. J. Biol. Chem. 287, 41288–41296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Khotskaya Y. B., Dai Y., Ritchie J. P., MacLeod V., Yang Y., Zinn K., Sanderson R. D. (2009) Syndecan-1 is required for robust growth, vascularization, and metastasis of myeloma tumors in vivo. J. Biol. Chem. 284, 26085–26095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Slomiany M. G., Dai L., Tolliver L. B., Grass G. D., Zeng Y., Toole B. P. (2009) Inhibition of Functional Hyaluronan-CD44 Interactions in CD133-positive Primary Human Ovarian Carcinoma Cells by Small Hyaluronan Oligosaccharides. Clin. Cancer Res. 15, 7593–7601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Purushothaman A., Uyama T., Kobayashi F., Yamada S., Sugahara K., Rapraeger A. C., Sanderson R. D. (2010) Heparanase-enhanced shedding of syndecan-1 by myeloma cells promotes endothelial invasion and angiogenesis. Blood 115, 2449–2457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schick B. P. (2010) Serglycin proteoglycan deletion in mouse platelets: physiological effects and their implications for platelet contributions to thrombosis, inflammation, atherosclerosis, and metastasis. Prog. Mol. Biol. Transl. Sci. 93, 235–287 [DOI] [PubMed] [Google Scholar]
- 23. Meen A. J., Øynebråten I., Reine T. M., Duelli A., Svennevig K., Pejler G., Jenssen T., Kolset S. O. (2011) Serglycin is a major proteoglycan in polarized human endothelial cells and is implicated in the secretion of the chemokine GROα/CXCL1. J. Biol. Chem. 286, 2636–2647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Falkowska-Hansen B., Oynebråten I., Uhlin-Hansen L., Smedsrød B. (2006) Endocytosis and degradation of serglycin in liver sinusoidal endothelial cells. Mol. Cell Biochem. 287, 43–52 [DOI] [PubMed] [Google Scholar]
- 25. Toyama-Sorimachi N., Kitamura F., Habuchi H., Tobita Y., Kimata K., Miyasaka M. (1997) Widespread expression of chondroitin sulfate-type serglycins with CD44 binding ability in hematopoietic cells. J. Biol. Chem. 272, 26714–26719 [DOI] [PubMed] [Google Scholar]
- 26. Toyama-Sorimachi N., Sorimachi H., Tobita Y., Kitamura F., Yagita H., Suzuki K., Miyasaka M. (1995) A novel ligand for CD44 is serglycin, a hematopoietic cell lineage-specific proteoglycan. Possible involvement in lymphoid cell adherence and activation. J. Biol. Chem. 270, 7437–7444 [DOI] [PubMed] [Google Scholar]
- 27. Toole B. P. (2009) Hyaluronan-CD44 Interactions in Cancer: Paradoxes and Possibilities. Clin. Cancer Res. 15, 7462–7468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ridley R. C., Xiao H., Hata H., Woodliff J., Epstein J., Sanderson R. D. (1993) Expression of syndecan regulates human myeloma plasma cell adhesion to type I collagen. Blood 81, 767–774 [PubMed] [Google Scholar]
- 29. Sengupta S., Gherardi E., Sellers L. A., Wood J. M., Sasisekharan R., Fan T. P. (2003) Hepatocyte growth factor/scatter factor can induce angiogenesis independently of vascular endothelial growth factor. Arterioscler. Thromb. Vasc. Biol. 23, 69–75 [DOI] [PubMed] [Google Scholar]
- 30. Iwasaki T., Hamano T., Ogata A., Hashimoto N., Kitano M., Kakishita E. (2002) Clinical significance of vascular endothelial growth factor and hepatocyte growth factor in multiple myeloma. Br. J. Haematol. 116, 796–802 [DOI] [PubMed] [Google Scholar]
- 31. Seidel C., Børset M., Turesson I., Abildgaard N., Sundan A., Waage A. (1998) Elevated serum concentrations of hepatocyte growth factor in patients with multiple myeloma. The Nordic Myeloma Study Group. Blood 91, 806–812 [PubMed] [Google Scholar]
- 32. Andersen N. F., Standal T., Nielsen J. L., Heickendorff L., Borset M., Sørensen F. B., Abildgaard N. (2005) Syndecan-1 and angiogenic cytokines in multiple myeloma: correlation with bone marrow angiogenesis and survival. Br. J. Haematol. 128, 210–217 [DOI] [PubMed] [Google Scholar]
- 33. Holt R. U., Fagerli U. M., Baykov V., Rø T. B., Hov H., Waage A., Sundan A., Børset M. (2008) Hepatocyte growth factor promotes migration of human myeloma cells. Haematologica 93, 619–622 [DOI] [PubMed] [Google Scholar]
- 34. Vallet S., Mukherjee S., Vaghela N., Hideshima T., Fulciniti M., Pozzi S., Santo L., Cirstea D., Patel K., Sohani A. R., Guimaraes A., Xie W., Chauhan D., Schoonmaker J. A., Attar E., Churchill M., Weller E., Munshi N., Seehra J. S., Weissleder R., Anderson K. C., Scadden D. T., Raje N. (2010) Activin A promotes multiple myeloma-induced osteolysis and is a promising target for myeloma bone disease. Proc. Natl. Acad. Sci. U.S.A. 107, 5124–5129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Klüppel M. (2010) The roles of chondroitin-4-sulfotransferase-1 in development and disease. Prog. Mol. Biol. Transl. Sci. 93, 113–132 [DOI] [PubMed] [Google Scholar]
- 36. Bret C., Hose D., Reme T., Sprynski A. C., Mahtouk K., Schved J. F., Quittet P., Rossi J. F., Goldschmidt H., Klein B. (2009) Expression of genes encoding for proteins involved in heparan sulphate and chondroitin sulphate chain synthesis and modification in normal and malignant plasma cells. Br. J. Haematol. 145, 350–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Malla N., Berg E., Theocharis A. D., Svineng G., Uhlin-Hansen L., Winberg J. O. (2013) In vitro reconstitution of complexes between pro-matrix metalloproteinase-9 and the proteoglycans serglycin and versican. Febs J. 280, 2870–2887 [DOI] [PubMed] [Google Scholar]
- 38. Abécassis I., Olofsson B., Schmid M., Zalcman G., Karniguian A. (2003) RhoA induces MMP-9 expression at CD44 lamellipodial focal complexes and promotes HMEC-1 cell invasion. Exp. Cell Res. 291, 363–376 [DOI] [PubMed] [Google Scholar]
- 39. Schick B. P., Senkowski-Richardson S. (1992) Proteoglycan synthesis in human erythroleukaemia (HEL) cells. Biochem. J. 282, 651–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pejler G., Abrink M., Ringvall M., Wernersson S. (2007) Mast cell proteases. Adv. Immunol. 95, 167–255 [DOI] [PubMed] [Google Scholar]
- 41. Serafin W. E., Katz H. R., Austen K. F., Stevens R. L. (1986) Complexes of heparin proteoglycans, chondroitin sulfate E proteoglycans, and [3H]diisopropyl fluorophosphate-binding proteins are exocytosed from activated mouse bone marrow-derived mast cells. J. Biol. Chem. 261, 15017–15021 [PubMed] [Google Scholar]
- 42. Zernichow L., Abrink M., Hallgren J., Grujic M., Pejler G., Kolset S. O. (2006) Serglycin is the major secreted proteoglycan in macrophages and has a role in the regulation of macrophage tumor necrosis factor-α secretion in response to lipopolysaccharide. J. Biol. Chem. 281, 26792–26801 [DOI] [PubMed] [Google Scholar]
- 43. Winberg J. O., Kolset S. O., Berg E., Uhlin-Hansen L. (2000) Macrophages secrete matrix metalloproteinase 9 covalently linked to the core protein of chondroitin sulphate proteoglycans. J. Mol. Biol. 304, 669–680 [DOI] [PubMed] [Google Scholar]
- 44. Sali A., Matsumoto R., McNeil H. P., Karplus M., Stevens R. L. (1993) Three-dimensional models of four mouse mast cell chymases. Identification of proteoglycan binding regions and protease-specific antigenic epitopes. J. Biol. Chem. 268, 9023–9034 [PubMed] [Google Scholar]
- 45. Pejler G., Abrink M., Wernersson S. (2009) Serglycin proteoglycan: regulating the storage and activities of hematopoietic proteases. Biofactors 35, 61–68 [DOI] [PubMed] [Google Scholar]
- 46. Chen L., Sanderson R. D. (2009) Heparanase regulates levels of syndecan-1 in the nucleus. PLoS One 4, e4947. [DOI] [PMC free article] [PubMed] [Google Scholar]


