Abstract
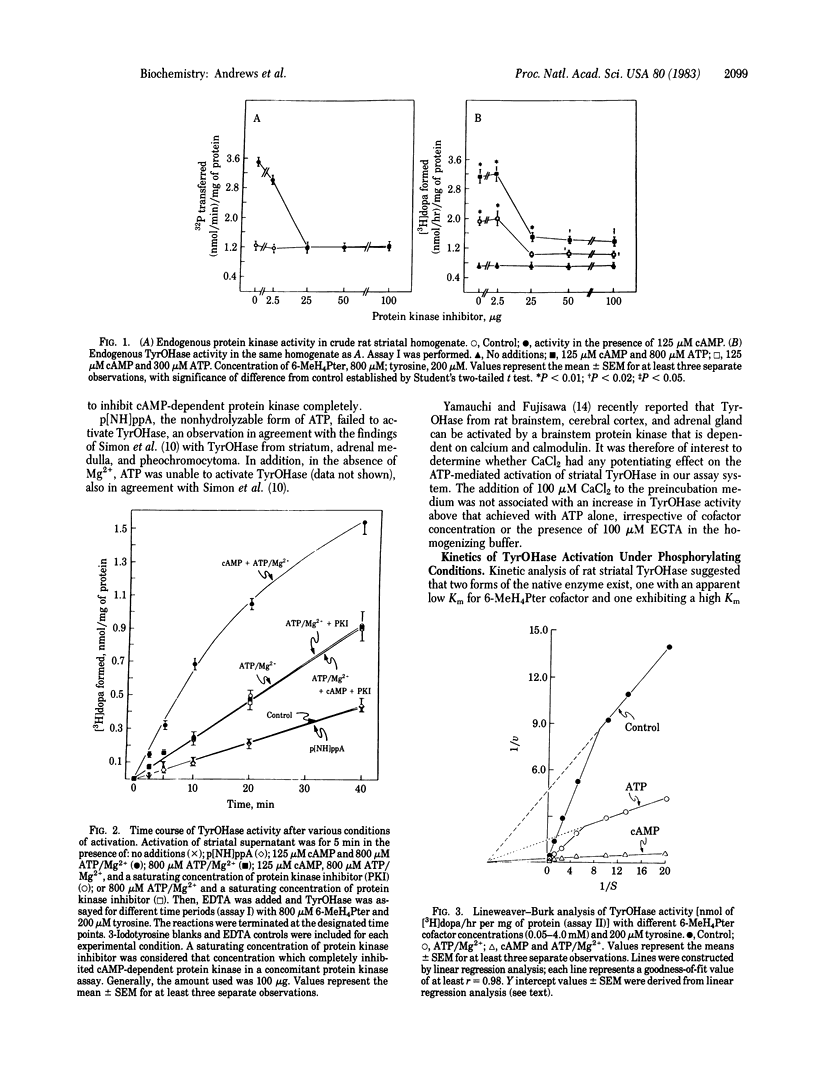
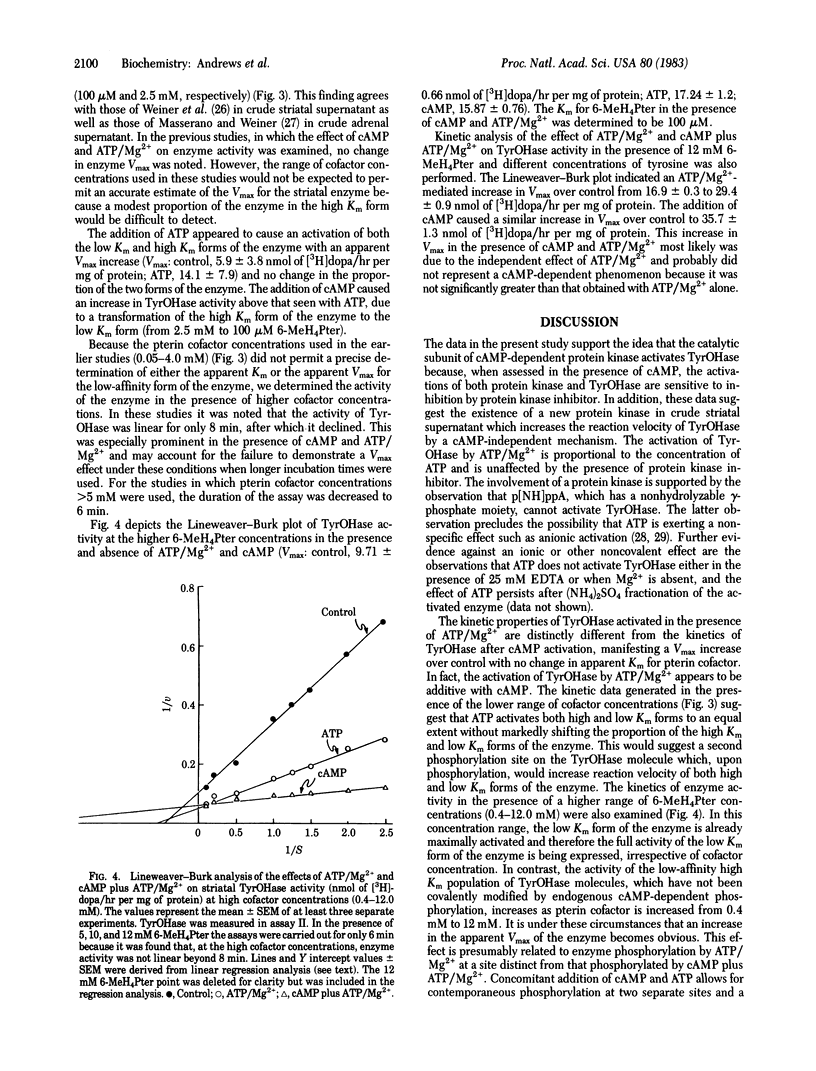
Activation of rat striatal tyrosine hydroxylase [TyrOHase; tyrosine monooxygenase; L-tyrosine, tetrahydropteridine:oxygen oxidoreductase (3-hydroxylating), EC 1.14.16.2] by ATP/Mg2+ and endogenous protein kinase can be produced without the addition of cAMP. This activation is not due to endogenous free catalytic subunit derived from cAMP-dependent protein kinase. In the presence of amounts of protein kinase inhibitor sufficient for complete inhibition of striatal cAMP-dependent protein kinase and the cAMP-mediated activation of TyrOHase, addition of ATP/Mg2+ results in an enhancement of TyrOHase activity. Enzyme activation does not occur when the nonhydrolyzable form of ATP, adenylyl imidodiphosphate, is substituted for ATP. When TyrOHase is assayed in the presence of ATP/Mg2+ and different concentrations of either tyrosine or 6-methyltetrahydropterin co-factor, a 2-fold increase in enzyme Vmax is demonstrable, with no change in the Km for either substrate or cofactor. In contrast, in the presence of cAMP and ATP/Mg2+, both an increase in Vmax and an enhanced affinity for pterin cofactor are demonstrable. In the latter circumstance, the 2-fold increase in Vmax can be attributed entirely to the action of cAMP-independent protein kinase. The addition of either EGTA or CaCl2 does not modify the effect seen in the presence of ATP, suggesting that the effect of ATP/Mg2+ is not mediated by a Ca2+-dependent protein kinase. These data support the existence of a cAMP-independent striatal protein kinase that can catalyze the activation of TyrOHase.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alousi A., Weiner N. The regulation of norepinephrine synthesis in sympathetic nerves: effect of nerve stimulation, cocaine, and catecholamine-releasing agents. Proc Natl Acad Sci U S A. 1966 Nov;56(5):1491–1496. doi: 10.1073/pnas.56.5.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames M. M., Lerner P., Lovenberg W. Tyrosine hydroxylase. Activation by protein phosphorylation and end product inhibition. J Biol Chem. 1978 Jan 10;253(1):27–31. [PubMed] [Google Scholar]
- Anagnoste B., Shirron C., Friedman E., Goldstein M. Effect of dibutyryl cyclic adenosine monophosphate on 14C-dopamine biosynthesis in rat brain striatal slices. J Pharmacol Exp Ther. 1974 Dec;191(3):370–376. [PubMed] [Google Scholar]
- Andrews D. N., Weiner N. Evidence for the involvement of a cyclic AMP independent protein kinase in the activation of rat striatal tyrosine hydroxylase. Proc West Pharmacol Soc. 1979;22:163–167. [PubMed] [Google Scholar]
- Coyle J. T. Tyrosine hydroxylase in rat brain--cofactor requirements, regional and subcellular distribution. Biochem Pharmacol. 1972 Jul 15;21(14):1935–1944. doi: 10.1016/0006-2952(72)90006-8. [DOI] [PubMed] [Google Scholar]
- Craine J. E., Hall E. S., Kaufman S. The isolation and characterization of dihydropteridine reductase from sheep liver. J Biol Chem. 1972 Oct 10;247(19):6082–6091. [PubMed] [Google Scholar]
- Demaille J. G., Peters K. A., Fischer E. H. Isolation and properties of the rabbit skeletal muscle protein inhibitor of adenosine 3',5'-monophosphate dependent protein kinases. Biochemistry. 1977 Jul 12;16(14):3080–3086. doi: 10.1021/bi00633a006. [DOI] [PubMed] [Google Scholar]
- Glowinski J., Iversen L. L. Regional studies of catecholamines in the rat brain. I. The disposition of [3H]norepinephrine, [3H]dopamine and [3H]dopa in various regions of the brain. J Neurochem. 1966 Aug;13(8):655–669. doi: 10.1111/j.1471-4159.1966.tb09873.x. [DOI] [PubMed] [Google Scholar]
- Greene L. A., Rein G. Short-term regulation of catecholamine biosynthesis in a nerve growth factor responsive clonal line of rat pheochromocytoma cells. J Neurochem. 1978 Mar;30(3):549–555. doi: 10.1111/j.1471-4159.1978.tb07808.x. [DOI] [PubMed] [Google Scholar]
- Harris J. E., Baldessarini R. J., Morgenroth V. H., 3rd, Roth R. H. Activation by cyclic 3':5'-adenosine monophosphate of tyrosine hydroxylase in the rat brain. Proc Natl Acad Sci U S A. 1975 Mar;72(3):789–793. doi: 10.1073/pnas.72.3.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joh T. H., Park D. H., Reis D. J. Direct phosphorylation of brain tyrosine hydroxylase by cyclic AMP-dependent protein kinase: mechanism of enzyme activation. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4744–4748. doi: 10.1073/pnas.75.10.4744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz I. R., Yamauchi T., Kaufman S. Activation of tyrosine hydroxylase by polyanions and salts. An electrostatic effect. Biochim Biophys Acta. 1976 Mar 11;429(1):84–95. doi: 10.1016/0005-2744(76)90032-2. [DOI] [PubMed] [Google Scholar]
- Krueger B. K., Forn J., Greengard P. Depolarization-induced phosphorylation of specific proteins, mediated by calcium ion influx, in rat brain synaptosomes. J Biol Chem. 1977 Apr 25;252(8):2764–2773. [PubMed] [Google Scholar]
- Kuczenski R. T., Mandell A. J. Allosteric activation of hypothalamic tyrosine hydroxylase by ions and sulphated mucopolysaccharides. J Neurochem. 1972 Jan;19(1):131–137. doi: 10.1111/j.1471-4159.1972.tb01262.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Langan T. A. Isolation of histone kinases. Methods Cell Biol. 1978;19:143–152. doi: 10.1016/s0091-679x(08)60019-9. [DOI] [PubMed] [Google Scholar]
- Lerner P., Nosé P., Ames M. M., Lovenberg W. Modification of the tyrosine hydroxylase assay. Increased enzyme activity in the presence of ascorbic acid. Neurochem Res. 1978 Oct;3(5):641–651. doi: 10.1007/BF00963765. [DOI] [PubMed] [Google Scholar]
- Lloyd T., Kaufman S. Evidence for the lack of direct phosphorylation of bovine caudate tyrosine hydroxylase following activation by exposure to enzymatic phosphorylating conditions. Biochem Biophys Res Commun. 1975 Oct 6;66(3):907–917. doi: 10.1016/0006-291x(75)90726-3. [DOI] [PubMed] [Google Scholar]
- Lovenberg W., Bruckwick E. A., Hanbauer I. ATP, cyclic AMP, and magnesium increase the affinity of rat striatal tyrosine hydroxylase for its cofactor. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2955–2958. doi: 10.1073/pnas.72.8.2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masserano J. M., Weiner N. The rapid activation of adrenal tyrosine hydroxylase by decapitation and its relationship to a cyclic AMP-dependent phosphorylating mechanism. Mol Pharmacol. 1979 Sep;16(2):513–528. [PubMed] [Google Scholar]
- Morgenroth V. H., 3rd, Hegstrand L. R., Roth R. H., Greengard P. Evidence for involvement of protein kinase in the activation by adenosine 3':5'-monophosphate of brain tyrosine 3-monooxygenase. J Biol Chem. 1975 Mar 10;250(5):1946–1948. [PubMed] [Google Scholar]
- Morita K., Oka M. Activation by cyclic AMP of soluble tyrosine hydroxylase in bovine adrenal medulla. FEBS Lett. 1977 Apr 15;76(2):148–150. doi: 10.1016/0014-5793(77)80141-5. [DOI] [PubMed] [Google Scholar]
- Raese J. D., Edelman A. M., Makk G., Bruckwick E. A., Lovenberg W., Barchas J. D. Brain striatal tyrosine hydroxylase: activation of the enzyme by cyclic AMP-independent phosphorylation. Commun Psychopharmacol. 1979;3(5):295–301. [PubMed] [Google Scholar]
- Renson J., Weissbach H., Udenfriend S. On the mechanism of oxidative cleavage of aryl-alkyl ethers by liver microsomes. Mol Pharmacol. 1965 Sep;1(2):145–148. [PubMed] [Google Scholar]
- Simon J. R., Hegstrand L. R., Roth R. H. Regulation of tyrosine hydroxylase from human pheochromocytoma, bovine adrenal and rat striatum. Life Sci. 1978 Feb;22(5):421–428. doi: 10.1016/0024-3205(78)90289-8. [DOI] [PubMed] [Google Scholar]
- Takimoto G. S., Weiner N. Evidence for the transsynaptic activation of tyrosine hydroxylase in rabbit portal vein mediated through a cyclic AMP-independent mechanism. J Pharmacol Exp Ther. 1981 Oct;219(1):97–106. [PubMed] [Google Scholar]
- Vulliet P. R., Langan T. A., Weiner N. Tyrosine hydroxylase: a substrate of cyclic AMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1980 Jan;77(1):92–96. doi: 10.1073/pnas.77.1.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner N., Lee F. L., Dreyer E., Barnes E. The activation of tyrosine hydroxylase in noradrenergic neurons during acute nerve stimulation. Life Sci. 1978 Apr 3;22(13-15):1197–1215. doi: 10.1016/0024-3205(78)90088-7. [DOI] [PubMed] [Google Scholar]
- Weiner N. Regulation of norepinephrine biosynthesis. Annu Rev Pharmacol. 1970;10:273–290. doi: 10.1146/annurev.pa.10.040170.001421. [DOI] [PubMed] [Google Scholar]
- Yamauchi T., Fujisawa H. Evidence for phosphorylation of bovine adrenal tyrosine hydroxylase by cyclic AMP-dependent protein kinase. Biochem Biophys Res Commun. 1978 May 30;82(2):514–517. doi: 10.1016/0006-291x(78)90904-x. [DOI] [PubMed] [Google Scholar]



