Background: The molecular mechanism of smooth muscle cell (SMC) migration, a crucial event in atherosclerosis, is not well understood.
Results: The de novo matricellular protein Cyr61 bridges lysophosphatidic acid (LPA) and integrin pathways, activating focal adhesion kinase (FAK) and leading to cell migration.
Conclusion: The LPA-Cyr61-integrin-FAK axis controls SMC migration.
Significance: This study provides new insights into mechanisms underlying cell migration-related disorders.
Keywords: Migration, Protein Kinases, Signal Transduction, Signaling, Vascular Biology
Abstract
Lysophosphatidic acid (LPA), a potent bioactive lipid found in atherosclerotic lesions, markedly induces smooth muscle cell (SMC) migration, which is an important process in atherogenesis. Therefore, understanding the mechanism of LPA-induced SMC migration is important. Several microarray databases suggest that the matricellular protein Cyr61 is highly induced by LPA. We hypothesized that Cyr61 mediates LPA-induced cell migration. Our data show that LPA induced temporal and spatial expression of Cyr61, which promptly accumulated in the cellular Golgi apparatus and then translocated to the extracellular matrix. Cyr61 antibody blockade and siRNA inhibition both diminished LPA-induced SMC migration, indicating a novel regulatory role of Cyr61. SMCs derived from LPA receptor 1 (LPA1) knock-out mice lack the ability of Cyr61 induction and cell migration, supporting the concept that LPA1 is required for Cyr61 expression and migration. By contrast, PPARγ was not found to be involved in LPA-mediated effects. Furthermore, focal adhesion kinase (FAK), a nonreceptor tyrosine kinase important for regulating cell migration, was activated by LPA at a late time frame coinciding with Cyr61 accumulation. Interestingly, knockdown of Cyr61 blocked LPA-induced FAK activation, indicating that an LPA-Cyr61-FAK axis leads to SMC migration. Our results further demonstrate that plasma membrane integrins α6β1 and ανβ3 transduced the LPA-Cyr61 signal toward FAK activation and migration. Taken together, these data reveal that de novo Cyr61 in the extracellular matrix bridges LPA and integrin pathways, which in turn, activate FAK, leading to cell migration. The current study provides new insights into mechanisms underlying cell migration-related disorders, including atherosclerosis, restenosis, and cancers.
Introduction
Lysophosphatidic acid (LPA)3 is a potent bioactive lipid component in oxidized LDL (1) and is likely the principal lipid component responsible for markedly inducing vascular smooth muscle cell (SMC) migration (2); SMC migration is one of the most important processes in the vascular lesion formation involved in atherosclerosis and restenosis. Although the phenomenon of LPA induction of cell migration is well known, the molecular mechanism by which LPA mediates cell migration is not yet fully understood. In particular, the functional role of matricellular proteins in LPA-induced cell migration has not been elucidated.
To explore the involvement of specific extracellular molecules in the LPA signaling pathway, we analyzed available published transcriptomic data and found that LPA markedly induces matricellular protein Cyr61 (CCN1) expression in fibroblasts and gastric adenocarcinoma cells (3–5). These microarray data were from the European Bioinformatics Institute (EBI)/ArrayExpress database (accession numbers E-NCMF-16, E-NCMF-17, E-NCMF-18, and E-NCMF-19) and GEO Microarray Database (accession numbers GSE10226 and GSE26309). Cyr61/Cef10 is a cysteine-rich matricellular protein. Chicken Cef10 and mouse Cyr61 were originally identified as growth factor-inducible genes by two groups (6, 7). Exogenous recombinant Cyr61 has been reported to induce angiogenesis (8) and promote cell proliferation, migration, adhesion, and differentiation (9, 10). In the present study, we hypothesized that endogenous Cyr61, induced by LPA, mediates LPA signaling, leading to SMC migration. To determine possible extracellular matrix (ECM) protein involvement in LPA-induced cell migration, we used primary SMCs isolated from wild type C57BL/6J mice and examined the effect of LPA on Cyr61 temporal and spatial expression in SMCs. We observed specific and dramatic induction of Cyr61 expression stimulated by LPA in vascular SMCs. We further explored the role of Cyr61 in LPA-induced SMC migration and related molecular mechanisms.
LPA elicits cellular responses mainly via its cognate G-protein coupled receptors (11). At least six specific G-protein-coupled receptors that trigger LPA signaling pathways have been identified: LPA1–6 (12). It has also been shown that LPA binds the nuclear receptor peroxisome proliferator-activated receptor (PPARγ) and induces PPARγ-dependent gene expression (13). In the present study, the specific PPARγ antagonist and primary SMCs isolated from wild type and LPA receptor knock-out mice were used to determine the specific role of LPA receptors and PPARγ involvement in LPA-induced Cyr61 expression and SMC migration.
Cyr61 transduces signals through cell surface integrins (14). In SMCs, Cyr61 binds to integrin α6β1 (15). The possibility that de novo Cyr61, induced by LPA, could signal through integrin engagement led us to further hypothesize that de novo Cyr61 produced by LPA might serve as a novel bridging molecule for LPA and integrin signaling.
The studies presented here demonstrate that LPA, via the activation of a specific cell membrane receptor, regulates Cyr61 expression. We further show that the induced Cyr61 proteins transiently accumulate in the Golgi apparatus and translocate to the ECM. Several approaches employed in determining the role of de novo Cyr61 in LPA-induced cell migration established the novel function of Cyr61. Notably, depletion of Cyr61 expression or integrin expression with siRNA largely blocked LPA-induced cell migration. Neutralizing extracellular Cyr61 with Cyr61 antibody or the use of integrin antibodies inhibited LPA-induced cell migration. These data reveal a new mechanism by which the de novo Cyr61, an extracellular signaling molecule, bridges LPA and integrin signal cascades and thus outlines a new pathway controlling cell migration.
EXPERIMENTAL PROCEDURES
Reagents
LPA (1-oleoyl-2-hydroxy-sn-glycero-3-phosphate) was purchased from Avanti Polar Lipids. TRIzol reagent and the ThermoScript RT-PCR system were from Invitrogen. The RNeasy kit was from Qiagen. GeneAmp PCR core reagents were from Applied Biosystems. Antibodies against mouse Cyr61 were from R&D Systems; antibody against LPA3 was from Cayman Chemical; antibody against β-actin was from Sigma; and antibody against γ-adaptin was from BD Transduction Laboratories. Antibodies against integrins β1, β3, β4, and β5, were from Cell Signaling Technology. Antibody against α6 was from Santa Cruz Biotechnology. Antibody against ανβ3 was from Millipore. GoTaq Flexl DNA polymerase and the reverse transcription system were from Promega. Recombinant Cyr61 protein was from Abcam. The LPA receptor primers used for conventional PCR were as follows: LPA1, 5′-AGC TGC CTC TAC TTC CAG C-3′ (forward) and 5′-TTG CTG TGA ACT CCA GCC AG-3′ (reverse); LPA2, 5′-ATG GGC CAG TGC TAC TAC AAC G-3′ (forward) and 5′ AGG GTG GAG TCC ATC AGT G-3′ (reverse); LPA3, 5′-GAC AAG CGC ATG GAC TTT-3′ (forward) and 5′-CAT GTC CTC GTC CTT GTA CG-3′ (reverse); LPA4, 5′-GTT GTA TTC ATC CTG GGT CT-3′ (forward) and 5′-AGC GAC TCC ATC CTT ATA TG-3′ (reverse); and LPA5, 5′-TGC TCT GAC CTT GTT GTT CC-3′ (forward) and 5′-AGC AAC CCA TAT ACA GCC AGC G-3′ (reverse).
RT-PCR Assay
mRNA expression levels of various LPA receptors were evaluated. Total RNA was isolated from SMCs using TRIzol reagent. The first strand of cDNA was reverse-transcribed using a reverse transcription system. The cDNA products were amplified using GoTaq Flexl DNA polymerase. Amplification conditions were as follows: 5 min at 95 °C and 27–33 cycles of 30 s at 95 °C, 30 s at 55 °C, and 1 min at 72 °C. The reaction was followed by a final extension for 10 min at 72 °C. The PCR products were analyzed by electrophoresis on a 1.0% agarose gel.
Tissue Culture
Mouse aortic SMCs were prepared from explants of excised aortas of mice as described previously (16). Cells were maintained in DMEM containing 10% fetal bovine serum. Cells were made quiescent by incubation in serum-free DMEM for 48 h. LPA was dissolved in PBS. Human aortic endothelial cells were from Life Technologies. Cells were cultured according to the manufacturer's instructions. The production and characterization of mice deficient in LPA receptors 1, 2, and 3 have previously been described (17–19).
Western Blot Analysis
Cultured mouse SMCs were rinsed with cold PBS and lysed in Western blot lysis buffer (50 mm Tris-HCl, pH 6.8, 8 m urea, 5% mercaptoethanol, 2% SDS, and protease/phosphatase inhibitors) with sonication for 30 s on ice. Cellular proteins were separated by 10% SDS-PAGE and transferred to a polyvinylidene fluoride membrane (Immobilon-P, Millipore). Membranes were then probed with the specific antibodies, and the specific protein bands were viewed using ECL Plus (GE Healthcare).
Northern Blot Analysis
Total cellular RNA was isolated using TRIzol reagent according to the manufacturer's instructions. Total RNA (8–10 μg) was subjected to denaturing electrophoresis on formaldehyde-agarose gels. RNA was blotted onto Nytran membranes (Schleicher & Schüll) and hybridized with radiolabeled cDNA probes. 18 S and 28 S ribosomal RNA were used as internal controls.
Immunoprecipitation
Cells were lysed in immunoprecipitation lysis buffer (1% CHAPS, 30 mm Tris-HCl, pH 8.0, 150 mm NaCl, 5 mm EDTA, and protease/phosphatase inhibitor mixture). After sonication for 20 s, total cell lysates were centrifuged at 14,000 × g for 5 min at 4 °C to remove cell debris, and the supernatants were incubated with Cyr61 antibody (R&D Systems) for 3 h with rotation at 4 °C. Protein A-Sepharose beads were then added and incubated with rotation overnight at 4 °C. After washing five times with cold PBS, the immunoprecipitates were separated by SDS-PAGE and probed with Cyr61 antibody.
Immunofluorescence
SMCs grown on cover glass slides were fixed in 4% ice-cold paraformaldehyde solution for 30 min followed by treatment with or without 0.3% Triton X-100 in PBS for 5 min at room temperature. The cells were then incubated for 1 h in 5% goat serum blocking buffer (Sigma) plus 0.1% Tween 20 in PBS and incubated with Cyr61 antibody or γ-adaptin in 1/100 dilution overnight at 4 °C. After being washed with PBS three times (5 min each), the cells were incubated with the secondary antibody, goat anti-sheep IgG Alexa Fluor 488, or Rhodamine Red-X-conjugated AffiniPure goat anti-mouse IgG for 2 h at room temperature. Then the cells were washed with PBS four times (5 min each) at room temperature, incubated with DAPI for 2 min, and washed with PBS three times (5 min each) at room temperature. Subsequently, the coverslips were mounted on slides with permanent aqueous mounting medium (Biogenex), and the labeled cells were analyzed by fluorescence microscopy with a Nikon Eclipse E600 microscope.
siRNA Treatment
Cells were transfected with nonsilencing or specific siRNA (Qiagen) for 48 h using Lipofectamine RNAiMAX reagent (Invitrogen) following the instructions provided by the manufacturer. On day 3, cells were starved for 48 h followed by treatment either with or without LPA.
Preparation of Detached Cells and Extracellular Matrix
SMCs were grown in 60-mm dishes. After removal of the culture medium and rinsing with PBS, cells were detached from the dish by incubation with 1 mm EDTA. The cells were then rinsed twice with 1 mm EDTA to remove remaining cells. Cellular fractions were lysed as described under “Western Blot Analysis.” Extracellular material remaining on the dishes after removal of the cellular components was extracted by scraping at 90 °C in 1× Laemmli sample buffer (60 mm Tris-HCl, pH 6.8, 2% SDS, 5% β-mercaptoethanol, 5% glycerol). These fractions were designated as ECM as described previously (20).
Cell Migration Assay
Cell migration was performed by trypsinizing SMCs and plating them onto Transwell migration plates from Corning for migration assays. 2 × 105 cells were added to the upper chamber. Cells were allowed to migrate through filters (8-μm pore size), which had been precoated on both sides with gelatin, in the presence of either medium (600 μl) alone or medium with LPA at designated concentrations in the lower chamber. Cell migration was carried out at 37 °C in 5% CO2 for 6 h. Cells remaining on the upper surface of the filter were carefully removed by mechanical scraping. Cells that migrated to the lower side were fixed with methanol and then stained with Harris hematoxylin and eosin. The number of cells that had migrated to the lower surface of the filter was counted in four random objective fields (200×magnification) using a Nikon Eclipse E600 microscope.
Statistical Analysis
Results are means ± S.E. Comparisons between multiple groups were performed using one-way analysis of variance with post hoc t tests. Single comparisons were made using two-tailed, unpaired Student t tests. A p value of 0.05 was considered statistically significant.
RESULTS
LPA Markedly Induces Cyr61 Expression in Vascular SMCs
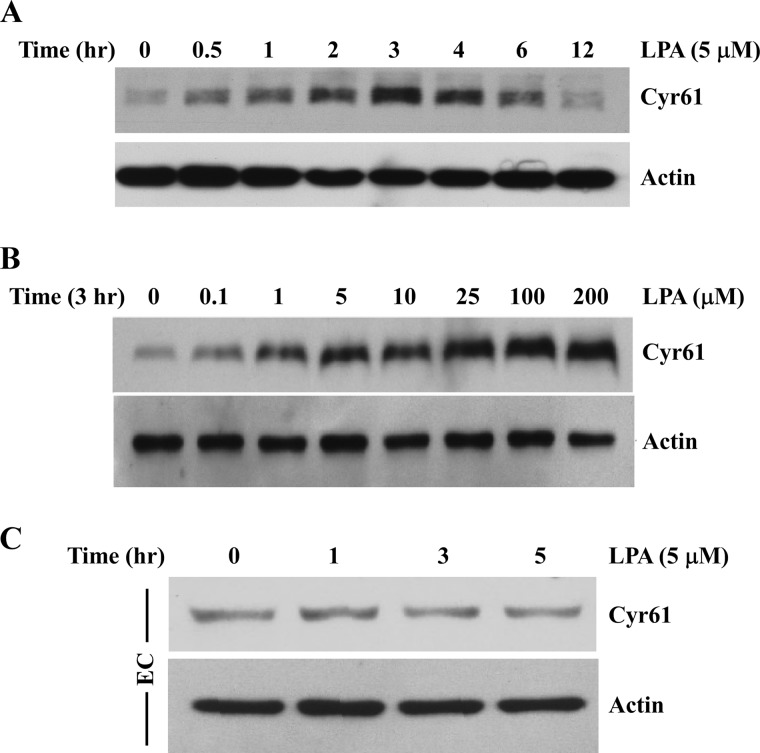
The molecular basis for the migration effect of LPA has not been fully elucidated. In particular, whether and how LPA-induced specific matricellular proteins are involved in cell migration has been largely undetermined. Searching the available RNA array databases indicated that LPA markedly induces matricellular protein Cyr61 expression in several cell types (3–5). To explore the possible role of extracellular molecule Cyr61 in LPA-induced cell migration, we first determined whether LPA affects the expression profile of Cyr61 in SMCs. Cultured mouse aortic SMCs were serum-starved for 48 h and then treated with 5 μm LPA for various time periods. Cell lysates were analyzed by SDS-PAGE, and Cyr61 protein expression was determined by Western blotting. As shown in Fig. 1A, we found that LPA markedly induced Cyr61 protein expression, peaking at around 3 h. LPA induction of Cyr61 was in a dose-dependent manner (Fig. 1B). At concentrations above 1 μm, LPA significantly induced Cyr61 protein expression. In the following studies, 5 or 10 μm LPA was used because these concentrations are in the range of LPA concentrations found in pathological conditions (1, 21). To address the specificity of LPA induction of Cyr61 in vascular SMCs, we compared Cyr61 expression in response to LPA in vascular endothelial cells. As shown in Fig. 1C, we found that LPA does not significantly induce Cyr61 expression in aortic endothelial cells, indicating that LPA specifically induces Cyr61 expression in aortic SMCs.
FIGURE 1.
LPA markedly induced Cyr61 protein expression in SMCs. A, time course of LPA induction of Cyr61 protein expression in mouse aortic SMCs. Cultured cells were starved for 48 h prior to LPA (5 μm) stimulation for various times as indicated. Cell lysates were subjected to Western blot analysis. The same membrane was reprobed with β-actin antibody to assess protein loading. B, Western blot analysis showing dose-dependent LPA induction of Cyr61 expression in SMCs. C, Western blot analysis showing the effect of LPA on Cyr61 expression in human aortic endothelial cells (EC). β-Actin served as the loading control.
Temporal and Spatial Expression of Cyr61 in Vascular SMCs in Response to LPA Stimulation
Although growth factor induction of Cyr61 expression has been reported (7, 22), the dynamics of intracellular trafficking of endogenous Cyr61 have not been documented. To investigate the intracellular dynamic expression of the induced Cyr61 protein, we used immunofluorescence microscopy technology to trace the induced Cyr61 protein intracellularly. Cells were cultured on cover glass slides for the selected time frames in response to LPA stimulation as indicated under “Experimental Procedures” and then fixed in 4% paraformaldehyde followed by permeabilization with 0.3% Triton X-100. Green fluorescence indicates Cyr61 protein location. Interestingly, we found a dynamic expression of Cyr61 (green), which was concentrated in specific intracellular compartments after 1 h of LPA treatment (Fig. 2A, top panel). However, the accumulation was transient and disappeared after 3 h of LPA treatment (Fig. 2A). It has been postulated that Cyr61 contains an N-terminal secretory signal sequence (7), suggesting that Cyr61 proteins pass through a secretory pathway. Given that after synthesis, secretory proteins are transported to the Golgi apparatus, where they intensively concentrate (23, 24), we assessed whether LPA-induced Cyr61 accumulates in the Golgi apparatus. Merged images shown in Fig. 2A (bottom panel) indicate that at 1 h of LPA stimulation, Cyr61 colocalized with the Golgi marker γ-adaptin, appearing in yellow. These data demonstrate that LPA-induced Cyr61 transiently and highly accumulates in the SMC Golgi apparatus, peaking at 1 h and disappearing after 3 h. We further detected extracellular Cyr61 accumulation in response to 3 h of LPA stimulation (Fig. 2B). Cells with or without LPA treatment were fixed in 4% paraformaldehyde without Triton permeabilization. Our data demonstrated that LPA induced Cyr61 via an intracellular secretary pathway through accumulation at the Golgi apparatus and translocation to the extracellular compartment.
FIGURE 2.
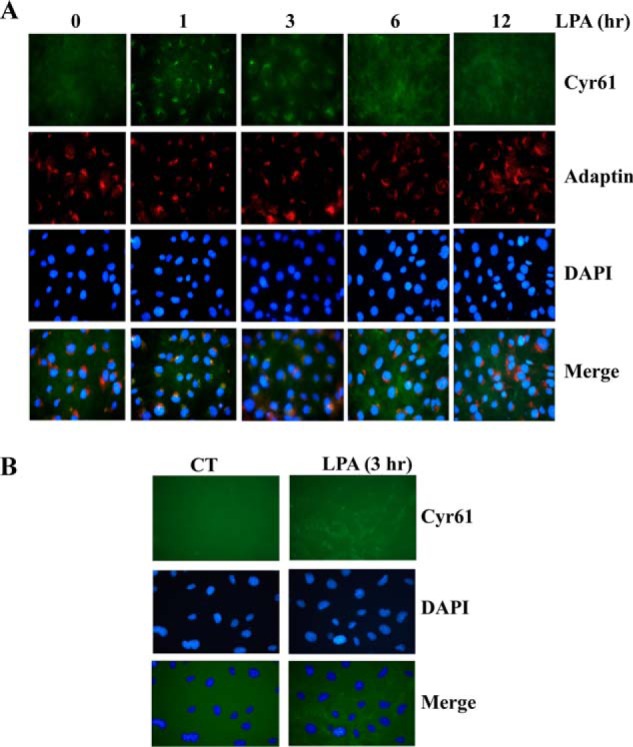
Temporal and spatial expression of Cyr61 protein induced by LPA in SMCs. A, immunofluorescence data revealing the dynamics of Cyr61 expression in SMCs in response to LPA stimulation. Quiescent SMCs on slides were stimulated with LPA (5 μm) for various times as indicated. After paraformaldehyde fixation, cells were treated with 0.3% Triton X-100 for permeabilization of the plasma membrane and then immunostained with specific antibodies against Cyr61, DAPI (nuclear marker), and γ-adaptin (Golgi marker). The expression of Cyr61 (green) and γ-adaptin (red) was examined by fluorescence microscopy; areas of co-localization are shown in the merged images (yellow). B, immunofluorescence data showing LPA induction of Cyr61 accumulation in the extracellular SMCs. Cultured SMCs on slides were stimulated with LPA (5 μm) for 3 h and then fixed with paraformaldehyde solution without Triton X-100 treatment. Extracellular Cyr61 protein was stained with the specific Cyr61 antibody. CT, control.
The Destination of LPA-induced Cyr61 Protein Is in the ECM
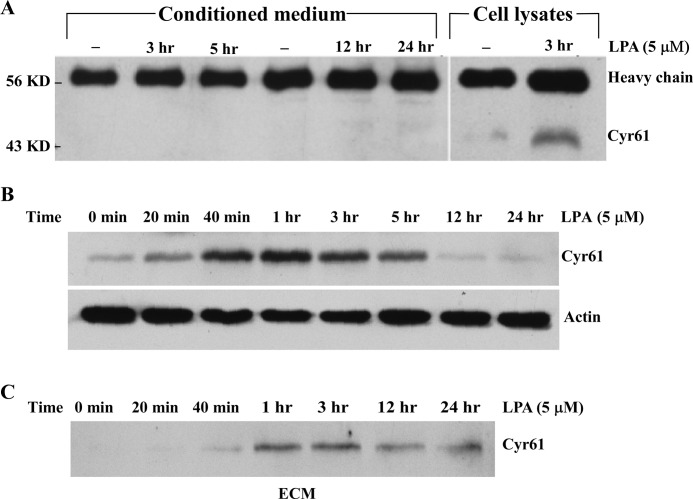
The above data suggest that LPA-induced Cyr61 via the secretory pathway is translocated extracellularly. To determine whether LPA-induced Cyr61 is located in ECM or in medium, we collected conditioned medium of SMCs at various time points after LPA stimulation; the collected medium were immunoprecipitated with the specific Cyr61 antibody. Western analysis results indicated that LPA-induced Cyr61 was not secreted into conditioned medium (Fig. 3A). To determine whether LPA-induced Cyr61 is secreted into ECM, we assessed Cyr61 accumulation in ECM as compared with that accumulated intracellularly. At various time points, cells stimulated with or without LPA were detached from the cultured dishes with 1 mm EDTA. After removal of the cellular fraction, the ECM was collected by incubation in Laemmli sample buffer at 90 °C for 1 h followed by scraping of the dish as described previously (20). As shown in Fig. 3, B and C, Western analysis from the paired groups of detached cell lysates and ECM indicate that the de novo synthesized Cyr61 was first highly accumulated intracellularly at 40 min, peaked around 1 h, and disappeared after 5 h. Accompanying this dynamic intracellular process, Cyr61 started to accumulate in the ECM at 1 h, peaking at 1–3 h, and lasting after 24 h. Therefore, LPA-induced Cyr61 protein went through the intracellular pathway, was promptly secreted into the ECM compartment, and bound to the matrix without release from the matrix into the medium.
FIGURE 3.
LPA-induced Cyr61 protein was secreted into the ECM. A, LPA-induced Cyr61 protein was detected in cell lysates but not in conditioned medium. Conditioned medium or cell lysates were collected after LPA stimulation of SMCs for various times, and then the conditioned medium and cell lysates were immunoprecipitated with the specific Cyr61 antibody. Western blot analysis of Cyr61 protein was carried out using a specific Cyr61 antibody. IgG heavy chain and Cyr61 bands were detected. B, Western blot analysis of SMCs detached from culture dishes. Cells were detached from the dishes with 1 mm EDTA at various times as indicated and lysed in lysis buffer followed by Western blot analysis. β-Actin was used to assess protein loading. C, Western blot analysis of ECM proteins. ECM remaining on the culture dishes was extracted in Laemmli sample buffer (described under “Experimental Procedures”) and then subjected to Western blot analysis.
LPA Receptor 1 (LPA1) Mediates Cyr61 Expression
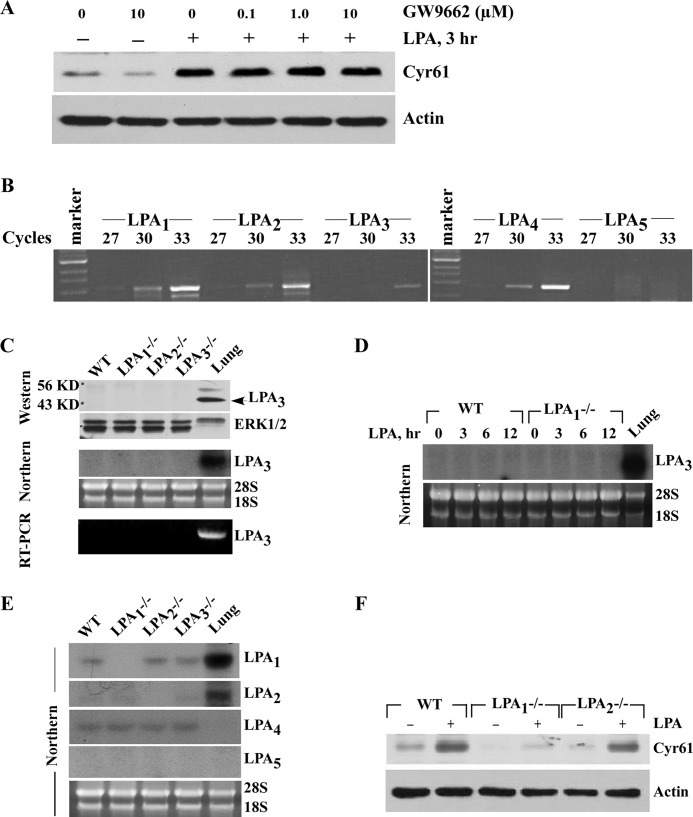
Although LPA transduces its signal mainly via its cognate cell membrane receptors (11), the possibility of the involvement of nuclear receptor PPARγ that binds to LPA has also been reported (13). To determine the involvement of PPARγ, we first tested whether PPARγ-specific antagonist GW9662 had an effect on LPA-induced Cyr61 expression. As shown in Fig. 4A, GW9662 had no effect on LPA-induced Cyr61 expression. A dose of 1 μm was reported to efficiently block PPARγ activation in vascular SMCs (53). Our data indicate that PPARγ was not involved in the LPA-induced Cyr61 expression.
FIGURE 4.
LPA1, but not PPARγ, mediated LPA-induced Cyr61 protein expression. A, pretreatment with the PPARγ-specific antagonist GW9662 had no effect on LPA-induced Cyr61 protein expression. Quiescent SMCs were pretreated with GW9662 at the concentrations indicated for 45 min, and then 5 μm LPA was added for 3 h. Cyr61 protein level was determined by Western blotting with 10% SDS-PAGE. β-Actin was used as the loading control. B, the expression of LPA1–5 receptor mRNA in SMCs was determined by RT-PCR. Total RNA from SMCs was extracted with TRIzol reagent. After reverse transcription, cDNA was used to perform PCR analysis with mouse LPA1–5 primers for various cycles as indicated. The RT-PCR results were evaluated in 1.2% agarose gels. DNA markers are indicated on the left side of the gel. C, results from Western blotting, Northern blotting, and RT-PCR (30 cycles) indicated that LPA3 levels were not enhanced in either LPA1-deficient or LPA2-deficient SMCs as compared with levels in wild type SMCs. Mouse lung tissue samples were included as positive controls for LPA3 expression. ERK and 28 S/18 S served as internal controls. D, LPA has no effect on LPA3 expression in wild type (WT) and LPA1-deficient SMCs (Northern blot). Mouse lung tissue sample was included as a positive control for LPA3 expression. E, Northern data demonstrated that expression levels of other LPA receptors were not changed in various LPA receptor-deficient SMCs. Mouse lung tissue samples were used as positive controls. F, LPA induced Cyr61 expression in wild type, LPA1−/−, or LPA2−/− SMCs. 5 μm LPA was added to quiescent wild type, LPA1−/−, or LPA2−/− SMCs for 3 h. Cyr61 protein level was determined by Western blotting with 10% SDS-PAGE. β-Actin was used as the loading control.
Thus far, at least six G-protein-coupled LPA receptors (LPA1–6) have been reported (12). To determine the role of cell surface G-protein-coupled LPA receptors in Cyr61 expression, we first analyzed the expression levels of the well established LPA receptors (LPA1–5) in mouse SMCs by RT-PCR. The results show the expression levels as follows: LPA1 > LPA2 = LPA4 >> LPA3 (Fig. 4B); clearly, the expression levels of LPA3 are very low, and LPA5 is not expressed in SMCs. We next sought to examine the expression relationship among these LPA receptors to evaluate 1) whether one LPA receptor deficiency alters expression of the other LPA receptors and 2) whether LPA receptor expression is influenced by LPA using SMCs isolated from wild type or LPA receptor knock-out mice. A previous study speculated that deficiency in LPA1 enhanced LPA3 expression in mouse SMCs based on a real-time PCR result (25). We employed three approaches (RT-PCR, Northern blotting, and Western blotting) to examine whether deficiency in LPA1 or LPA2 affects LPA3 expression at both the mRNA (indirect and direct levels) and the protein levels in mouse SMCs. As shown in Fig. 4C, neither LPA1 deficiency nor LPA2 deficiency has an effect on LPA3 expression. We observed that LPA does not up-regulate LPA3 expression in either wild type SMCs or LPA1-deficient SMCs (Fig. 4D). Furthermore, our Northern results indicate that deficiency of any one LPA receptor (LPA1–3) does not affect expression of other LPA receptors in SMCs (Fig. 4E). Altogether, these results support a notion that no one kind of endogenous LPA receptor expression (LPA1–5) in SMCs is influenced by deficiency of any one other kind of LPA receptor (LPA1–3).
To address the role of LPA receptors in Cyr61 expression, we used SMCs from LPA receptor knock-out mice and compared Cyr61 expression in response to LPA in wild type SMCs with those isolated from either the LPA1 or the LPA2 knock-out mice. As shown in Fig. 4F, knock-out of LPA1 nearly completely blocked LPA induction of Cyr61 expression as compared with wild type SMCs; however, knock-out of LPA2 expression had almost no effect on Cyr61 expression, indicating that LPA1 was the major responsible receptor mediating LPA signaling leading to Cyr61 expression.
De Novo Expression of Cyr61 Induced by LPA Mediates LPA-induced Cell Migration
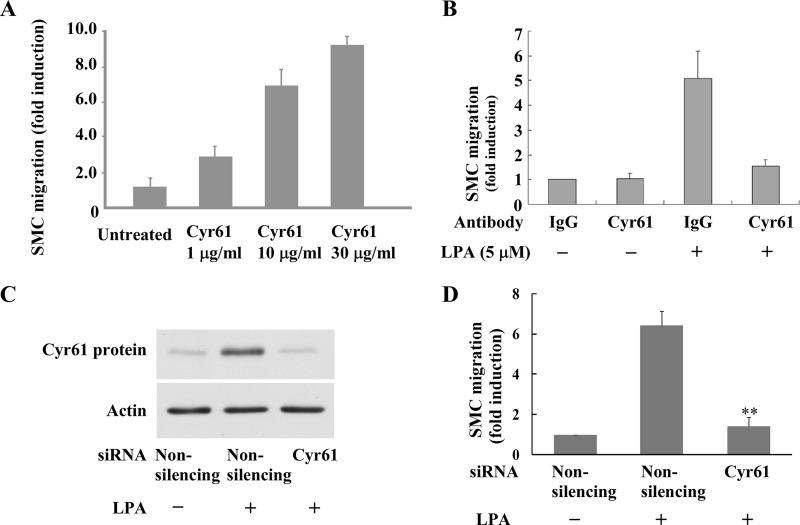
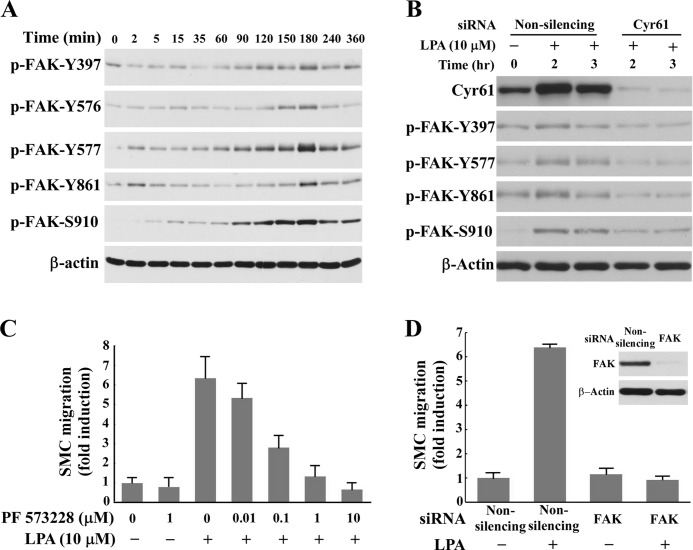
We next pursued the biological function of the de novo synthesized Cyr61 by LPA in SMCs. LPA has been shown to induce SMC migration (26); however, whether de novo, LPA-synthesized Cyr61 plays a role in LPA pathway mediation of cell migration is undocumented. We hypothesized that LPA-induced de novo Cyr61 contributes to LPA signaling for SMC migration in light of evidence that the exogenous addition of recombinant Cyr61 stimulates bovine SMC chemotaxis (15). To test our hypothesis, we first determined whether recombinant Cyr61 induced SMC migration. As shown in Fig. 5A, recombinant Cyr61 dose-dependently induced SMC migration. Secondly, we determined whether neutralizing Cyr61 function by Cyr61 antibody affected LPA-induced SMC migration. As shown in Fig. 5B, our data clearly demonstrate that pretreatment with Cyr61 antibody largely blocked LPA-induced SMC migration. Thirdly, we tested whether depletion of the newly synthesized Cyr61 with Cyr61 siRNA had any effect on LPA-induced SMC migration. As shown in Fig. 5, C and D, our results reveal that knockdown of the newly synthesized Cyr61 nearly completely blocked LPA signaling for cell migration. Together, these data strongly support a new role for the de novo synthesized Cyr61 in LPA-induced cell migration. These data further indicate that the newly synthesized extracellular Cyr61 is an important component of the LPA signaling pathway leading to LPA-stimulated cellular function.
FIGURE 5.
LPA-induced SMC migration was mediated by Cyr61 protein. A, recombinant Cyr61 protein dose-dependently induced SMC migration. The migration of SMCs was examined using a Transwell chamber. Various doses of recombinant protein Cyr61 were added to the bottom chamber. Quiescent SMCs were added to the upper Transwell chambers and allowed to migrate for 6 h. Relative migration rates were means ± S.E. of three experiments. B, pretreatment with Cyr61-specific antibody blocked LPA-induced SMC migration. Quiescent SMCs were pretreated with either IgG or the specific Cyr61 antibody for 45 min prior to LPA stimulation. Cell migration assay was performed as indicated in panel A. C, Western blot results show that Cyr61 siRNA blocked LPA-induced Cyr61 expression. β-Actin was used as the loading control. D, knockdown of Cyr61 expression using the specific Cyr61 siRNA blocked LPA-induced SMC migration. Relative migration rates were means ± S.E. of three experiments. Data were analyzed using one-way analysis of variance with post hoc t tests. **, p < 0.01 versus control.
LPA1, but Not the Nuclear Receptor PPARγ, Mediates LPA-induced SMC Migration in SMCs
The above data indicate that LPA1 mediates Cyr61 expression in SMCs and that Cyr61 mediates LPA signaling, leading to cell migration. Next we addressed whether LPA1 is the major LPA receptor that mediates LPA-induced SMC migration. We demonstrated that SMCs from LPA1 knock-out mice failed to respond to LPA stimulation for migration. However, we did not observe significant changes in migration rate in the LPA2 knock-out SMCs as compared with the wild type SMCs, indicating that LPA1 but not LPA2 mainly controls SMC migration (Fig. 6, A and B). These data are consistent with our observation that upstream LPA1 mediates Cyr61 expression and that synthesized Cyr61 contributes to LPA-induced SMC migration. We next evaluated the possibility of nuclear receptor PPARγ involvement in LPA-induced SMC migration. The PPARγ-specific antagonist GW9662 at effective dose ranges had no effect on LPA-induced SMC migration (Fig. 6C), indicating that PPARγ has no role in LPA-induced SMC migration.
FIGURE 6.
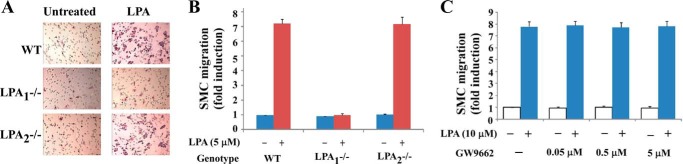
LPA-induced SMC migration was mediated by the LPA cognate plasma membrane receptor. A and B, LPA1 mediated SMC migration. Quiescent wild type (WT), LPA1−/−, and LPA2−/− SMCs were stimulated with 5 μm LPA; migration results are illustrated in A (representative Transwell chamber assay results) and B (bar graphs of migration data). C, the PPARγ-specific antagonist GW9662, at various doses, was used to treat SMCs for 45 min prior to LPA stimulation. Pretreatment with GW9662 did not affect LPA-induced SMC migration.
Focal Adhesion Kinase (FAK) Is a Downstream Component of Cyr61 in the LPA Signaling Pathway, and Cyr61 Mediates FAK Activation in SMCs
To determine the molecular mechanism by which Cyr61 mediates LPA-induced cell migration, we examined 1) whether FAK activation is the late event of LPA signaling, 2) whether Cyr61 mediates FAK activation, and 3) whether inhibition of FAK activity and down-regulation of FAK expression affect LPA-induced SMC migration. Intracellular FAK activation is important in cell migration because it regulates dynamics of cell adhesion, actin polymerization, and cytoskeleton reorganization (27–29). Several sites of tyrosine phosphorylation have been identified in FAK to mediate FAK activity or FAK interaction with SH2 domain-containing proteins. The major autophosphorylation site Tyr-397 is essential for the majority of FAK functions (30). The phosphorylated Tyr-397 site, via other protein mediators, phosphorylates Tyr-576 and Tyr-577 in the activation loop of the FAK enzyme (31) and Tyr-861 at the C-terminal domain (32). We found that stimulation with LPA induced late-phase FAK activation, peaking around 2–3 h (Fig. 7A) and suggesting that FAK activation may be the downstream factor of Cyr61 in response to LPA. To test our hypothesis, we examined whether depletion of Cyr61 using specific Cyr61 siRNA had an effect on FAK activation. As shown in Fig. 7B, down-regulation of Cyr61 effectively blocked the activation of FAK, indicating that LPA-induced Cyr61 mediates LPA-induced FAK activation. We next examined the functional role of FAK in LPA-induced SMC migration. Pretreatment of SMCs with PF573228, the specific inhibitor of FAK, dose-dependently blocked LPA-induced migration (Fig. 7C), suggesting that FAK is involved in LPA-induced cell migration. Knockdown of FAK expression with the specific siRNA completely blocked LPA-induced SMC migration (Fig. 7D), indicating that FAK is a key regulatory molecule in the LPA signaling pathway mediating SMC migration.
FIGURE 7.
Cyr61 mediated FAK activation in the LPA signaling pathway. A, LPA induced late-phase FAK activation. Quiescent SMCs were stimulated with LPA (5 μm) for various times. Cell lysates were subjected to Western blot analysis using antibodies against FAK phosphorylated (P) at specific sites. B, knockdown of Cyr61 expression with specific Cyr61 siRNA blocked LPA-induced FAK activation. β-Actin was used as the loading control. C, pretreatment (40 min) with PF 573228, a specific inhibitor of FAK, dose-dependently blocked LPA-induced SMC migration. D, knockdown of FAK expression with the specific FAK siRNA blocked LPA-induced SMC migration. Inset: Western blot results of knockdown of FAK expression.
Integrins α6β1 and αvβ3 Are Mediators of the LPA-Cyr61 Pathway Modulating LPA-induced FAK Activation and Cell Migration
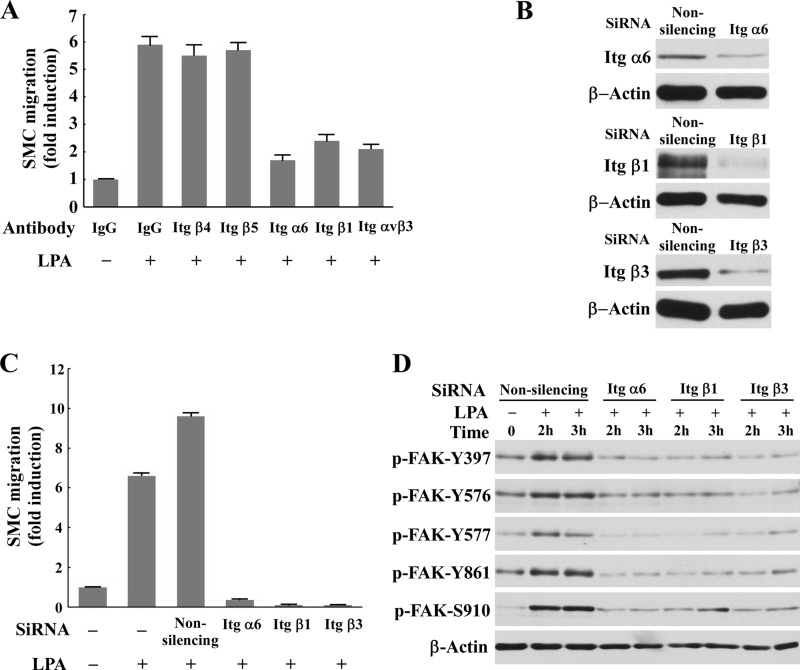
To further explore the molecular mechanisms by which the LPA-Cyr61 signaling pathway leads to SMC migration, we examined the role of cell membrane integrins in the LPA-Cyr61-FAK axis because integrins have been shown to mediate growth factor-induced FAK activation and cell migration (33–35) and integrin α6β1 was reported to interact with exogenous recombinant Cyr61 (15). Currently, whether and how integrins mediate LPA-induced SMC migration is unknown. To assess the role of integrins in LPA-induced SMC migration, we evaluated the effect of specific integrin antibodies on LPA-induced SMC migration. A number of integrin subunits such as α5, α6, β1, β3, β5, α6β1, and ανβ3 are expressed in SMCs (36–38). We found that the specific antibodies against α6, β1, or ανβ3 blocked LPA-induced SMC migration, but the antibodies against β4 and β5 had no effect on LPA-induced SMC migration (Fig. 8A), suggesting that integrins α6β1 and ανβ3 are required for LPA-induced SMC migration. To confirm the role of α6β1 and ανβ3 in LPA-induced SMC migration, we took another approach to knock down the expression of the α6, β1, and β3 subunits using specific siRNAs against each subunit. As shown in Fig. 8, B and C, knockdown of α6, β1, or β3 completely blocked LPA-induced SMC migration, indicating that integrins α6β1 and ανβ3 mediate LPA-induced SMC migration. To explore the mechanism by which these integrins mediate that migration, we examined whether knockdown of these integrins had an effect on LPA-induced phosphorylation of FAK. As shown in Fig. 8D, knockdown of α6, β1, or β3 nearly completely blocked LPA-induced FAK activation. These results indicate that integrins α6β1 and ανβ3 at the cytoplasmic membrane transduce the LPA-Cyr61 signal to intracellular FAK, leading to SMC migration.
FIGURE 8.
The LPA-Cyr61 pathway mediated LPA-induced FAK activation and cell migration via integrins α6β1 and ανβ3. A, The specific antibodies against integrin (Itg) α6, β1, or ανβ3, but not the β4 and β5 antibodies, blocked LPA-induced SMC migration. IgG was used as a control. B and C, knockdown of integrins (Itg) α6, β1, and β3 with specific siRNAs blocked LPA-induced SMC migration. Knockdown efficiency of integrin expression was assessed with Western analysis (B). Cell migration analysis was performed using a Transwell chamber assay. D, Western blot results indicate that knockdown of integrins α6, β1, or β3 blocked LPA-induced FAK activation. P, phosphorylated.
Taken together, as summarized in Fig. 9, the current data provide the first evidence that the de novo matricellular protein Cyr61 in the ECM bridges LPA and integrin pathways, which in turn, activate FAK, leading to cell migration.
FIGURE 9.
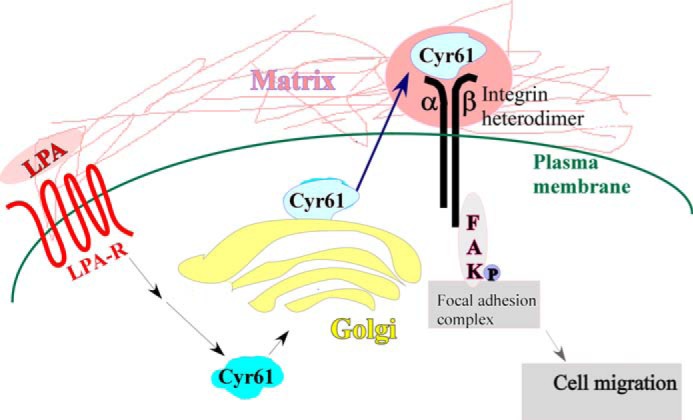
Summary illustration. De novo matricellular protein Cyr61 bridges the LPA signaling pathway and the integrin signaling pathway, leading to SMC migration. LPA-R, LPA receptor.
DISCUSSION
Aortic SMC migration from media to intima is an important process in the development of atherosclerosis. Many factors contribute to SMC migration (39), including oxidized LDL and its oxidative lipid components. We previously found that of the tested lipids of oxidized LDL, LPA is the prominent chemotactic lipid component (2) involved in SMC migration.
As illustrated in Fig. 9, in the current study, we identified that matricellular protein Cyr61 is a key molecule in the LPA signaling pathway, controlling LPA-induced SMC migration. Our data further reveal that Cyr61, via activation of FAK, leads to SMC migration and that plasma membrane receptor integrins α6β1 and ανβ3 are essential molecules mediating LPA-induced extracellular molecule Cyr61 signaling; these integrins transduce Cyr61 signaling toward intracellular FAK activation. Our results, to the best of our knowledge, show the first evidence that Cyr61 bridges LPA and integrin signaling pathways leading to cell migration. Previously, a study demonstrated that Cyr61 mediates thrombin-induced astrocytoma cell proliferation (40). A recent study described that Cyr61 mediates LPA-induced prostate tumor cell invasion, suggesting that Cyr61 is a potential biomarker correlating with prostate cancer aggressiveness (41). Our results that Cyr61 bridges LPA signaling and integrin signaling, leading to SMC migration, demonstrate that Cyr61 is a potentially important molecule that connects intracellular and extracellular information and thus contributes to atherogenesis.
Our data demonstrate that LPA markedly induces Cyr61 expression in SMCs in intracellular compartments and the ECM. LPA-induced Cyr61 proteins, via the secretion pathway, transiently accumulate in the Golgi apparatus and then translocate to the ECM. These results provide the first evidence that endogenous Cyr61 is through the intracellular Golgi secretory pathway before its translocation to the ECM.
Although nuclear receptor PPARγ has been reported to be an intracellular receptor for LPA (13), its role in LPA-dependent effects was not observed, consistent with other studies (42). Instead, we found that LPA mediates Cyr61 expression and SMC migration via a cell membrane-specific, receptor-mediated LPA1 signaling pathway. Using primary SMCs isolated from specific LPA receptor knock-out mice, our results reveal that LPA1 is required for Cyr61 induction and SMC migration.
Data from a series of experimental results support our conclusion that Cyr61 is the key molecule mediating LPA-induced SMC migration (Fig. 5). 1) Recombinant protein Cyr61 dose-dependently induced SMC migration; 2) pretreatment with Cyr61-specific antibody from various sources (Fig. 5 and data not shown) blocked LPA-induced SMC migration; and 3) pretreatment with Cyr61-specific siRNA nearly completely blocked LPA-induced SMC migration. Therefore, de novo Cyr61 protein synthesized by LPA in the ECM appears to be a key mediator continually transducing LPA signals to the intracellular compartment to execute the effect of LPA.
Integrins are transmembrane receptors and have been reported to transmit “inside-out” and “outside-in” signaling; for review, see Ref. 43. As compared with the well documented fact that integrins serve as receptors to interact with ECM proteins such as laminin, fibronectin, collagen, and vitronectin, the relationship between matricellular protein Cyr61 and integrins, as well as the connected signaling pathways and the derived biological consequences from these interactions, remains largely unelucidated. LPA signaling has diverse effects on adhesion molecules including focal adhesions (44) and integrins (45). Studies conducted in the Lau laboratory have revealed various properties of integrin interaction with Cyr61, as follows: interaction of integrin ανβ3 with Cyr61 mediates fibroblast proliferation and endothelial cell adhesion and migration (8, 46, 47); interaction of integrin αIIbβ3 with Cyr61 mediates blood platelet adhesion (48); interaction of integrin ανβ5 with Cyr61 mediates fibroblast migration (46); interaction of integrin α6β1 with Cyr61 mediates human skin fibroblast adhesion (49), bovine aortic SMC migration (15), and fibroblast senescence (50); and interaction of both ανβ5 and α6β1 with Cyr61 mediates apoptosis (51). These results indicate that the specific interactions between Cyr61 and integrins mediate diverse and specific cell functions. A previous study described that integrin subunit β1 is involved in LPA signaling pathway mediating fibroblastic cell migration (52). However, whether the integrin pathway is connected with the LPA-Cyr61 pathway has been unknown. Our data demonstrate that integrins α6β1 and ανβ3 are downstream components of the LPA-Cyr61 axis, which mediates LPA-induced SMC migration. Therefore, the results of this study reveal that the LPA-Cyr61-integrin-FAK axis is an important pathway mediating SMC migration.
In summary, our study provides new evidence that de novo Cyr61 protein in the ECM serves as a novel bridging molecule that connects the two pathways, namely the LPA and integrin signaling pathways, leading to cell migration. The finding from this study provides new insights into molecular mechanisms underlying the development of many cell migration-related disorders, including atherosclerosis, restenosis, and cancers.
Acknowledgment
We thank Misty Bailey for critical reading of the manuscript.
This work was supported, in whole or in part, by National Institutes of Health Grants HL107466 (to M. Z. C.), AG026640 (to X. X.), and NS082092 (to J. C.). This work was also supported by an American Health Assistance Foundation Grant (to X. X.)
- LPA
- lysophosphatidic acid
- ECM
- extracellular matrix
- SMC
- smooth muscle cell
- LPA1–6
- LPA receptors 1–6
- PPARγ
- peroxisome proliferator-activated receptor
- FAK
- focal adhesion kinase.
REFERENCES
- 1. Siess W., Zangl K. J., Essler M., Bauer M., Brandl R., Corrinth C., Bittman R., Tigyi G., Aepfelbacher M. (1999) Lysophosphatidic acid mediates the rapid activation of platelets and endothelial cells by mildly oxidized low density lipoprotein and accumulates in human atherosclerotic lesions. Proc. Natl. Acad. Sci. U.S.A. 96, 6931–6936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cui M.-Z. (2011) Lysophosphatidic acid effects on atherosclerosis and thrombosis. Clin. Lipidol. 6, 413–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Stortelers C., Kerkhoven R., Moolenaar W. H. (2008) Multiple actions of lysophosphatidic acid on fibroblasts revealed by transcriptional profiling. BMC Genomics 9, 387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mili S., Moissoglu K., Macara I. G. (2008) Genome-wide screen reveals APC-associated RNAs enriched in cell protrusions. Nature 453, 115–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bennett G., Sadlier D., Doran P. P., Macmathuna P., Murray D. W. (2011) A functional and transcriptomic analysis of NET1 bioactivity in gastric cancer. BMC Cancer 11, 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Simmons D. L., Levy D. B., Yannoni Y., Erikson R. L. (1989) Identification of a phorbol ester-repressible v-src-inducible gene. Proc. Natl. Acad. Sci. U.S.A. 86, 1178–1182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. O'Brien T. P., Yang G. P., Sanders L., Lau L. F. (1990) Expression of cyr61, a growth factor-inducible immediate-early gene. Mol. Cell. Biol. 10, 3569–3577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Babic A. M., Kireeva M. L., Kolesnikova T. V., Lau L. F. (1998) CYR61, a product of a growth factor-inducible immediate early gene, promotes angiogenesis and tumor growth. Proc. Natl. Acad. Sci. U.S.A. 95, 6355–6360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kireeva M. L., MO F. E., Yang G. P., Lau L. F. (1996) Cyr61, a product of a growth factor-inducible immediate-early gene, promotes cell proliferation, migration, and adhesion. Mol. Cell. Biol. 16, 1326–1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wong M., Kireeva M. L., Kolesnikova T. V., Lau L. F. (1997) Cyr61, product of a growth factor-inducible immediate-early gene, regulates chondrogenesis in mouse limb bud mesenchymal cells. Dev. Biol. 192, 492–508 [DOI] [PubMed] [Google Scholar]
- 11. Houben A. J., Moolenaar W. H. (2011) Autotaxin and LPA receptor signaling in cancer. Cancer Metastasis Rev. 30, 557–565 [DOI] [PubMed] [Google Scholar]
- 12. Mutoh T., Rivera R., Chun J. (2012) Insights into the pharmacological relevance of lysophospholipid receptors. Br. J. Pharmacol. 165, 829–844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. McIntyre T. M., Pontsler A. V., Silva A. R., St Hilaire A., Xu Y., Hinshaw J. C., Zimmerman G. A., Hama K., Aoki J., Arai H., Prestwich G. D. (2003) Identification of an intracellular receptor for lysophosphatidic acid (LPA): LPA is a transcellular PPARγ agonist. Proc. Natl. Acad. Sci. U.S.A. 100, 131–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lau L. F., Lam S. C. (1999) The CCN family of angiogenic regulators: the integrin connection. Exp. Cell Res. 248, 44–57 [DOI] [PubMed] [Google Scholar]
- 15. Grzeszkiewicz T. M., Lindner V., Chen N., Lam S. C., Lau L. F. (2002) The angiogenic factor cysteine-rich 61 (CYR61, CCN1) supports vascular smooth muscle cell adhesion and stimulates chemotaxis through integrin α6β1 and cell surface heparan sulfate proteoglycans. Endocrinology 143, 1441–1450 [DOI] [PubMed] [Google Scholar]
- 16. Brock T. A., Alexander R. W., Ekstein L. S., Atkinson W. J., Gimbrone M. A., Jr. (1985) Angiotensin increases cytosolic free calcium in cultured vascular smooth muscle cells. Hypertension 7, I105–I109 [DOI] [PubMed] [Google Scholar]
- 17. Contos J. J., Fukushima N., Weiner J. A., Kaushal D., Chun J. (2000) Requirement for the lpA1 lysophosphatidic acid receptor gene in normal suckling behavior. Proc. Natl. Acad. Sci. U.S.A. 97, 13384–13389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Contos J. J., Ishii I., Fukushima N., Kingsbury M. A., Ye X., Kawamura S., Brown J. H., Chun J. (2002) Characterization of lpa2 (Edg4) and lpa1/lpa2 (Edg2/Edg4) lysophosphatidic acid receptor knockout mice: signaling deficits without obvious phenotypic abnormality attributable to lpa2. Mol. Cell. Biol. 22, 6921–6929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ye X., Hama K., Contos J. J., Anliker B., Inoue A., Skinner M. K., Suzuki H., Amano T., Kennedy G., Arai H., Aoki J., Chun J. (2005) LPA3-mediated lysophosphatidic acid signalling in embryo implantation and spacing. Nature 435, 104–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bradley R. S., Brown A. M. (1990) The proto-oncogene int-1 encodes a secreted protein associated with the extracellular matrix. EMBO J. 9, 1569–1575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rother E., Brandl R., Baker D. L., Goyal P., Gebhard H., Tigyi G., Siess W. (2003) Subtype-selective antagonists of lysophosphatidic acid receptors inhibit platelet activation triggered by the lipid core of atherosclerotic plaques. Circulation 108, 741–747 [DOI] [PubMed] [Google Scholar]
- 22. Pendurthi U. R., Allen K. E., Ezban M., Rao L. V. (2000) Factor VIIa and thrombin induce the expression of Cyr61 and connective tissue growth factor, extracellular matrix signaling proteins that could act as possible downstream mediators in factor VIIa·tissue factor-induced signal transduction. J. Biol. Chem. 275, 14632–14641 [DOI] [PubMed] [Google Scholar]
- 23. Jamieson J. D., Palade G. E. (1966) Role of the Golgi complex in the intracellular transport of secretory proteins. Proc. Natl. Acad. Sci. U.S.A. 55, 424–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Palade G. (1975) Intracellular aspects of the process of protein synthesis. Science 189, 347–358 [DOI] [PubMed] [Google Scholar]
- 25. Panchatcharam M., Miriyala S., Yang F., Rojas M., End C., Vallant C., Dong A., Lynch K., Chun J., Morris A. J., Smyth S. S. (2008) Lysophosphatidic acid receptors 1 and 2 play roles in regulation of vascular injury responses but not blood pressure. Circ. Res. 103, 662–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kim J., Keys J. R., Eckhart A. D. (2006) Vascular smooth muscle migration and proliferation in response to lysophosphatidic acid (LPA) is mediated by LPA receptors coupling to Gq. Cell. Signal. 18, 1695–1701 [DOI] [PubMed] [Google Scholar]
- 27. Tomar A., Schlaepfer D. D. (2009) Focal adhesion kinase: switching between GAPs and GEFs in the regulation of cell motility. Curr. Opin. Cell Biol. 21, 676–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schaller M. D. (2010) Cellular functions of FAK kinases: insight into molecular mechanisms and novel functions. J. Cell Sci. 123, 1007–1013 [DOI] [PubMed] [Google Scholar]
- 29. Zhao X., Guan J. L. (2011) Focal adhesion kinase and its signaling pathways in cell migration and angiogenesis. Adv. Drug Deliv. Rev. 63, 610–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schaller M. D., Hildebrand J. D., Shannon J. D., Fox J. W., Vines R. R., Parsons J. T. (1994) Autophosphorylation of the focal adhesion kinase, pp125FAK, directs SH2-dependent binding of pp60src. Mol. Cell. Biol. 14, 1680–1688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Calalb M. B., Polte T. R., Hanks S. K. (1995) Tyrosine phosphorylation of focal adhesion kinase at sites in the catalytic domain regulates kinase activity: a role for Src family kinases. Mol. Cell. Biol. 15, 954–963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Calalb M. B., Zhang X., Polte T. R., Hanks S. K. (1996) Focal adhesion kinase tyrosine-861 is a major site of phosphorylation by Src. Biochem. Biophys. Res. Commun. 228, 662–668 [DOI] [PubMed] [Google Scholar]
- 33. Sieg D. J., Hauck C. R., Ilic D., Klingbeil C. K., Schaefer E., Damsky C. H., Schlaepfer D. D. (2000) FAK integrates growth-factor and integrin signals to promote cell migration. Nat. Cell Biol. 2, 249–256 [DOI] [PubMed] [Google Scholar]
- 34. Streblow D. N., Vomaske J., Smith P., Melnychuk R., Hall L., Pancheva D., Smit M., Casarosa P., Schlaepfer D. D., Nelson J. A. (2003) Human cytomegalovirus chemokine receptor US28-induced smooth muscle cell migration is mediated by focal adhesion kinase and Src. J. Biol. Chem. 278, 50456–50465 [DOI] [PubMed] [Google Scholar]
- 35. Mitra S. K., Hanson D. A., Schlaepfer D. D. (2005) Focal adhesion kinase: in command and control of cell motility. Nat. Rev. Mol. Cell Biol. 6, 56–68 [DOI] [PubMed] [Google Scholar]
- 36. Witzenbichler B., Kureishi Y., Luo Z., Le Roux A., Branellec D., Walsh K. (1999) Regulation of smooth muscle cell migration and integrin expression by the Gax transcription factor. J. Clin. Invest. 104, 1469–1480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Marek I., Volkert G., Jahn A., Fahlbusch F., Zürn C., Ozcan Z., Goppelt-Struebe M., Hilgers K. F., Rascher W., Hartner A. (2010) Lack of α8 integrin leads to morphological changes in renal mesangial cells, but not in vascular smooth muscle cells. BMC Cell Biol. 11, 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Moiseeva E. P. (2001) Adhesion receptors of vascular smooth muscle cells and their functions. Cardiovasc. Res. 52, 372–386 [DOI] [PubMed] [Google Scholar]
- 39. Gerthoffer W. T. (2007) Mechanisms of vascular smooth muscle cell migration. Circ. Res. 100, 607–621 [DOI] [PubMed] [Google Scholar]
- 40. Walsh C. T., Radeff-Huang J., Matteo R., Hsiao A., Subramaniam S., Stupack D., Brown J. H. (2008) Thrombin receptor and RhoA mediate cell proliferation through integrins and cysteine-rich protein 61. FASEB J. 22, 4011–4021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Terada N., Shiraishi T., Zeng Y., Mooney S. M., Yeater D. B., Mangold L. A., Partin A. W., Kulkarni P., Getzenberg R. H. (2012) Cyr61 is regulated by cAMP-dependent protein kinase with serum levels correlating with prostate cancer aggressiveness. Prostate 72, 966–976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Simon M. F., Daviaud D., Pradère J. P., Grès S., Guigné C., Wabitsch M., Chun J., Valet P., Saulnier-Blache J. S. (2005) Lysophosphatidic acid inhibits adipocyte differentiation via lysophosphatidic acid 1 receptor-dependent down-regulation of peroxisome proliferator-activated receptor γ2. J. Biol. Chem. 280, 14656–14662 [DOI] [PubMed] [Google Scholar]
- 43. Shen B., Delaney M. K., Du X. (2012) Inside-out, outside-in, and inside-outside-in: G protein signaling in integrin-mediated cell adhesion, spreading, and retraction. Curr. Opin. Cell Biol. 24, 600–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Weiner J. A., Fukushima N., Contos J. J., Scherer S. S., Chun J. (2001) Regulation of Schwann cell morphology and adhesion by receptor-mediated lysophosphatidic acid signaling. J. Neurosci. 21, 7069–7078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sakai T., de la Pena J. M., Mosher D. F. (1999) Synergism among lysophosphatidic acid, β1A integrins, and epidermal growth factor or platelet-derived growth factor in mediation of cell migration. J. Biol. Chem. 274, 15480–15486 [DOI] [PubMed] [Google Scholar]
- 46. Grzeszkiewicz T. M., Kirschling D. J., Chen N., Lau L. F. (2001) CYR61 stimulates human skin fibroblast migration through Integrin αvβ5 and enhances mitogenesis through integrin αvβ3, independent of its carboxyl-terminal domain. J. Biol. Chem. 276, 21943–21950 [DOI] [PubMed] [Google Scholar]
- 47. Kireeva M. L., Lam S. C., Lau L. F. (1998) Adhesion of human umbilical vein endothelial cells to the immediate-early gene product Cyr61 is mediated through integrin αvβ3. J. Biol. Chem. 273, 3090–3096 [DOI] [PubMed] [Google Scholar]
- 48. Jedsadayanmata A., Chen C. C., Kireeva M. L., Lau L. F., Lam S. C. (1999) Activation-dependent adhesion of human platelets to Cyr61 and Fisp12/mouse connective tissue growth factor is mediated through integrin αIIbβ3. J. Biol. Chem. 274, 24321–24327 [DOI] [PubMed] [Google Scholar]
- 49. Chen N., Chen C. C., Lau L. F. (2000) Adhesion of human skin fibroblasts to Cyr61 is mediated through integrin α6β1 and cell surface heparan sulfate proteoglycans. J. Biol. Chem. 275, 24953–24961 [DOI] [PubMed] [Google Scholar]
- 50. Jun J. I., Lau L. F. (2010) The matricellular protein CCN1 induces fibroblast senescence and restricts fibrosis in cutaneous wound healing. Nat. Cell Biol. 12, 676–685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Chen C. C., Young J. L., Monzon R. I., Chen N., Todorović V., Lau L. F. (2007) Cytotoxicity of TNFα is regulated by integrin-mediated matrix signaling. EMBO J. 26, 1257–1267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sakai T., Peyruchaud O., Fässler R., Mosher D. F. (1998) Restoration of β1A integrins is required for lysophosphatidic acid-induced migration of β1-null mouse fibroblastic cells. J. Biol. Chem. 273, 19378–19382 [DOI] [PubMed] [Google Scholar]
- 53. Fu M., Zhang J., Zhu X., Myles D. E., Willson T. M., Liu X., Chen Y. E. (2001) Peroxisome proliferator-activated receptor γ inhibits transforming growth factor β-induced connective tissue growth factor expression in human aortic smooth muscle cells by interfering with Smad3. J. Biol. Chem. 276, 45888–45894 [DOI] [PubMed] [Google Scholar]