Abstract
Background
Exposure to stress in early life is correlated with the development of anxiety disorders in adulthood. The underlying mechanisms are not fully understood, but an imbalance in corticosteroid receptor (CR) expression in the limbic system, particularly the hippocampus, has been implicated in the etiology of anxiety disorders. However, little is known about how prepubertal stress in the so called “juvenile” period might alter the expression of these receptors.
Aims
Therefore, the aim of this study was to investigate how stress experienced in the juvenile phase of life altered hippocampal expression of CRs and anxiety behaviors in adulthood.
Materials and methods
We used a rodent model to assess the effects of juvenile stress on hippocampal CR expression, and performance in three behavioral tests of anxiety in adulthood.
Results
Juvenile stress (JS) increased anxiety-like behavior on the elevated plus maze, increased mineralocorticoid receptor (MR) expression, and decreased the ratio of glucocorticoid receptor (GR) to MR expression in the hippocampus of adult animals. Females demonstrated lower levels of anxiety-type behavior and increased activity in three behavioral tests, and had greater expression of GR and GR:MR ratio than males, regardless of treatment.
Discussion and conclusion
These results demonstrate that JS can alter the expression and balance of CRs, providing a potential mechanism for the corresponding increase in anxiety behavior observed in adulthood. Further evidence for the role of CR expression in anxiety is provided by sex differences in anxiety behavior and corresponding alterations in CR expression.
Keywords: Anxiety, glucocorticoid receptor, juvenile stress, mineralocorticoid receptor, sex differences
Introduction
In humans, the risk of developing neuropsychiatric disorders such as posttraumatic stress disorder (PTSD), depression, and anxiety in adulthood is increased when stress is experienced earlier in life (Anda et al. 2006; Heim et al. 2008; Bale et al. 2010; Meewisse et al. 2011; Pechtel and Pizzagalli 2011). Major mediators of the effects of early life stress are thought to be corticosteroid hormones and their receptors in the brain (glucocorticoid receptors [GR] and mineralocorticoid receptors [MR]). During a stress response, glucocorticoids (mainly corticosterone in rodents and cortisol in humans) are released as a consequence of activation of the hypothalamic–pituitary–adrenal (HPA) axis. As these stress hormones can pass through the blood–brain barrier, the HPA axis is one of the major pathways through which stress can alter brain development. Indeed, previous work suggests that prenatal stress can program the HPA axis, and may be related to adult pathophysiology (Meaney et al. 2007; Seckl and Holmes 2007).
In the central nervous system, GR and MR receptor densities are highest in the hippocampus (Herman 1993). The hippocampus is an important regulator of behavioral measures of anxiety (Mirescu et al. 2004), and clinical and basic research has identified alterations in the hippocampus in mood disorders (Mayberg 2009; Arnone et al. 2012). Early life stress can structurally and functionally alter the hippocampus (Fenoglio et al. 2006; Tottenham and Sheridan 2010), and stress in the prenatal and neonatal phases alters MR and GR expression in adult animals (rats, primates, and birds). However, the direction of change varies with the exact paradigm used and between sexes, and many effects are GR or MR specific (Welberg et al. 2001; Kapoor et al. 2006; Patel et al. 2008; Lupien et al. 2009; Belay et al. 2011; Wynne et al. 2011; Banerjee et al. 2012; van Hasselt et al. 2012).
Although there is a wealth of information on the adulthood consequences of perinatal stress, comparatively little is known about the effects of juvenile (prepubertal or childhood) stress. The juvenile brain experiences dramatic changes in structure and function as it matures (Romeo and McEwen 2006), and epidemiological studies have linked juvenile stress (JS) with the development of depression, anxiety, and PTSD, as well as suicide attempts later in life (Morgan et al. 2003; Kausch et al. 2006; Weich et al. 2009). In animal models, JS increases anxiety behavior, alters fear conditioning, learning, and memory (Avital and Richter-Levin 2005; Toledo-Rodriguez and Sandi 2007; Tsoory et al. 2007; Jacobson-Pick and Richter-Levin 2010; Brydges et al. 2012, 2013), remodels corticolimbic architecture (Eiland et al. 2012), and alters neural gene expression in adulthood (Jacobson-Pick et al. 2008; Tsoory et al. 2010). Effects on behavior are observed when animals experience stress in adulthood, but they are significantly enhanced when stress is given in the juvenile phase (Avital and Richter-Levin 2005; Tsoory and Richter-Levin 2006), demonstrating phase specific changes.
To date, the effects of JS on the expression of corticosteroid receptor (CR) expression in the adult brain have not been investigated. Therefore, the aims of this study were to investigate the effects of JS on anxiety behavior, and analyze corresponding changes in hippocampal CR expression in adulthood. We hypothesized that JS would increase anxiety behavior in adulthood, and alter the expression of MR and GR. To date, most rodent studies have used rat models to investigate the effects of JS (see Peleg-Raibstein and Feldon 2011, for an exception), but given the large number and availability of transgenic mouse models for the study of genetic components of psychiatric disorders, we aimed to expand this research through use of a mouse model.
Material and Methods
Ethics statement
All procedures were carried out in strict accordance with and permission of the local ethics committee, and under the aegis of the UK Home Office Animals (Scientific Procedures) Act, 1986.
Animals
C57BL/6 mice were bred from eight stressed and seven control adult pairs (Harlan, Oxfordshire, U.K.) at the University of Edinburgh. After weaning (postnatal day [PND] 21), 22 female and 23 male offspring were housed in standard, same-sex, same-litter cages lined with wood shavings (Lillico, Hookwood, Surrey, U.K.), on a 12:12 h light/dark cycle with food (RM1 diet, Special Diet Services, Witham, U.K.) and water provided ad libitum. Humidity and temperature were maintained at 60% and between 19°C and 21°C, respectively. Eight litters were randomly assigned to JS, the other seven served as controls. Age and sex ratios were evenly distributed between the groups.
JS protocol
The JS protocol follows that used in Brydges et al. (2012, 2013). Eight litters were exposed to variable short-term stress on PND 25, 26, and 27. On PND 25, animals were given a forced swim in a swim tank (15 cm high, 11 cm diameter, 1 L capacity filled with 500 mL water, water temperature 25 ± 1°C) for 10 min. On PND 26, animals received restraint stress; they were placed into plastic restraint tubes (26 mm diameter) for three sessions of 30 min, separated by 30 min breaks. On PND 27, they were given six mild electric footshocks (0.3 mA) over 3 min (1 every 30 sec) in a mouse shock chamber.
Adult behavioral tests
All tests were performed in the same sequence (elevated plus maze [EPM], open field, emergence test), at the same age (mean age 99 days) and in the light phase for all mice.
Elevated plus maze
On day one, animals were tested in the EPM. The EPM was raised 100 cm above the floor, made of black plastic, and comprised two open and opposite arms (28 × 6 cm) and two closed and opposite arms (28 × 6 cm with 14 cm high walls) arranged in a cross shape. The arms were connected by a central square (6 × 6 cm). During testing, an animal was placed in the central square of the maze facing an open arm. Behavior was recorded for 5 min via a video recorder mounted above the maze, and tracking software (Limelight; Actimetrics, Wilmette, IL) was used to analyze the amount of time animals spent in the open versus the closed arms (minus time spent in the central square), and the number of times animals crossed from one arm to another. Data from five male and five female mice which fell off the apparatus before testing was complete were excluded. Numbers used for each experiment can be found in Table 1.
Table 1.
Number of animals used in each behavioral test
| Analysis | Con females | JS females | Con males | JS males |
|---|---|---|---|---|
| EPM | 11 | 6 | 7 | 11 |
| Open field | 11 | 11 | 11 | 12 |
| Emergence | 11 | 11 | 11 | 12 |
Con, control; JS, juvenile stress; EPM, elevated plus maze.
Open field
Twenty-four hours after testing in the EPM, animals were tested in the open field. The open field consisted of a white plastic box (50 × 50 × 15 cm high) divided into 16 equal sized squares. During testing, animals were placed into the center of the open field, and filmed for 5 min via a video camera mounted above the maze. Tracking software (Limelight; Actimetrics) was used to analyze the number of crossings animals made between the 16 squares, and the percentage of time spent in the central four compared to the outer 12 squares of the maze.
Emergence test
Twenty-four hours after testing in the open field, animals were tested in the emergence test. The apparatus was made of Perspex, and consisted of two compartments, one covered and dark (15 × 17 × 26.5 cm, 0.01 lux), the other light and open (27 × 26.5 × 26.5 cm, 66 lux). A sliding door connected the two. Animals were placed into the dark compartment, given 1 min to settle, the door was raised and time to emerge into the light compartment was recorded. This is another test of anxiety behavior in rodents (Frye et al. 2000; Walf et al. 2009).
Tissue extraction
One week after behavioral testing, mice were killed by CO2 and brains removed and snap frozen for hippocampal mRNA extraction.
Real time-polymerase chain reaction
The QIAGEN RNeasy system (QIAGEN Ltd., Crawley, U.K.) was used to extract total hippocampal RNA, which was reverse transcribed using Promega reverse transcription kit (Promega UK Ltd., Southampton, U.K.). Triplicate samples of cDNA (the equivalent of 1 ng of total RNA) were incubated with fluorescent probes (using predesigned systems from Applied Biosystems [Warrington, U.K.]) and gene-specific primers (GR [NR3C1]: forward 5′-GTGGAAGGACAGCACAATTACCT-3′ and reverse 5′-GCGGCATGCTGGACAGTT-3′, MR [NR3C2]: forward 5′-CCCTACCATGTCCTAGAAAAGC-3′ and reverse: 5′-AGAACGCTCCAAGGTCTGAG-3′) in 1x Roche LightCycler 40 probes mastermix (Roche Diagnostics, West Sussex, U.K.). A Roche LightCycler 480 was used for polymerase chain reaction (PCR) cycling and detection of fluorescent signal, and a serial dilution of cDNA pooled from all samples was used to create a standard curve for each primer–probe set. Results were standardized using the housekeeping gene HPRT1 (forward sequence: 5′-TCCTCCTCAGACCGCTTTT-3′, reverse sequence: 5′-CCTGGTTCATCATCGCTAATC-3′).
Data analysis
Data were analyzed by linear models. All data were checked for normality of distribution and homogeneity of variance and were transformed to provide the best approximation to a normal distribution when in violation of these assumptions (Box and Cox 1964). The first two models investigated the effects of group (Con, JS), sex, and group × sex interaction on the percentage of time spent in the open arms and number of crossing made in the EPM. A third and fourth model looked at the effects of group, sex, and group × sex interaction on percentage of time spent in the center and number of crossing made in the open field maze. Another model investigated the effect of group, sex, and group × sex on time to emerge from the dark to the light side of the emergence box. Separate models were set up to investigate the effects of group, sex, and group × sex on the hippocampal mRNA expression levels of HPRT1, GR, MR, and GR:MR ratio. Between one and five animals were used per litter, so litter was nested within group, and fitted as a random factor in all models to account for litter effects.
Results
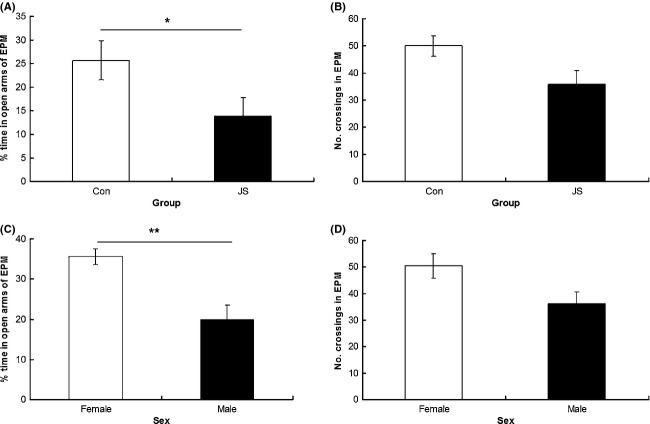
As a group, JS animals spent a greater proportion of time in the closed arms of the EPM than control animals (F1,8.28 = 9.17, P = 0.02, data square root transformed, Fig. 1A), and there was no interaction between group × sex on percentage of time in the closed arms (F1,28.13 = 3.67, P = 0.7). There was no difference between groups (F1,9.19 = 1.86, P = 0.21), and no group × sex interaction (F1,29.88 = 0.29, P = 0.6) on number of arm entries made in the EPM task (Fig. 1B). There was no difference between groups (F1,17.68 = 0.87, P = 0.36) or a group × sex interaction (F1,39.75 = 1.92, P = 0.17) on percentage of time spent in the center of the open field (Fig. 2A), and no difference between groups (F1,12.5 = 0.8, P = 0.39) or a group × sex interaction (F1,36.83 = 0.43, P = 0.52) on number of crossings in the open field (Fig. 2B). Similarly, there was no difference between groups (F1,11.5 = 0.55, P = 0.47) or a group × sex interaction (F1,35.42 = 0.13, P = 0.72) on time to emerge from the emergence box (Fig. 3A).
Figure 1.
Elevated plus maze. (A) Percentage of time spent in the open arms and (B) number of crossings made in the elevated plus maze (EPM) by control (Con) and juvenile stress (JS) animals. (C) Percentage of time spent in the open arms and D) number of crossing made in the EPM by female and male animals. Error bars represent 1 SE, bars connected by an asterisk are significantly different to one another. (*P < 0.05, **P < 0.01, ***P < 0.001).
Figure 2.
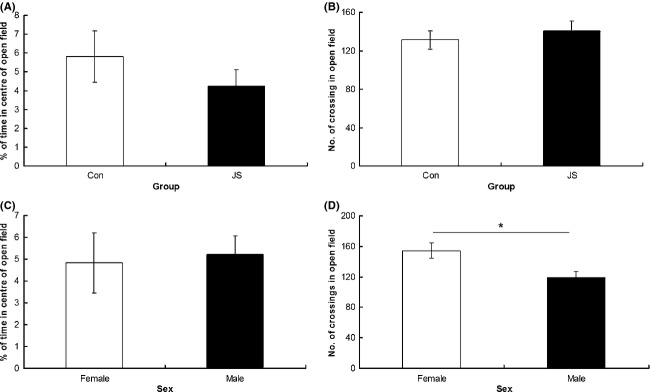
Open field test. (A) Percentage of time spent in the center of the open field maze and (B) number of crossings made in the open field by control (Con) and juvenile stress (JS) animals. (C) Percentage of time spent in the center of the open field maze and (D) number of crossings made in the open field by female and male animals. Error bars represent 1 SE, bars connected by an asterisk are significantly different to one another. (*P < 0.05, **P < 0.01, ***P < 0.001).
Figure 3.
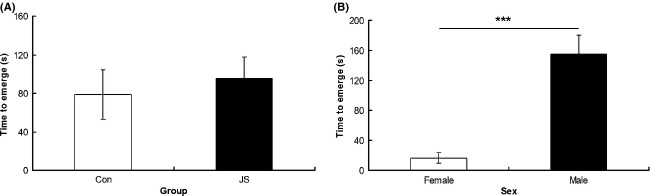
Emergence test. Time to emerge from the emergence box for (A) control (Con) and juvenile stress (JS) and (B) female and male animals. Error bars represent 1 SE, bars connected by an asterisk are significantly different to one another. (*P < 0.05, **P < 0.01, ***P < 0.001).
Females showed less anxiety-related behavior than males, spending a greater proportion of time on the open arms of the EPM (F1,28.13 = 8.67, P < 0.01, data square root transformed, Fig. 1C), and showing a trend toward making more arm entries in the EPM (F1,29.88 = 2.88, P = 0.09, Fig. 1D), as well as making more crossings in the open field (F1,36.83 = 6.81, P = 0.01, Fig. 2D) and emerging sooner from the dark side of the emergence apparatus (F1,35.42 = 27.25, P < 0.0001 Fig. 3B). However, there was no difference between the sexes in the amount of time spent in the center of the open field (F1,39.75 = 0.3, P = 0.86, Fig. 1C).
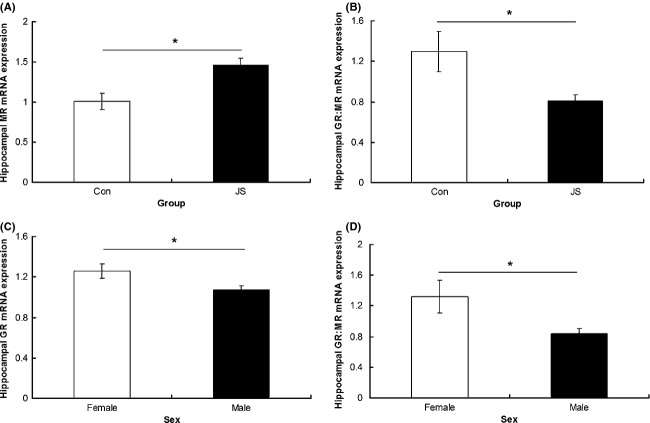
Overall, JS animals had higher expression of hippocampal MR mRNA than controls (F1,2.81 = 25.13, P = 0.02, Fig. 4A) and lower GR:MR ratio (F1,2.58 = 22.78, P = 0.02, data box-cox transformed, Fig. 4B) than control animals. There was no interaction between group and sex on hippocampal MR expression (F1,16.3 = 0.01, P = 0.91) or GR:MR ratio (data box-cox transformed, F1,16.15 = 0.003, P = 0.95). There was no difference between control and JS animals in hippocampal GR expression (F1,9.48 = 0.23, P = 0.64) and no group × sex interaction (F1,19.94 = 0.26, P = 0.62).
Figure 4.
Hippocampal mRNA expression. (A) MR and (B) GR:MR ratio in control (Con) and juvenile Stress (JS) animals. Hippocampal mRNA expression of (C) GR, (D) GR:MR ratio in males and females. Error bars represent 1 SE, bars connected by an asterisk are significantly different to one another. (*P < 0.05, **P < 0.01, ***P < 0.001).
Females expressed greater hippocampal mRNA levels of GR (F1,19.94 = 5.88, P = 0.02, Fig. 4C) and a higher GR:MR ratio (F1,16.15 = 7.04, P = 0.02, data box-cox transformed, Fig. 4D) than males. There were no sex differences in hippocampal MR expression (F1,16.3 = 2.9, P = 0.1).
There were no differences in the expression of the housekeeping gene, HPRT1, between groups (F1,8.59 = 1.33, P = 0.28), sexes (F1,14.38 = 0.17, P = 0.69), or group × sex interaction (F1,14.38 = 0.48, P = 0.5).
Discussion
Juvenile stress
The experience of stress in the juvenile phase increased anxiety-like behavior on the EPM in adulthood. This result is in agreement with previous studies in rats, (Avital and Richter-Levin 2005; Tsoory et al. 2007; Ilin and Richter-Levin 2009), suggesting that the effects of JS on anxiety are conserved across rats and mice. However, it contrasts with results from one study using mice, in which no effects of JS were found on adulthood behavior in an EPM (Peleg-Raibstein and Feldon 2011). One possible explanation for the disagreement between studies is differences in the type and duration of JS protocols used, as well as sample sizes. In particular, the previous study used 5 days of variable stress (including exposure to a shaking platform, water deprivation, and exposure to a predator, stressors not used in the present study) whereas 3 days were used in this study. Furthermore, sample sizes were significantly smaller (n = 7/group) in the Peleg-Raibstein and Feldon (2011) study, whereas the present study used 18 control and 17 stressed animals (combined over males and females). Our result also reflects the findings in human populations, in which juvenile or childhood stress shows a strong correlation with anxiety disorders in adulthood (Green et al. 2010). The specific mechanisms underlying these disruptions are not well understood, although existing studies suggest that reprogramming of the HPA axis may be involved (Meaney et al. 2007; McGowan et al. 2009; Belay et al. 2011; van Hasselt et al. 2012). Therefore, we investigated hippocampal mRNA expression of genes involved in the stress response (specifically, CRs) in adult animals that had experienced JS.
Compared to control animals, hippocampal MR mRNA expression was upregulated in adults that had experienced JS, and the GR:MR ratio was lower. Previous studies have revealed mixed results regarding the effects of stress on corticosteroid expression in the hippocampus (Welberg et al. 2001). Acute forced swim and novelty exposure increased MR expression in the hippocampus 24 h later in adult rats (Reul et al. 2000), and neonatal stress increased hippocampal MR expression and anxiety behavior in adulthood (Gill et al. 2012). In contrast, predator stress in adulthood decreased hippocampal MR expression 4 months later (Wang et al. 2012), and environmental enrichment restored chronic cerebral hypoperfusion induced reductions in hippocampal MR and GR in adult rats (Zhang et al. 2013). Furthermore, exposure to stress in the prenatal period resulted in decreased MR and GR expression in the hippocampus, and increased GR expression in the amygdala in adulthood (Levitt et al. 1996). The discrepancies between studies are likely due to differences in experimental protocols as well as timing and type of stress exposure.
Glucocorticoid receptors and MR are involved in regulating the stress response via the HPA axis, and are abundantly expressed in the hippocampus (Reul et al. 2000). Nuclear MR has a high affinity for glucocorticoids, and is thought to maintain the stress response, setting thresholds for its activation (vanHaarst et al. 1997; Joels et al. 2008). Membrane bound MR has a lower affinity for glucocorticoids, and is thought to mediate fast nongenomic actions, playing a crucial role at the onset of the stress reaction (Karst et al. 2005; Joels et al. 2008). Specifically, in the hippocampus, nongenomic presynaptic MR increases excitability through promoting glutamate release, and postsynaptic nuclear MR enhances potential probability (Karst et al. 2005; Joels et al. 2008). Following this, GR-mediated mechanisms dampen the initial stress response, normalizing brain activity and promoting recovery, with nonnuclear postsynaptic GR receptors decreasing excitation (Joels et al. 2008). In the present experiment, increased levels of MR in the hippocampus of stressed animals could result in a greater magnitude of initial stress response, with the lower GR:MR ratio resulting in a decreased magnitude of or longer duration to GR-mediated dampening. This could be a potential mechanism underlying the increased anxiety behavior observed in this model, although further experiments are needed to investigate this hypothesis further. In agreement with these findings, blocking the action of MR receptors with an antagonist has been found to decrease anxiety behavior in rats (Smythe et al. 1997), and MR/GR imbalances have been found in patients with psychiatric disorders (Baes et al. 2012). Some studies have found increased GR and MR expression in depressed individuals, others decreased, and it has therefore been suggested that any alteration in these receptors should be considered as a biomarker of disease (McGowan et al. 2009; Berardelli et al. 2013; Medina et al. 2013). Furthermore, human carriers of certain MR alleles are more reactive to stress, showing enhanced amygdala activation and HPA activation in response to stress (van Leeuwen et al. 2011; Bogdan et al. 2012).
It should be noted that behavioral alterations between JS and control animals were only found in one measure of anxiety behavior, the EPM, and not in two subsequent tests (open field and emergence test). A possible reason for this is that experience of the EPM (first test encountered) could have affected subsequent performance in the open field and emergence tests, and suggests caution when performing multiple behavioral tests on the same animal, something which remains an unresolved issue in behavioral test batteries (Paylor et al. 2006; Blokland et al. 2012). Alternatively, it has been suggested that these three tests measure subtly different aspects of anxiety-like behavior (Ramos 2008), with the current results suggesting a selective deficit on the EPM. A further option is that stress effects on anxiety are subtle, with effects seen in only one out of the three tests performed.
Sex differences
Sex differences were found in all three behavioral tests performed, with female animals displaying lower levels of anxiety-like behavior and greater levels of activity. Female mice and rats typically display less anxiety than males in the EPM (Zimmerberg and Farley 1993; Voikar et al. 2001). In the present study, we find that hippocampal GR expression is higher in females, suggesting a role for CRs in differences in anxiety behavior between the sexes. This result adds to recent findings of sex differences in forebrain GR (including hippocampus) on HPA axis regulation and depression-type behaviors (Solomon et al. 2012). As sex differences are found in the development of neuropsychiatric disorders (Bao and Swaab 2010), this highlights further that males and females need to be considered separately in basic research models, and suggests different MR/GR between sexes may contribute to sex differences in vulnerability to stress-related disorders.
Conclusion
Experiencing stress in the prepubertal or juvenile phase increased anxiety-like behavior and altered the expression of MR and GR:MR in the hippocampus in adulthood. This alteration in CR expression provides a potential mechanism for the observed increase in anxiety-like behavior observed in adulthood. Further evidence for the involvement of CR receptors in adult anxiety-like behavior is provided by the finding that females demonstrated greater GR and GR:MR expression in the hippocampus, with corresponding decreases in anxiety-type behaviors when compared to males. These results demonstrate the potential role of CR in mediating later anxiety-type behavior when stress is experienced early in life.
Acknowledgments
We would like to thank the Mortimer and Theresa Sackler Foundation for funding. Additionally, J. H. was supported by a Scottish Senior Clinical Fellowship.
Conflict of Interest
None declared.
Funding Information
We would like to thank the Dr. Mortimer and Theresa Sackler Foundation for funding. Additionally, J. H. was supported by a Scottish Senior Clinical Fellowship.
References
- Anda RF, Felitti VJ, Bremner JD, Walker JD, Whitfield C, Perry BD, et al. The enduring effects of abuse and related adverse experiences in childhood. A convergence of evidence from neurobiology and epidemiology. Eur. Arch. Psychiatry Clin. Neurosci. 2006;256:174–186. doi: 10.1007/s00406-005-0624-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnone D, McIntosh AM, Ebmeier KP, Munafo MR, Anderson IM. Magnetic resonance imaging studies in unipolar depression: systematic review and meta-regression analyses. Eur. Neuropsychopharmacol. 2012;22:1–16. doi: 10.1016/j.euroneuro.2011.05.003. [DOI] [PubMed] [Google Scholar]
- Avital A, Richter-Levin G. Exposure to juvenile stress exacerbates the behavioural consequences of exposure to stress in the adult rat. Int. J. Neuropsychopharmacol. 2005;8:163–173. doi: 10.1017/S1461145704004808. [DOI] [PubMed] [Google Scholar]
- Baes CV, Tofoli SMD, Martins CMS, Juruena MF. Assessment of the hypothalamic-pituitary-adrenal axis activity: glucocorticoid receptor and mineralocorticoid receptor function in depression with early life stress – a systematic review. Acta Neuropsychiatr. 2012;24:4–15. doi: 10.1111/j.1601-5215.2011.00610.x. [DOI] [PubMed] [Google Scholar]
- Bale TL, Baram TZ, Brown AS, Goldstein JM, Insel TR, McCarthy MM, et al. Early life programming and neurodevelopmental disorders. Biol. Psychiatry. 2010;68:314–319. doi: 10.1016/j.biopsych.2010.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee SB, Arterbery AS, Fergus DJ, Adkins-Regan E. Deprivation of maternal care has long-lasting consequences for the hypothalamic-pituitary-adrenal axis of zebra finches. Proc. Biol. Sci. 2012;279:759–766. doi: 10.1098/rspb.2011.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao A-M, Swaab DF. Sex differences in the brain, behavior, and neuropsychiatric disorders. Neuroscientist. 2010;16:550–565. doi: 10.1177/1073858410377005. [DOI] [PubMed] [Google Scholar]
- Belay H, Burton CL, Lovic V, Meaney MJ, Sokolowski M, Fleming AS. Early adversity and serotonin transporter genotype interact with hippocampal glucocorticoid receptor mRNA expression, corticosterone, and behavior in adult male rats. Behav. Neurosci. 2011;125:150–160. doi: 10.1037/a0022891. [DOI] [PubMed] [Google Scholar]
- Berardelli R, Karamouzis I, D'Angelo V, Zichi C, Fussotto B, Giordano R, et al. Role of mineralocorticoid receptors on the hypothalamus-pituitary-adrenal axis in humans. Endocrine. 2013;43:51–58. doi: 10.1007/s12020-012-9750-8. [DOI] [PubMed] [Google Scholar]
- Blokland A, ten Oever S, van Gorp D, van Draanen M, Schmidt T, Nguyen E. The use of a test battery assessing affective behavior in rats: order effects. Behav. Brain Res. 2012;228:16–21. doi: 10.1016/j.bbr.2011.11.042. [DOI] [PubMed] [Google Scholar]
- Bogdan R, Williamson DE, Hariri AR. Mineralocorticoid receptor Iso/Val (rs5522) genotype moderates the association between previous childhood emotional neglect and amygdala reactivity. Am. J. Psychiatry. 2012;169:515–522. doi: 10.1176/appi.ajp.2011.11060855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Box GEP, Cox DR. An analysis of transformations. J. R. Stat. Soc. Series B Stat. Methodol. 1964;26:211–252. [Google Scholar]
- Brydges NM, Hall L, Nicolson R, Holmes MC, Hall J. The effects of juvenile stress on anxiety, cognitive bias and decision making in adulthood: a rat model. PLoS ONE. 2012;7:e48143. doi: 10.1371/journal.pone.0048143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brydges NM, Whalley HC, Jansen MA, Merrifield GD, Wood ER, Lawrie SM, et al. Imaging conditioned fear circuitry using awake rodent FMRI. PLoS ONE. 2013;8:e54197. doi: 10.1371/journal.pone.0054197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiland L, Ramroop J, Hill MN, Manley J, McEwen BS. Chronic juvenile stress produces corticolimbic dendritic architectural remodeling and modulates emotional behavior in male and female rats. Psychoneuroendocrinology. 2012;37:39–47. doi: 10.1016/j.psyneuen.2011.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenoglio KA, Brunson KL, Baram TZ. Hippocampal neuroplasticity induced by early-life stress: functional and molecular aspects. Front. Neuroendocrinol. 2006;27:180–192. doi: 10.1016/j.yfrne.2006.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Petralia SM, Rhodes ME. Estrous cycle and sex differences in performance on anxiety tasks coincide with increases in hippocampal progesterone and 3 alpha,5 alpha-THP. Pharmacol. Biochem. Behav. 2000;67:587–596. doi: 10.1016/s0091-3057(00)00392-0. [DOI] [PubMed] [Google Scholar]
- Gill DA, Perry MA, McGuire EP, Perez-Gomez A, Tasker RA. Low-dose neonatal domoic acid causes persistent changes in behavioural and molecular indicators of stress response in rats. Behav. Brain Res. 2012;230:409–417. doi: 10.1016/j.bbr.2012.02.036. [DOI] [PubMed] [Google Scholar]
- Green JG, McLaughlin KA, Berglund PA, Gruber MJ, Sampson NA, Zaslavsky AM, et al. Childhood adversities and adult psychiatric disorders in the national comorbidity survey replication I associations with first onset of DSM-IV disorders. Arch. Gen. Psychiatry. 2010;67:113–123. doi: 10.1001/archgenpsychiatry.2009.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- vanHaarst AD, Oitzl MS, deKloet ER. Facilitation of feedback inhibition through blockade of glucocorticoid receptors in the hippocampus. Neurochem. Res. 1997;22:1323–1328. doi: 10.1023/a:1022010904600. [DOI] [PubMed] [Google Scholar]
- van Hasselt FN, Cornelisse S, Zhang TY, Meaney MJ, Velzing EH, Krugers HJ, et al. Adult hippocampal glucocorticoid receptor expression and dentate synaptic plasticity correlate with maternal care received by individuals early in life. Hippocampus. 2012;22:255–266. doi: 10.1002/hipo.20892. [DOI] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Mletzko T, Miller AH, Hemeroff CB. The link between childhood trauma and depression: insights from HPA axis studies in humans. Psychoneuroendocrinology. 2008;33:693–710. doi: 10.1016/j.psyneuen.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Herman JP. Regulation of adrenocorticosteroid receptor messenger-RNA expression in the central nervous system. Cell. Mol. Neurobiol. 1993;13:349–372. doi: 10.1007/BF00711577. [DOI] [PubMed] [Google Scholar]
- Ilin Y, Richter-Levin G. Enriched environment experience overcomes learning deficits and depressive-like behavior induced by juvenile stress. PLoS ONE. 2009;4:e4329. doi: 10.1371/journal.pone.0004329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson-Pick S, Richter-Levin G. Differential impact of juvenile stress and corticosterone in juvenility and in adulthood, in male and female rats. Behav. Brain Res. 2010;214:268–276. doi: 10.1016/j.bbr.2010.05.036. [DOI] [PubMed] [Google Scholar]
- Jacobson-Pick S, Elkobi A, Vander S, Rosenblum K, Richter-Levin G. Juvenile stress-induced alteration of maturation of the GABA(A) receptor alpha subunit in the rat. Int. J. Neuropsychopharmacol. 2008;11:891–903. doi: 10.1017/S1461145708008559. [DOI] [PubMed] [Google Scholar]
- Joels M, Karst H, DeRijk R, de Kloet ER. The coming out of the brain mineralocorticoid receptor. Trends Neurosci. 2008;31:1–7. doi: 10.1016/j.tins.2007.10.005. [DOI] [PubMed] [Google Scholar]
- Kapoor A, Dunn E, Kostaki A, Andrews MH, Matthews SG. Fetal programming of hypothalamo-pituitary-adrenal function: prenatal stress and glucocorticoids. J. Physiol. 2006;572:31–44. doi: 10.1113/jphysiol.2006.105254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karst H, Berger S, Turiault M, Tronche F, Schutz G, Joels M. Mineralocorticoid receptors are indispensable for nongenomic modulation of hippocampal glutamate transmission by corticosterone. Proc. Natl. Acad. Sci. USA. 2005;102:19204–19207. doi: 10.1073/pnas.0507572102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kausch O, Rugle L, Rowland DY. Lifetime histories of trauma among pathological gamblers. Am. J. Addict. 2006;15:35–43. doi: 10.1080/10550490500419045. [DOI] [PubMed] [Google Scholar]
- van Leeuwen N, Bellingrath S, Zitman ER, de Kloet FG, DeRijk RH, Kudielka BM, et al. Human mineralocorticoid receptor (MR) gene haplotypes modulate MR expression and transactivation: implication for the stress response. Psychoneuroendocrinology. 2011;36:699–709. doi: 10.1016/j.psyneuen.2010.10.003. [DOI] [PubMed] [Google Scholar]
- Levitt NS, Lindsay RS, Holmes MC, Seckl JR. Dexamethasone in the last week of pregnancy attenuates hippocampal glucocorticoid receptor gene expression and elevates blood pressure in the adult offspring in the rat. Neuroendocrinology. 1996;64:412–418. doi: 10.1159/000127146. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat. Rev. Neurosci. 2009;10:434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- Mayberg HS. Targeted electrode-based modulation of neural circuits for depression. J. Clin. Investig. 2009;119:717–725. doi: 10.1172/JCI38454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan PO, Sasaki A, D'Alessio AC, Dymov S, Labonte B, Szyf M, et al. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nat. Neurosci. 2009;12:342–348. doi: 10.1038/nn.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaney MJ, Szyf M, Seckl JR. Epigenetic mechanisms of perinatal programming of hypothalamic-pituitary-adrenal function and health. Trends Mol. Med. 2007;13:269–277. doi: 10.1016/j.molmed.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Medina A, Seasholtz AF, Sharma V, Burke S, Bunney W, Jr, Myers RM, et al. Glucocorticoid and mineralocorticoid receptor expression in the human hippocampus in major depressive disorder. J. Psychiatr. Res. 2013;47:307–314. doi: 10.1016/j.jpsychires.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meewisse ML, Olff M, Kleber R, Kitchiner NJ, Gersons BPR. The course of mental health disorders after a disaster: predictors and comorbidity. J. Trauma. Stress. 2011;24:405–413. doi: 10.1002/jts.20663. [DOI] [PubMed] [Google Scholar]
- Mirescu C, Peters JD, Gould E. Early life experience alters response of adult neurogenesis to stress. Nat. Neurosci. 2004;7:841–846. doi: 10.1038/nn1290. [DOI] [PubMed] [Google Scholar]
- Morgan L, Scourfield J, Williams D, Jasper A, Lewis G. The Aberfan disaster: 33-year follow-up of survivors. Br. J. Psychiatry. 2003;182:532–536. doi: 10.1192/bjp.182.6.532. [DOI] [PubMed] [Google Scholar]
- Patel PD, Katz M, Karssen AM, Lyons DM. Stress-induced changes in corticosteroid receptor expression in primate hippocampus and prefrontal cortex. Psychoneuroendocrinology. 2008;33:360–367. doi: 10.1016/j.psyneuen.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paylor R, Spencer CM, Yuva-Paylor LA, Pieke-Dahl S. The use of behavioral test batteries, II: effect of test interval. Physiol. Behav. 2006;87:95–102. doi: 10.1016/j.physbeh.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Pechtel P, Pizzagalli DA. Effects of early life stress on cognitive and affective function: an integrated review of human literature. Psychopharmacology. 2011;214:55–70. doi: 10.1007/s00213-010-2009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peleg-Raibstein D, Feldon J. Differential effects of post-weaning juvenile stress on the behaviour of C57BL/6 mice in adolescence and adulthood. Psychopharmacology. 2011;214:339–351. doi: 10.1007/s00213-010-1991-8. [DOI] [PubMed] [Google Scholar]
- Ramos A. Animal models of anxiety: do I need multiple tests? Trends Pharmacol. Sci. 2008;29:493–498. doi: 10.1016/j.tips.2008.07.005. [DOI] [PubMed] [Google Scholar]
- Reul J, Gesing A, Droste S, Stec ISM, Weber A, Bachmann C, et al. The brain mineralocorticoid receptor: greedy for ligand, mysterious in function. Eur. J. Pharmacol. 2000;405:235–249. doi: 10.1016/s0014-2999(00)00677-4. [DOI] [PubMed] [Google Scholar]
- Romeo RD, McEwen BS. Stress and the adolescent brain. Ann. N. Y. Acad. Sci. 2006;1094:202–214. doi: 10.1196/annals.1376.022. [DOI] [PubMed] [Google Scholar]
- Seckl JR, Holmes MC. Mechanisms of disease: glucocorticoids, their placental metabolism and fetal'programming' of adult pathophysiology. Nat. Clin. Pract. Endocrinol. Metab. 2007;3:479–488. doi: 10.1038/ncpendmet0515. [DOI] [PubMed] [Google Scholar]
- Smythe JW, Murphy D, Timothy C, Costall B. Hippocampal mineralocorticoid, but not glucocorticoid, receptors modulate anxiety-like behavior in rats. Pharmacol. Biochem. Behav. 1997;56:507–513. doi: 10.1016/s0091-3057(96)00244-4. [DOI] [PubMed] [Google Scholar]
- Solomon MB, Furay AR, Jones K, Packard AEB, Packard BA, Wulsin AC, et al. Deletion of forebrain glucocorticoid receptors imapirs neuroendocrine stress responses and induces depression-like behavior in males but not females. Neuroscience. 2012;203:135–143. doi: 10.1016/j.neuroscience.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledo-Rodriguez M, Sandi C. Stress before puberty exerts a sex-and age-related impact on auditory and contextual fear conditioning in the rat. Neural Plast. 2007;2007:71203. doi: 10.1155/2007/71203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Sheridan MA. A review of adversity, the amygdala and the hippocampus: a consideration of developmental timing. Front. Hum. Neurosci. 2010;3:18. doi: 10.3389/neuro.09.068.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsoory M, Richter-Levin G. Learning under stress in the adult rat is differentially affected by ‘juvenile’ or ‘adolescent’ stress. Int. J. Neuropsychopharmacol. 2006;9:713–728. doi: 10.1017/S1461145705006255. [DOI] [PubMed] [Google Scholar]
- Tsoory M, Cohen H, Richter-Levin G. Juvenile stress induces a predisposition to either anxiety or depressive-like symptoms following stress in adulthood. Eur. Neuropsychopharmacol. 2007;17:245–256. doi: 10.1016/j.euroneuro.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Tsoory MM, Guterman A, Richter-Levin G. “Juvenile Stress” alters maturation-related changes in expression of the neural cell adhesion molecule L1 in the limbic system: relevance for stress-related psychopathologies. J. Neurosci. Res. 2010;88:369–380. doi: 10.1002/jnr.22203. [DOI] [PubMed] [Google Scholar]
- Voikar V, Koks S, Vasar E, Rauvala H. Strain and gender differences in the behavior of mouse lines commonly used in transgenic studies. Physiol. Behav. 2001;72:271–281. doi: 10.1016/s0031-9384(00)00405-4. [DOI] [PubMed] [Google Scholar]
- Walf AA, Koonce C, Manley K, Frye CA. Proestrous compared to diestrous wildtype, but not estrogen receptor beta knockout, mice have better performance in the spontaneous alternation and object recognition tasks and reduced anxiety-like behavior in the elevated plus and mirror maze. Behav. Brain Res. 2009;196:254–260. doi: 10.1016/j.bbr.2008.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Yu K, Wang J, Lin H, Wu Y, Wang W. Predator stress-induced persistent emotional arousal is associated with alterations of plasma corticosterone and hippocampal steroid receptors in rat. Behav. Brain Res. 2012;230:167–174. doi: 10.1016/j.bbr.2012.01.051. [DOI] [PubMed] [Google Scholar]
- Weich S, Patterson J, Shaw R, Stewart-Brown S. Family relationships in childhood and common psychiatric disorders in later life: systematic review of prospective studies. Br. J. Psychiatry. 2009;194:392–398. doi: 10.1192/bjp.bp.107.042515. [DOI] [PubMed] [Google Scholar]
- Welberg LAM, Seckl JR, Holmes MC. Prenatal glucocorticoid programming of brain corticosteroid receptors and corticotrophin-releasing hormone: Possible implications for behaviour. Neuroscience. 2001;104:71–79. doi: 10.1016/s0306-4522(01)00065-3. [DOI] [PubMed] [Google Scholar]
- Wynne O, Horvat JC, Kim RY, Ong LK, Smith R, Hansbro PM, et al. Neonatal respiratory infection and adult re-infection: effect on glucocorticoid and mineralocorticoid receptors in the hippocampus in BALB/c mice. Brain Behav. Immun. 2011;25:1214–1222. doi: 10.1016/j.bbi.2011.03.014. [DOI] [PubMed] [Google Scholar]
- Zhang L, Zhang J, Sun H, Zhu H, Liu H, Yang Y. An enriched environment elevates corticosteroid receptor levels in the hippocampus and restores cognitive function in a rat model of chronic cerebral hypoperfusion. Pharmacol. Biochem. Behav. 2013;103:693–700. doi: 10.1016/j.pbb.2012.12.023. [DOI] [PubMed] [Google Scholar]
- Zimmerberg B, Farley MJ. Sex-differences in anxiety behaviour in rats: role of gonadal hormones. Physiol. Behav. 1993;54:1119–1124. doi: 10.1016/0031-9384(93)90335-d. [DOI] [PubMed] [Google Scholar]