Abstract
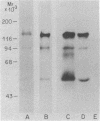
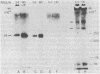
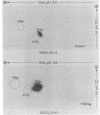
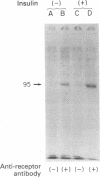
Highly purified human placental insulin receptors were obtained by sequential affinity chromatography on wheat germ agglutinin and insulin-agarose. The preparation had an insulin binding capacity of 4,700 pmol/mg of protein approaching theoretical purity. The purified receptor revealed three major bands of Mr 135,000, 95,000, and 52,000 in NaDodSO4/polyacrylamide gel electrophoresis after reduction by dithiothreitol. All three bands were immunoprecipitated by anti-insulin-receptor antibodies. When this preparation was incubated with [gamma-32P]ATP in the presence of MnCl2 (2 mM) and analyzed in NaDodSO4/acrylamide gel electrophoresis, only the Mr 95,000 band was labeled. Preincubation with several concentrations of insulin increased the 32P incorporation into this peptide in dose-dependent fashion, whereas insulin-like growth factors were approximately equal to 2% as potent and epidermal growth factor had little or no effect, consistent with their known affinities for the insulin receptor. Insulin stimulation of phosphorylation of the Mr 95,000 subunit of the receptor was observed also in immunoprecipitates of this highly purified insulin receptor by anti-insulin-receptor antibodies. Phosphoamino acid determination revealed only phosphotyrosine in both the basal and insulin-stimulated states. These data suggest that a tyrosine-specific protein kinase activity is closely associated with insulin receptor, and this may be important in the signal transmission required for insulin action.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avruch J., Nemenoff R. A., Blackshear P. J., Pierce M. W., Osathanondh R. Insulin-stimulated tyrosine phosphorylation of the insulin receptor in detergent extracts of human placental membranes. Comparison to epidermal growth factor-stimulated phosphorylation. J Biol Chem. 1982 Dec 25;257(24):15162–15166. [PubMed] [Google Scholar]
- Bhaumick B., Bala R. M., Hollenberg M. D. Somatomedin receptor of human placenta: solubilization, photolabeling, partial purification, and comparison with insulin receptor. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4279–4283. doi: 10.1073/pnas.78.7.4279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinkers M., Cohen S. Purified EGF receptor-kinase interacts specifically with antibodies to Rous sarcoma virus transforming protein. Nature. 1981 Apr 9;290(5806):516–519. doi: 10.1038/290516a0. [DOI] [PubMed] [Google Scholar]
- Cohen S., Carpenter G., King L., Jr Epidermal growth factor-receptor-protein kinase interactions. Co-purification of receptor and epidermal growth factor-enhanced phosphorylation activity. J Biol Chem. 1980 May 25;255(10):4834–4842. [PubMed] [Google Scholar]
- Cohen S., Ushiro H., Stoscheck C., Chinkers M. A native 170,000 epidermal growth factor receptor-kinase complex from shed plasma membrane vesicles. J Biol Chem. 1982 Feb 10;257(3):1523–1531. [PubMed] [Google Scholar]
- Collett M. S., Purchio A. F., Erikson R. L. Avian sarcoma virus-transforming protein, pp60src shows protein kinase activity specific for tyrosine. Nature. 1980 May 15;285(5761):167–169. doi: 10.1038/285167a0. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P. Affinity chromatography and purification of the insulin receptor of liver cell membranes. Proc Natl Acad Sci U S A. 1972 May;69(5):1277–1281. doi: 10.1073/pnas.69.5.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denton R. M., Brownsey R. W., Belsham G. J. A partial view of the mechanism of insulin action. Diabetologia. 1981 Oct;21(4):347–362. doi: 10.1007/BF00252681. [DOI] [PubMed] [Google Scholar]
- Ek B., Westermark B., Wasteson A., Heldin C. H. Stimulation of tyrosine-specific phosphorylation by platelet-derived growth factor. Nature. 1982 Feb 4;295(5848):419–420. doi: 10.1038/295419a0. [DOI] [PubMed] [Google Scholar]
- Erikson E., Shealy D. J., Erikson R. L. Evidence that viral transforming gene products and epidermal growth factor stimulate phosphorylation of the same cellular protein with similar specificity. J Biol Chem. 1981 Nov 25;256(22):11381–11384. [PubMed] [Google Scholar]
- Feldman R. A., Hanafusa T., Hanafusa H. Characterization of protein kinase activity associated with the transforming gene product of Fujinami sarcoma virus. Cell. 1980 Dec;22(3):757–765. doi: 10.1016/0092-8674(80)90552-8. [DOI] [PubMed] [Google Scholar]
- Fewtrell C., Davis C. L., Metzger H. Phosphorylation of the receptor of immunoglobulin E. Biochemistry. 1982 Apr 27;21(9):2004–2010. doi: 10.1021/bi00538a005. [DOI] [PubMed] [Google Scholar]
- Glenn K., Bowen-Pope D. F., Ross R. Platelet-derived growth factor. III. Identification of a platelet-derived growth factor receptor by affinity labeling. J Biol Chem. 1982 May 10;257(9):5172–5176. [PubMed] [Google Scholar]
- Gordon A. S., Davis C. G., Milfay D., Diamond I. Phosphorylation of acetylcholine receptor by endogenous membrane protein kinase in receptor-enriched membranes of Torpedo californica. Nature. 1977 Jun 9;267(5611):539–540. doi: 10.1038/267539a0. [DOI] [PubMed] [Google Scholar]
- Harrison L. C., Itin A. Purification of the insulin receptor from human placenta by chromatography on immobilized wheat germ lectin and receptor antibody. J Biol Chem. 1980 Dec 25;255(24):12066–12072. [PubMed] [Google Scholar]
- Hempstead B. L., Kulczycki A., Jr, Parker C. W. Phosphorylation of the IgE receptor from ionophore A23187 stimulated intact rat mast cells. Biochem Biophys Res Commun. 1981 Feb 12;98(3):815–822. doi: 10.1016/0006-291x(81)91184-0. [DOI] [PubMed] [Google Scholar]
- Hunter T., Sefton B. M. Transforming gene product of Rous sarcoma virus phosphorylates tyrosine. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1311–1315. doi: 10.1073/pnas.77.3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs S., Chang K. J., Cuatrecasas P. Antibodies to purified insulin receptor have insulin-like activity. Science. 1978 Jun 16;200(4347):1283–1284. doi: 10.1126/science.663609. [DOI] [PubMed] [Google Scholar]
- Jacobs S., Hazum E., Shechter Y., Cuatrecasas P. Insulin receptor: covalent labeling and identification of subunits. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4918–4921. doi: 10.1073/pnas.76.10.4918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs S., Shechter Y., Bissell K., Cuatrecasas P. Purification and properties of insulin receptors from rat liver membranes. Biochem Biophys Res Commun. 1977 Aug 8;77(3):981–988. doi: 10.1016/s0006-291x(77)80074-0. [DOI] [PubMed] [Google Scholar]
- Kasuga M., Hedo J. A., Yamada K. M., Kahn C. R. The structure of insulin receptor and its subunits. Evidence for multiple nonreduced forms and a 210,000 possible proreceptor. J Biol Chem. 1982 Sep 10;257(17):10392–10399. [PubMed] [Google Scholar]
- Kasuga M., Kahn C. R., Hedo J. A., Van Obberghen E., Yamada K. M. Insulin-induced receptor loss in cultured human lymphocytes is due to accelerated receptor degradation. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6917–6921. doi: 10.1073/pnas.78.11.6917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasuga M., Karlsson F. A., Kahn C. R. Insulin stimulates the phosphorylation of the 95,000-dalton subunit of its own receptor. Science. 1982 Jan 8;215(4529):185–187. doi: 10.1126/science.7031900. [DOI] [PubMed] [Google Scholar]
- Kasuga M., Van Obberghen E., Nissley S. P., Rechler M. M. Demonstration of two subtypes of insulin-like growth factor receptors by affinity cross-linking. J Biol Chem. 1981 Jun 10;256(11):5305–5308. [PubMed] [Google Scholar]
- Kasuga M., Zick Y., Blith D. L., Karlsson F. A., Häring H. U., Kahn C. R. Insulin stimulation of phosphorylation of the beta subunit of the insulin receptor. Formation of both phosphoserine and phosphotyrosine. J Biol Chem. 1982 Sep 10;257(17):9891–9894. [PubMed] [Google Scholar]
- Kasuga M., Zick Y., Blithe D. L., Crettaz M., Kahn C. R. Insulin stimulates tyrosine phosphorylation of the insulin receptor in a cell-free system. Nature. 1982 Aug 12;298(5875):667–669. doi: 10.1038/298667a0. [DOI] [PubMed] [Google Scholar]
- Kawai S., Yoshida M., Segawa K., Sugiyama H., Ishizaki R., Toyoshima K. Characterization of Y73, an avian sarcoma virus: a unique transforming gene and its product, a phosphopolyprotein with protein kinase activity. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6199–6203. doi: 10.1073/pnas.77.10.6199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudlow J. E., Buss J. E., Gill G. N. Anti-pp60src antibodies are substrates for EGF-stimulated protein kinase. Nature. 1981 Apr 9;290(5806):519–521. doi: 10.1038/290519a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Machicao F., Urumow T., Wieland O. H. Phosphorylation--dephosphorylation of purified insulin receptor from human placenta. Effect of insulin. FEBS Lett. 1982 Nov 22;149(1):96–100. doi: 10.1016/0014-5793(82)81079-x. [DOI] [PubMed] [Google Scholar]
- Massagué J., Czech M. P. The subunit structures of two distinct receptors for insulin-like growth factors I and II and their relationship to the insulin receptor. J Biol Chem. 1982 May 10;257(9):5038–5045. [PubMed] [Google Scholar]
- Morrissey J. H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981 Nov 1;117(2):307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- Petruzzelli L. M., Ganguly S., Smith C. J., Cobb M. H., Rubin C. S., Rosen O. M. Insulin activates a tyrosine-specific protein kinase in extracts of 3T3-L1 adipocytes and human placenta. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6792–6796. doi: 10.1073/pnas.79.22.6792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilch P. F., Czech M. P. The subunit structure of the high affinity insulin receptor. Evidence for a disulfide-linked receptor complex in fat cell and liver plasma membranes. J Biol Chem. 1980 Feb 25;255(4):1722–1731. [PubMed] [Google Scholar]
- Porath J., Axen R., Ernback S. Chemical coupling of proteins to agarose. Nature. 1967 Sep 30;215(5109):1491–1492. doi: 10.1038/2151491a0. [DOI] [PubMed] [Google Scholar]
- Reynolds F. H., Jr, Todaro G. J., Fryling C., Stephenson J. R. Human transforming growth factors induce tyrosine phosphorylation of EGF receptors. Nature. 1981 Jul 16;292(5820):259–262. doi: 10.1038/292259a0. [DOI] [PubMed] [Google Scholar]
- Rosenfeld R. G., Baldwin D., Jr, Dollar L. A., Hintz R. L., Olefsky J. M., Rubenstein A. Simultaneous inhibition of insulin and somatomedin-C binding to cultured IM-9 lymphocytes by naturally occurring antireceptor antibodies. Diabetes. 1981 Nov;30(11):979–982. doi: 10.2337/diab.30.11.979. [DOI] [PubMed] [Google Scholar]
- Roth R. A., Cassell D. J. Insulin receptor: evidence that it is a protein kinase. Science. 1983 Jan 21;219(4582):299–301. doi: 10.1126/science.6849137. [DOI] [PubMed] [Google Scholar]
- Siegel T. W., Ganguly S., Jacobs S., Rosen O. M., Rubin C. S. Purification and properties of the human placental insulin receptor. J Biol Chem. 1981 Sep 10;256(17):9266–9273. [PubMed] [Google Scholar]
- Ushiro H., Cohen S. Identification of phosphotyrosine as a product of epidermal growth factor-activated protein kinase in A-431 cell membranes. J Biol Chem. 1980 Sep 25;255(18):8363–8365. [PubMed] [Google Scholar]
- Van Obberghen E., Kowalski A. Phosphorylation of the hepatic insulin receptor: stimulating effect of insulin on intact cells and in a cell-free system. FEBS Lett. 1982 Jul 5;143(2):179–182. doi: 10.1016/0014-5793(82)80094-x. [DOI] [PubMed] [Google Scholar]
- Witte O. N., Dasgupta A., Baltimore D. Abelson murine leukaemia virus protein is phosphorylated in vitro to form phosphotyrosine. Nature. 1980 Feb 28;283(5750):826–831. doi: 10.1038/283826a0. [DOI] [PubMed] [Google Scholar]
- Zick Y., Kasuga M., Kahn C. R., Roth J. Characterization of insulin-mediated phosphorylation of the insulin receptor in a cell-free system. J Biol Chem. 1983 Jan 10;258(1):75–80. [PubMed] [Google Scholar]