Abstract
The blue–green phenazine, Pyocyanin (PYO), is a well-known virulence factor produced by Pseudomonas aeruginosa, notably during cystic fibrosis lung infections. It is toxic to both eukaryotic and bacterial cells and several mechanisms, including the induction of oxidative stress, have been postulated. However, the mechanism of PYO toxicity under the physiological conditions of oxygen limitation that are encountered by P. aeruginosa and by target organisms in vivo remains unclear. In this study, wild-type and mutant strains of the yeast Saccharomyces cerevisiae were used as an effective eukaryotic model to determine the toxicity of PYO (100–500 μmol/L) under key growth conditions. Under respiro-fermentative conditions (with glucose as substrate), WT strains and certain H2O2-hypersensitive strains showed a low-toxic response to PYO. Under respiratory conditions (with glycerol as substrate) all the strains tested were significantly more sensitive to PYO. Four antioxidants were tested but only N-acetylcysteine was capable of partially counteracting PYO toxicity. PYO did not appear to affect short-term respiratory O2 uptake, but it did seem to interfere with cyanide-poisoned mitochondria through a complex III-dependent mechanism. Therefore, a combination of oxidative stress and respiration disturbance could partly explain aerobic PYO toxicity. Surprisingly, the toxic effects of PYO were more significant under anaerobic conditions. More pronounced effects were observed in several strains including a ‘petite’ strain lacking mitochondrial DNA, strains with increased or decreased levels of ABC transporters, and strains deficient in DNA damage repair. Therefore, even though PYO is toxic for actively respiring cells, O2 may indirectly protect the cells from the higher anaerobic-linked toxicity of PYO. The increased sensitivity to PYO under anaerobic conditions is not unique to S. cerevisiae and was also observed in another yeast, Candida albicans.
Keywords: Aerobiosis, anaerobiosis, oxidative stress, phenazine, pyocyanin, Saccharomyces cerevisiae, yeast mutants.
Introduction
Pyocyanin (PYO, 1-hydroxy-N-methylphenazine) is a blue–green redox-active secondary metabolite produced by the common opportunistic pathogen Pseudomonas aeruginosa. This pigment is an important virulence factor (Lau et al. 2004a,2004b; Caldwell et al. 2009; Hunter et al. 2012). It is toxic to a broad range of target organisms including bacteria, yeast, and mammalian cells (Hassan and Fridovitch 1980; Baron and Rowe 1981; Kerr et al. 1999; Muller 2006).
Many of the cytotoxic effects of PYO result from its ability to undergo a redox cycle (Gloyne et al. 2011; Fig. 1). PYO can be reduced nonenzymatically by NAD(P)H and this reduced form can react with O2 to produce superoxide (O2·−), and by dismutation, hydrogen peroxide (Hassan and Fridovitch 1980; David and Thornalley 1983; Müller et al. 1989; Britigan et al. 1992; Muller 2011). Moreover, it can affect host antioxidant mechanisms by inactivating catalase (CAT) and depleting glutathione levels (Muller 2002; O'Malley et al. 2003a). It is noteworthy that PYO directly reacts with several fluorescent probes (e.g., 2′,7′-dichlorodihydrofluorescein, dihydrorhodamine), which are commonly used to detect reactive oxygen species (ROS) (O'Malley et al. 2004b). Data correlating oxidative stress with PYO toxicity should therefore be taken with caution. It has also been suggested that PYO interferes with the respiratory chain and diverts electrons from it, potentially lowering cellular ATP content (Friedheim 1934; Armstrong and Stewart-Tull 1971; Baron et al. 1989). O'Malley et al. (2003b) showed that the addition of PYO has a dramatic impact on the ultrastructure of mitochondria from epithelial lung cells and these authors suggested that the redox cycling of PYO takes place in or near mitochondria. However, despite numerous studies, the subcellular site(s) of PYO redox cycling and the mechanisms underlying PYO toxicity remain unclear. Data published on the toxic effects of PYO in eukaryotic cells were performed under aerobic conditions. The few studies conducted under hypoxia or anaerobiosis were on bacteria (Hassan and Fridovitch 1980; Baron and Rowe 1981; Baron et al. 1989; Yoon et al. 2006; Price-Whelan et al. 2007; Wang et al. 2010). Yet, in vivo, both the producer of PYO (P. aeruginosa) and the eukaryotic target cells are subject to oxygen limitation, at least at certain periods (Hassett et al. 2002; Worlitzsch et al. 2002; Yoon et al. 2006).
Figure 1.
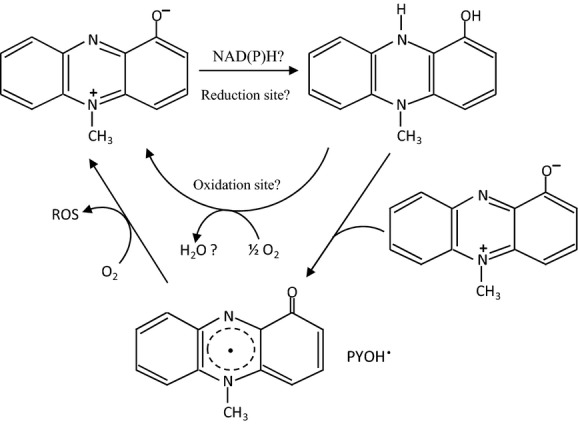
Hypothetical pyocyanin redox cycling (adapted from Jacob et al. 2011). Oxidized pyocyanin (blue) can be reduced nonenzymatically by NAD(P)H. The reduced pyocyanin (colorless) reacts with the oxidized form to generate the highly reactive pyocyanin radical (PYOH·). In the presence of O2, the auto-oxidation of pyocyanin leads to reactive oxygen species (ROS) production (O2, H2O2). Both oxidized and reduced pyocyanin may interfere with the respiratory chain.
Saccharomyces cerevisiae is an eminently suitable model system to study PYO–eukaryote interactions because of its metabolic plasticity and the wide availability of mutants. Ran et al. (2003) used S. cerevisiae as a surrogate host to screen and identify mammalian ortholog genes conferring sensitivity to PYO. These authors showed that PYO targets, identified using yeast mutants, in particular, mutations in the vacuolar H+-ATPase gene, were also inhibited in human lung epithelial cells. In 2006, Angell et al. assessed the transcriptional effects of PYO in S. cerevisiae. However, in both studies, only respiro-fermentative conditions (in YPD medium) with the WT-BY4741 strain (MATa) and derived mutants were used. It should be noted that the WT-BY4741 strain carries a mutation at the HAP1 locus, which encodes a major heme-responsive transcription factor that regulates gene expression in response to O2 (Gaisne et al. 1999). This strain is impaired in its respiratory capacity and phenomena related to respiration could therefore have been underestimated.
In this study, we examined PYO toxicity in S. cerevisiae cells and mitochondria. Different aerobic and anaerobic physiological conditions were used. The effect of PYO was quantified in several strains (including mutants impaired in respiration and/or oxidative stress resistance) under metabolic conditions of oxidation, respiro-fermentation, or pure fermentation.
Materials and Methods
Strains
The S. cerevisiae strains used in this study were WT-W303 (MATα), WT-BY4742 (MATα), mutants and constructions derived from WT-BY4742, and others strains differing in pleiotropic drug resistance or affected in DNA damage repair. The RWT-BY4742 strain restored for Hap1p (Gaisne et al. 1999) was also used as WT control. The main characteristics of the strains are summarized in Table S1. Many strains were purchased from EUROSCARF collection. The erg1Δ, hem1Δ, and rho0 mutants were constructed in the present study. The erg1Δ (TBY24805) haploid strain was obtained from Y24805 (EUROSCARF) by random spore analysis (Dawes and Hardie 1974) and anaerobic selection on the YPD medium supplemented with ergosterol (80 μg/mL), oleate (1% v/v Tween 80), and geneticin (G418 sulfate) 200 mg L−1. This mutant did not grow aerobically and microaerobically on this medium. The hem1Δ (TBY23591) haploid strain was obtained from Y23591 (EUROSCARF) by random spore analysis and selection on YPD supplemented with δ-aminolevulinate 80 mg/L. The rho0 respiratory-deficient mutant was obtained by ethidium bromide induction (Slonimski et al. 1968). We ensured that the strain rho0 was completely mDNA-free by staining the cells with the fluorescent dye, DAPI (4′-6-diamidine-2-phenylindole). For stock culture maintenance and storage, the RWT-BY47442 was grown on yeast nitrogen base (Sigma-Aldrich, Lyon, France) glucose 2% (w/v) devoid of uracil, and the hem1Δ mutant was grown in the presence of δ-aminolevulinate 80 mg L−1 to avoid the enrichment in spontaneous rho−/0 cells.
One strain of Candida albicans was used in this study, namely C. albicans ATCC 10231™ (American type culture collection, Manassa, VA).
Media and growth conditions
Yeast extract peptone (YEP) medium consisting of 1% (w/v) yeast extract, 2% (w/v) bacto-peptone (both from Difco Laboratories, Detroit, MI), 0.1% (w/v) KH2PO4, and 0.12% (w/v) NH4(SO4)2 was used as the basic medium supplemented with 2% (w/v) glucose (YPD) or 2% (w/v) glycerol (YPGly). The pH was adjusted to 5.5 with H2SO4 (10% w/v) before sterilization (115°C, 20 min). For growth of the WT-W303 strain, adenine 100 mg L−1 was added to the culture medium. For the hem1Δ mutant and/or for growth under anaerobiosis, the following anaerobic growth factors (AF) were added to the medium after sterilization (per liter): 15 mg ergosterol dissolved in 1 mL, Tween 80: pure ethanol (50:50, v/v).
The yap1Δ, skn7Δ, ctt1Δ, sod1Δ, zwf1Δ, erg1Δ, and hem1Δ mutants were precultivated in the presence of geneticin (G418 sulfate, 100 mg L−1). Aerobic or anaerobic precultures were performed at 30°C under shaking (180 rpm) in 100 mL Erlenmeyer flasks using a liquid to gas ratio of 1:10. Anaerobic precultures were incubated in anaerobic jars under an H2–CO2 atmosphere generated by Oxoid® gas-generating kits (Oxoid, Thermoscientific, Dardilly, France). Anaerobic Indicators (Oxoid®) were used to verify the absence of oxygen.
Aerobic cultures were performed in 24-well microtiter plates (Greiner, Frickenhausen, Germany). The initial optical density (OD600 nm) was 0.05 with an optical path length of 0.49 cm. At time zero, cells were treated with PYO (500 μmol/L) by adding a small volume (2.5% v/v) of a stock solution (20 mmol/L) freshly prepared in methanol. Control wells were used to verify the absence of effect of the solvent alone (methanol). Cells were incubated for 24 h at 30°C under continuous stirring at 180 rpm in H2O-enriched air.
Anaerobic cultures were performed in 15 mL Hungate tubes filled with 3 mL YPD supplemented with AF. The culture medium was desaerated by bubbling pure sterile argon for 2 min prior inoculation at an initial OD600 nm of 0.05. PYO (100–500 μmol/L) was added and anaerobic conditions were reached by bubbling again pure argon for 2 min. Fermentation processes were carried out at 30°C and 180 rpm for 24 or 48 h.
For anaerobic growth of C. albicans, YPD medium was supplemented with 0.07% (w/v) L-cysteine and adjusted to pH 6.0 (Rosa et al. 2008). AF (ergosterol, tween 80) were added to the medium after sterilization.
Chemicals
Purified PYO was purchased from Bertin Pharma (Montigny Le Bretonneux, France). G418 sulfate, H2O2, bovine superoxide dismutase (SOD) and catalase (CAT), potassium cyanide (KCN), antimycin A, Myxothiazol, HQNO (2-n-heptyl-4-hydroxyquinoline-N-oxide), L-ascorbate, tiron, resveratrol, L-cysteine hydrochloride monohydrate, and N-acetylcysteine (NAC) were purchased from Sigma-Aldrich (Saint-Quentin Fallavier, France).
Stock solutions were freshly prepared in distilled water or methanol or dimethylsulfoxide at appropriate concentrations. For each experiment, solvent controls were used.
Cell-density measurements
Growth was monitored by measuring the optical density at 600 nm (OD600 nm) using 24-well microtiter plates (Greiner) and the FLUOstar OPTIMA microplate reader from (BMG Labtech GmbH, Ortenberg, Germany). Wells were filled with 1 mL of sample (optical path length = 0.49 cm). If needed, cultures and culture supernatants (controls) were diluted in saline water (NaCl 0.9% w/v). The final absorbance of PYO was systematically taken into account for cell-density determinations.
Glucose and glycerol quantification
Glucose and glycerol were quantified using a Waters high pressure liquid chromatography system. Five microliters of filtered supernatant (on a 0.2 μm pore filters) were loaded on a Phenomenex Rezex ROA-organic acid H+ column (300 by 7.8 mm) subjected to an isocratic method with 2.5 mmol/L H2SO4 as an eluant (flow rate of 0.5 mL min−1). Compounds were detected by a Waters differential refractometer. Products were identified by the use of glucose, glycerol, pyruvate, acetate, and lactate as external standard and concentrations were calculated using standard curves.
Ethanol quantification
Ethanol quantification was performed on a Gas Chromatograph (Hewlett Packard model 5890 A; Hewlett Packard, Agilent Technologies France SAS, Les Ulis, France), equipped with a flame ionization detector (FID). The column used was an OV-1701 fused silica capillary column (25 m × 0.25 mm i.d. × 0.25 μm film thickness; Chrompack, France). The split ratio was 43/2. The injector was kept at 220°C, and the detector was kept at 250°C. The column temperature was held at 50°C for 15 min. Nitrogen was used as carrier gas and the flow rate in the column was 2 mL min−1. Hydrogen and air were supplied to the FID at 38 and 398 mL min−1, respectively. 1-Propanol was used as internal standard.
PYO quantification
The amount of PYO remaining in the rich medium after incubation was assessed spectrophotometrically at 690 nm, as previously reported (Price-Whelan et al. 2007). Culture supernatants incubated in the presence of PYO were vigorously reoxygenated until the 690 nm-signal stabilized. The absorbance of remaining oxidized PYO was then compared to the absorbance of 500 μmol/L PYO freshly dissolved in culture supernatants incubated without PYO. Absorbance spectra from 300 to 720 nm were measured to verify more precisely the recovery of typical peaks in the 376 nm and 690 nm regions. Media pH values were adjusted at the same values before measurement.
Cell viability
Cell viability was assessed by trypan blue staining. 200 μL of cell suspension was mixed and incubated for 3 min at room temperature with an equal volume of 0.4% (w/v) trypan blue solution prepared in 0.81% (w/v) NaCl and 0.06% (w/v) dipotassium phosphate.
Isolation of mitochondria and high-resolution oxygraphy
Mitochondria were isolated from the WT-W303 strain cultivated aerobically on YEP medium supplemented with adenine (100 mg L−1) and 2% (w/v) lactate. This carbon source was used as it allows the production of numerous mitochondria. Cells were harvested in the midexponential phase. Mitochondria were isolated using the procedure described by Avéret et al. (1998). Mitochondrial proteins were quantified by the Biuret method using bovine serum albumin (BSA) as standard.
For respiration measurements, mitochondria were resuspended at a final concentration of 0.1 mg protein mL−1 in the following buffer: mannitol 0.65 mol/L, ethylene glycol-bis aminoethylether N,N,N′,N′ tetraacetic acid 0.36 mmol/L, Tris/maleate 10 mmol/L (pH 6.7), BSA 0.3% (w/v). Oxygen concentrations were measured by high-resolution respirometry with the Oroboros Oxygraph-2k (Hutter et al. 2006) in standard configuration, with a 2 mL volume for the two chambers at 30°C and 500 rpm stirrer speed (Dufour et al. 2013). Data were recorded at 1 sec intervals using the Datlab 4 Acquisition software (Oroboros, Innsbruck, Austria). Standardized calibration procedures of the oxygen signal were carried out using the mannitol/Tris-maleate buffer. Respiration was automatically corrected for contributions of the polarographic oxygen sensor and of oxygen diffusion to total apparent respiration as a continuous function of oxygen concentration. P/O ratios (ATP formed vs. oxygen atoms consumed) were assessed by measuring the decrease in [O2] during the rapid burst of state 3 respiration after adding 0.1 mmol/L ADP. Oxygen consumption was expressed as pmoles of O2 consumed per second per unit of mitochondrial protein. Similar conditions were used for respiration measurements with whole cells, washed or not with Tris-maleate (100 mmol/L) buffer, KH2PO4 10 mmol/L, pH 5.5. Data shown are the means (± SD) of six independent measurements resulting from two independent experiments.
Results
Oxidative stress only partially explains PYO toxicity
PYO toxicity is generally attributed to the induction of oxidative stress (O'Malley et al. 2004a; Rada and Leto 2009; Muller 2011). The sensitivity of the different strains to oxidative stress was first assessed by measuring growth inhibition in the presence of 2 mmol/L H2O2, in YPD medium (Fig. 2A, gray bars). WT strains and certain mutants (described in Fig. 2 legend and Table S1) showed little, if any, sensitivity to 2 mmol/L H2O2. As expected, the yap1Δ, hem1Δ, and skn7Δ mutants were hypersensitive to H2O2.
Figure 2.
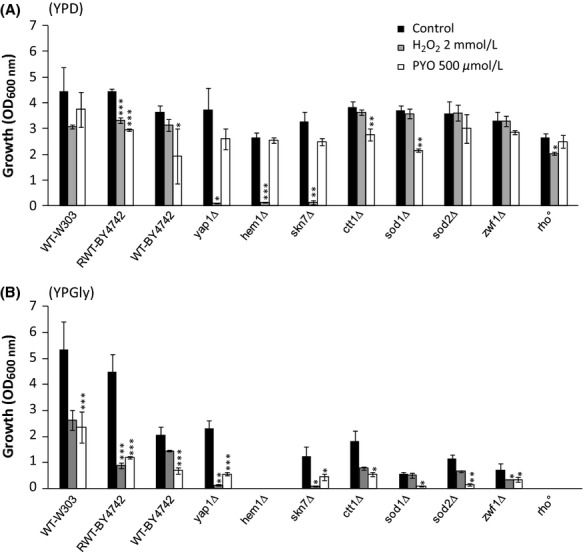
Effect of H2O2 and pyocyanin (PYO) on the aerobic growth (optical density [OD]600 nm) of several Saccharomyces cerevisiae strains. Yeast strains were cultivated aerobically for 24 h at 30°C on YPD medium (A) or on YPGly medium (B) in the presence of 2 mmol/L H2O2 or 500 μmol/L PYO. Black bars correspond to the control experiments. OD600 nm were measured with an optical path length of 0.49 cm. Cultures supernatants (diluted or not) were used as blank controls. Control wells were used to check the absence of effect of methanol 2.5% v/v (solvent of PYO). Results represent the means (± SD) of three to five separate experiments (12–20 absorbance values). Statistical significances of PYO effects (compared to the control) are indicated by the P-values: *P < 0.05; **P < 0.01; ***P < 0.001. Final pH values were about 4.6–4.9 and 6.0–7.1 in YPD and YPGly, respectively. Short description of the strains used (For further information, see Table S1 and Saccharomyces genome database at http://www.yeastgenome.org/): Wild-type W303 and BY-4742 are commonly used lab strains. WT-BY4742 is partially impaired in respiration as it is mutated in the HAP1 locus which encodes a major heme-responsive transcription factor that regulates O2-responsive genes. The RWT-BY4742 strain is restored for Hap1p (Gaisne et al. 1999). The yap1Δ and skn7Δ strains are hypersensitive to oxidative stress due to deletions of major transcription factors required for oxidative stress tolerance. The hem1Δ strain cannot synthesize heme and it is therefore unable to respire, devoid of catalase and cytochrome c peroxydase activities and impaired in lipid metabolism. The strains ctt1Δ, sod1Δ, sod2Δ, and zwf1Δ (directly derived from WT-BY4742) are deleted in cytosolic catalase, cytosolic Cu,Zn superoxide dismutase (SOD), mitochondrial Mn SOD, and cytosolic NADP(H)-dependent glucose-6-phosphate dehydrogenase, respectively. The rho0 strain is a respiratory-deficient mutant devoid of mitochondrial DNA.
In a parallel series, 500 μmol/L PYO was added to YPD medium (Fig. 2A, white bars). PYO had a minor toxic effect on most strains, including the H2O2-hypersensitive yap1Δ and skn7Δ mutant strains. In addition, the rho0 mutant, which is devoid of the mitochondrial respiratory chain, was almost entirely resistant to PYO.
It is noteworthy that final pH values (4.6–4.9) were just below the pKa value of PYO (pKa = 4.9, O'Malley et al. 2004a). Nevertheless, we verified that the relative resistance of YPD-grown WT cells to PYO was pH-independent by using either alkalinized (pH 6.5)-, alkalinized (pH 6.5)-phosphate buffered (100 mmol/L)-or acidified-(pH 4.5) YPD media (data not shown).
These data suggest that the low-PYO toxic effects observed in certain strains are mediated in part by oxidative stress, however, caused by species other than H2O2, and that additional phenomena are probably also involved. The data also suggest that respiration may reinforce PYO toxicity, as shown in bacteria (Hassan and Fridovitch 1980; Baron and Rowe 1981). YPD glucose-containing medium represses mitochondrial respiration and glucose is mainly catabolized by fermentation. We therefore studied the impact of H2O2 and PYO uniquely in oxidative conditions, using glycerol as growth substrate (YPGly medium). Under both YPD and YPgly conditions, PYO inhibited growth kinetics without affecting cell survival at any growth stage. Examples of growth kinetics are shown in Figure S1.
As expected, the respiratory-deficient hem1Δ and rho0 mutants did not grow on YPGly (Fig. 2B). All the mutant strains were growth impaired. Exposure to H2O2 caused greater growth inhibition for all the strains compared to similar conditions in YPD media, with the exception of yap1Δ and skn7Δ strains who were severely inhibited in both culture media (Fig. 2A and B, gray bars). Growth sensitivity was markedly increased (more than fivefold) for ctt1Δ, sod2Δ, and zwf1Δ mutants. In parallel, we examined PYO toxicity and found that the effect on growth was more than doubled compared to that observed on YPD medium for all the strains examined (Fig. 2B, white bars). Interestingly, the sod1Δ and sod2Δ mutants were found to be the most PYO-sensitive strains, suggesting the involvement of cytosolic and mitochondrial superoxide anions in PYO toxicity. However, little differences were found between the strains (hypersensitive or not to oxidative stress) in terms of response to PYO toxin. The data obtained using YPGly culture medium confirm the notion that actively respiring cells are more sensitive to PYO and that its effect is probably only partially due to oxidative stress.
Antioxidants only slightly protect against PYO toxicity
The addition of L-ascorbate (Muller 2009) or NAC can attenuate PYO toxicity in epithelial lung cells (Gloyne et al. 2011). We examined the effects of several cell-permeable antioxidants on PYO toxicity under oxidative conditions in YPGly medium. The antioxidants, L-ascorbate (10 mmol/L), resveratrol (200 μmol/L), and tiron (2 mmol/L) had no effect or even slightly increased the inhibitory effect of PYO (data not shown). This suggests that the use of such antioxidants to counteract PYO toxicity needs to be reexamined. As shown in Figure 3, a protective effect was, however, observed in WT strains with NAC, a precursor of glutathione biosynthesis and a radical scavenger. A similar protective effect of NAC was found for the yap1Δ mutant that is hypersensitive to oxidative stress. The NAC effect was only partial and was identical for both WT and yap1Δ strains, which again suggests that PYO toxicity is due in part to an oxidative stress-independent process.
Figure 3.
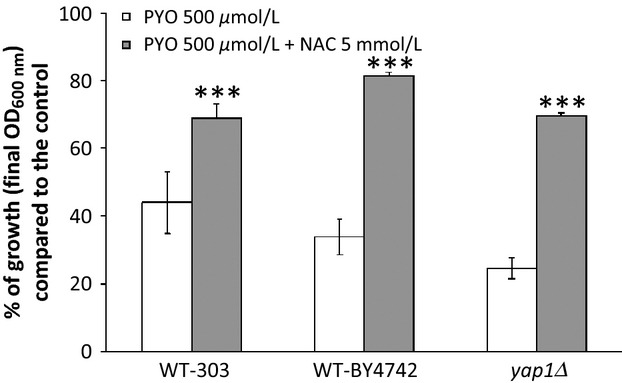
Protective effect of N-acetyl cystein against aerobic pyocyanin toxicity in WT-303, WT-BY4742 and yap1Δ Saccharomyces cerevisiae strains. Bars represent the percentages of growth observed compared to the controls. Yeast were cultivated aerobically for 48 h at 30°C on YPGly medium. Statistical significances of the NAC protective effects are indicated by the P-value: ***P < 0.001. All initial pH were adjusted to 5.5. Used alone, NAC had no effect on yeast growth (data not shown). The protective effect of NAC observed in WT-BY4742 (this figure) was similar in the complemented RWT-BY4742 strain and the zwf1Δ mutant (data not shown).
PYO appears to interact with S. cerevisiae respiratory chain
We were unable to detect any effect of PYO (100–500 μmol/L) on the instant respiration of YPGly-grown cells (data not shown). We, therefore, used isolated yeast mitochondria and examined whether PYO at 100 μmol/L concentration interferes with the respiratory chain and whether a redox cycling occurs at this level. Succinate was added to intact mitochondria (Fig. 4). Our data suggest that the addition of PYO has no apparent effect on “state 4” respiration, that is, in the absence of ADP or after the conversion of ADP (100 μmol/L) to ATP (Fig. 4A and B). It is possible that PYO alters the efficiency of ATP synthesis, therefore, we also measured the P/O ratios (ATP formed vs. oxygen atoms consumed). Similar P/O values were obtained with ADP (0.05 or 0.1 mmol/L) in the presence (1.64 ± 0.32) or absence (1.73 ± 0.33) of PYO. Moreover, the addition of PYO had no significant effect on the actively respiring “state 3” (excess of ADP, 3 mmol/L): specific mitochondrial O2 consumption rates were 1636 ± 61 and 1520 ± 175 pmoles O2 min−1 mg protein−1 in the absence or presence of PYO, respectively.
Figure 4.
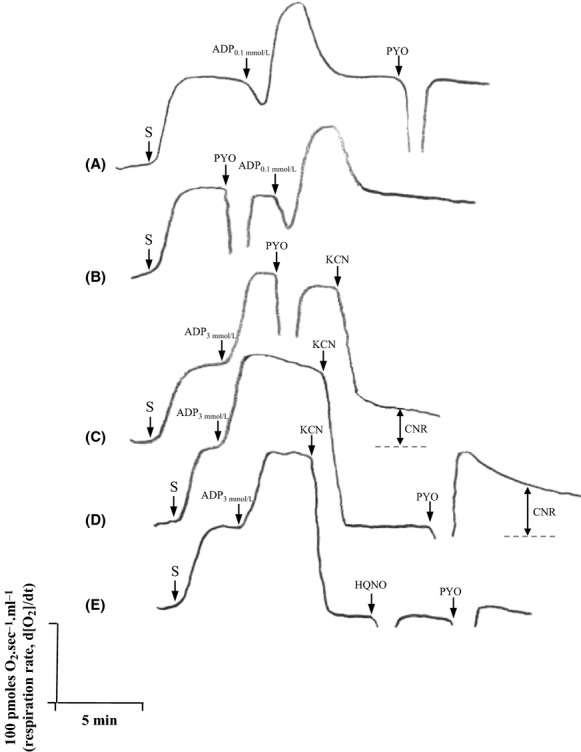
Effects of pyocyanin on respiration rates of isolated Saccharomyces cerevisiae mitochondria. O2 fluxes were measured by high-resolution respirometry (at [O2] up to 50 μmol/L) by successive additions of substrate (S, succinate 7.5 mmol/L), ADP (0.1 or 3 mmol/L), pyocyanin (PYO 100 μmol/L) and other poisons (potassium cyanide [KCN] 1 mmol/L and HQNO 20 μmol/L1). Three separate experiments (six acquisitions) were performed but only typical acquisitions are shown. In (A and B), a limited amount of ADP (0.1 mmol/L) was added (conditions used for P/O determinations). In (C, D, and E),2 Effects were measured at state 3 respiration (excess of ADP, 3 mmol/L). CNR, cyanide-resistant respiration.
1Myxothiazol (10 μmol/L) and antimycin A (10 μmol/L) acted similarly to HQNO.
2Similar results were obtained in the absence of ADP (state 4) and/or with α-ketoglutarate as substrate.
We also examined the effect of PYO together with two well-known respiratory poisons, cyanide and HQNO, also secreted by P. aeruginosa (Williams et al. 2007). An unusual cyanide-resistant respiration was detected (Fig. 4C). When PYO was added after cyanide (Fig. 4D), this cyanide-resistant respiration was again observed and was even stimulated. A peak of O2 consumption was seen just after the pulse of PYO but the signal stabilized after 8–10 min. As shown in Figure 4E, PYO did not induce this form of respiration in HQNO-poisoned mitochondria. Although no significant effect was detected on states 4 and 3 respirations, these results suggest that PYO can accept electrons from the respiratory chain through a complex III-dependent process.
In the actively respiring state 3, we assessed the release of ROS from mitochondria by adding CAT and/or SOD (both at 500 U/mL) after the consumption of 200 μmol/L O2 and when the [O2] reached zero in the oxygraph chambers. We found that the addition of PYO (± cyanide) did not cause the release of detectable amounts of O2 originating from H2O2 or O2·− (data not shown).
PYO strongly affects growth of S. cerevisiae under anaerobiosis
Our data suggest that, in vivo, PYO could divert a minor part of the electron flow from the respiratory chain. This effect, as well as oxidative stress, could impede growth but both effects should vanish under anaerobiosis. Therefore, we tested the effect of PYO under fermentative anaerobic conditions, as has already been done for bacteria (Hassan and Fridovitch 1980; Baron and Rowe 1981).
Surprisingly, PYO (500 μmol/L) was found to be highly toxic for yeast cells under anaerobiosis (Fig. 5). PYO toxicity (80% inhibition) increased fourfold for both WT-W303 and WT-BY4742 strains (Fig. 5A), compared to aerobic YPD conditions (Fig. 2A). Moreover, toxicity was also high (about 40% inhibition) with 100 μmol/L PYO and was dose-dependent. PYO, at 100 or 500 μmol/L concentrations, was fully reduced by yeast cells within a number of hours after the start of anaerobic growth. At 100 μmol/L, PYO was colorless within less than 4 h (Fig. S2). At 500 μmol/L, PYO was colorless at about 6 h (data not shown). Several control experiments were carried out in order to verify anaerobiosis and to clarify anaerobic PYO toxicity. We paid special attention to the potential involvement of trace amounts of O2. Yeast growth was not observed with glycerol as substrate. The erg1Δ mutant, which requires strict anaerobiosis to develop and therefore acted as a positive control, was readily able to grow under our conditions. In addition, anaerobic PYO toxicity was unaffected by NAC 5 mmol/L. These data (not shown) thus make it unlikely that there was an involvement of trace O2 in our anaerobic PYO toxicity measurements. We also verified that the effect of PYO was not influenced by the presence and composition of AF. The addition of AF and PYO did not increase toxicity under aerobiosis. The addition of 2 mg/L nicotinic acid (another AF, potentially present in limiting amounts in YPD) did not reduce anaerobic PYO toxicity (data not shown).
Figure 5.
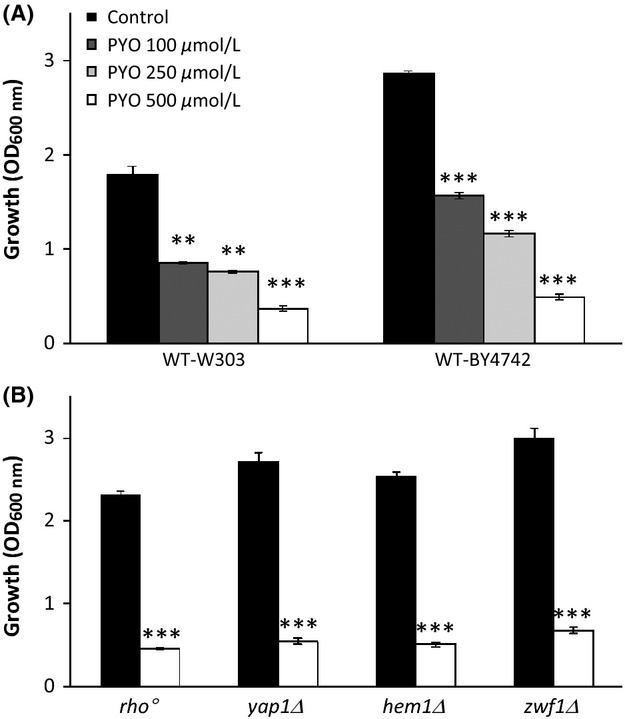
Effect of pyocyanin on the anaerobic growth of WT (A) and mutant strains (B) of Saccharomyces cerevisiae. Yeast were cultivated anaerobically for 24 h at 30°C in YPD medium supplemented with anaerobic growth factors, in the absence of pyocyanin (PYO) (black bars) or in the presence of PYO 100, 250, or 500 μmol/L. Results represent the means ± SD of three separate experiments. Similar results were obtained with the RWT-BY4742 strain (83% inhibition with PYO 500 μmol/L). The H2O2-hypersensitive trait of yap1Δ and hem1Δ mutants was also checked in anaerobic conditions (data not shown). Statistical significances of the pyocyanin effects are indicated by the P-values: **P < 0.01; ***P < 0.001.
If anaerobic PYO toxicity is independent of ROS formation, the different mutants used previously should behave in a similar manner under anaerobiosis. This hypothesis was tested. The growth of the yap1Δ, zwf1Δ, and hem1Δ mutants in YPD medium exhibited comparable sensitivity to PYO as the WT strains (Fig. 5B). Interestingly the rho0 mutant, which has no mitochondrial DNA and for which the same behavior under aerobiosis and anaerobiosis should therefore be expected, was sensitive to PYO only under anaerobiosis. The presence of O2 may indirectly protect the cells from an alternative mechanism of PYO toxicity operating under anaerobiosis.
PYO does not affect anaerobic fermentation parameters
We attempted to define, more precisely, the effects of PYO in anaerobic cells by examining several parameters during glucose fermentation (Table 1). After 48 h of growth, the cells incubated with PYO (500 μmol/L) were almost all viable. This suggests that PYO affects anaerobic growth kinetics rather than killing the cells. As a consequence of growth inhibition, glucose consumption and ethanol production were strongly reduced in the presence of PYO. The yields of ethanol and glycerol production did not show major changes and no other by-products, such as pyruvate, could be detected in the culture medium. We conclude that the end-product formation pathways are probably not the site of action of PYO. No loss of PYO could be detected (aerobically) after 24 h (Fig. S3) and 48 h (data not shown) of anaerobic growth, suggesting that the effect is probably due to PYO itself and not to the accumulation of a degradation product. From these data, we hypothesize that a relatively high level of PYO radical is produced during anaerobic glucose fermentation and/or that this radical has higher toxicity than ROS.
Table 1.
Effect of pyocyanin (PYO 500 μmol/L) on anaerobic growth parameters.
| Strains | WT-W303 |
WT-BY4742 |
||
|---|---|---|---|---|
| −PYO | +PYO | −PYO | +PYO | |
| Growth (final OD600 nm)1 | 1.78 | 0.39 | 2.66 | 0.365 |
| Glucose consumed (moles) | 111 | 63 | 110 | 47 |
| Ethanol produced (moles) | 177 | 102 | 186 | 73 |
| Y ethanol/glucose | 0.80 | 0.82 | 0.85 | 0.76 |
| Glycerol produced (moles) | 11.9 | 7.9 | 9.4 | 5.6 |
| Y glycerol/glucose | 0.054 | 0.063 | 0.043 | 0.059 |
| Viability (%) | 99 | 99 | 99 | 99 |
| Final pH | 4.99 | 5.3 | 4.93 | 5.02 |
Yeast cells were grown under anaerobiosis for 48 h at 30°C.
Measured with an optical path length of 0.49 cm. All the cultures were in stationary phase.
PYO toxicity is not correlated to multidrug resistance
We asked the question whether the high toxicity of PYO under anaerobiosis could be explained by a decrease in the expression of ABC transporters involved in multidrug resistance. We compared the effects of PYO, under aerobic and anaerobic conditions, on WT-222-95-C strain and both AD1-9 and US50-18C mutant strains. AD1-9 strain carries multiple deletions in 7 ABC genes (YOR1, SNQ2, PDR5, YCF1, PDR10, PDR11, and PDR15) and in two transcription activation factors (PDR1 and PDR3). These mutations render the cells 2–200 times more sensitive to numerous toxic compounds including small heterocyclic compounds such as resazurin and 8-hydroxyquinoline that share some similarities with PYO (Rogers et al. 2001; Decottignies et al. 2002). The US50-18C strain carries the pdr1-3 activating mutation that confers considerable multiple drug resistance. As shown in Figure 6, the three strains exhibited similar patterns of PYO inhibition. Toxic effects on yeast growth were low during aerobic fermentation (YPD), especially in the case of the US50-18C strain. Toxicity was increased significantly during aerobic respiration (YPGly) and anaerobic fermentation (YPD + AF) for all strains tested. These data suggest that pleiotropic drug resistance-related ABC transporters in the yeast S. cerevisiae are not involved in PYO toxicity.
Figure 6.
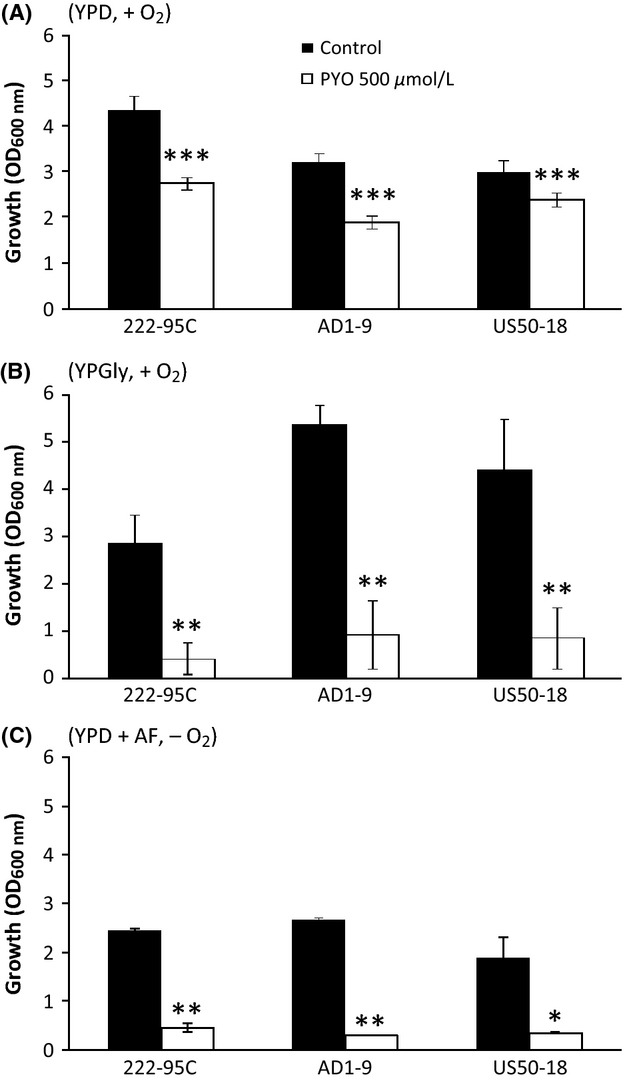
Effect of pyocyanin on the aerobic and anaerobic growth of WT-222-95C and mutant strains of Saccharomyces cerevisiae. AD1-9 strain has a decreased resistance to multiple drugs and US50-18 strain has an increased resistance to drugs. Yeast were cultivated at 30°C aerobically for 24 h on YPD medium (A) and for 48 h on YPGly (B) or for 24 h on YPD supplemented with anaerobic growth factors (C). Statistical significances of the pyocyanin effects are indicated by the P-values: *P < 0.05; **P < 0.01; ***P < 0.001.
PYO toxicity is not linked to DNA intercalation
PYO binds directly and intercalates the DNA (Hollstein and Van Gemert 1971; Das et al. 2013). Although DNA intercalation occurs via noncovalent interactions and is reversible, PYO could cause substantial changes in DNA structure under our conditions. The potential link between anaerobic toxicity and DNA damage was therefore explored using S. cerevisiae mutants, which are deficient in homologous recombination and DNA damage repair (nucleotide or base excision repair). Experiments were carried out as for Figures 2, 5. As shown in Figure S4, the mutants did not show increased sensitivity to PYO, when 500 and 100 μmol/L PYO were added under aerobic and anaerobic conditions, respectively. Under these conditions, no link was found between PYO toxicity and DNA damage.
Candida albicans demonstrates similar PYO sensitivity under anaerobiosis to S. cerevisiae
We examined whether PYO can affect the anaerobic growth of another species of yeast. The pathogenic crabtree-negative yeast C. albicans was chosen because this fungus, together with Aspergillus fumigates, may coexist with P. aeruginosa in the lungs of cystic fibrosis (CF) patients (McAlester et al. 2008). This species is capable of anaerobic growth in the presence of L-cysteine (Rosa et al. 2008) and AF. Importantly, we found that high anaerobic PYO toxicity was also elicited in C. albicans (Fig. 7). PYO toxicity was lower under aerobiosis in the presence or absence of L-cysteine.
Figure 7.
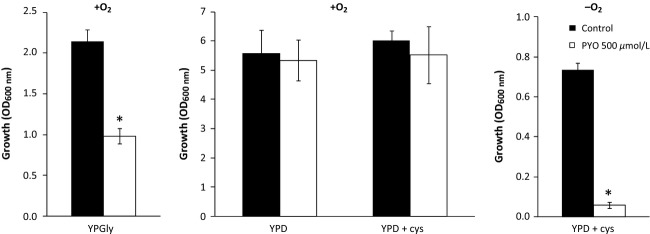
Effect of pyocyanin 500 μmol/L on the aerobic and anaerobic growth of Candida albicans. Yeast were cultivated at 30°C aerobically for 24 h on YPGly, YPD or YPD supplemented with 0.07% (w/v) L-cysteine and anaerobically for 48 h on YPD supplemented with 0.07% (w/v) L-cysteine and anaerobic growth factors. Statistical significance of the pyocyanin (PYO) effect is indicated by the P-value: *P < 0.05.
Altogether, these data strongly suggest that PYO is an anaerobic poison.
Discussion
As recently discussed by Okegbe et al. (2012), the understanding of the biological roles of redox-active metabolites such as the phenazine PYO requires characterization of their specific biological effects in a condition-dependent manner. Numerous studies have reported the toxic effects of PYO. However, to our knowledge, the relationships between PYO toxicity, respiratory capacities, oxidative stress, and O2 tension have not yet been examined in a eukaryotic organism. The interaction of PYO with mitochondrial respiration has only been documented to a small extent (Friedheim 1931; Armstrong and Stewart-Tull 1971; O'Malley et al., 2003b). In this study, we first reexamined PYO toxicity using the standard medium (YPD) for growth of several S. cerevisiae strains. For these experiments, we used concentrations of PYO (500 μmol/L ≡ 105 μg/mL) that are similar to the concentrations previously tested in S. cerevisiae by other authors (Ran et al. 2003 [25–100 μg/mL]; Angell et al. 2006 [25–250 μg/mL]). These concentrations are higher than the concentration of PYO detected in infected lungs of CF patients (Wilson et al. 1988; Hunter et al. 2012). Notwithstanding, a relative toxin resistance to 500 μmol/L PYO was observed for several genetic backgrounds. We verified that this was not caused by acidification of the medium that could modify the color and protonation state of PYO at pH ≤ 4.9. For the first time, we provide data on PYO toxicity in actively respiring S. cerevisiae cells (in YPGly medium) and during strict fermentation (anaerobiosis). We found that oxidized PYO (100 μmol/L) did not affect respiration of yeast cells or of isolated mitochondria. However, using cyanide and HQNO (and other complex III inhibitors), we found that PYO could interfere with mitochondrial respiration at the level of complex III or of a downstream target. PYO can, therefore, be considered as another mitochondria-targeted agent, together with the well-known poisons, cyanide and HQNO, also produced by P. aeruginosa under hypoxia (Machan et al. 1992; Williams et al. 2007). Despite the apparent absence of a short-term effect of PYO on cellular and “state 3” O2 fluxes, it is still unknown whether a futile redox cycling of PYO occurs in vivo in mitochondria. In this study, we obtained a panel of data showing a role for mitochondrial respiration in triggering aerobic PYO toxicity. PYO might also target specific steps in the oxidative metabolism of glycerol (e.g., glycerol-3-phosphaste dehydrogenase).
Despite the inhibition of PYO toxicity by NAC, the role of oxidative stress remains unclear. For instance, the yap1Δ and skn7Δ mutants, both of whom are hypersensitive to oxidative stress, were not PYO-hypersensitive.
The most striking result of this study is the high toxicity observed with PYO under anaerobiosis (argon atmosphere). Increased toxicity of PYO under anaerobiosis has already been observed in bacteria under denitrifying conditions, but not under fermentative conditions (Baron and Rowe 1981). The authors concluded that active respiration, but not necessarily the presence of O2, was required for PYO toxicity and that under anaerobiosis, a nitrogen radical instead of ROS could be involved. They also proposed that PYO could divert electron flow at a specific site of the respiratory chain, thus disturbing oxidative phosphorylation. As recently shown in Escherichia coli by Gu and Imlay (2011), redox drugs such as phenazine methosulfate (PMS) quickly accumulate in a reduced inactive form during anaerobic fermentation (when oxygen is unavailable to reoxidize them). The anaerobic toxicity of PMS requires its reoxidation by a terminal electron acceptor in the respiratory chain. In both S. cerevisiae and C. albicans (this work), anaerobic PYO toxicity is entirely independent of the respiratory chain, as demonstrated here with rho and hem1Δ mutants. Recently, Imlay (2013) pointed out that, even under anoxic conditions, redox-cycling compounds can be toxic because of their ability to destabilize Fe-S cluster containing enzymes, especially not only dehydratases involved in amino acid biosynthesis but also aconitase and fumarase. In S. cerevisiae, PYO inhibited anaerobic growth without any change in cell viability and without a significant effect on the apparent redox balance. We hypothesize a mechanism similar to Imlay to explain the anaerobic PYO toxicity in S. cerevisiae. In the absence of O2 and at high fermentative glycolytic fluxes (Gancedo et Serrano, 1989; Beauvoit et al., 1993), the cross-reaction between NAD(P)H-reduced and oxidized PYO could lead to elevated levels of the radical form (Fig. 1). We propose that this form reacts with Fe-S-dependent enzymes and impairs growth more efficiently than the ROS formed under aerobiosis. To solve some of these questions, specific electronic paramagnetic resonance (EPR) experiments are currently being designed to quantify the PYO radical, in both aerobic and anaerobic S. cerevisiae cells. This approach will then be extended to C. albicans, other fungi, and to mammalian cells under transient anaerobiosis.
Certain bacteria and fungi are susceptible to PYO and other phenazines at low concentrations (μmol/L range) (Hassan and Fridovitch 1980; Baron and Rowe 1981). This is not the case in S. cerevisiae. 100 μmol/L were required for 50% inhibition of anaerobic growth. This difference reinforces the idea that there are probably many different mechanisms of PYO toxicity in living organisms. Nevertheless, it should be kept in mind that the concentration used in anaerobiosis (100 μmol/L) is of the same order as those observed (˜10–130 μmol/L or 25–50 μmol/L) in the lungs of CF patients infected by P. aeruginosa (Wilson et al. 1988; Hunter et al. 2012).
PYO synthesis helps P.aeruginosa's growth and survival under O2-limitations (Price-Whelan et al. 2007; Wang et al. 2010). In addition, O2-limitations are encountered in vivo by eucaryotic target cells: during cystic fibrosis (CF), P. aeruginosa actively produces a microaerobic environment even at high aeration rates due to the overproduction of alginate (Sabra et al. 2002). Very steep hypoxic gradients or even anaerobic conditions can be generated in the airway mucus of CF patients (Stutts et al. 1986; Worlitzsch et al. 2002; Yoon et al. 2002; Hassett et al. 2009). In this type of anaerobic microenvironment, target host cells or infectious agents (C. albicans or other fungi) may be exposed to increased PYO toxicity. Taking into account these data and the results of this study, further research is clearly needed to fully understand the impact of hypoxia and anoxia on PYO toxicity and on P. aeruginosa pathogenicity.
Acknowledgments
We greatly thank Anne Devin for providing the Restored WT-BY strain. The authors are indebted to A. Goffeau for his advice on multidrug resistance yeast mutants. E. R. tenderly acknowledges the special contribution of Laura Rosenfeld Pereira. This work was supported by a Région Poitou-Charentes grant.
Conflict of Interest
None declared.
Funding Information
This work was supported by a Région Poitou-Charentes grant.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Figure S1. Typical aerobic growth kinetics of Saccharomyces cerevisiae strains in the absence (▪) or presence (□) of PYO 500 μgmol/L. W303 (A), BY-4742 (B) hem↿ (C) yap↿ (D) and rho0 (E) strains were grown at 30°C on YPD medium. W303 (F) BY-4742 (G) yap↿ (H) strain were grown at 30°C on YPGly medium. Results are the means of four determinations. For the control experiments (▪), we checked the absence of effect of the solvent alone (methanol). Using trypan blue, we also checked that PYO did not increase cell mortality (at detectable levels) and did not induce significant changes in yeast cell morphology at any growth stage (data not shown). Even minor on YPD medium, PYO toxicity was visible in middle or late growth stages. In the particular hem↿ mutant (C) cumulating several severe dysfunctions (respiratorydeficiency, strong impairments of lipid metabolism and of O2 and ROS adaptations), growth was delayed in the presence of PYO but final growth (after 24 h) was unaffected (see also Fig. 22A).
Figure S2. Typical photographs showing the decoloration of pyocyanin (PYO) during anaerobic cultures in Hungate tubes. In this example, the WT-303 strain was used. The culture medium was YPD supplemented with anaerobic growth factors. At t = 0, OD600 nm was 0.05 (l = 0.49 cm). Depending on the initial concentration, PYO became colorless after a few hours of cultivation. At time 4 h, PYO 100 μmol/L was colorless while PYO 500 μmol/L was only partially discolored. At time 24 h, the blue color of PYO 500 μmol/L was entirely recovered after a vigorous oxygenation of the tube. PYO recovery was also measured by spectrophotometry as described in Material and Methods (Fig. S3 and data not shown).
Figure S3. Assessment of the amount of pyocyanin (PYO) remaining in the rich medium after 24 h of anaerobic growth (WT-BY4742 strain). Culture supernatants incubated in the presence of PYO 500 μmol/L were vigorously reoxygenated until the 690 nm-signal stabilized. The absorbance of the remaining oxidized PYO was then compared to the absorbance of 500 μmol/L pyocyanin freshly dissolved in culture supernatants incubated without pyocyanin. Media pH were adjusted at the same values before measurement. Three comparative assays were performed.
Figure S4. Effect of pyocyanin 500 μmol/L and 100 μmol/L on the aerobic and anaerobic growth of WT-BY4742 and mutant strains of Saccharomyces cerevisiae deficient in DNA damage repair ability. Yeast were cultivated at 30°C aerobically for 24 h on YPD medium (A) and for 48 h on YPGly medium (B) or anaerobically for 24 h on YPD supplemented with anaerobic growth factors (C). Rad mutants (derived from WT-BY4742) are affected in nucleotide excision repair or recombinational repair of double-strand breaks in DNA. The mutant lar013 (derived from the control strain lar009), deleted in Ntg1, Ntg2 and Apn1 genes, is affected in base excision repair and repair of DNA damage caused by oxidation and alkylating agents (see Table S1 for further details).
Table S1. Saccharomyces cerevisiae strains used in this study.
References
- Angell S, Bench BJ, Williams H, Watanabe CM. Pyocyanin isolated from a marine microbial population: synergistic production between two distinct bacterial species and mode of action. Chem. Biol. 2006;13:1349–1359. doi: 10.1016/j.chembiol.2006.10.012. [DOI] [PubMed] [Google Scholar]
- Armstrong AV, Stewart-Tull DE. The site of the activity of extracellular products of Pseudomonas aeruginosa in the electron-transport chain in mammalian cell respiration. J. Med. Microbiol. 1971;4:263–270. doi: 10.1099/00222615-4-2-263. [DOI] [PubMed] [Google Scholar]
- Avéret N, Fitton V, Bunoust O, Rigoulet M, Guérin B. Yeast mitochondrial metabolism: from in vitro to in situ quantitative study. Mol. Cell. Biochem. 1998;184:67–79. [PubMed] [Google Scholar]
- Baron S, Rowe J. Antibiotic action of pyocyanin. Antimicrob. Agents Chemother. 1981;20:814–820. doi: 10.1128/aac.20.6.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron S, Terranova G, Rowe J. Molecular mechanism of the antimicrobial action of pyocyanin. Curr. Microbiol. 1989;18:223–230. [Google Scholar]
- Beauvoit B, Rigoulet M, Bunoust O, Raffard G, Canioni P, Guérin B. Interactions between glucose metabolism and oxidative phosphorylations on respiratory-competent Saccharomyces cerevisiae cells. Eur. J. Biochem. 1993;214:163–172. doi: 10.1111/j.1432-1033.1993.tb17909.x. [DOI] [PubMed] [Google Scholar]
- Britigan BE, Roeder TL, Rasmussen GT, Shasby DM, McCormick ML, Cox C. Interaction of the Pseudomonas aeruginosa secretory products pyocyanin and pyochelin generates hydroxyl radical and causes synergistic damage to endothelial cells. J. Clin. Invest. 1992;90:2187–2196. doi: 10.1172/JCI116104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell CC, Chen Y, Goetzmann HS, Hao Y, Borchers MT, Hasset DJ, et al. Pseudomonas aeruginosa exotoxin pyocyanin causes cystic fibrosis airway pathogenesis. Am. J. Pathol. 2009;175:2473–2488. doi: 10.2353/ajpath.2009.090166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das T, Kutty SK, Kumar N, Manefield M. Pyocyanin facilitates extracellular DNA binding to Pseudomonas aeruginosa influencing cell surface properties and aggregation. PLoS One. 2013;8:e58299. doi: 10.1371/journal.pone.0058299. doi: 10.1371/journal.pone.0058299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David G, Thornalley PJ. Free radical production from the aerobic oxidation of reduced pyridine nucleotides catalyzed by phenazine derivatives. Biochim. Biophys. Acta. 1983;724:456–464. doi: 10.1016/0005-2728(83)90106-8. [DOI] [PubMed] [Google Scholar]
- Dawes IW, Hardie ID. Selective killing of vegetative cells in sporulated yeast cultures by exposure to diethyl ether. Mol. Genet. Genomics. 1974;131:281–289. doi: 10.1007/BF00264859. [DOI] [PubMed] [Google Scholar]
- Decottignies A, Rogers B, Kolaczkowski M, Carvajal E, Balzi E, Conseil G, Paulsen IT, Lewis K, et al. Microbial multidrug efflux. Wymondham, U.K: Horizon Scientific Press; 2002. The pleiotropic drug ABC transporters from Saccharomyces cerevisiae; p. 301. (Chapter 11, Pp. 157–176). [Google Scholar]
- Dufour V, Li J, Flint A, Rosenfeld E, Rivoal K, Georgeault S, et al. Inactivation of the LysR regulator Cj1000 of Campylobacter jejuni affects host colonisation and respiration. Microbiology. 2013;159:1165–1178. doi: 10.1099/mic.0.062992-0. [DOI] [PubMed] [Google Scholar]
- Friedheim EA. Pyocyanin, an accessory respiratory enzyme. J. Exp. Med. 1931;54:207–221. doi: 10.1084/jem.54.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedheim EA. The effect of pyocyanin on the respiration of some normal tissues and tumours. Biochem. J. 1934;28:173–179. doi: 10.1042/bj0280173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaisne M, Bécam AM, Verdière J, Herbert CJ. A ‘natural’ mutation in Saccharomyces cerevisiae strains derived from S288c affects the complex regulatory gene HAP1 (CYP1) Curr. Genet. 1999;36:195–200. doi: 10.1007/s002940050490. [DOI] [PubMed] [Google Scholar]
- Gancedo C, Serrano R, Harisson JS, Rose AH. The yeasts. NewYork: Academic Press; 1989. Energy-yielding metabolism. 2nd ed. Vol. 3; pp. 205–259. [Google Scholar]
- Gloyne L, Grant GD, Perkins AV, Mc Dermott CM, Jonhson PV, Anderson GJ. Pyocyanin-induced toxicity in A549 respiratory cells is causally linked to oxidative stress. Toxicol. In Vitro. 2011;25:1353–1358. doi: 10.1016/j.tiv.2011.05.004. [DOI] [PubMed] [Google Scholar]
- Gu M, Imlay JA. The SoxRS response of Escherichia coli is directly activated by redox-cycling drugs rather than by superoxide. Mol. Microbiol. 2011;79:1136–1150. doi: 10.1111/j.1365-2958.2010.07520.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan HM, Fridovitch I. Mechanism of the antibiotic action of pyocyanin. J. Bacteriol. 1980;141:156–163. doi: 10.1128/jb.141.1.156-163.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassett DJ, Cuppolettib J, Trapnellc B, Lymard SV, Rowee JJ, Yoona SS, et al. Anaerobic metabolism and quorum sensing by Pseudomonas aeruginosa biofilms in chronically infected cystic fibrosis airways: rethinking antibiotic treatment strategies and drug targets. Adv. Drug Deliv. Rev. 2002;54:1425–1443. doi: 10.1016/s0169-409x(02)00152-7. [DOI] [PubMed] [Google Scholar]
- Hassett DJ, Sutton MD, Schurr MJ, Herr AB, Caldwell CC, Matu JO. Pseudomonas aeruginosa hypoxic or anaerobic biofilm infections within cystic fibrosis airways. Trends Microbiol. 2009;17:130–138. doi: 10.1016/j.tim.2008.12.003. [DOI] [PubMed] [Google Scholar]
- Hollstein U, Van Gemert RJ. Interaction of phenazines with polydeoxyribonucleotides. Biochemistry. 1971;10:497–504. doi: 10.1021/bi00779a023. [DOI] [PubMed] [Google Scholar]
- Hunter RC, Klepac-Ceraj V, Lorenzi MM, Grotzinger H, Martin TR, Newman DK. Phenazine content in the cystic fibrosis respiratory tract negatively correlates with lung function and microbial complexity. Am. J. Respir. Cell Mol. Biol. 2012;47:738–745. doi: 10.1165/rcmb.2012-0088OC. [DOI] [PubMed] [Google Scholar]
- Hutter E, Unterluggauer H, Garedew A, Jansen-Durr P, Gnaiger E. High resolution respirometry – a modern tool in aging research. Exp. Gerontol. 2006;41:103–109. doi: 10.1016/j.exger.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Imlay JA. The molecular mechanisms and physiological consequences of oxidative stress: lessons from a model bacterium. Nat. Rev. Microbiol. 2013;11:443–454. doi: 10.1038/nrmicro3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob C, Jamier V, Ba LA. Redox active secondary metabolites. Curr. Opin. Chem. Biol. 2011;15:149–155. doi: 10.1016/j.cbpa.2010.10.015. [DOI] [PubMed] [Google Scholar]
- Kerr JR, Taylor GW, Rutman A, Høiby N, Cole PJ, Wilson R. Pseudomonas aeruginosa pyocyanin and 1-hydroxyphenazine inhibit fungal growth. J. Clin. Pathol. 1999;52:385–387. doi: 10.1136/jcp.52.5.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau GW, Ran H, Kong F, Hassett DJ, Mavrodi D. Pseudomonas aeruginosa pyocyanin is critical for lung infection in mice. Infect. Immun. 2004a;72:4275–4278. doi: 10.1128/IAI.72.7.4275-4278.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau GW, Hassett DJ, Ran H, Kong F. The role of pyocyanin in Pseudomonas aeruginosa infection. Trends Mol. Med. 2004b;10:599–606. doi: 10.1016/j.molmed.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Machan ZA, Taylor GW, Pitt TL, Cole PJ, Wilson R. 2-Heptyl-4-hydroxyquinoline N-oxide, an antistaphylococcal agent produced by Pseudomonas aeruginosa. J. Antimicrob. Chemother. 1992;30:615–623. doi: 10.1093/jac/30.5.615. [DOI] [PubMed] [Google Scholar]
- McAlester G, O'Gara F, Morrissey JP. Signal-mediated interactions between Pseudomonas aeruginosa and Candida albicans. J. Med. Microbiol. 2008;57:563–569. doi: 10.1099/jmm.0.47705-0. [DOI] [PubMed] [Google Scholar]
- Minard KI, McAlister-Henn L. Antioxidant function of cytosolic sources of NADPH in yeast. Free Radic. Biol. Med. 2001;31:832–843. doi: 10.1016/s0891-5849(01)00666-9. [DOI] [PubMed] [Google Scholar]
- Muller M. Pyocyanin induces oxidative stress in human endothelial cells and modulates the glutathione redox cycle. Free Radic. Biol. Med. 2002;33:1527–1533. doi: 10.1016/s0891-5849(02)01087-0. [DOI] [PubMed] [Google Scholar]
- Muller M. Premature cellular senescence induced by pyocyanin, a redox-active Pseudomonas aeruginosa toxin. Free Radic. Biol. Med. 2006;41:1670–1677. doi: 10.1016/j.freeradbiomed.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Muller M. Polyphenol cytotoxicity induced by the bacterial toxin pyocyanin: role of NQO1. Free Radic. Biol. Med. 2009;47:84–91. doi: 10.1016/j.freeradbiomed.2009.04.011. [DOI] [PubMed] [Google Scholar]
- Muller M. Glutathione modulates the toxicity of, but is not a biologically relevant reductant for, the Pseudomonas aeruginosa redox toxin pyocyanin. Free Radic. Biol. Med. 2011;50:971–977. doi: 10.1016/j.freeradbiomed.2011.01.012. [DOI] [PubMed] [Google Scholar]
- Müller PK, Krohn K, Mühlradt P. Effects of pyocyanin, a phenazine dye from Pseudomonas aeruginosa, on oxidative burst and bacterial killing in human neutrophils. Infect. Immun. 1989;57:2591–2596. doi: 10.1128/iai.57.9.2591-2596.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okegbe C, Sakhtah H, Sekedat MD, Price-Whelan A, Dietrich LE. Redox eustress: roles for redox-active metabolites in bacterial signaling and behavior. Antioxid. Redox Signal. 2012;16:658–667. doi: 10.1089/ars.2011.4249. [DOI] [PubMed] [Google Scholar]
- O'Malley YQ, Reszka KJ, Rasmussen GT, Abdalla MY, Denning GM, Britigan BE. The Pseudomonas secretory product pyocyanin inhibits catalase activity in human lung epithelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2003a;285:L1077–L1086. doi: 10.1152/ajplung.00198.2003. [DOI] [PubMed] [Google Scholar]
- O'Malley YQ, Abdalla MY, McCormick ML, Reszka KJ, Denning GM, Britigan BE. Subcellular localization of Pseudomonas pyocyanin cytotoxicity in human lung epithelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2003b;284:L420–L430. doi: 10.1152/ajplung.00316.2002. [DOI] [PubMed] [Google Scholar]
- O'Malley YQ, Reszka KJ, Spitz DR, Denning GM, Britigan BE. Pseudomonas aeruginosa pyocyanin directly oxidizes glutathione and decreases its levels in airway epithelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2004a;287:L94–L103. doi: 10.1152/ajplung.00025.2004. [DOI] [PubMed] [Google Scholar]
- O'Malley YQ, Reszka KJ, Britigan BE. Direct oxidation of 2′,7′-dichlorodihydrofluorescein by pyocyanin and other redox-active compounds independent of reactive oxygen species production. Free Radic. Biol. Med. 2004b;36:90–100. doi: 10.1016/j.freeradbiomed.2003.09.021. [DOI] [PubMed] [Google Scholar]
- Price-Whelan A, Dietrich LEP, Newman DK. Pyocyanin alters redox homeostasis and carbon flux through central metabolic pathways in Pseudomonas aeruginosa PA14. J. Bacteriol. 2007;189:6372–6381. doi: 10.1128/JB.00505-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rada B, Leto TL. Redox warfare between airway epithelial cells and Pseudomonas: dual oxidase versus pyocyanin. Immunol. Res. 2009;43:198–209. doi: 10.1007/s12026-008-8071-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ran H, Hassett DJ, Lau GW. Human targets of Pseudomonas aeruginosa pyocyanin. Proc. Natl Acad. Sci. USA. 2003;100:14315–14320. doi: 10.1073/pnas.2332354100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers B, Decottignies A, Kolaczkowski M, Carvajal E, Balzi E, Goffeau A. The pleitropic drug ABC transporters from Saccharomyces cerevisiae. J. Mol. Microbiol. Biotechnol. 2001;3:207–214. [PubMed] [Google Scholar]
- Rosa EA, Rached RN, Ignácio SA, Rosa RT, José da Silva W, Yau JY, et al. Phenotypic evaluation of the effect of anaerobiosis on some virulence attributes of Candida albicans. J. Med. Microbiol. 2008;57:1277–1281. doi: 10.1099/jmm.0.2008/001107-0. [DOI] [PubMed] [Google Scholar]
- Rosenfeld E, Beauvoit B. Role of the non-respiratory pathways in the utilization of molecular oxygen by Saccharomyces cerevisiae. Yeast. 2003;20:1115–1144. doi: 10.1002/yea.1026. [DOI] [PubMed] [Google Scholar]
- Sabra W, Ki EJ, Zeng A-P. Physiological responses of Pseudomonas aeruginosa PAO1 to oxidative stress in controlled microaerobic and aerobic cultures. Microbiology. 2002;148:3195–3202. doi: 10.1099/00221287-148-10-3195. [DOI] [PubMed] [Google Scholar]
- Slonimski PP, Perrodin G, Croft JH. Ethidium bromide induced mutation of yeast mitochondria: complete transformation of cells into respiratory-deficient non-chromosomal ‘petites’. Biochem. Biophys. Res. Commun. 1968;30:232–239. doi: 10.1016/0006-291x(68)90440-3. [DOI] [PubMed] [Google Scholar]
- Stutts MJ, Knowles MR, Gatzy JT, Boucher RC. Oxygen consumption and ouabain binding sites in cystic fibrosis nasal epithelium. Pediatr. Res. 1986;20:1316–1320. doi: 10.1203/00006450-198612000-00026. [DOI] [PubMed] [Google Scholar]
- Wang Y, Kern S, Newman DK. Endogenous phenazine antibiotics promote anaerobic survival of Pseudomonas aeruginosa via extracellular electron transfer. J. Bacteriol. 2010;192:365–369. doi: 10.1128/JB.01188-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams HD, Zlosnik JE, Ryall B. Oxygen, cyanide and energy generation in the cystic fibrosis pathogen Pseudomonas aeruginosa. Adv. Microb. Physiol. 2007;52:1–71. doi: 10.1016/S0065-2911(06)52001-6. [DOI] [PubMed] [Google Scholar]
- Wilson R, Sykes DA, Watson D, Rutman A, Taylor GW, Cole PJ. Measurement of Pseudomonas aeruginosa phenazine pigments in sputum and assessment of their contribution to sputum sol toxicity for respiratory epithelium. Infect. Immun. 1988;56:2515–2517. doi: 10.1128/iai.56.9.2515-2517.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worlitzsch D, Tarran R, Ulrich M, Schwab U, Cekici A, Meyer KC, et al. Effects of reduced mucus oxygen concentration in airway Pseudomonas infections of cystic fibrosis patients. J. Clin. Invest. 2002;109:317–325. doi: 10.1172/JCI13870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon S, Coakley R, Lau GW, Lymar SV, Gaston B, Karabulut AC, et al. Anaerobic killing of mucoid Pseudomonas aeruginosa by acidified nitrite derivatives under cystic fibrosis airway conditions. J. Clin. Invest. 2006;116:436–446. doi: 10.1172/JCI24684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon SS, Henningan RF, Hilliard GM, Ochsner UA, Parvatiyar K, Kamani MC, et al. Pseudomonas aeruginosa anaerobic respiration in biofilms: relationship to cystic fibrosis pathogenesis. Dev. Cell. 2002;3:593–603. doi: 10.1016/s1534-5807(02)00295-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Typical aerobic growth kinetics of Saccharomyces cerevisiae strains in the absence (▪) or presence (□) of PYO 500 μgmol/L. W303 (A), BY-4742 (B) hem↿ (C) yap↿ (D) and rho0 (E) strains were grown at 30°C on YPD medium. W303 (F) BY-4742 (G) yap↿ (H) strain were grown at 30°C on YPGly medium. Results are the means of four determinations. For the control experiments (▪), we checked the absence of effect of the solvent alone (methanol). Using trypan blue, we also checked that PYO did not increase cell mortality (at detectable levels) and did not induce significant changes in yeast cell morphology at any growth stage (data not shown). Even minor on YPD medium, PYO toxicity was visible in middle or late growth stages. In the particular hem↿ mutant (C) cumulating several severe dysfunctions (respiratorydeficiency, strong impairments of lipid metabolism and of O2 and ROS adaptations), growth was delayed in the presence of PYO but final growth (after 24 h) was unaffected (see also Fig. 22A).
Figure S2. Typical photographs showing the decoloration of pyocyanin (PYO) during anaerobic cultures in Hungate tubes. In this example, the WT-303 strain was used. The culture medium was YPD supplemented with anaerobic growth factors. At t = 0, OD600 nm was 0.05 (l = 0.49 cm). Depending on the initial concentration, PYO became colorless after a few hours of cultivation. At time 4 h, PYO 100 μmol/L was colorless while PYO 500 μmol/L was only partially discolored. At time 24 h, the blue color of PYO 500 μmol/L was entirely recovered after a vigorous oxygenation of the tube. PYO recovery was also measured by spectrophotometry as described in Material and Methods (Fig. S3 and data not shown).
Figure S3. Assessment of the amount of pyocyanin (PYO) remaining in the rich medium after 24 h of anaerobic growth (WT-BY4742 strain). Culture supernatants incubated in the presence of PYO 500 μmol/L were vigorously reoxygenated until the 690 nm-signal stabilized. The absorbance of the remaining oxidized PYO was then compared to the absorbance of 500 μmol/L pyocyanin freshly dissolved in culture supernatants incubated without pyocyanin. Media pH were adjusted at the same values before measurement. Three comparative assays were performed.
Figure S4. Effect of pyocyanin 500 μmol/L and 100 μmol/L on the aerobic and anaerobic growth of WT-BY4742 and mutant strains of Saccharomyces cerevisiae deficient in DNA damage repair ability. Yeast were cultivated at 30°C aerobically for 24 h on YPD medium (A) and for 48 h on YPGly medium (B) or anaerobically for 24 h on YPD supplemented with anaerobic growth factors (C). Rad mutants (derived from WT-BY4742) are affected in nucleotide excision repair or recombinational repair of double-strand breaks in DNA. The mutant lar013 (derived from the control strain lar009), deleted in Ntg1, Ntg2 and Apn1 genes, is affected in base excision repair and repair of DNA damage caused by oxidation and alkylating agents (see Table S1 for further details).
Table S1. Saccharomyces cerevisiae strains used in this study.


