Abstract
In response to diverse environmental stimuli at different infection sites, Pseudomonas aeruginosa, a serious nosocomial pathogen, coordinates the production of different virulence factors through a complicated network of the hierarchical quorum-sensing (QS) systems including the las, rhl, and the 2-alkyl-4-quinolone-related QS systems. We recently showed that at early stages of growth serum alters the expression of numerous P. aeruginosa genes. In this study, we utilized transcriptional analysis and enzyme assays to examine the effect of serum on the QS and QS-controlled virulence factors during early and late phases of growth of the P. aeruginosa strain PAO1. At early phase, serum repressed the transcription of lasI, rhlI, and pqsA but not lasR or rhlR. However, at late phase, serum enhanced the expression of all QS genes. Serum produced a similar effect on the synthesis of the autoinducers 3OC12-HSL, C4-HSL, and HHQ/PQS. Additionally, serum repressed the expression of several QS-controlled genes in the early phase, but enhanced them in the late phase. Furthermore, serum influenced the expression of different QS-positive (vqsR, gacA, and vfr) as well as QS-negative (rpoN, qscR, mvaT, and rsmA) regulatory genes at either early or late phases of growth. However, with the exception of PAOΔvfr, we detected comparable levels of lasI/lasR expression in PAO1 and PAO1 mutants defective in these regulatory genes. At late stationary phase, serum failed to enhance lasI/lasR expression in PAOΔvfr. These results suggest that depending on the phase of growth, serum differentially influenced the expression of P. aeruginosa QS and QS-controlled virulence genes. In late phase, serum enhanced the expression of las genes through vfr.
Keywords: Pseudomonas aeruginosa, quorum sensing, transcription regulation, virulence factors
Introduction
Pseudomonas aeruginosa is a versatile Gram-negative opportunistic pathogen that survives under different environmental conditions (Lyczak et al. 2000). Pseudomonas aeruginosa causes a wide range of infections in immunocompromised individuals including human immunodeficiency virus (HIV)-infected patients and cancer patients undergoing chemotherapy (Driscoll et al. 2007; Pier and Ramphal 2010). Pseudomonas aeruginosa also causes serious infections in patients with acute or chronic wounds, individuals with cystic fibrosis (CF), and patients in intensive care units (Sadikot et al. 2005; Branski et al. 2009; Pier and Ramphal 2010). Damage produced by P. aeruginosa at different infection sites is due to the production of numerous cell-associated and extracellular virulence factors (van Delden 2004; Sadikot et al. 2005). Cell-associated virulence factors include pili, flagella, and lipopolysaccharide; extracellular virulence factors include exotoxin A, pyocyanin, proteases, and elastase (Lyczak et al. 2000; van Delden 2004; Sadikot et al. 2005; Pier and Ramphal 2010).
Pseudomonas aeruginosa coordinates the production of different virulence factors and biofilm formation through the cell density–dependent signaling system, quorum sensing (QS) (de Kievit and Iglewski 2000; Rumbaugh et al. 2000; Yoon et al. 2002; Hentzer et al. 2003; Smith and Iglewski 2003; Schuster and Greenberg 2006). The signaling is accomplished through diffusible N-acylhomoserine lactone (AHL) signal molecules termed autoinducers. Pseudomonas aeruginosa possesses two well-characterized QS systems, las and rhl, which are hierarchically arranged, with the entire QS cascade being controlled by the las system (Latifi et al. 1996; de Kievit and Iglewski 2000; Rumbaugh et al. 2000). Each system consists of a transcriptional activator (LasR, RhlR) and an autoinducer synthase (LasI, RhlI). LasI directs the synthesis of N-(3-oxododecanoyl)-l-homoserine lactone (3OC12-HSL), whereas RhlI directs the synthesis of N-(butyryl)-l-homoserine lactone (C4-HSL) (de Kievit and Iglewski 2000; Rumbaugh et al. 2000). The two systems control the production of numerous virulence factors including LasB, LasA, rhamnolipids, alkaline protease, exotoxin A, pyocyanin, hydrogen cyanide, and the cytotoxic lectin LecA (Winson et al. 1995; de Kievit and Iglewski 2000; Rumbaugh et al. 2000; Yoon et al. 2002; Hentzer et al. 2003; Schuster et al. 2003; Wagner et al. 2003; Schuster and Greenberg 2006). Besides the las and the rhl systems, P. aeruginosa contains a third QS system that functions through the 2-alkyl-4-quinolone (AQ) signaling molecules (Lepine et al. 2004). Although P. aeruginosa produces numerous AQs, the two main AQs that function as QS signals are 2-heptyl-3-hydroxy-4-quinonlone (Pseudomonas quinolone signal, PQS) and its precursor 2-heptyl-4-quinolone (HHQ) (Pesci et al. 1999; Deziel et al. 2004; Diggle et al. 2007). Both AQs activate MvfR/PqsR, which enhances expression of the biosynthetic genes pqsA-E that synthesize HHQ (Xiao et al. 2006; Diggle et al. 2007). Previous studies identified several positive and negative regulators that regulate the P. aeruginosa QS systems including, Vfr, VqsR, MvaT, GacA, RpoN, RpoS, and RsmA (Albus et al. 1997; Reimmann et al. 1997; Chugani et al. 2001; Pessi et al. 2001; Diggle et al. 2002; Heurlier et al. 2003; Juhas et al. 2004).
Several previous studies demonstrated the influence of QS systems on the pathogenesis of P. aeruginosa. For example, multiple studies strongly suggested that the P. aeruginosa QS systems within the lung of chronically infected CF patients are fully functional (Storey et al. 1997, 1998; Wu et al. 2000; Erickson et al. 2002). Analysis of sputum samples from CF patients indicated the presence of either 3OC12-HSL or C4-HSL, or both (Geisenberger et al. 2000; Singh et al. 2000). Additionally, transcriptional analysis of P. aeruginosa strains from the sputum samples of CF patients showed the presence of lasI and rhlI transcripts in these samples (Storey et al. 1998; Erickson et al. 2002). The level of lasI and rhlI transcripts correlated well with the level of lasB, lasA, and toxA transcripts (Storey et al. 1998; Erickson et al. 2002). Using different animal models, other studies demonstrated the relevance of the QS systems to the virulence of P. aeruginosa (Rumbaugh et al. 1999; Pearson et al. 2000; Wu et al. 2000). Using the thermally injured mouse model and the mouse models of acute and chronic lung infections, investigators compared the virulence of P. aeruginosa mutants defective in QS systems with that of their parent strains (Rumbaugh et al. 1999; Pearson et al. 2000). The mortality rate among thermally injured mice infected with P. aeruginosa defective in either the las, rhl, or both was significantly lower than in mice infected with the wild-type strain (Rumbaugh et al. 1999). Similarly, the mortality rate, as well as lung damage, produced in mice infected with QS mutants was significantly lower than those infected with the wild-type strain (Pearson et al. 2000). The role of the AQ-related system in P. aeruginosa virulence was also demonstrated in multiple studies. Using the thermally injured mouse model and the Arabidopsis leaf infiltration assay, Cao et al. (2001) showed that the virulence of a P. aeruginosa mutant defective in mvfR/pqsR was significantly lower than its parent strain. Besides functioning as a signal molecule, PQS produces an oxidative stress response (Haussler and Becker 2008), plays a role in biofilm development (Allesen-Holm et al. 2006), and chelates iron (Bredenbruch et al. 2006; Diggle et al. 2007).
Interestingly, by constructing a PAO1 isogenic mutant defective in lasR, lasI, rhlR, and rhlI genes, Lazenby et al. (2013) recently produced evidence indicating that the loss of the las/rhl systems does not completely compromise the virulence of P. aeruginosa. Compared with its parent strain, the mutant produced reduced levels of elastase and proteases (Lazenby et al. 2013). However, in the mouse model of lung infection, the mutant was not defective; its persistence within the lung was equivalent to that of its parent strain (Lazenby et al. 2013). Additionally, the neutrophil infiltration as well as the expression of inflammatory cytokines within the lung was not diminished (Lazenby et al. 2013).
We previously showed that by enhancing twitching motility, serum interfered with biofilm development by P. aeruginosa (Hammond et al. 2010). Additionally, using transcriptome analysis, we recently investigated the effect of serum on the expression of different P. aeruginosa genes (Kruczek et al. 2012). At early stages of growth, serum repressed the expression of numerous P. aeruginosa iron-controlled genes (Kruczek et al. 2012). In this study, we examined the effect of serum on the expression of P. aeruginosa QS and QS-controlled genes.
Experimental Procedures
Bacterial strains, plasmids, media, and growth conditions
Bacterial strains and plasmids used in this study are listed in Table 1. Overnight cultures were grown in Luria Bertani (LB) broth at 37°C with shaking at 230 rpm. Aliquots of overnight cultures were pelleted, washed, and resuspended to a final OD600 of 0.02–0.03. Cultures were grown in LB or LB supplemented with 10% (v/v) adult bovine serum (LB/S) under conditions described above and samples taken at the indicated time points. We established a growth index of OD600 1.0–1.2 achieved approximately 6-h postinoculation, as early exponential phase of growth (early phase) and a growth index of OD600 3.0–4.0 achieved approximately 16-h postinoculation as late stationary phase of growth (late phase).
Table 1.
Strains and plasmids utilized in this study.
| Strains and plasmids | Description | Reference |
|---|---|---|
| Pseudomonas aeruginosa | ||
| PAO1 | Prototrophic PAO1 | Holloway et al. (1979) |
| PAO1lecA::lux | lecA:luxCDABE genomic reporter fusion in PAO1; Tcr | Winzer et al. (2000) |
| PAOΔvfr | vfr deletion of PAO1; Gmr | Runyen-Janecky et al. (1997) |
| PAOΔmvaT | mvaT isogenic mutant of PAO1 | Westfall et al. (2004) |
| PAO1pqsA CTX-lux::pqsA | pqsA mutant containing a copy of the pqsA promoter linked to the luxCDABE genes and inserted into a neutral site in the chromosome | Fletcher et al. (2007) |
| PW5352 | vqsR-F12::ISphoA/hah in PAO1; out of frame; Tcr | Jacobs et al. (2003) |
| Plasmids | ||
| pPCS223 | lasI-lasZ transcriptional fusion in pLP170; Cbr | Preston et al. (1997) |
| pSB1142 | AHL (C12) reporter plasmid; Tcr | Wang et al. (2007) |
| pSB536 | AHL (C4) biosensor; ahyR''::luxCDABE in pAHP13; Cbr | Swift et al. (1997) |
Gm, gentamicin; Tc, tetracycline; Cb, carbenicillin; r, resistant.
Assay for gene expression by β-galactosidase activity
Assays for β-galactosidase activity were performed as previously described (Miller 1972; Stachel et al. 1985; Gaines et al. 2005). The calculation for units of β-galactosidase activity takes into account the amount of growth. Results represent the averages of three independent experiments ± SEM.
Detection of 3OC12-HSL, C4-HSL, and HHQ/PQS
Pseudomonas aeruginosa PAO1 was grown in triplicate in LB or LB/S until the indicated time points. The supernatant fraction was collected and passed through a 0.2-μm syringe filter (VWR, Arlington Heights, IL). Autoinducers were separated from the supernatant via three successive rounds of acidified ethyl acetate (high performance liquid chromatography [HPLC] grade) extraction (Pearson et al. 1994). The final extracts were then dried in an Eppendorf 5301 concentrator (Eppendorf, Hauppauge, NY) and resuspended in 30 μL of HPLC-grade methanol. HHQ is oxidized by the putative monooxygenase PqsH to produce PQS (Gallagher et al. 2002; Deziel et al. 2004). Therefore, the reporter strain detects both HHQ and PQS. Overnight cultures of the 3OC12-HSL, C4-HSL, and HHQ/PQS reporter strains (Table 1) were diluted to an OD600 of 0.5 in fresh LB. Aliquots (100 μL) of the diluted reporter strains were pipetted into a 96-well clear flat-bottom microtiter plate (Costar, Corning, NY). Five-microliter aliquots of the methanol-dissolved autoinducer samples were then quickly added to the microtiter plate and the plate was incubated in the dark at 37°C for 3 h. Luminescence was detected using a luminometer (Modulus Microplate Reader, Turner Biosystems, Promega, Madison, WI). Values were standardized based on the OD600 value of each respective culture.
Analysis of lecA expression
PAO1lecA::lux was grown in triplicate in LB or LB/S to the indicated time points and the OD600 values were obtained. A 100-μL aliquot of each culture was added to a 96-well microtiter plate, and 20 μL of 0.3% (v/v) decanal solution in water was then added to each well and quickly mixed with each culture. Luminescence was immediately measured using a luminometer (1 sec exposure). Reported values were standardized by dividing the relative light units (RLU) by the OD600 of the culture.
RNA extraction and qRT-PCR analysis of gene expression
Overnight cultures of P. aeruginosa strains were subcultured in fresh LB or LB/S to an OD600 of 0.02 and incubated at 37°C with shaking to early and late phases of growth (˜6 and 16 h, respectively). Cultures were then mixed with twice of their volume of RNAprotect Bacteria Reagent (QIAGEN, Valencia, CA) for 5 min at room temperature. The cells were pelleted and were stored at −80°C. Bacterial pellets were first lysed with lysozyme (15 mg/mL) and proteinase K (1 mg/mL) for 15 min at room temperature and the RNA was subsequently extracted using the RNeasy Mini Kit (QIAGEN) according to the manufacturer's recommendations. The RNA solution was then digested with the RNase-free DNase Set (QIAGEN) to remove residual genomic DNA. RNA was purified from DNase by the RNA cleanup protocol (QIAGEN) with the exception that on-column DNase digestion was applied to eliminate any remaining traces of genomic DNA. RNA was quantified by NanoDrop® spectrophotometer (NanoDrop Products, Wilmington, DE) and the integrity of the RNA was assessed using RNA Nano Chip on an Agilent 2100 Bioanalyzer (Agilent, Santa Clara, CA).
Synthesis of cDNA from the extracted RNA was performed using the QuantiTect Reverse Transcription Kit (QIAGEN). A 200-ng aliquot of cDNA was mixed with SYBR Green PCR Master Mix (Life Technologies, Carlsbad, CA) and 250 nmol/L of specific primer (Table 2). Amplification and detection of the product were conducted using StepOne Plus real-time polymerase chain reaction (PCR) system (Life Technologies). For each experiment, we used three independent biological replicates for RNA extraction. Additionally, each PCR reaction was set up in triplicate. The quantity of cDNA in different samples was normalized using 30S ribosomal RNA (rplS) as an internal standard. Gene expression analysis was performed using StepOne Plus software version 2.2.2 (Life Technologies).
Table 2.
Primers utilized in this study.
| Gene | Forward primer | Reverse primer |
|---|---|---|
| lasI | 5′-TTCCGACTGTACGCTGGAG-3′ | 5′-ATCTGGGTCTTGGCATTGAG-3′ |
| lasR | 5′-TTCTGGGAACCGTCCATCTA-3′ | 5′-CAGTGCGTAGTCCTTGAGCA-3′ |
| lasB | 5′-GTTCTATCCGCTGGTGTCG-3′ | 5′-GCCCTTGATGTCGTAGC-3′ |
| lasA | 5′-CGTTCCTCTTCGTCTTGCTG-3′ | 5′-GCTCCAGGTATTCGCTCTTG-3′ |
| rsaL | 5′-GAGAGAACACAGCCCCAAAA-3′ | 5′-GATTGGCTTATCCCGAAGC-3′ |
| rhlI | 5′-CTCTCTGAATCGCTGGAAGG-3′ | 5′-CGACGATGTAGCGGGTTT-3′ |
| rhlR | 5′-GTTGCATGATCGAGTTGCTG-3′ | 5′-TGGATGTTCTTGTGGTGGAA-3′ |
| rhlA | 5′-CTGAAAGCCAGCAACCATC-3′ | 5′-GGCGGTGGTGTATTCGTC-3′ |
| pqsA | 5′-CAATACACCTCGGGTTCCAC-3′ | 5′-TGCCATAGCCGAAGAACATC-3′ |
| phzC1 | 5′-GACCCTGCCGGTCTATCG-3′ | 5′-CAGCATCGACAGCTCGTAGT-3′ |
| hcnB | 5′-CTGACGGAAGTGACGGTAGC-3′ | 5′-CAGGTGGATGTGCGGTTG-3′ |
| rsmA | 5′-TGACGGTACTGGGTGTCAAA-3′ | 5′-CTTTCTGGATGCGCTGGTAA-3′ |
| gacA | 5′-GGTCGTGGTAGTCACCGTCT-3′ | 5′-AAGGACTTCAGCGCCAGTT-3′ |
| vfr | 5′-TACCCACACACCCAAACTCA-3′ | 5′-GTTCGCTGCCTTCCTTTTC-3′ |
| vqsR | 5′-TTATGTGGTGACGGACGAAG-3′ | 5′-CTCGAAGTGGATGCGTTTTT-3′ |
| qscR | 5′-TCAAGAATAACAACCGAGGAAGA-3′ | 5′-CGGGTCGATGGATGTGTAGT-3′ |
| rpoN | 5′-AACGCATACCCAGCGAGTT-3′ | 5′-TCGAGGTAGCCATCGTTGTT-3′ |
| mvaT | 5′-ACGCTGATGGGCACTTAC-3′ | 5′-CACTTGGCTTTCCACTCTTT-3′ |
Effect of serum on P. aeruginosa virulence factor production
Overnight cultures of P. aeruginosa PAO1 were subcultured in fresh LB or LB/S to an OD600 of 0.02 and incubated at 37°C with shaking to the early and late phases of growth. For the detection of elastolytic activity we utilized the elastin plate assay as described by Ohman et al. (1980). Elastin plates prepared with and without 10% serum were incubated for 24 h at 37°C and then 48 h at room temperature (Ohman et al. 1980). Staphylolytic activity was determined as previously described in Diggle et al. (2002). Phospholipase C activity was assayed as previously described using nitrophenylphosphoryl choline as a substrate (Berka et al. 1981; Barker et al. 2004).
Statistical analyses
Statistical analyses were done using GraphPad InStat 3.06 (GraphPad Software, San Diego, CA). One-way analysis of variance (ANOVA) with the Tukey–Kramer multiple comparisons posttest was used to determine significant differences across time. The two-tailed t-test was used to compare pairs of strains grown in LB with those grown in LB/S.
Results
Serum differentially regulates the expression of PAO1 QS-related genes during growth
Through transcriptome analysis, we previously identified several PAO1 genes whose expression is significantly altered by serum. Most of these genes were iron-controlled genes. However, we also identified the QS gene lasI whose expression was repressed 20-fold by serum at early stages of growth. We confirmed the negative regulation of the lasI gene by serum using PAO1 carrying the lasI-lacZ transcriptional fusion plasmid pPCS223. To optimize QS gene expression, cells were grown in LB or LB/S for 16 h. Samples were obtained at the indicated time points throughout the growth cycle and the level of β-galactosidase activity was determined. During the early to midexponential phases growth (4–8 h, OD600 0.8–1.8), lasI expression was reduced in the presence of serum (Fig. 1). However, at the late stationary phase of growth (16 h, OD600 3.0–4.0), lasI expression was significantly enhanced by serum (Fig. 1). For the remainder of the study, we used a growth index of OD600 1.0–1.2 (early phase) and a growth index of OD600 3.0–4.0 (late phase) to provide consistency of results.
Figure 1.
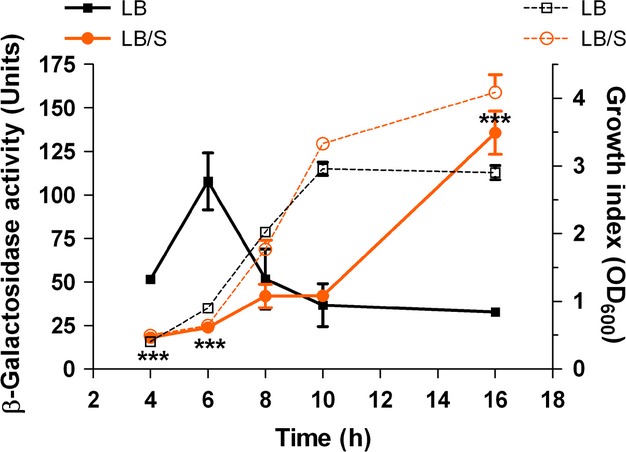
Serum represses lasI expression during early stages of growth, but enhances expression during late stages of growth. PAO1 carrying the lasI-lacZ fusion plasmid pPCS223 (Table 1) was grown in either LB or LB/S. Samples were obtained at the indicated time points, and the level of β-galactosidase activity and the growth index (OD600) were determined. Solid lines indicate units of β-galactosidase activity, whereas dashed lines indicate cell growth. Values represent the means of three independent experiments ± SEM; ***P < 0.001.
Pseudomonas aeruginosa has three QS systems – las, rhl, and the AQ-related system. Therefore, we decided to comprehensively analyze the effect of serum on the expression of different PAO1 QS genes within each system. To avoid problems associated with the presence of multiple copies of the regulated genes in a single cell, we examined the expression of the QS genes by qRT-PCR, rather than using lacZ fusion constructs carried on plasmids. We grew PAO1 in either LB or LB/S and obtained triplicate samples at early and late phases of growth and analyzed the expression of QS genes. To analyze the las system, we examined the level of expression of lasR, lasI, and lasB, a virulence gene that is stringently regulated by the las system. At early phase, the level of lasI and lasB expression in LB/S was significantly lower than that in LB, but the level of lasR expression was essentially unchanged (Fig. 2A). In contrast, at the late phase, the level of expression of all three genes was significantly enhanced in LB/S compared with LB (Fig. 2B).
Figure 2.
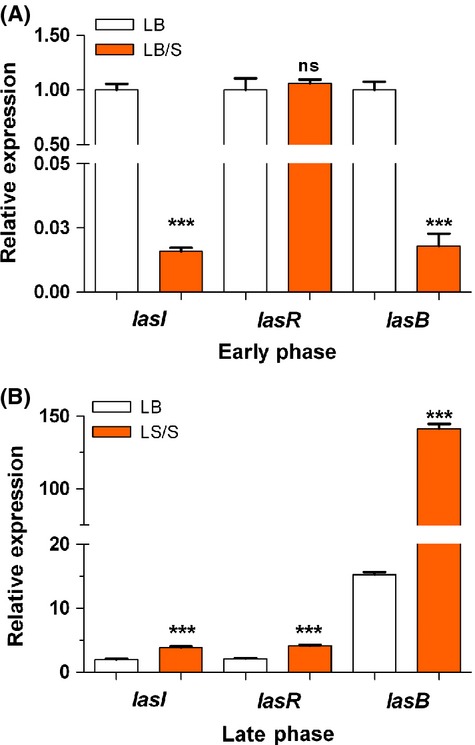
Serum represses lasI and lasB expression during early phase (A) but enhances lasI, lasR, and lasB expression during late phase (B) of growth. PAO1 was grown in either LB or LB/S. Samples were collected at early phase (OD600 1.0–1.2) or late phase (OD600 3.0–4.0) and the level of gene expression was determined by qRT-PCR. Values represent the means of three independent experiments ± SEM; ***P < 0.001.
Using the same approach, we analyzed the effect of serum on the expression of the rhl genes, rhlI, rhlR, and rhlA. Similarly to its effects on the las genes, serum repressed rhlI and rhlA expression significantly in the early phase but enhanced their expression significantly in the late phase, although the enhancement in rhlA expression was not as pronounced as that seen with lasB (Fig. 3A and B). As we observed with lasR expression, serum had no significant effect on rhlR expression at early phase, but enhanced its expression at late phase (Fig. 3A and B). HHQ and PQS are synthesized by proteins encoded by the pqsA-D genes of the pqsA-E operon. Specifically, these proteins synthesize HHQ, which is then converted into PQS by the potential monooxygenase encoded by pqsH (Diggle et al. 2002; Deziel et al. 2004). The potential monooxygenase encoded by pqsH converts HHQ into PQS (Gallagher et al. 2002; Deziel et al. 2004). The pqsA-E operon is positively regulated by MvfR/PqsR (Cao et al. 2001; Gallagher et al. 2002). Therefore, we examined the expression of pqsA as a representative gene from the operon. Similar to the pattern of lasI and rhlI expression, serum significantly repressed pqsA expression at early phase, but significantly enhanced it at the late phase (Fig. 3C). These results suggest that, depending on the stage of growth, serum differentially regulates the expression of its target las, rhl, and pqs genes, repressing their expression at early stages of growth but enhancing it at late stages of growth. Additionally, the regulation at early stages of growth is limited to the lasI and rhlI genes.
Figure 3.
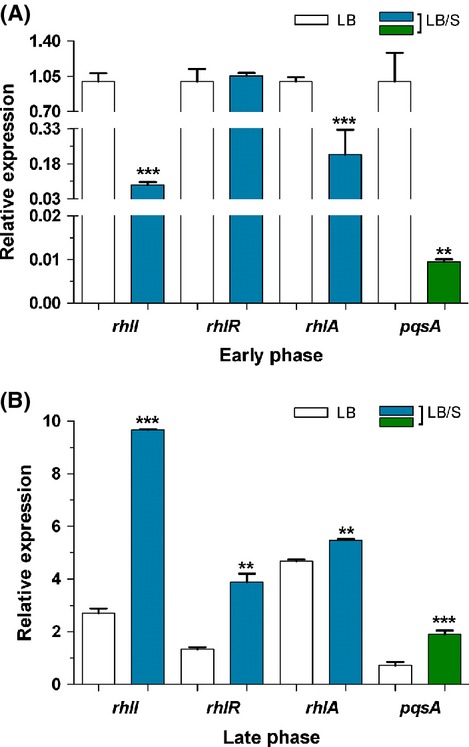
Serum represses rhlI, rhlA, and pqsA expression during early phase (A) but enhances rhlI, rhlR, rhlA, and pqsA expression during late phase (B) of growth. PAO1 was grown in either LB or LB/S. Samples were collected at early and late phases and the level of gene expression was determined by qRT-PCR. Values represent the means of three independent experiments ± SEM; ***P < 0.001; **P < 0.01.
We excluded the possibility that serum interacts with the autoinducers and either modifies or alters their function. We incubated the autoinducers with either LB or LB/S and reextracted them. The reextracted autoinducers maintained their ability to significantly enhance lasB expression in PAO1 (data not shown).
Serum differentially regulates the production of PAO1 autoinducers 3OC12-HSL, C4-HSL, and HHQ/PQS at early and late phases of growth
The lasI, rhlI, and pqsA-E genes encode synthases that produce the 3OC12-HSL, C4-HSL, and HHQ autoinducers, respectively. Therefore, we analyzed the effect of serum on the level of 3OC12-HSL, C4-HSL, and HHQ/PQS produced by PAO1. Cells were grown in either LB or LB/S to early and late phases of growth and the level of autoinducer was detected using their respective autoinducer assays as previously described (Swift et al. 1997; Winson et al. 1998; Diggle et al. 2002). At early phase and in the presence of serum, PAO1 produced significantly lower levels of 3OC12-HSL and C4-HSL than PAO1 grown in LB only (Fig. 4A). However, serum had no significant effect on HHQ/PQS production (Fig. 4A). At late phase, serum significantly enhanced the production of all three autoinducers (Fig. 4B). These results suggest that serum differentially regulates the production of the three autoinducers by significantly repressing 3OC12-HSL and C4-HSL production at earlier stages of growth but significantly enhancing them at late stages of growth.
Figure 4.
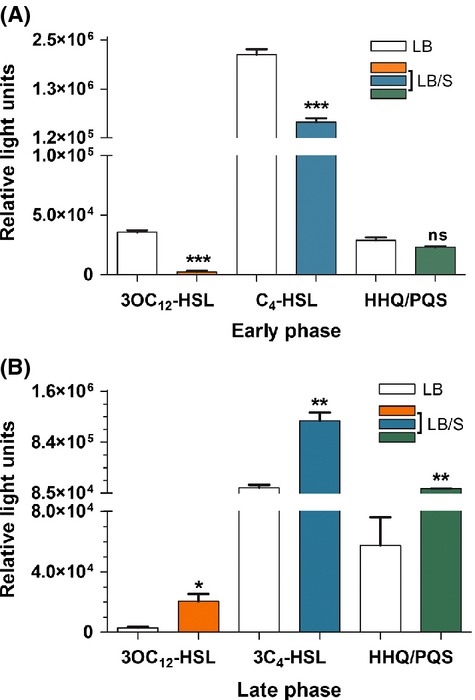
Serum represses 3OC12-HSL and C4-HSL production during early phases (A) but enhances 3OC12-HSL, C4-HSL, and HHQ/PQS production during late phases (B) of growth. PAO1 was grown in either LB or LB/S. Samples were collected at early and late phases, autoinducers were extracted from the supernatant and their levels were determined as described in Experimental Procedures. Values represent the means of three independent experiments ± SEM; ***P < 0.001; **P < 0.01; *P < 0.05; ns, not significant.
We ruled out the possibility that serum contains certain factors or molecules that affect the level of the luminescence produced by the reporter strains. We prepared ethyl acetate extracts from either LB or LB/S. Compared with the negative control, to which no extract was added, the addition of ethyl acetate extract from LB or LB/S did not alter the level of the luminescence activity produced by the reporter strains (data not shown).
Serum influences the expression of QS-controlled virulence genes
The three QS systems control the expression of numerous virulence genes (de Kievit and Iglewski 2000; Rumbaugh et al. 2000; Smith and Iglewski 2003). In addition to the LasB elastase gene lasB, the expression of lasA, which encodes the staphylolytic protein LasA, is regulated by the las system (de Kievit and Iglewski 2000; Rumbaugh et al. 2000; Smith and Iglewski 2003). Besides rhlAB, expression of the hcn operon, which encodes proteins that direct production of hydrogen cyanide, is primarily regulated by the rhl system; whereas expression of plcB, which encodes the third P. aeruginosa phospholipase C protein PlcB, is controlled by both las and rhl (Reimmann et al. 1997; Barker et al. 2004). Pyocyanin production by P. aeruginosa is positively regulated by pqsE which is part of the pqsA-E operon (Farrow et al. 2008; Rampioni et al. 2010). Both HHQ and PQS bind to and activate MvfR/PqsR, which in turn binds to the pqsA promoter and enhances pqsA-E expression (McGrath et al. 2004; Wade et al. 2005; Xiao et al. 2006; Diggle et al. 2007). Therefore, HHQ and PQS indirectly regulate pyocyanin production by enhancing pqsE expression. To determine if serum affects additional QS-controlled virulence genes (besides lasB and rhlA), we analyzed the expression of lasA, hcnB, plcB, and phzC using qRT-PCR. The expression of phzC and hcnB served to represent the phz and hcn operons, respectively. As observed with lasB and rhlA, in the presence of serum, the expression of all four genes was significantly reduced in the early phase of growth and significantly enhanced in the late phase (Fig. 5). We also examined the effect of serum on the expression of lecA which encodes a cytotoxic lectin (Diggle et al. 2006). This gene is regulated by the QS-controlled sigma factor RpoS and the rhl QS system (Winzer et al. 2000). To determine the effect of serum on lecA expression, we utilized the P. aeruginosa strain PAO1lecA::lux which carries a lecA::lux chromosomal fusion (Winzer et al. 2000; Diggle et al. 2002). At the early phase of growth, lecA expression was relatively low (Fig. 6). Therefore, we grew PAO1lecA::lux for 8 h to an OD600 of 2.0–2.4, or mid-to-late exponential phase (midlate exponential phase). At both early and midlate exponential phases, serum significantly reduced the level of lecA expression (Fig. 6). In contrast, by late phase, serum significantly enhanced lecA expression (Fig. 6). These results suggest that in response to the effect of serum and depending on the stage of growth of PAO1 QS-controlled genes are differentially expressed in the same manner as their cognate QS genes.
Figure 5.
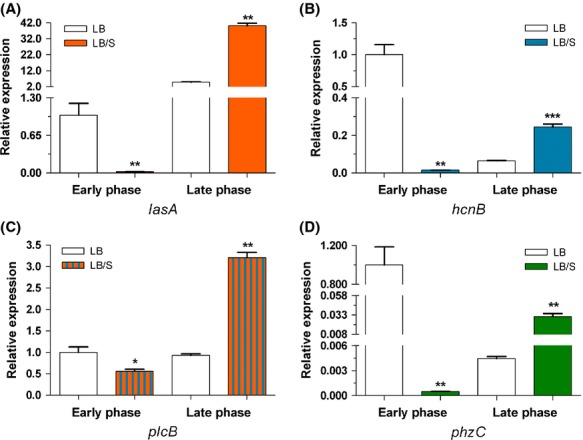
Serum differentially regulates the expression of the QS-controlled virulence genes (A) lasA, (B) hcnB, (C) plcB, and (D) phzC during both early and late stages of growth. PAO1 was grown in either LB or LB/S, samples were collected at early and late phases, and the level of gene expression was determined by qRT-PCR. Values represent the means of three independent experiments ± SEM; ***P < 0.001, **P < 0.01, *P < 0.05.
Figure 6.
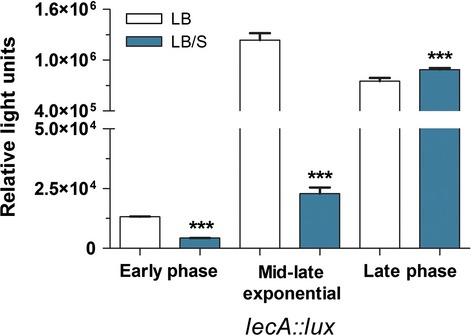
In the presence of serum, lecA expression is reduced at early phase but increased at late phase of growth of PAO1. PAO1lecA::lux was grown in LB or LB/S for 16 h. Samples were collected at early phase, midlate exponential phase (OD600 2.0–2.2), and late phase. Relative luminescence values were determined as described in Experimental Procedures. Values represent the means of three independent experiments ± SEM; ***P < 0.001.
QS-controlled virulence factor production is altered in the presence of serum
Using standard assays, we examined the effects of serum on the production of QS-controlled P. aeruginosa virulence factors. However, different components of serum interfered with many of these assays. Despite that, we obtained results that support our above described transcriptional studies at either early or late stages of growth. For example, we attempted to analyze lasB activity using the elastin Congo red assay, but serum contains strong endogenous elastolytic activity that interfered with our analysis (data not shown). We were also unsuccessful in determining the level of LasB protein in the presence and absence of serum using either enzyme-linked immunosorbent assay (ELISA) or immunoblotting as serum contains high-molecular-weight proteins (80–150 kDa) that strongly interacted with the LasB antibody (data not shown). In a last attempt, we compared the elastolytic activity produced by PAO1 in the presence and absence of serum using the previously described elastin plate assay (Ohman et al. 1980). After 24 h of incubation at 37°C and 48 h at room temperature, PAO1 colonies on the serum-containing elastin plates produced a zone of elastin proteolysis that was larger than that produced around PAO1 colonies on a regular elastin plate (Fig. 7A). Although these results support the assumption that serum affects LasB production through QS genes at late stages of growth of PAO1, it does not address the potential reduction in LasB production at early stages of growth. Serum also interfered with the detection of pyocyanin within the supernatant of PAO1. However, we were able to examine the effect of serum on LasA production using the previously described staphylolytic assay (Diggle et al. 2002). In the presence of serum and at early phase, PAO1 staphylolytic activity was significantly reduced (Fig. 7B). Using the phospholipase C assay (Berka et al. 1981), we determined the level of phospholipase C activity within the supernatant of PAO1 in the presence and absence of serum. At late phase, serum significantly enhanced phospholipase C production (Fig. 7C).
Figure 7.
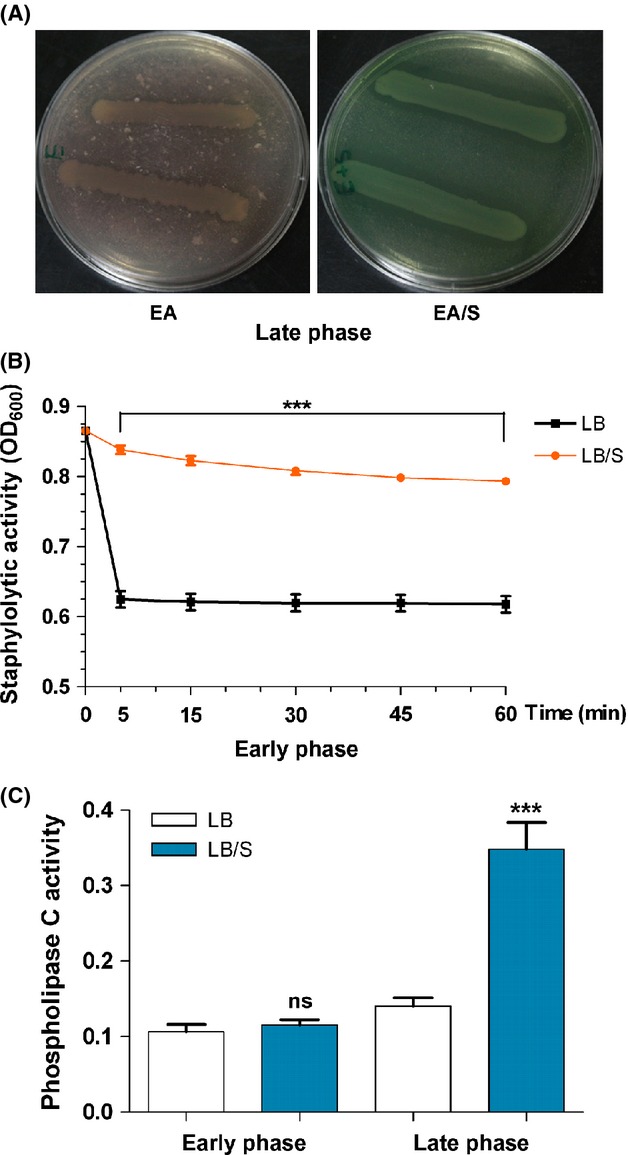
Serum influences the production of the PAO1 QS-controlled virulence factors, LasB, LasA, and phospholipase C. (A) At late phase of growth, serum enhances LasB production. PAO1 was streaked on elastin agar (EA) or elastin agar with 10% serum (EA/S). The plates were incubated at 37°C for 24 h and then at room temperature for 48 h before examination. Images are representative of three independent experiments. (B) Serum represses LasA production at early phase. PAO1 was grown in either LB or LB/S and samples were collected at the early phase. The supernatant fractions were isolated and the level of LasA activity in each fraction was determined using the staphylolytic assay as described in Experimental Procedures. (C) In the presence of serum, PAO1 produced increased levels of phospholipase C activity at late phase. Cells were grown as described in (B). Samples were collected at early and late phases, the supernatant fractions were isolated, and the level of phospholipase C activity in each fraction was determined as described in Experimental Procedures. For (B) and (C), values represent the means of three independent experiments ± SEM; ***P < 0.001.
Serum influences the expression of PAO1 genes that regulate the QS systems
Previous studies identified and characterized numerous P. aeruginosa genes that positively or negatively regulate the las, rhl, and the AQ-related QS systems. Among the positive regulators are vfr, gacA, and vqsR and among the negative regulators are mvaT, rsmA, qscR, rpoS, and rpoN (Albus et al. 1997; Reimmann et al. 1997; Chugani et al. 2001; Pessi et al. 2001; Diggle et al. 2002; Heurlier et al. 2003; Juhas et al. 2004, 2005). Therefore, we determined if serum affected the expression of any of the QS genes (las, rhl, and PQS) through one or more of these regulators. At early phase, serum significantly reduced expression of the positive regulator vqsR, but enhanced the expression of the negative regulators mvaT and rsmA (Fig. 8A). Serum produced no significant effect on the expression of gacA, rpoN, qscR, or rpoS at early phase (data not shown). In the late phase, serum significantly enhanced the expression of the QS-positive regulators vqsR, gacA, and vfr (Fig. 8B). While serum minimally reduced expression of the negative regulators rpoN and qscR, this reduction was not significant (data not shown). Again, there was no significant effect on the expression of mvaT, rsmA, or rpoS (data not shown). These results suggest that at either early or late stages of growth, serum affects the expression of one or more of the QS gene regulators.
Figure 8.
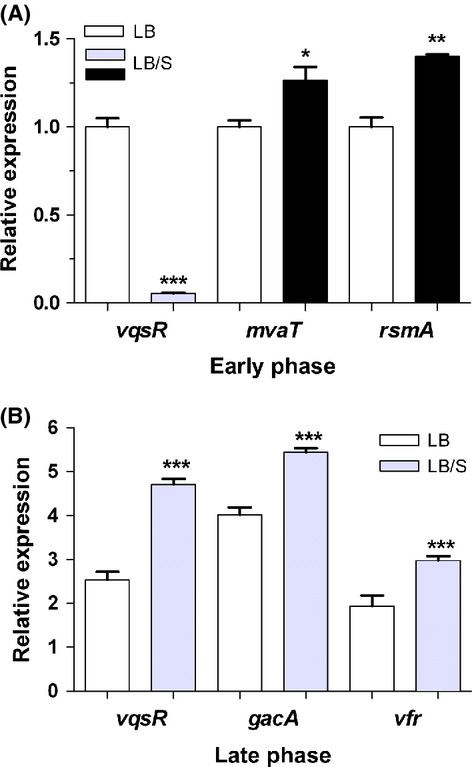
Serum differentially regulates the expression of the QS regulatory genes during early and late phases. PAO1 was grown in either LB or LB/S. Samples were obtained at early and late phases and the level of expression was determined by qRT-PCR analysis. (A) At early phase, serum reduced the expression of the PAO1 QS-positive regulator vqsR, but enhanced the expression of the QS-negative regulators mvaT and rsmA. (B) At late phase, serum enhanced the expression of the QS-positive regulators vqsR, gacA, and vfr. Values represent the means of three independent experiments ± SEM; ***P < 0.001, **P < 0.01, *P < 0.05.
At late stages of growth of PAO1 serum enhances lasI and lasR expression through vfr
To determine if serum produces its effect on the las/rhl genes through vfr, we compared the level of lasI, lasR, rhlI, and rhlR expression between PAO1 and the vfr deletion mutant PAOΔvfr at early and late phases of growth. At early phase and in LB, the level of expression of all these genes in PAOΔvfr was significantly lower than that in PAO1 confirming the importance of vfr in regulating the QS genes (Fig. 9). Serum significantly reduced lasI expression in PAO1 and PAOΔvfr at early phase (Fig. 9A). As we previously observed, serum did not affect lasR expression in PAO1 at early phase, however, its presence significantly repressed the expression of lasR in PAOΔvfr (Fig. 9B). This suggests that at early stages of growth serum does not repress lasI expression through vfr, which supports our above finding regarding vfr regulation by serum in the early phase of growth. Additionally, at this stage of growth, intact vfr may be required to prevent the repression of lasR expression by serum (Fig. 9B). At late phase and in LB, the expression of lasI and lasR is reduced in PAOΔvfr compared with PAO1 (Fig. 9C and D). However, serum enhancement of lasI and lasR expression was abrogated in PAOΔvfr (Fig. 9C and D). These results suggest that in the late phase of growth, serum enhances the expression of las genes through vfr.
Figure 9.
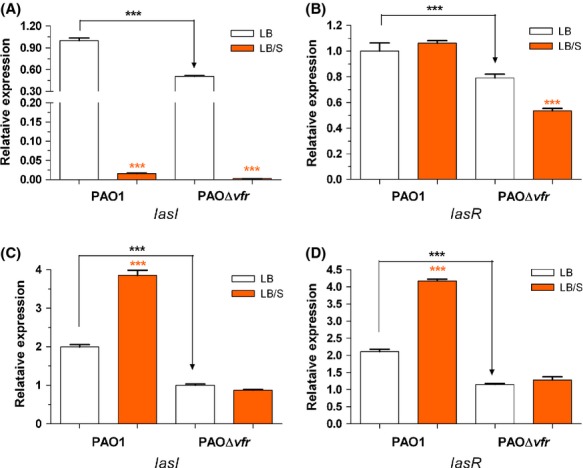
Deletion within vfr eliminated the effect of serum on lasI/lasR expression at late but not early phase. PAO1 and PAOΔvfr were grown in either LB or LB/S to either early phase (A and C) or late phase (B and D). Samples were collected at each phase and the level of lasI (A and C) and lasR (B and D) expression was determined by qRT-PCR analysis. Values represent the means of three independent experiments ± SEM; ***P < 0.001.
At early stages of growth serum does not enhance lasI expression through vqsR or mvaT
Juhas et al. (2004) previously suggested that VqsR positively regulates lasI expression and AHL synthesis. As we demonstrated in Figure 9, serum significantly reduced vqsR at the early phase of growth of PAO1. To determine if serum potentially regulates lasI/lasR expression at this stage through VqsR, we utilized the vqsR transposon insertion mutant PW5352 (Table 1). In LB and compared with PAO1, the expression of lasI in PW5352 was significantly reduced indicating that VqsR is a positive regulator of the QS systems (Fig. 10A). However, in LB/S the expression of lasI was significantly reduced in both PAO1 and PW5352 indicating that serum represses the expression of these genes independently of VqsR (Fig. 10A). MvaT, which belongs to the family of histone-like nucleoid-structuring proteins, controls the timing of QS-dependent gene expression (Diggle et al. 2002; Tendeng et al. 2003; Vallet et al. 2004). Mutations in the mvaT gene cause a premature expression of the QS genes (Diggle et al. 2002). We showed that serum enhances mvaT expression at the early phase of growth (Fig. 8A). Therefore, to determine if serum reduces the expression of lasI at this stage of growth through mvaT, we compared the level of lasI expression between PAO1 and its mvaT mutant. In agreement with the results of previous studies, in LB the level of lasI expression in PAOΔmvaT was significantly higher than that in PAO1 (Fig. 10B). However, in the presence of serum, the level of lasI expression was significantly reduced in PAO1 and PAOΔmvaT suggesting that serum does not repress lasI expression through mvaT (Fig. 10B).
Figure 10.
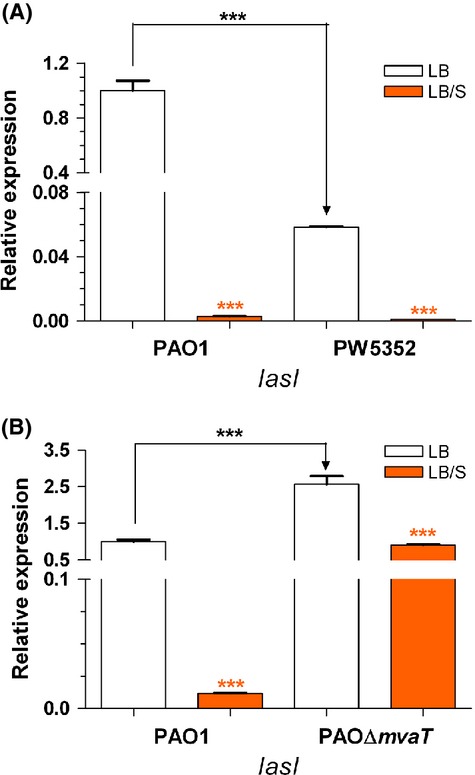
A mutation in either vqsR or mvaT does not interfere with the ability of serum to repress lasI expression during early phase of growth. (A) PAO1 and PW5352 (vqsR::phoA) were grown in either LB or LB/S to early phase. (B) PAO1 and PAOΔmvaT were grown in either LB or LB/S to early phase. The level of lasI and lasR expression was determined by qRT-PCR analysis. Values represent the means of three independent experiments ± SEM; ***P < 0.001.
Discussion
In this study, we demonstrated that depending on the stage of growth of P. aeruginosa, serum differentially regulates the expression of different QS-controlled genes. In the early phase of growth, serum repressed the expression of lasI, lasB, rhlI, rhlA, and pqsA (Figs. 2A, 3A). At the late phase, serum enhanced the expression of lasI, lasR, lasB, rhlI, rhlR, rhlA, and pqsA (Figs. 2B, 3B and C). Similarly to its effect on the QS systems, serum reduces the production of 3OC12-HSL and C4-HSL autoinducers at the early phase and enhances the production of 3OC12-HSL, C4-HSL, and HHQ/PQS at late phase of growth (Fig. 4). In the early phase of growth, serum does not accomplish its effect through any of the known QS regulators. However, in the late phase, serum utilizes vfr to influence the QS systems (Fig. 9).
With respect to the las and rhl QS systems and at early phase, the serum-induced reduction in the expression of QS-controlled virulence genes occurs by reducing the autoinducer synthases but not the transcriptional activators (Figs. 2A, 3A). Expression of lasI and 3OC12-HSL production were reduced but not lasR transcription (Figs. 2A, 4A). Similarly, the reduction involves rhlI transcription and C4-HSL production but not rhlR transcription (Figs. 3A, 4A). Synthesis of LasR and RhlR is regulated by the growth conditions that affect the metabolic activity of P. aeruginosa as well as the growth phase (Pesci et al. 1997; Medina et al. 2003). Such regulation is accomplished through several previously identified regulators. For example, RpoS, MvaT, and QscR regulate the timing of activation of the QS genes; rather than repressing the activated QS systems, these regulators prevent their premature activation (Whiteley et al. 2000; Chugani et al. 2001; Diggle et al. 2002). However, no previous study described either growth or an environmental condition that represses the expression of the already activated QS system. In this study, serum does not interfere with the timing of activation of the QS genes. Rather, serum represses the expression of lasI and rhlI genes at the same time when both genes are maximally expressed in PAO1 grown in LB only (Fig. 1, data not shown). In this regard, serum basically shuts down the QS systems at the time of their maximum function. This effect may be related to the interaction of different components of the QS system in response to an environmental stimulus as the growth phase changes. Upon its multimerization by 3OC12-HSL, LasR activates the transcription of its target genes (Kiratisin et al. 2002). RhlR, on the other hand, requires C4-HSL for its activation but does not require it for dimerization (Pearson et al. 1995). Additionally, through a positive feedback system, both LasR and RhlR activate the transcription of lasI and rhlI, respectively, thereby amplifying the activation signal (Latifi et al. 1996; Pesci et al. 1997). The autoinducers HHQ/PQS activate the LysR transcriptional activator MvfR/PqsR, which enhances the transcription of the pqsA-E operon (McGrath et al. 2004; Wade et al. 2005; Xiao et al. 2006). As P. aeruginosa reaches quorum, the initial response involves an increase in lasI and rhlI transcription which leads to an increased production of 3OC12-HSL and C4-HSL. At this stage, both LasR and RhlR are only activated by their respective autoinducers. This activation at this stage may not involve an increase in either lasR or rhlR transcription.
One possible scenario to explain the observed effect of serum at early growth phases is that at quorum and rather than reducing the expression of QS genes, serum interferes with lasI and rhlI activation (Fig. 11). Consequently, the amount of synthesized 3OC12-HSL and C4-HSL is not sufficient to activate LasR and RhlR and no increase in the transcription of the QS-controlled genes occurs. In contrast, in LB and at quorum, induction of lasI and rhlI expression produces sufficient amounts of 3OC12-HSL and C4-HSL to fully activate LasR and RhlR. Activated LasR and RhlR in turn produce maximum expression of the QS-controlled virulence genes. Our results suggest that the effect of serum is direct. Serum is less likely to interfere with the QS systems by binding the autoinducer extracellularly and reducing their availability to activate LasR and RhlR. Rather, serum appears to prevent LasI and RhlI activation and reduces the amount of the synthase required to produce the autoinducers (Fig. 11).
Figure 11.
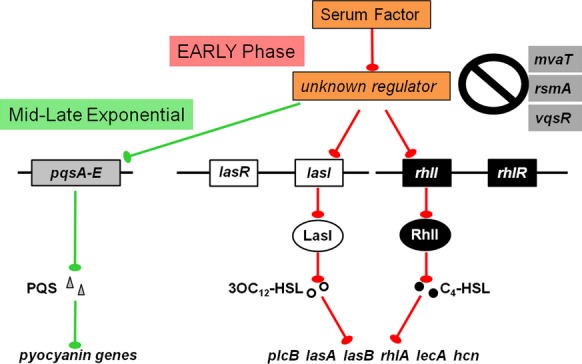
Diagram illustrating the possible mechanism through which serum represses the expression of PAO1 QS and QS-controlled virulence genes. The specific gene(s) through which serum regulates the QS systems is not known at this time. Blunted arrows indicate repression.
To determine the mechanism by which serum affects QS gene expression at early phases of growth, we sought to identify the gene(s) through which this may occur. We compared the expression of several QS regulatory genes in the presence and absence of serum. Although the expression of many of these genes was not affected, the expression of vqsR, rsmA, and mvaT was affected (Fig. 8). The QS genes are positively regulated by vqsR and negatively regulated by rsmA and mvaT (Pessi et al. 2001; Diggle et al. 2002; Juhas et al. 2004). To determine if serum regulation of QS systems occurs through any of these genes, we compared the level of lasI expression between PAO1 and PAO1 mutants defective in mvaT and vqsR in LB and LB/S. Serum still repressed lasI expression in a PAO1 mutant defective in mvaT as well as a PAO1 mutant defective in vqsR suggesting that neither gene is the potential candidate (Fig. 10). Therefore, the potential gene through which serum regulates the QS systems is not known at this time (Fig. 11).
At late stationary phases of growth, serum enhances the expression of the QS genes as well as QS-controlled virulence genes through vfr. At this stage of growth, serum increases the transcription of the QS-positive regulators gacA, vqsR, and vfr but reduced the transcription of the QS-negative regulator rpoN (Fig. 8, data not shown). Among the PAO1 mutants defective in each of these genes, only vfr deletion strains showed no change in lasR or lasI expression in response to serum (Fig. 9C and D). All or most of the serum effect is likely to occur through vfr. At late stationary phase of growth and compared with PAO1, the enhancement in lasR and lasI expression in PAOΔvfr by serum was abrogated (Fig. 9C and D). Vfr, which is a member of the cyclic cAMP receptor protein family of transcriptional regulators, positively regulates the expression of the lasR and rhlR genes and numerous QS-dependent and QS-independent P. aeruginosa virulence genes (West et al. 1994; Albus et al. 1997). The flagellar genes only are negatively regulated by Vfr (Vfr represses the expression of fleQ, which controls the expression of many flagellar genes) (Dasgupta et al. 2002). Vfr regulates its target genes by specifically binding to a vfr consensus sequence within the promoter region of these genes including lasR (Kanack et al. 2006). Upon its activation by cyclic AMP (cAMP), Vfr binds to most of these promoters (West et al. 1994; Suh et al. 2002). No Vfr binding has been demonstrated to either lasI or rhlI (Albus et al. 1997; Kanack et al. 2006). Therefore, the likely scenario to explain how serum affects the QS genes at late phase is that serum enhances vfr expression which increases the level of Vfr protein (Fig. 12). Consequently, the lasR gene is activated which enhances lasI transcription and increases 3OC12-HSL production (Fig. 12). Additionally, LasR activates rhlR/rhlI transcription, increasing C4-HSL levels. Furthermore, LasR enhances mvfR expression, inducing the transcription of the pqsA-E operon leading to increased levels of HHQ and PQS. Activation of these three systems would increase the transcription of different QS-controlled genes. It is important to note that in contrast to its effect at early growth phases, which is LasR independent, serum appears to accomplish its effects at late stationary phase mainly through LasR.
Figure 12.
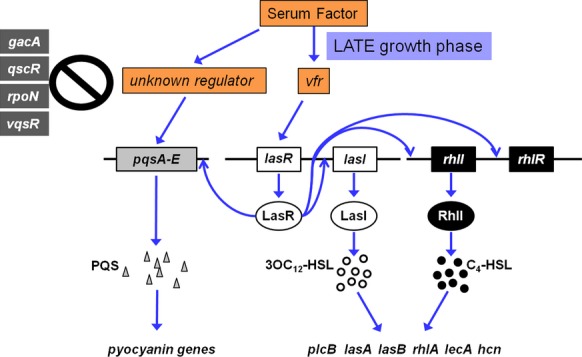
Diagram illustrating the possible mechanism through which serum enhances the expression of PAO1 QS and QS-controlled virulence genes. Our results suggest that serum regulates the QS genes through vfr. Serum enhances vfr expression, which increases the level of Vfr. Consequently, lasR expression is increased and LasR is activated, which enhances lasI transcription and rhlI and rhlR transcription is activated. Additionally, activated LasR induces pqsA-E expression.
The serum-induced growth phase-dependent regulation of P. aeruginosa QS-controlled virulence factors has been previously described. We previously demonstrated that serum induces the expression of numerous PAO1 iron-regulated genes at early stages of growth (OD600 0.7–1) (Kruczek et al. 2012). Serum had no effect on the expression of these genes at later stages of growth (Kruczek et al. 2012). Further analysis revealed that serum albumin induced the expression of these iron-regulated genes (Kruczek et al. 2012). Serum is a complex medium that contains proteins and factors that may positively or negatively regulate the expression of QS and QS-controlled genes. Depending on the efficiency of this regulation by each factor, the net regulation may either be positive or negative. These potential factors exist in the LB/S throughout the growth cycle. Therefore, the PAO1 response to serum at early or late stages of growth is likely dictated by certain metabolites or structural products that are induced at that specific stage of growth.
With respect to the enhancement in QS and QS-controlled virulence genes by serum, several previous studies support this observation. Juhas et al. (2004) previously showed that PAO1 QS and QS-controlled genes were among the 113 genes whose expression is enhanced by serum. Such an effect was eliminated by either rhlI or rhlR mutation (Juhas et al. 2004). Additionally, Wu et al. (2005) suggested that a specific binding of interferon gamma to the P. aeruginosa outer membrane protein OmpF triggers the effect on rhlI/rhlR and the enhancement in the production of the rhl-dependent lecA and pyocyanin. It is interesting to note that the effect of interferon gamma was detected during the late stationary phase of growth of PAO1 (high cell density) (Wu et al. 2005). Similarly, we detected the serum-induced enhancement in QS systems at the late stationary phase of growth of PAO1 (Figs. 2B, 3B and C). Other previously reported factors that enhance PAO1 QS systems are the circulating natriuretic peptides (NP). Blier et al. (2011) reported that NP, a family of eukaryotic hormones produced physiologically by atrial and endothelial cells, modulates the QS and QS-controlled factors in PAO1. Pretreatment of PAO1 with two of the peptides, the brain NP and C-type NP, significantly enhanced the production of 3OC12-HSL and C4-HSL, hydrogen cyanide, and exotoxin A (Blier et al. 2011). This effect appears to occur through the cAMP-activated Vfr and the P. aeruginosa global regulator PtxR (Blier et al. 2011). However, unlike our present findings, the C-type NP strongly inhibited pyocyanin production (Blier et al. 2011). Additionally, the NPs are produced by the host in response to certain stressful conditions such as bacterial infections and sepsis (Blier et al. 2011). In this study, the observed changes in the QS systems occurred in response to potential innate factors that exist in adult bovine serum (Figs. 2–4).
The clinical relevance of our findings is realized if one considers P. aeruginosa infected serum-containing tissues such as wounds. Within these localized infections, P. aeruginosa bacteria grow, reach a quorum, and communicate with each other through the autoinducer molecules. At this initial stage of quorum, and as part of the innate host defense, a potential serum factor(s) significantly reduces the synthesis of the autoinducer by repressing the autoinducer synthase genes (lasI/rhlI). However, as the infection progresses and the number of bacteria within the wound increase dramatically, this host defense may be overwhelmed. As a result and instead of being inhibited by serum, P. aeruginosa uses serum factors to enhance its virulence by increasing the production of different QS-controlled virulence factors.
Acknowledgments
The authors thank Stephen P. Diggle for the contribution of the PAO1lecA::lux and PAO1pqsACTX-lux::pqsA strains and the AHL reporter plasmids pSB1142 and pSB536, Barbara Iglewski for the lasI-lacZ plasmid pPCS223, and Susan West for the strain PAOΔvfr. The authors also thank Joanna E. Swickard for critical reading of the manuscript. Strain PW5352 was made available through grant # NIH P30 DK089507.
Conflict of Interest
None declared.
Funding Information
Strain PW5352 was made available through grant National Institutes of Health (NIH) P30 DK089507.
References
- Albus AM, Pesci EC, Runyen-Janecky LJ, West SE, Iglewski BH. Vfr controls quorum sensing in Pseudomonas aeruginosa. J. Bacteriol. 1997;179:3928–3935. doi: 10.1128/jb.179.12.3928-3935.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allesen-Holm M, Barken KB, Yang L, Klausen M, Webb JS, Kjelleberg S, et al. A characterization of DNA release in Pseudomonas aeruginosa cultures and biofilms. Mol. Microbiol. 2006;59:1114–1128. doi: 10.1111/j.1365-2958.2005.05008.x. [DOI] [PubMed] [Google Scholar]
- Barker AP, Vasil AI, Filloux A, Ball G, Wilderman PJ, Vasil ML. A novel extracellular phospholipase C of Pseudomonas aeruginosa is required for phospholipid chemotaxis. Mol. Microbiol. 2004;53:1089–1098. doi: 10.1111/j.1365-2958.2004.04189.x. [DOI] [PubMed] [Google Scholar]
- Berka RM, Gray GL, Vasil ML. Studies of phospholipase C (heat-labile hemolysin) in Pseudomonas aeruginosa. Infect. Immun. 1981;34:1071–1074. doi: 10.1128/iai.34.3.1071-1074.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blier AS, Veron W, Bazire A, Gerault E, Taupin L, Vieillard J, et al. C-type natriuretic peptide modulates quorum sensing molecule and toxin production in Pseudomonas aeruginosa. Microbiology. 2011;157:1929–1944. doi: 10.1099/mic.0.046755-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branski LK, Al-Mousawi A, Rivero H, Jeschke MG, Sanford AP, Herndon DN. Emerging infections in burns. Surg. Infect. (Larchmt) 2009;10:389–397. doi: 10.1089/sur.2009.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredenbruch F, Geffers R, Nimtz M, Buer J, Haussler S. The Pseudomonas aeruginosa quinolone signal (PQS) has an iron-chelating activity. Environ. Microbiol. 2006;8:1318–1329. doi: 10.1111/j.1462-2920.2006.01025.x. [DOI] [PubMed] [Google Scholar]
- Cao H, Krishnan G, Goumnerov B, Tsongalis J, Tompkins R, Rahme LG. A quorum sensing-associated virulence gene of Pseudomonas aeruginosa encodes a LysR-like transcription regulator with a unique self-regulatory mechanism. Proc. Natl Acad. Sci. USA. 2001;98:14613–14618. doi: 10.1073/pnas.251465298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chugani SA, Whiteley M, Lee KMKM, D'Argenio D, Manoil C, Greenberg EP. QscR, a modulator of quorum-sensing signal synthesis and virulence in Pseudomonas aeruginosa. Proc. Natl Acad. Sci. USA. 2001;98:2752–2757. doi: 10.1073/pnas.051624298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasgupta N, Ferrell EP, Kanack KJ, West SE, Ramphal R. fleQ, the gene encoding the major flagellar regulator of Pseudomonas aeruginosa, is sigma70 dependent and is downregulated by Vfr, a homolog of Escherichia coli cyclic AMP receptor protein. J. Bacteriol. 2002;184:5240–5250. doi: 10.1128/JB.184.19.5240-5250.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Delden C, Ramos J-L. Pseudomonas: virulence and gene regulation. Vol. 2. New York, NY: Kluwer Academic/Plenum Publishers; 2004. Virulence factors in Pseudomonas aeruginosa; pp. 3–45. [Google Scholar]
- Deziel E, Lepine F, Milot S, He J, Mindrinos MN, Tompkins RG, et al. Analysis of Pseudomonas aeruginosa 4-hydroxy-2-alkylquinolines (HAQs) reveals a role for 4-hydroxy-2-heptylquinoline in cell-to-cell communication. Proc. Natl Acad. Sci. USA. 2004;101:1339–1344. doi: 10.1073/pnas.0307694100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diggle SP, Winzer K, Lazdunski A, Williams P, Camara M. Advancing the quorum in Pseudomonas aeruginosa: MvaT and the regulation of N-acylhomoserine lactone production and virulence gene expression. J. Bacteriol. 2002;184:2576–2586. doi: 10.1128/JB.184.10.2576-2586.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diggle SP, Stacey RE, Dodd C, Camara M, Williams P, Winzer K. The galactophilic lectin, LecA, contributes to biofilm development in Pseudomonas aeruginosa. Environ. Microbiol. 2006;8:1095–1104. doi: 10.1111/j.1462-2920.2006.001001.x. [DOI] [PubMed] [Google Scholar]
- Diggle SP, Matthijs S, Wright VJ, Fletcher MP, Chhabra SR, Lamont IL, et al. The Pseudomonas aeruginosa 4-quinolone signal molecules HHQ and PQS play multifunctional roles in quorum sensing and iron entrapment. Chem. Biol. 2007;14:87–96. doi: 10.1016/j.chembiol.2006.11.014. [DOI] [PubMed] [Google Scholar]
- Driscoll JA, Brody SL, Kollef MH. The epidemiology, pathogenesis and treatment of Pseudomonas aeruginosa infections. Drugs. 2007;67:351–368. doi: 10.2165/00003495-200767030-00003. [DOI] [PubMed] [Google Scholar]
- Erickson DL, Endersby R, Kirkham A, Stuber K, Vollman DD, Rabin HR, et al. Pseudomonas aeruginosa quorum-sensing systems may control virulence factor expression in the lungs of patients with cystic fibrosis. Infect. Immun. 2002;70:1783–1790. doi: 10.1128/IAI.70.4.1783-1790.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrow JM, III, Sund ZM, Ellison ML, Wade DS, Coleman JP, Pesci EC. PqsE functions independently of PqsR-Pseudomonas quinolone signal and enhances the rhl quorum-sensing system. J. Bacteriol. 2008;190:7043–7051. doi: 10.1128/JB.00753-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher MP, Diggle SP, Camara M, Williams P. Biosensor-based assays for PQS, HHQ and related 2-alkyl-4-quinolone quorum sensing signal molecules. Nat. Protoc. 2007;2:1254–1262. doi: 10.1038/nprot.2007.158. [DOI] [PubMed] [Google Scholar]
- Gaines JM, Carty NL, Colmer-Hamood JA, Hamood AN. Effect of static growth and different levels of environmental oxygen on toxA and ptxR expression in the Pseudomonas aeruginosa strain PAO1. Microbiology. 2005;151:2263–2275. doi: 10.1099/mic.0.27754-0. [DOI] [PubMed] [Google Scholar]
- Gallagher LA, McKnight SL, Kuznetsova MS, Pesci EC, Manoil C. Functions required for extracellular quinolone signaling by Pseudomonas aeruginosa. J. Bacteriol. 2002;184:6472–6480. doi: 10.1128/JB.184.23.6472-6480.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisenberger O, Givskov M, Riedel K, Hoiby N, Tummler B, Eberl L. Production of N-acyl-l-homoserine lactones by P. aeruginosa isolates from chronic lung infections associated with cystic fibrosis. FEMS Microbiol. Lett. 2000;184:273–278. doi: 10.1111/j.1574-6968.2000.tb09026.x. [DOI] [PubMed] [Google Scholar]
- Hammond A, Dertien J, Colmer-Hamood JA, Griswold JA, Hamood AN. Serum inhibits P. aeruginosa biofilm formation on plastic surfaces and intravenous catheters. J. Surg. Res. 2010;159:735–746. doi: 10.1016/j.jss.2008.09.003. [DOI] [PubMed] [Google Scholar]
- Haussler S, Becker T. The Pseudomonas quinolone signal (PQS) balances life and death in Pseudomonas aeruginosa populations. PLoS Pathog. 2008;4:e1000166. doi: 10.1371/journal.ppat.1000166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hentzer M, Wu H, Andersen JB, Riedel K, Rasmussen TB, Bagge N, et al. Attenuation of Pseudomonas aeruginosa virulence by quorum sensing inhibitors. EMBO J. 2003;22:3803–3815. doi: 10.1093/emboj/cdg366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heurlier K, Denervaud V, Pessi G, Reimmann C, Haas D. Negative control of quorum sensing by RpoN (sigma54) in Pseudomonas aeruginosa PAO1. J. Bacteriol. 2003;185:2227–2235. doi: 10.1128/JB.185.7.2227-2235.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloway BW, Krishnapillai V, Morgan AF. Chromosomal genetics of Pseudomonas. Microbiol. Rev. 1979;43:73–102. doi: 10.1128/mr.43.1.73-102.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs MA, Alwood A, Thaipisuttikul I, Spencer D, Haugen E, Ernst S, et al. Comprehensive transposon mutant library of Pseudomonas aeruginosa. Proc. Natl Acad. Sci. USA. 2003;100:14339–14344. doi: 10.1073/pnas.2036282100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juhas M, Wiehlmann L, Huber B, Jordan D, Lauber J, Salunkhe P, et al. Global regulation of quorum sensing and virulence by VqsR in Pseudomonas aeruginosa. Microbiology. 2004;150:831–841. doi: 10.1099/mic.0.26906-0. [DOI] [PubMed] [Google Scholar]
- Juhas M, Wiehlmann L, Salunkhe P, Lauber J, Buer J, Tummler B. GeneChip expression analysis of the VqsR regulon of Pseudomonas aeruginosa TB. FEMS Microbiol. Lett. 2005;242:287–295. doi: 10.1016/j.femsle.2004.11.020. [DOI] [PubMed] [Google Scholar]
- Kanack KJ, Runyen-Janecky LJ, Ferrell EP, Suh SJ, West SE. Characterization of DNA-binding specificity and analysis of binding sites of the Pseudomonas aeruginosa global regulator, Vfr, a homologue of the Escherichia coli cAMP receptor protein. Microbiology. 2006;152:3485–3496. doi: 10.1099/mic.0.29008-0. [DOI] [PubMed] [Google Scholar]
- de Kievit TR, Iglewski BH. Bacterial quorum sensing in pathogenic relationships. Infect. Immun. 2000;68:4839–4849. doi: 10.1128/iai.68.9.4839-4849.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiratisin P, Tucker KD, Passador L. LasR, a transcriptional activator of Pseudomonas aeruginosa virulence genes, functions as a multimer. J. Bacteriol. 2002;184:4912–4919. doi: 10.1128/JB.184.17.4912-4919.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruczek C, Wachtel M, Alabady MS, Payton PR, Colmer-Hamood JA, Hamood AN. Serum albumin alters the expression of iron-controlled genes in Pseudomonas aeruginosa. Microbiology. 2012;158:353–367. doi: 10.1099/mic.0.053371-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latifi A, Foglino M, Tanaka K, Williams P, Lazdunski A. A hierarchical quorum-sensing cascade in Pseudomonas aeruginosa links the transcriptional activators LasR and RhlR (VsmR) to expression of the stationary-phase sigma factor RpoS. Mol. Microbiol. 1996;21:1137–1146. doi: 10.1046/j.1365-2958.1996.00063.x. [DOI] [PubMed] [Google Scholar]
- Lazenby JJ, Griffin PE, Kyd J, Whitchurch CB, Cooley MA. A quadruple knockout of lasIR and rhlIR of Pseudomonas aeruginosa PAO1 that retains wild-type twitching motility has equivalent infectivity and persistence to PAO1 in a mouse model of lung infection. PLoS One. 2013;8:e60973. doi: 10.1371/journal.pone.0060973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepine F, Milot S, Deziel E, He J, Rahme LG. Electrospray/mass spectrometric identification and analysis of 4-hydroxy-2-alkylquinolines (HAQs) produced by Pseudomonas aeruginosa. J. Am. Soc. Mass Spectrom. 2004;15:862–869. doi: 10.1016/j.jasms.2004.02.012. [DOI] [PubMed] [Google Scholar]
- Lyczak JB, Cannon CL, Pier GB. Establishment of Pseudomonas aeruginosa infection: lessons from a versatile opportunist. Microbes Infect. 2000;2:1051–1060. doi: 10.1016/s1286-4579(00)01259-4. [DOI] [PubMed] [Google Scholar]
- McGrath S, Wade DS, Pesci EC. Dueling quorum sensing systems in Pseudomonas aeruginosa control the production of the Pseudomonas quinolone signal (PQS) FEMS Microbiol. Lett. 2004;230:27–34. doi: 10.1016/S0378-1097(03)00849-8. [DOI] [PubMed] [Google Scholar]
- Medina G, Juarez K, Diaz R, Soberon-Chavez G. Transcriptional regulation of Pseudomonas aeruginosa rhlR, encoding a quorum-sensing regulatory protein. Microbiology. 2003;149:3073–3081. doi: 10.1099/mic.0.26282-0. [DOI] [PubMed] [Google Scholar]
- Miller JH. Experiments in molecular genetics. New York, NY: Cold Spring Harbor Laboratory Press; 1972. [Google Scholar]
- Ohman DE, Cryz SJ, Iglewski BH. Isolation and characterization of Pseudomonas aeruginosa PAO mutant that produces altered elastase. J. Bacteriol. 1980;143:836–842. doi: 10.1128/jb.142.3.836-842.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson JP, Gray KM, Passador L, Tucker KD, Eberhard A, Iglewski BH, et al. Structure of the autoinducer required for expression of Pseudomonas aeruginosa virulence genes. Proc. Natl Acad. Sci. USA. 1994;91:197–201. doi: 10.1073/pnas.91.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson JP, Passador L, Iglewski BH, Greenberg EP. A second N-acylhomoserine lactone signal produced by Pseudomonas aeruginosa. Proc. Natl Acad. Sci. USA. 1995;92:1490–1494. doi: 10.1073/pnas.92.5.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson JP, Feldman M, Iglewski BH, Prince A. Pseudomonas aeruginosa cell-to-cell signaling is required for virulence in a model of acute pulmonary infection. Infect. Immun. 2000;68:4331–4334. doi: 10.1128/iai.68.7.4331-4334.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesci EC, Pearson JP, Seed PC, Iglewski BH. Regulation of las and rhl quorum sensing in Pseudomonas aeruginosa. J. Bacteriol. 1997;179:3127–3132. doi: 10.1128/jb.179.10.3127-3132.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesci EC, Milbank JB, Pearson JP, McKnight S, Kende AS, Greenberg EP, et al. Quinolone signaling in the cell-to-cell communication system of Pseudomonas aeruginosa. Proc. Natl Acad. Sci. USA. 1999;96:11229–11234. doi: 10.1073/pnas.96.20.11229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessi G, Williams F, Hindle Z, Heurlier K, Holden MT, Camara M, et al. The global posttranscriptional regulator RsmA modulates production of virulence determinants and N-acylhomoserine lactones in Pseudomonas aeruginosa. J. Bacteriol. 2001;183:6676–6683. doi: 10.1128/JB.183.22.6676-6683.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pier GB, Ramphal R, Mandell GL, Bennett JE, Dolin R. Mandell, douglas, and Bennett's principles and practice of infectious diseases. 7th ed. Vol. 2. Philadelphia, PA: Churchill Livingstone; 2010. Pseudomonas aeruginosa; pp. 2835–2860. [Google Scholar]
- Preston MJ, Seed PC, Toder DS, Iglewski BH, Ohman DE, Gustin JK, et al. Contribution of proteases and LasR to the virulence of Pseudomonas aeruginosa during corneal infections. Infect. Immun. 1997;65:3086–3090. doi: 10.1128/iai.65.8.3086-3090.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rampioni G, Pustelny C, Fletcher MP, Wright VJ, Bruce M, Rumbaugh KP, et al. Transcriptomic analysis reveals a global alkyl-quinolone-independent regulatory role for PqsE in facilitating the environmental adaptation of Pseudomonas aeruginosa to plant and animal hosts. Environ. Microbiol. 2010;12:1659–1673. doi: 10.1111/j.1462-2920.2010.02214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimmann C, Beyeler M, Latifi A, Winteler H, Foglino M, Lazdunski A, et al. The global activator GacA of Pseudomonas aeruginosa PAO positively controls the production of the autoinducer N-butyryl-homoserine lactone and the formation of the virulence factors pyocyanin, cyanide, and lipase. Mol. Microbiol. 1997;24:309–319. doi: 10.1046/j.1365-2958.1997.3291701.x. [DOI] [PubMed] [Google Scholar]
- Rumbaugh KP, Griswold JA, Iglewski BH, Hamood AN. Contribution of quorum sensing to the virulence of Pseudomonas aeruginosa in burn wound infections. Infect. Immun. 1999;67:5854–5862. doi: 10.1128/iai.67.11.5854-5862.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumbaugh KP, Griswold JA, Hamood AN. The role of quorum sensing in the in vivo virulence of Pseudomonas aeruginosa. Microbes Infect. 2000;2:1721–1731. doi: 10.1016/s1286-4579(00)01327-7. [DOI] [PubMed] [Google Scholar]
- Runyen-Janecky LJ, Sample AK, Maleniak TC, West SE. A divergently transcribed open reading frame is located upstream of the Pseudomonas aeruginosa vfr gene, a homolog of Escherichia coli crp. J. Bacteriol. 1997;179:2802–2809. doi: 10.1128/jb.179.9.2802-2809.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadikot RT, Blackwell TS, Christman JW, Prince AS. Pathogen-host interactions in Pseudomonas aeruginosa pneumonia. Am. J. Respir. Crit. Care Med. 2005;171:1209–1223. doi: 10.1164/rccm.200408-1044SO. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster M, Greenberg EP. A network of networks: quorum-sensing gene regulation in Pseudomonas aeruginosa. Int. J. Med. Microbiol. 2006;296:73–81. doi: 10.1016/j.ijmm.2006.01.036. [DOI] [PubMed] [Google Scholar]
- Schuster M, Lostroh CP, Ogi T, Greenberg EP. Identification, timing, and signal specificity of Pseudomonas aeruginosa quorum-controlled genes: a transcriptome analysis. J. Bacteriol. 2003;185:2066–2079. doi: 10.1128/JB.185.7.2066-2079.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh PK, Schaefer AL, Parsek MR, Moninger TO, Welsh MJ, Greenberg EP. Quorum-sensing signals indicate that cystic fibrosis lungs are infected with bacterial biofilms. Nature. 2000;407:762–764. doi: 10.1038/35037627. [DOI] [PubMed] [Google Scholar]
- Smith RS, Iglewski BH. P. aeruginosa quorum-sensing systems and virulence. Curr. Opin. Microbiol. 2003;6:56–60. doi: 10.1016/s1369-5274(03)00008-0. [DOI] [PubMed] [Google Scholar]
- Stachel SE, An G, Flores C, Nester EW. A Tn3 lacZ transposon for the random generation of beta-galactosidase gene fusions: application to the analysis of gene expression in Agrobacterium. EMBO J. 1985;4:891–898. doi: 10.1002/j.1460-2075.1985.tb03715.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey DG, Ujack EE, Mitchell I, Rabin HR. Positive correlation of algD transcription to lasB and lasA transcription by populations of Pseudomonas aeruginosa in the lungs of patients with cystic fibrosis. Infect. Immun. 1997;65:4061–4067. doi: 10.1128/iai.65.10.4061-4067.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey DG, Ujack EE, Rabin HR, Mitchell I. Pseudomonas aeruginosa lasR transcription correlates with the transcription of lasA lasB, and toxA in chronic lung infections associated with cystic fibrosis. Infect. Immun. 1998;66:2521–2528. doi: 10.1128/iai.66.6.2521-2528.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh SJ, Runyen-Janecky LJ, Maleniak TC, Hager P, MacGregor CH, Zielinski-Mozny NA, et al. Effect of vfr mutation on global gene expression and catabolite repression control of Pseudomonas aeruginosa. Microbiology. 2002;148:1561–1569. doi: 10.1099/00221287-148-5-1561. [DOI] [PubMed] [Google Scholar]
- Swift S, Karlyshev AV, Fish L, Durant EL, Winson MK, Chhabra SR, et al. Quorum sensing in Aeromonas hydrophila and Aeromonas salmonicida: identification of the LuxRI homologs AhyRI and AsaRI and their cognate N-acylhomoserine lactone signal molecules. J. Bacteriol. 1997;179:5271–5281. doi: 10.1128/jb.179.17.5271-5281.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tendeng C, Soutourina OA, Danchin A, Bertin PN. MvaT proteins in Pseudomonas spp.: a novel class of H-NS-like proteins. Microbiology. 2003;149:3047–3050. doi: 10.1099/mic.0.C0125-0. [DOI] [PubMed] [Google Scholar]
- Vallet I, Diggle SP, Stacey RE, Camara M, Ventre I, Lory S, et al. Biofilm formation in Pseudomonas aeruginosa: fimbrial cup gene clusters are controlled by the transcriptional regulator MvaT. J. Bacteriol. 2004;186:2880–2890. doi: 10.1128/JB.186.9.2880-2890.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade DS, Calfee MW, Rocha ER, Ling EA, Engstrom E, Coleman JP, et al. Regulation of Pseudomonas quinolone signal synthesis in Pseudomonas aeruginosa. J. Bacteriol. 2005;187:4372–4380. doi: 10.1128/JB.187.13.4372-4380.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner VE, Bushnell D, Passador L, Brooks AI, Iglewski BH. Microarray analysis of Pseudomonas aeruginosa quorum-sensing regulons: effects of growth phase and environment. J. Bacteriol. 2003;185:2080–2095. doi: 10.1128/JB.185.7.2080-2095.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Zhang YD, Hu Y, Yang B, Chen S. Effects of quorum sensing autoinducer degradation gene on virulence and biofilm formation of Pseudomonas aeruginosa. Sci. China C. Life Sci. 2007;50:385–391. doi: 10.1007/s11427-007-0044-y. [DOI] [PubMed] [Google Scholar]
- West SE, Sample AK, Runyen-Janecky LJ. The vfr gene product, required for Pseudomonas aeruginosa exotoxin A and protease production, belongs to the cyclic AMP receptor protein family. J. Bacteriol. 1994;176:7532–7542. doi: 10.1128/jb.176.24.7532-7542.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westfall LW, Luna AM, San Francisco M, Diggle SP, Worrall KE, Williams P, et al. The Pseudomonas aeruginosa global regulator MvaT specifically binds to the ptxS upstream region and enhances ptxS expression. Microbiology. 2004;150:3797–3806. doi: 10.1099/mic.0.27270-0. [DOI] [PubMed] [Google Scholar]
- Whiteley M, Parsek MR, Greenberg EP. Regulation of quorum sensing by RpoS in Pseudomonas aeruginosa. J. Bacteriol. 2000;182:4356–4360. doi: 10.1128/jb.182.15.4356-4360.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winson MK, Camara M, Latifi A, Foglino M, Chhabra SR, Daykin M, et al. Multiple N-acyl-l-homoserine lactone signal molecules regulate production of virulence determinants and secondary metabolites in Pseudomonas aeruginosa. Proc. Natl Acad. Sci. USA. 1995;92:9427–9431. doi: 10.1073/pnas.92.20.9427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winson MK, Swift S, Fish L, Throup JP, Jorgensen F, Chhabra SR, et al. Construction and analysis of luxCDABE-based plasmid sensors for investigating N-acyl homoserine lactone-mediated quorum sensing. FEMS Microbiol. Lett. 1998;163:185–192. doi: 10.1111/j.1574-6968.1998.tb13044.x. [DOI] [PubMed] [Google Scholar]
- Winzer K, Falconer C, Garber NC, Diggle SP, Camara M, Williams P. The Pseudomonas aeruginosa lectins PA-IL and PA-IIL are controlled by quorum sensing and by RpoS. J. Bacteriol. 2000;182:6401–6411. doi: 10.1128/jb.182.22.6401-6411.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Song Z, Hentzer M, Andersen JB, Heydorn A, Mathee K, et al. Detection of N-acylhomoserine lactones in lung tissues of mice infected with Pseudomonas aeruginosa. Microbiology. 2000;146:2481–2493. doi: 10.1099/00221287-146-10-2481. [DOI] [PubMed] [Google Scholar]
- Wu L, Estrada O, Zaborina O, Bains M, Shen L, Kohler JE, et al. Recognition of host immune activation by Pseudomonas aeruginosa. Science. 2005;309:774–777. doi: 10.1126/science.1112422. [DOI] [PubMed] [Google Scholar]
- Xiao G, Deziel E, He J, Lepine F, Lesic B, Castonguay MH, et al. MvfR, a key Pseudomonas aeruginosa pathogenicity LTTR-class regulatory protein, has dual ligands. Mol. Microbiol. 2006;62:1689–1699. doi: 10.1111/j.1365-2958.2006.05462.x. [DOI] [PubMed] [Google Scholar]
- Yoon SS, Hennigan RF, Hilliard GM, Ochsner UA, Parvatiyar K, Kamani MC, et al. Pseudomonas aeruginosa anaerobic respiration in biofilms: relationships to cystic fibrosis pathogenesis. Dev. Cell. 2002;3:593–603. doi: 10.1016/s1534-5807(02)00295-2. [DOI] [PubMed] [Google Scholar]


