SUMMARY
One of the most promising approaches in the efforts to produce a malaria vaccine involves the use of attenuated whole sporozoite immunizations. Attenuation may be achieved by the use of genetic modification, irradiation, chemical attenuation, or by the contemporaneous administration of antimalarial drugs that target only the erythrocytic stages of the parasite. Most research to date has focused on the efficacy of these approaches upon challenge with parasites homologous to those used for the initial immunizations. We, as have others, have previously shown that a component of the immunity achieved against the erythrocytic stages of the rodent malaria parasite Plasmodium chabaudi chabaudi is strain-specific, with a stronger immune response targeting the immunizing strain than genetically distinct strains. Here, we show that the immunity induced by infection with the pre-erythrocytic stages of these parasites, achieved via inoculation of sporozoites contemporaneously with mefloquine, also has a strain-specific component.
Keywords: Plasmodium chabaudi, rodent malaria parasites, sporozoites, strain-specific immunity
INTRODUCTION
Antigenic variation between strains of malaria parasites has been shown to lead to the phenomenon of ‘strain-specific immunity’ in which the protective effect of exposure-induced immunity, both in the laboratory (1) and in the field (2), is stronger against a homologous strain than against genetically distinct heterologous strains. One of the most comprehensive studies of this phenomenon to date was conducted using the rodent malaria parasite Plasmodium chabaudi chabaudi, for which it was shown that the major genetic determinant of the strain-specificity of the immunity achieved via immunization with blood-stage parasites is the merozoite surface protein 1 gene (msp1) (3). Natural malaria infections of both rodents and humans are initiated by the bite of malaria parasite-infected Anopheles mosquitoes, which inoculate sporozoites into the skin during blood feeding. Very effective protective immunity against malaria can be achieved by immunization with sporozoites that have been attenuated by irradiation (4). More recently, other methods of sporozoite attenuation such as genetic modification (5) and chemical attenuation (6) have also been shown to confer protective immunity against re-infection. A similar approach in which live sporozoites are inoculated contemporaneously with anti-erythrocytic stage drugs such as chloroquine (CQ) has recently been shown to confer sterile protective immunity against Plasmodium falciparum in human volunteers (7). The protective efficacies of these vaccine strategies have, most commonly, been assessed using parasites homologous to the vaccinating strain. Those few studies which have assessed the level of protection against heterologous challenge have almost exclusively assessed the degree of cross-protection between malaria parasite species (8-15) and are generally inconsistent in their conclusions. Should it occur, parasite strain-specificity to the induction of immunity by live sporozoites of P. falciparum will need to be understood if such vaccination is to be used effectively. Here, we present the results of experiments to test for and determine the degree of cross protection between strains of Plasmodium chabaudi immunized by inoculation of live sporozoites in conjunction with mefloquine (MF) treatment.
MATERIALS AND METHODS
All experiments were carried out in compliance with the British Home Office Animals (Scientific Procedures) Act 1986. For sporozoite immunizations, two groups of 20 inbred female CBA/Ca mice (6 weeks old at the time of first immunization) were inoculated via intraperitoneal (IP) injection with known numbers of sporozoites of P. c. chabaudi clones AJ or CB diluted in a 50 : 50 mixture of Foetal Calf Serum (FCS) and Ringer’s solution contemporaneously with oral MF treatment (20 mg/kg/day for 5 days). Immunizations were performed twice with an interval of 3 weeks between inoculations. Each mouse received an inoculation of ~400 sporozoites of each strain in the first immunization, and ~2000 in the second. Twenty control mice were inoculated with 50 : 50 FCS: Ringer’s solution only, and also drug treated. Five weeks following the second immunization, mice were each challenged IP with 2400 sporozoites of either strain, or with 1 × 106 parasite-infected red blood cells (iRBCs). Immunized and mock-immunized control mice were separated into groups of five and received either a homologous or heterologous challenge strain of sporozoites or iRBCs. The mock-immunized group that received an AJ challenge were reduced to two mice in the group because of a technical error during challenge. The resulting blood-stage infections were followed by microscopic examination of Giemsa’s solution-stained thin blood smears taken daily using venous blood from the tail. In order to determine the day at which parasites first became detectable in the blood, at least 10 000 red blood cells were examined per smear. For the generation of sporozoites, Anopheles stephensi mosquitoes were allowed to feed on anaesthetized mice that had been inoculated with 1 × 106 iRBCs IP 6 days previously. Prior to feeding, mouse blood was checked for the presence of gametocytes, and their viability assessed by the observation of exflagellation of microgametocytes in fresh blood preparations. Seven to 10 days post-feed, mosquito mid-guts were dissected and the presence of oocysts confirmed. Sixteen days post-feed, mosquito salivary glands were dissected into a 50 : 50 solution of FCS and Ringer’s solution, crushed in a glass and Teflon tissue homogeniser, and the numbers of sporozoites in the homogenate assessed by counting with a haemocytometer. In order to assess sporozoite viability, only those sporozoites displaying circular gliding motility were considered viable. There were no discernable differences in the viability of CB and AJ sporozoites, and sporozoites of both strains were handled in exactly the same manner prior to immunization and challenge inoculation. All mice were kept on 0·05% para-aminobenzoic acid (PABA)-supplemented water ad libitum and were housed at 21°C on a 12 h-light–dark cycle. Anopheles stephensi mosquitoes were fed with 0·05% PABA-supplemented 10% glucose solution and were housed at 27°C and 70% humidity on a 12-h light–dark cycle. We used R version 2·7·0; The R Foundation for Statistical Computing; http://www.R-project.org) for data analysis. To analyse patterns of parasitaemia during infections, we used mixed effects models because, by treating each infection as a ‘random’ effect, we can account for repeated measures from each infection and overcome pseudoreplication problems associated with such data. These models were fitted with Poisson error distributions and minimized following stepwise deletion of the least significant term, using log-likelihood ratio tests to evaluate the change in model deviance, until only significant terms remained. We present F-ratios for fixed effects remaining in minimal models. Mann–Whitney tests were used to compare patency data. Cumulative, summary data were analysed with linear models, using anova (F ratios) to evaluate significance of terms.
RESULTS
The days on which parasites became detectable by microscopy (patent) in the blood of mice subjected to various immunization and challenge regimens are shown in Table 1. In mice that had been immunized with the CB strain by inoculation with live sporozoites contemporaneously with MF administration, the onset of patent parasitaemia following homologous strain (CB) sporozoite challenge was delayed by around 2 days relative to an equivalent CB sporozoite challenge in mock immunized mice (P = 0·017) (Table 1, cf rows 1 and 3). The prepatency period for CB immunized mice was significantly greater in the CB sporozoite-challenged group compared to AJ sporozoite-challenged group (P = 0·010) (Table 1, cf rows 1 and 2). This suggests that live sporozoite immunization under MF drug cover with the CB strain induced a strain-specific, anti-parasitic immunity against homologous CB sporozoite-induced infection, and that this anti-parasitic immunity was already acting before the appearance of a patent blood infection.
Table 1.
Day of patency of blood-stage parasites following sporozoite challenge (number of mice positive/total number in group)
| Immunizing strain | Challenge strain |
Day post-challenge |
||||
|---|---|---|---|---|---|---|
| 3 | 4 | 5 | 6 | 7 | ||
| CB | CB | 0/5 | 0/5 | 0/5 | 2/5 | 4/5 |
| CB | AJ | 0/5 | 0/5 | 5/5 | 5/5 | 5/5 |
| Mock-immunized | CB | 0/5 | 2/5 | 5/5 | 5/5 | 5/5 |
| Mock-immunized | AJ | 0/2 | 0/2 | 1/2 | 2/2 | 2/2 |
| AJ | CB | 1/5 | 4/5 | 5/5 | 5/5 | 5/5 |
| AJ | AJ | 0/5 | 3/5 | 5/5 | 5/5 | 5/5 |
In mice immunized with AJ strain sporozoites using the same protocol as described earlier and subsequently challenged with sporozoites of either CB or AJ, blood-stage parasites of both strains tended to appear even earlier following equivalent challenge in naïve mice (Table 1, cf rows 5 and 3, and cf rows 6 and 4), although this effect was not statistically significant (homologous (AJ sporozoite) challenge vs. mock immunized, P = 0·143; homologous (AJ sporozoite) challenge vs. heterologous (CB sporozoite) challenge, P = 0·403).
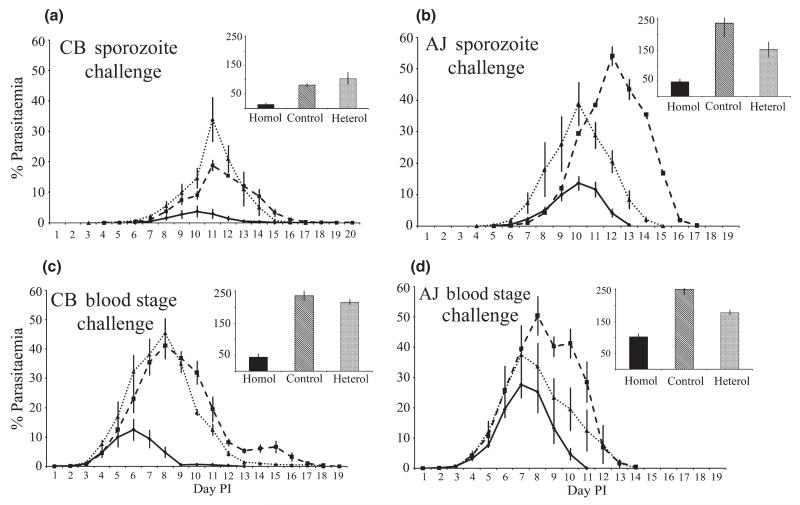
The course of blood infections in both sporozoite-induced and blood-stage parasite-induced infections in sporozoite-immunized and in mock-immunized control mice are shown in Figure 1. The infection dynamics reveal that sporozoite challenges result in significantly lower parasitaemias than blood-stage challenges (F1,50 = 21·96; P ≤ 0·0001). Immunization with CB reduced parasitaemias of challenge infections significantly more than AJ immunization for both challenge strains (F2,50 = 29·28; P ≤ 0·0001). Moreover, the reduction in parasitaemias following CB immunization were greater for homologous challenges (F2,50 = 6·05; P = 0·004), but this was not the case following immunization with AJ.
Figure 1.
Course of parasitaemia in challenge infections with Plasmodium chabaudi chabaudi in groups of mice twice immunized by sporozoite infection under a mefloquine cover with CB (solid lines) or AJ (light dotted lines) sporozoites of P. c. chabaudi and in mock-immunized control mice (heavy dashed lines). Cumulative proportion of parasitized red blood cells throughout the infections is shown in the bar chart insets. The challenge infections were with sporozoites of CB (Panel a) and AJ (b), and with blood-stage parasites of CB (c) and AJ (d). Error bars indicate one standard error above and below the mean.
Specific comparisons of the cumulative proportion of parasitized red blood cells for each type of challenge with its mock-immunized control group supported these findings for the effects of immunization. For CB sporozoite challenge, CB immunization strongly reduced parasitaemias but those achieved following AJ immunization were not significantly different to mock-immunized control infections (Figure 1a; F2,11 = 8·69; P = 0·005). For AJ sporozoite challenge, there was a similar trend in which the lowest parasitaemias were reached after CB immunization (Figure 1b; F2,8 = 0·01; P = 0·009). For CB blood-stage challenge, CB immunization strongly reduced parasitaemia and a slight reduction was achieved following AJ immunization (Figure 1c; F2,12 = 70·57; P ≤ 0·0001). For AJ blood-stage challenge, CB immunization strongly reduced parasitaemia relative to that in the mock immunised mice, but parasitaemias achieved following AJ immunization were not significantly different to mock-immunized control infections (Figure 1d; F2,12 = 10·82; P = 0·002).
DISCUSSION
Immunization of female CBA mice by infection with live sporozoites of a single strain, CB or AJ, of the malaria parasite P. c. chabaudi, under the cover of the anti-bloodstage antimalarial drug, MF, induced responses that were variously effective before and/or during patent blood infection following challenge with either sporozoites or blood-stage parasites of one or the other of these two strains of parasite. The effects of immunization with live sporozoites under MF cover included strain-specific suppression of pre-patent parasite growth (CB sporozoite-immunization suppressed pre-patent parasite growth in CB sporozoite–induced infections but not in those of AJ sporozoite–induced infections); strain-specific suppression of patent erythrocytic parasite growth (CB sporozoite–immunisation suppressed blood-parasite growth in sporozoite- and blood parasite-induced infections of CB more than it did to growth of blood parasites in corresponding AJ infections; AJ sporozoite–immunized mice partially suppressed growth of AJ blood parasites in sporozoite- and blood parasite-induced infections but did not suppress growth of CB blood parasites); pan-strain suppression of patent erythrocytic parasite growth (CB sporozoite–immunization suppressed growth of erythrocytic parasites in sporozoite- and blood parasite-induced infections of both AJ and CB).
It should also be noted that the parasites showed strain-specificity, or its absence, in their immunological properties not only as targets of immunity but also as inducers of immunity. While both AJ and CB were involved in the induction of strain-specific immunity against the blood-stage parasites, only CB, and not AJ, live sporozoite immunization induced powerful pan-strain effects in suppressing blood-stage parasites. Such strain-specific properties of the induction of immunity against blood-stage parasites have been recorded previously among strains of P. c. chabaudi (1). The two strains differed also in the immunity they induced against the parasites pre-blood patency. Experiments testing whether strains such as CB induce pan-strain immunity through broader antigen repertoire and whether this is linked to lower parasite densities in control infections are now required. Quantifying variation in strain-specificity and explaining the underlying mechanisms are central to predicting the success of interventions that work by inducing immunity.
It is conceivable that differences in the viabilities of CB and AJ sporozoites may have contributed to some of the effects observed in this study, as this would result in the development of differing numbers of exo-erythrocytic stage parasites for each strain during the immunization procedure. However, we found no evidence for any differences in viabilities when assessing sporozoite motility prior to inoculation. Nevertheless, as viabilities were not directly assessed, differences between strains remain possible.
The most significant findings of this study are, we suggest, the following. We show that immunity induced by sporozoites under a drug cover that should prevent development of the parasites in the blood, has a marked strain-specific component against both sporozoite and blood-stage parasite challenge. The strain-specific effect appears to apply to the parasites in sporozoite-induced infections at a stage before they are detectible in the blood by conventional blood smear microscopy. This could be because there is strain-specific anti parasite immunity acting against the parasites in the liver. However, our results also clearly show that strain-specific immunity is acting against the blood stage parasites themselves. We suggest that this anti-blood stage immunity arises either through the expression of antigens common to blood-stage parasites during exo-erythrocytic schizont development, as was shown previously for MSP1 (16), or by the exposure to the immune system of the exo-erythrocytic merozoites which are released, and invade red blood cells, before being killed by the effect of MF in our immunization protocol. Each P. chabaudi liver schizont is believed to release in the order of 20 000 merozoites (17). As the immunizing inocula in the present experiments probably delivered many tens, at least, of sporozoites successfully to the liver (based on an evaluation of the IP route for sporozoite inoculation, Inoue & Culleton, unpublished data), each sporozoite immunization under MF would probably have resulted in the release of the order of at least 105 blood stage merozoites. This, we suggest, is a likely basis for the induction of the immunity, both pan- and strain-specific, that we observed against the blood-stage parasites in these experiments.
The protective immunity achieved via immunization with both irradiation attenuated and genetically attenuated sporozoites is thought to be mediated mainly through CD8+ T-cell responses, at least for the Plasmodium berghei and Plasmodium yoelii parasites (18). There is also evidence for the involvement of other immune mechanisms in these systems, including those involving B cells, CD4+ T-cells and NK cells (19–21). When immunizations are performed with live sporozoites under the cover of anti-blood stage chemoprophylaxis, as in our study, it appears that both CD4+ and CD8+ T-cells are involved in the protective affect achieved, and there is little evidence for the role of antibodies (22). However, these experiments were performed with P. yoelii parasites, and it is possible that protective mechanisms differ between parasite species (10,23). The two P. c. chabaudi strains used in this study, AJ and CB, have previously been shown to differ considerably at the nucleotide level (24). This genetic diversity incorporates known antigen genes, such as MSP1 (25), which elicit strongly strain-specific immune responses (3). It is, therefore, highly likely that antigenic proteins expressed during the pre-erythrocytic stages of these parasite strains also differ significantly and may explain the strain-specific effects observed in this study. For example, there is a clear size polymorphism in the gene encoding the major sporozoite surface antigen circumsporozite protein (CSP), which indicates sequence variation between these strains (data not shown). CSP contains both T-cell and B-cell epitopes (26,27), and differences between strains at these domains could result in the strain-specific effects we have observed. Interestingly, it has recently been shown that CSP plays only a minimal role in the protection obtained with live sporozoites under anti-blood stage chemoprophylaxis, indicating the involvement of other, as yet uncharacterized major antigens (28). A future direction of this work is to utilize the strain-specificity of pre-erythrocytic stage immunity apparent between strains of P. c. chabaudi in genetic linkage analyses, including Linkage Group Selection (29), in order to identify these antigens.
ACKNOWLEDGEMENTS
We thank Les Steven for technical assistance and Sofia Trindade Borges for discussion. This work was supported by The Cunningham Trust of the UK (to R.C), A Royal Society Bilateral Grant for Co-operative Research (to R.C and R.L.C) and a Sasakawa Foundation Butterfield Award (to R.L.C).
Footnotes
Disclosure: None
REFERENCES
- 1.Cheesman S, Raza A, Carter R. Mixed strain infections and strain-specific protective immunity in the rodent malaria parasite Plasmodium chabaudi chabaudi in mice. Infect Immun. 2006;74:2996–3001. doi: 10.1128/IAI.74.5.2996-3001.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eisen DP, Saul A, Fryauff DJ, Reeder JC, Coppel RL. Alterations in Plasmodium falciparum genotypes during sequential infections suggest the presence of strain-specific immunity. Am J Trop Med Hyg. 2002;67:8–16. doi: 10.4269/ajtmh.2002.67.8. [DOI] [PubMed] [Google Scholar]
- 3.Cheesman S, O’Mahony E, Pattaradilokrat S, Degnan K, Knott S, Carter R. A single parasite gene determines strain-specific protective immunity against malaria: the role of the merozoite surface protein I. Int J Parasitol. 2010;40:951–961. doi: 10.1016/j.ijpara.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 4.Nussenzweig RS, Vanderberg J, Most H, Orton C. Protective immunity produced by the injection of x-irradiated sporozoites of Plasmodium berghei. Nature. 1967;216:160–162. doi: 10.1038/216160a0. [DOI] [PubMed] [Google Scholar]
- 5.VanBuskirk KM, O’Neill MT, De La Vega P, et al. Preerythrocytic, live-attenuated Plasmodium falciparum vaccine candidates by design. Proc Natl Acad Sci USA. 2009;106:13004–13009. doi: 10.1073/pnas.0906387106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Purcell LA, Yanow SK, Lee M, Spithill TW, Rodriguez A. Chemical attenuation of Plasmodium berghei sporozoites induces sterile immunity in mice. Infect Immun. 2008;76:1193–1199. doi: 10.1128/IAI.01399-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roestenberg M, McCall M, Hopman J, et al. Protection against a malaria challenge by sporozoite inoculation. N Eng J Med. 2009;361:468–477. doi: 10.1056/NEJMoa0805832. [DOI] [PubMed] [Google Scholar]
- 8.Purcell LA, Wong KA, Yanow SK, Lee M, Spithill TW, Rodriguez A. Chemically attenuated Plasmodium sporozoites induce specific immune responses, sterile immunity and cross-protection against heterologous challenge. Vaccine. 2008;26:4880–4884. doi: 10.1016/j.vaccine.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sedegah M, Weiss WW, Hoffman SL. Cross-protection between attenuated Plasmodium berghei and P. yoelii sporozoites. Parasite Immunol. 2007;29:559–565. doi: 10.1111/j.1365-3024.2007.00976.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Douradinha B, van Dijk MR, Ataide R, et al. Genetically attenuated P36p-deficient Plasmodium berghei sporozoites confer long-lasting and partial cross-species protection. Int J Parasitol. 2007;37:1511–1519. doi: 10.1016/j.ijpara.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 11.Hoffman SL, Goh LM, Luke TC, et al. Protection of humans against malaria by immunization with radiation-attenuated Plasmodium falciparum sporozoites. J Infect Dis. 2002;185:1155–1164. doi: 10.1086/339409. [DOI] [PubMed] [Google Scholar]
- 12.Collins WE, Jeffery GM. A retrospective examination of sporozoite- and trophozoite-induced infections with Plasmodium falciparum in patients previously infected with heterologous species of Plasmodium: effect on development of parasitologic and clinical immunity. Am J Trop Med Hyg. 1999;61:36–43. doi: 10.4269/tropmed.1999.61-036. [DOI] [PubMed] [Google Scholar]
- 13.Sina BJ, do Rosario VE, Woollett G, Sakhuja K, Hollingdale MR. Plasmodium falciparum sporozoite immunization protects against Plasmodium berghei sporozoite infection. Exp Parasitol. 1993;77:129–135. doi: 10.1006/expr.1993.1069. [DOI] [PubMed] [Google Scholar]
- 14.Sacci JB, Jr, Schriefer ME, Resau JH, et al. Mouse model for exoerythrocytic stages of Plasmodium falciparum malaria parasite. Proc Natl Acad Sci USA. 1992;89:3701–3705. doi: 10.1073/pnas.89.9.3701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nussenzweig RS, Vanderberg JP, Most H, Orton C. Specificity of protective immunity produced by x-irradiated Plasmodium berghei sporozoites. Nature. 1969;222:488–489. doi: 10.1038/222488a0. [DOI] [PubMed] [Google Scholar]
- 16.Kawabata Y, Udono H, Honma K, et al. Merozoite surface protein 1-specific immune response is protective against exoerythrocytic forms of Plasmodium yoelii. Infect Immun. 2002;70:6075–6082. doi: 10.1128/IAI.70.11.6075-6082.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Landau I, Boulard Y. In: Rodent Malaria. Killick-Kendrick R, Peters W, editors. Academic Press; London, New York, San Francisco: 1978. pp. 53–84. [Google Scholar]
- 18.Doolan DL, Hoffman SL. The complexity of protective immunity against liver-stage malaria. J Immunol. 2000;165:1453–1462. doi: 10.4049/jimmunol.165.3.1453. [DOI] [PubMed] [Google Scholar]
- 19.Doolan DL, Hoffman SL. IL-12 and NK cells are required for antigen-specific adaptive immunity against malaria initiated by CD8+ T cells in the Plasmodium yoelii model. J Immunol. 1999;163:884–892. [PubMed] [Google Scholar]
- 20.Schofield L, Villaquiran J, Ferreira A, Schellekens H, Nussenzweig R, Nussenzweig V. Gamma interferon, CD8+ T cells and antibodies required for immunity to malaria sporozoites. Nature. 1987;330:664–666. doi: 10.1038/330664a0. [DOI] [PubMed] [Google Scholar]
- 21.Weiss WR, Sedegah M, Berzofsky JA, Hoffman SL. The role of CD4+ T cells in immunity to malaria sporozoites. J Immunol. 1993;151:2690–2698. [PubMed] [Google Scholar]
- 22.Belnoue E, Costa FT, Frankenberg T, et al. Protective T cell immunity against malaria liver stage after vaccination with live sporozoites under chloroquine treatment. J Immunol. 2004;172:2487–2495. doi: 10.4049/jimmunol.172.4.2487. [DOI] [PubMed] [Google Scholar]
- 23.Butler NS, Schmidt NW, Harty JT. Differential effector pathways regulate memory CD8 T cell immunity against Plasmodium berghei versus P. yoelii sporozoites. J Immunol. 2010;184:2528–2538. doi: 10.4049/jimmunol.0903529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pattaradilokrat S, Cheesman SJ, Carter R. Linkage group selection: towards identifying genes controlling strain specific protective immunity in malaria. PLoS ONE. 2007;2:e857. doi: 10.1371/journal.pone.0000857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheesman S, Tanabe K, Sawai H, O’Mahony E, Carter R. Strain-specific immunity may drive adaptive polymorphism in the merozoite surface protein 1 of the rodent malaria parasite Plasmodium chabaudi. Infect Genet Evol. 2009;9:248–255. doi: 10.1016/j.meegid.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 26.Kumar S, Miller LH, Quakyi IA, et al. Cytotoxic T cells specific for the circumsporozoite protein of Plasmodium falciparum. Nature. 1988;334:258–260. doi: 10.1038/334258a0. [DOI] [PubMed] [Google Scholar]
- 27.Zavala F, Cochrane AH, Nardin EH, Nussenzweig RS, Nussenzweig V. Circumsporozoite proteins of malaria parasites contain a single immunodominant region with two or more identical epitopes. J Exp Med. 1983;157:1947–1957. doi: 10.1084/jem.157.6.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mauduit M, Tewari R, Depinay N, et al. Minimal role for the circumsporozoite protein in the induction of sterile immunity by vaccination with live rodent malaria sporozoites. Infect Immun. 2010;78:2182–2188. doi: 10.1128/IAI.01415-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Culleton R, Martinelli A, Hunt P, Carter R. Linkage group selection: rapid gene discovery in malaria parasites. Genome Res. 2005;15:92–97. doi: 10.1101/gr.2866205. [DOI] [PMC free article] [PubMed] [Google Scholar]