Abstract
Objective
The programmed cell death-1 receptor/programmed cell death-1 ligand (PD-1/PD-L1) pathway plays a crucial role in tumor evasion from host immunity. This study was designed to evaluate the association between circulating PD-L1 expression and prognosis in patients with advanced gastric cancer.
Methods
Totally 80 advanced gastric cancer patients and 40 health controls from Beijing Cancer Hospital were enrolled in the present study. Circulating PD-L1 expression was tested by enzyme-linked immunosorbent assay (ELISA). The associations between the expression level of PD-L1 and clinicopathological features and prognosis were analyzed statistically.
Results
Expression of PD-L1 in advanced gastric cancer patients was significantly up-regulated compared with health people (P=0.006). The expression of PD-L1 was significantly correlated with differentiation and lymph node metastasis (P=0.026 and P=0.041, respectively). Although we didn’t find significant difference in all advanced gastric cancer patients with different PD-L1 expression, the adenocarcinoma patients with higher up-regulated PD-L1 expression had much better prognosis than low expression patients (65.6% vs. 44.7%, P=0.028).
Conclusions
PD-L1 was elevated in advance gastric cancer patients and may play an important role in tumor immune evasion and patients prognosis.
Keywords: Programmed cell death-1 ligands (PD-L1), tumor immunity, advanced gastric cancer, enzyme-linked immunosorbent assay (ELISA)
Introduction
Gastric cancer is one of the most common cancers worldwide and has the second leading cancer-related mortality rate with much poorer survival (1,2). Even with advances in diagnosis and therapy, the total prognosis of gastric cancer patients is still poor (3). Cell-mediated immune responses against tumor are either present spontaneously in human cancer patients as a critical component of tumor immune surveillance or can be elicited by cancer vaccination and adoptive T-cell transfer (4-7). Recent evidence showed that experimental depletion of regulatory T cells (Tregs) improves immune-mediated tumor clearance and enhances the response to immune-based therapy (8,9). Tregs have been shown to suppress tumor-specific T cell immunity and therefore may contribute to the progression of human tumors (10,11).
At present, tumor-associated macrophages (TAMs) and T cells are the most commonly used immune cells within the tumor microenvironment for research. The family of T-cell inhibitory receptors (12-16) limits T-cell functions by negatively regulating signals in immune cells (e.g., T cells and natural killer cells), including activating signals mediated by the T cell receptor (TCR) (17). Recently, various molecules have been identified, that can modulate TCR signals, such as the well known CD28 and CTLA-4. Programmed cell death-1 (PD-1), an immunoinhibitory receptor of the CD28 family, which plays a major role in tumor immune escape (18,19), is an inhibitory receptor expressed on the surface of T cells that functions to physiologically limit T-cell activation and proliferation (20). Its ligand, PD-L1 (B7-H1/CD274), is expressed on antigen-presenting cells. The PD-1/PD-L1 pathway is another major receptor-ligand network that functions primarily to provide a coinhibitory signal. Binding of PD-L1 to its receptor inhibits T lymphocyte proliferation and effector functions (cytotoxicity, cytokine release), induces apoptosis of tumor-specific T cells, promotes the differentiation of CD4+ T cells into Foxp3+ Tregs, as well as the resistance of tumor cells to cytotoxic lymphocyte (CTL) attack (21-25). The expression of PD-L1 in tumors has been described in many histological types of carcinoma, such as renal cell carcinoma, breast cancer, pancreatic cancer, ovarian cancer, urothelial cancer, melanoma, esophageal cancer, hepatocellular carcinoma, as well as gastric carcinoma (26-34). Less invasive and more efficient biomarkers are needed for detection and evaluation of gastric cancer by mass screening. The current biomarkers, pepsinogen, carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9 (CA19-9), are not sufficient for accurately predicting gastric cancer (35-37). The aim of this study is to identify circulating PD-L1 level in patients with advanced gastric cancer by using enzyme-linked immunosorbent assay (ELISA) procedures, and evaluate the relation of the PD-L1 expression and clinicopathological features and their prognostic significance.
Materials and methods
Patients
A total of 80 patients with advanced gastric cancer, who were treated at the Department of Gastrointestinal Surgery of Beijing Cancer Hospital from 2006 to 2009, were included in this retrospective analysis, and 40 healthy subjects served as controls. Patients were excluded from this analysis due to incomplete clinical data or inadequate follow-up or inadequate blood sampling. All patients had histologically confirmed carcinoma of the stomach. Staging was based on clinical assessment and histopathological analysis by the International Union Against Cancer (UICC) TNM staging system recommended by the American Joint Committee on Cancer (AJCC) stage (7th edition) and Japanese Gastric Cancer Association (JGCA) guidelines. Clinicopathological characteristics of the patients were collected from our hospital electronic patient records. None of the gastric cancer patients had synchronous cancers, previous gastrointestinal diseases, abdominal surgery, chemotherapy or radiotherapy. All samples were taken from endoscopic biopsy or surgically resected material. Follow-up data of patients were retrieved from hospital records and by contacting the general practitioners. Three of them were lost to follow-up because of unable to contact. The study was approved by the Ethics Committee of Peking University Cancer Hospital. Informed consent was obtained from each patient.
Blood sampling
Blood samples were drawn prior to any treatment. Sera derived from advanced gastric cancer patients and from sex, age-matched controls were collected prior to definitive treatment using a standardized protocol. The serum was obtained by centrifugation (at 3,000 g for 10 min), aliquoted and stored at –80 °C until analysis. Blood collection and analyses were approved by the Ethics Committee of Peking University Cancer Hospital. All samples were analyzed retrospectively, and cases and controls were processed simultaneously. Patient recruitment and sample collection were performed within the guidelines of protocols approved by the institutional review boards. All cases have been analyzed by clinicians.
ELISA assay
Serum samples obtained from 80 advanced gastric cancer patients and 40 health controls were assayed by a commercially available ELISA Kit for PD-L1 (Uscn Life Science Inc.), following the manufacturer’s protocol. Briefly, 96-well plates were incubated with standards at different concentrations, and serum samples were incubated for 2 h at 37 °C. After covering biotinylated antibodies and several aspiration/wash processes, horseradish peroxidase (HRP)-conjugated streptavidin was prepared at 37 °C for 15-25 min, and protected from light. The liquid will turn blue by the addition of substrate solution. Enzymatic reactions were developed and the absorbance was measured at 450 nm (A450) in Bio-Rad Model 680 Microplate Reader immediately (Bio-Rad Laboratories, Inc., California, USA). Protein levels were calculated according to standard curves. For a correct evaluation of the results, parallel investigations were made in healthy non-treated controls.
Statistical analysis
Continuous variables were summarized using x±s and median (range). The χ2-test was used to determine the associations between PD-L1 expression and clinicopathological characteristics. The overall survival time was calculated from the date of gastric cancer treatment to the gastric cancer-specific death or censored at the time of death by other causes or the end of follow-up. The Kaplan-Meier method was used to determine the cumulative probability of survival. The log rank test was used for comparison of cumulative survival rate in the patient group. All the statistical analyses and graphics were performed with the SPSS 20.0 statistical package (SPSS Inc., Chicago, IL, USA). P<0.05 was considered statistically significant.
Results
Circulating level of PD-L1 in advanced gastric cancer patients and health controls
The overall data from the 80 patients and 40 health controls in Beijing Cancer Hospital were analyzed. The serum level of PD-L1 was tested by ELISA. The mean value of serum PD-L1 level in advanced gastric cancer was 0.8928±0.0900 ng/mL, and in health person was 0.5899±0.0617 ng/mL. The expression level of PD-L1 showed significant difference between normal controls and advanced gastric cancer patients (P=0.006). A cut-off value of 0.5993 ng/mL was best distinguished in patients without lymph node metastasis and with lymph node metastasis, which was used as a cut-off value for correlations and survival analysis, and the area under curve (AUC) value was 0.613 [95% confidence interval (95% CI): 0.509-0.717, P=0.044] (Figure 1).
Figure 1.
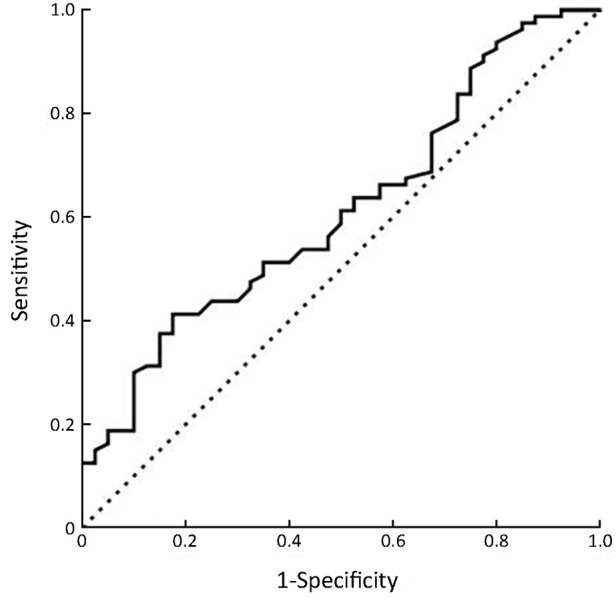
Receiver operating characteristics (ROC) curve for predicting lymph node metastasis for patients with gastric cancer.
Correlations between serum PD-L1 level and characteristics of advanced gastric cancer patients
The advanced gastric cancer cohort includes 62 men and 18 women and the median age was 59 years (range, 30-72 years). Twenty (25.0%) patients were at clinical stage I or II, and 60 (75.0%) were at stage III. Sixty-four (80.0%) patients had lymph node metastasis, and 16 (20.0%) didn’t. A total of 20 patients (25.0%) were well-moderately differentiated, and 60 (75.0%) were poorly differentiated/undifferentiated. The expression of PD-L1 in serum was found to be low up-regulated in 33 (41.2%) of 80 cases, whereas the remaining 47 cases (58.8%) were classified as having highly up-regulated expression. As shown in Table 1, the PD-L1 expression appeared to be significantly associated with differentiation and lymph node metastasis (P=0.026 and P=0.041, respectively) in univariate analysis. There were more patients with differentiated cancer and/or without lymph node metastasis had higher expression of PD-L1 in serum. No significant association was observed between serum PD-L1 level and other clinicopathological variables. Multivariate logistic regression analysis found that the differentiation of advanced gastric cancer was statistically significant with soluble PD-L1 (sPD-L1) expression (RR=0.267, 95% CI: 0.080-0.894, P=0.032).
Table 1. Correlations between serum programmed cell death-1 ligands (PD-L1) level and clinical characteristics of advanced gastric cancer patients.
| Characteristics | PD-L1 |
P | ||
|---|---|---|---|---|
| Low expression (≤0.5993 ng/mL) | High expression (>0.5993 ng/mL) | |||
| Gender | 0.392 | |||
| Male | 24 (38.7%) | 38 (61.3%) | ||
| Female | 9 (50.0%) | 9 (50.0%) | ||
| Age (year) | 0.446 | |||
| <60 | 19 (45.2%) | 23 (54.8%) | ||
| ≥60 | 14 (36.8%) | 24 (63.2%) | ||
| Borrmann type | 0.215 | |||
| I-II | 3 (25.0%) | 9 (75.0%) | ||
| III-IV | 30 (44.1%) | 38 (55.9%) | ||
| Location | 0.741 | |||
| Upper 1/3 | 10 (43.5%) | 13 (56.5%) | ||
| Middle 1/3 | 3 (30.0%) | 7 (70.0%) | ||
| Low 1/3 | 16 (40.0%) | 24 (60.0%) | ||
| Multiple site | 4 (57.1%) | 3 (42.9%) | ||
| Size (mm) | 0.290 | |||
| ≤50 | 15 (35.7%) | 27 (64.3%) | ||
| >50 | 18 (47.4%) | 20 (52.6%) | ||
| Differentiation | 0.026 | |||
| Differentiated | 4 (20.0%) | 16 (80.0%) | ||
| Undifferentiated | 29 (48.3%) | 31 (51.7%) | ||
| Histology | 0.931 | |||
| Adenocarcinoma | 25 (41.0%) | 36 (59.0%) | ||
| Others | 8 (42.1%) | 11 (57.9%) | ||
| Depth of invasion | 0.729 | |||
| T2-3 | 3 (33.3%) | 6 (66.7%) | ||
| T4 | 30 (42.3%) | 41 (57.7%) | ||
| Lymph node metastasis | ||||
| N0 | 3 (18.8%) | 13 (81.2%) | 0.098 | |
| N1 | 5 (27.8%) | 8 (61.5%) | ||
| N2 | 10 (52.6%) | 9 (47.4%) | ||
| N3 | 15 (46.9%) | 17 (53.1%) | ||
| No | 3 (18.8%) | 13 (81.2%) | 0.041 | |
| Yes | 30 (46.9%) | 34 (53.1%) | ||
| TNM stage | 0.183 | |||
| I | 0 (0.0%) | 2 (100.0%) | ||
| II | 6 (33.3%) | 12 (66.7%) | ||
| III | 27 (45.0%) | 33 (55.0%) | ||
Correlation of PD-L1 expression with prognosis of advanced gastric cancer patients
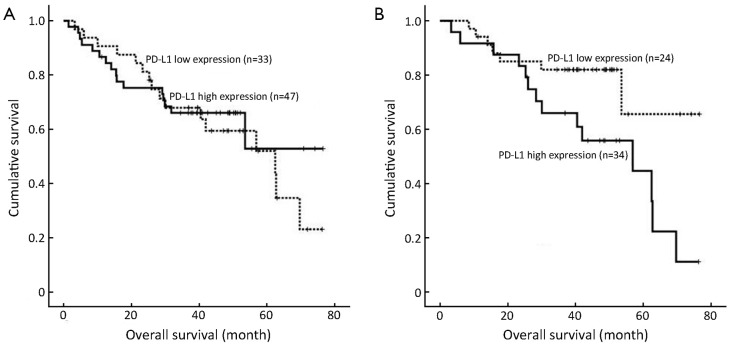
The overall survival rates of advanced gastric cancer patients were statistically estimated with the expression of serum PD-L1. The median follow-up duration since the time of diagnosis was 39.6 months (range, 1.5-76.6 months). The advanced gastric cancer patients with different PD-L1 expression had similar overall survival with no significant differences (P=0.636, Figure 2A). But the overall 5-year survival rate in advanced gastric adenocarcinoma patients with high and low up-regulated PD-L1 expression levels was nearly 65.6% and 44.7%, respectively. The survival difference between these two groups was statistically significant (P=0.028, Figure 2B). We found that there were no significant differences in the serum expression of PD-L1 between the differentiated cancer patients and undifferentiated patients (P>0.05).
Figure 2.
Kaplan-Meier overall survival curves. (A) Advanced gastric cancer patients; (B) Advanced gastric adenocarcinoma patients. Survival analysis was performed according to the expression of serum PD-L1.
Discussion
A lot of studies have shown that immune escape is an active process in which tumor cells and immune cells within the tumor microenvironment actively suppress the antitumor immune response. The most notably Tregs have been shown to suppress tumor-specific T cell immunity and account for tumor progression (38,39). PD-L1 (B7-H1), with its receptor PD-1, plays a critical role in suppressing T cell-based immunity and could mediate tumor immunosuppression (40). PD-1/PD-L1 interactions contribute to the maintenance of peripheral tolerance of self-antigens in normal hosts (41). One in vitro study showed that PD-L1 (B7-H1) specifically interacts with B7-1 to inhibit T and B cell activation and proliferation. Additionally, both B and T cell function can be modulated by engagement of cell-surface PD-L1 (42,43). Different from membrane-bound forms, soluble forms have been found for several members of B7 family (44,45), but no study has been reported soluble PD-L1 expression in gastric cancer patient until now. sPD-L1 may play an important role in the immunoregulation of PD-1/PD-L1 pathway, and could afford distant effect to the activated T cells by the intervention between PD-1/membrane PD-L1 (mPD-L1). The soluble ligands can bind receptors in a similar manner as their membrane-bound counterparts and as a result may play an important role in the regulation of receptor activity. Circulating sPD-L1 remains the biological activity and has the ability of binding to PD-1 receptor. The existence of sPD-L1 in human peripheral blood increases the complexity of PD-1/PD-L1 co-inhibitory signal in immune regulation.
To further explore the existence of sPD-L1 and evaluate the pathological significance of this circulating factor in human cancer serum, we developed this study for the detection and quantification of sPD-L1 in advanced gastric cancer patients. Although the sPD-L1 level in advanced gastric cancer is lower than some earlier reported in other cancers in the present study, but it also indicates that the sPD-L1 expression in advanced gastric cancer was much higher than health controls, which is in agreement with previous findings (46). The patients with differentiated tumor and/or without lymph node metastasis had higher sPD-L1 level. However, despite the lack of statistical significance, there was a lower level of sPD-L1 expression with increasing stage and/or deeper depth of gastric cancer. Therefore, it was considered to be an early event during tumor progression. In line with these findings, Wang et al. observed the similar results in hepatocellular carcinoma tissues (47), although there still have more different cancer observations (48,49). Several studies have shown that the PD-1/PD-L1 pathway plays critical roles in compromised chronic infections and tumor immunity. It is interesting that chronic infections and tumors may have exploited the PD-1 pathway to evade eradication by the immune system. PD-1 is up-regulated upon T cell activation in infection but declines with resolution of the infection and establishment of memory (50). Interactions between PD-L1 and PD-1 are thought to maintain peripheral tolerance. PD-L1/PD-1 has been shown to mediate T-cell exhaustion, an important mechanism underlying T-cell dysfunction during chronic inflammation and chronic infection (51,52). Therefore, the increased level of sPD-L1 occurred in the early staged tumor which was considered to be caused by longlasting chronic infections and may represent an important contribution to immune evasion during tumor progression. Recent reports demonstrated that PD-L1 expressed in tumor cells or peritumoral activated monocytes contributed to tumor aggressiveness and postoperative recurrence in hepatocellular carcinoma patients (53-55).
When we explored in depth the survival rates of patients according to different level of sPD-L1 expression, the most striking finding was that patients with advanced adenocarcinoma with higher sPD-L1 expression had better overall survival although all the patients in this study have similar prognosis. This observation indicates that sPD-L1 may be a novel and unexpected feature of advanced gastric adenocarcinoma, more importantly, and predict certain patients with completely different clinical outcomes. In spite of our findings, the relationship of PD-L1 expression with survival of gastric cancer patients has remained largely debated. Previous studies had indicated that the increase of PD-L1 expression in carcinomas can cause poor clinical prognosis of many tumors, as well as gastric cancer (32,34,35). These controversial results may have been affected by different strategies in selecting the study population and mostly by different kinds of test methods that we detect the PD-L1 in peripheral circulating serum using ELISA unlike other studies of carcinoma tissues. In light of our own findings, sPD-L1 may be a useful marker to define the survival of patients with advanced gastric adenocarcinoma.
This study observed the circulating level of PD-L1 expression in advanced gastric cancer. The potential limitations should be considered that the cohort is small, and we should enlarge the samples in order to confirm the results. Further prospective studies need to monitor the alteration of PD-L1 expression during perioperative period/chemotherapy and follow-up. Despite these limitations, the findings offer an effective and easy method to predict the special clinical feature and outcome of advanced gastric cancer patients.
In conclusion, high up-regulated expression of circulating serum PD-L1 in advanced gastric cancer was correlated with differentiated tumor and without lymph node metastasis. Furthermore, the expression level of sPD-L1 might be also a potential prognostic factor for advanced gastric adenocarcinoma patients.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- 1.Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69-90 [DOI] [PubMed] [Google Scholar]
- 2.Hartgrink HH, Jansen EP, van Grieken NC, et al. Gastric cancer. Lancet 2009;374:477-90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cunningham SC, Kamangar F, Kim MP, et al. Survival after gastric adenocarcinoma resection: eighteen-year experience at a single institution. J Gastrointest Surg 2005;9:718-25 [DOI] [PubMed] [Google Scholar]
- 4.Finn OJ. Cancer immunology. N Engl J Med 2008;358:2704-15 [DOI] [PubMed] [Google Scholar]
- 5.Galon J, Costes A, Sanchez-Cabo F, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 2006;313:1960-4 [DOI] [PubMed] [Google Scholar]
- 6.Sharma P, Shen Y, Wen S, et al. CD8 tumor-infiltrating lymphocytes are predictive of survival in muscle-invasive urothelial carcinoma. Proc Natl Acad Sci USA 2007;104:3967-72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee HE, Chae SW, Lee YJ, et al. Prognostic implications of type and density of tumour-infiltrating lymphocytes in gastric cancer. Br J Cancer 2008;99:1704-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li X, Kostareli E, Suffner J, et al. Efficient Treg depletion induces T-cell infiltration and rejection of large tumors. Eur J Immunol 2010;40:3325-35 [DOI] [PubMed] [Google Scholar]
- 9.Poehlein CH, Haley DP, Walker EB, et al. Depletion of tumor-induced Treg prior to reconstitution rescues enhanced priming of tumor-specific, therapeutic effector T cells in lymphopenic hosts. Eur J Immunol 2009;39:3121-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yuan XL, Chen L, Li MX, et al. Elevated expression of Foxp3 in tumor-infiltrating Treg cells suppresses T-cell proliferation and contributes to gastric cancer progression in a COX-2-depedent manner. Clin Immunol 2010;134:277-88 [DOI] [PubMed] [Google Scholar]
- 11.Antony PA, Piccirillo CA, Akpinarli A, et al. CD8+ T cell immunity against a tumor/self-antigen is augmented by CD4+ T helper cells and hindered by naturally occurring T regulatory cells. J Immunol 2005;174:2591-601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ahmadzadeh M, Johnson LA, Heemskerk B, et al. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood 2009;114:1537-44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chapon M, Randriamampita C, Maubec E, et al. Progressive upregulation of PD-1 in primary and metastatic melanomas associated with blunted TCR signaling in infiltrating T lymphocytes. J Invest Dermatol 2011;131:1300-7 [DOI] [PubMed] [Google Scholar]
- 14.Fourcade J, Sun Z, Benallaoua M, et al. Upregulation of Tim-3 and PD-1 expression is associated with tumor antigen-specific CD8+ T cell dysfunction in melanoma patients. J Exp Med 2010;207:2175-86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matsuzaki J, Gnjatic S, Mhawech Fauceglia P, et al. Tumor-infiltrating NY-ESO-1-specific CD8+ T cells are negatively regulated by LAG-3 and PD-1 in human ovarian cancer. Proc Natl Acad Sci USA 2010;107:7875-80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sakuishi K, Apetoh L, Sullivan JM, et al. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J Exp Med 2010;207:2187-94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vazquez-Cintron EJ, Monu NR, Frey AB. Tumor-induced disruption of proximal TCR-mediated signal transduction in tumor-infiltrating CD8+ lymphocytes inactivates antitumor effector phase. J Immunol 2010;185:7133-40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Okazaki T, Honjo T.The PD-1-PD-L pathway in immunological tolerance. Trends Immunol 2006;27:195-201 [DOI] [PubMed] [Google Scholar]
- 19.Keir ME, Liang SC, Guleria I, et al. Tissue expression of PD-L1 mediates peripheral T cell tolerance. J Exp Med 2006;203:883-95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Keir ME, Butte MJ, Freeman GJ, et al. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol 2008;26:677-704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tseng SY, Otsuji M, Gorski K, et al. B7-DC, a new dendritic cell molecule with potent costimulatory properties for T cells. J Exp Med 2001;193:839-46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dong H, Strome SE, Salomao DR, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med 2002;8:793-800 [DOI] [PubMed] [Google Scholar]
- 23.Wang L, Pino-Lagos K, de Vries VC, et al. Programmed death 1 ligand signaling regulates the generation of adaptive Foxp3+CD4+ regulatory T cells. Proc Natl Acad Sci U S A 2008;105:9331-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iwai Y, Ishida M, Tanaka Y, et al. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci U S A 2002;99:12293-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsushima F, Yao S, Shin T, et al. Interaction between B7-H1 and PD-1 determines initiation and reversal of T-cell anergy. Blood 2007;110:180-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thompson RH, Dong H, Kwon ED. Implications of B7-H1 expression in clear cell carcinoma of the kidney for prognostication and therapy. Clin Cancer Res 2007;13:709s-15s [DOI] [PubMed] [Google Scholar]
- 27.Ghebeh H, Mohammed S, Al Omair A, et al. The B7-H1 (PD-L1) T lymphocyte-inhibitory molecule is expressed in breast cancer patients with infiltrating ductal carcinoma: correlation with important high-risk prognostic factors. Neoplasia 2006;8:190-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nomi T, Sho M, Akahori T, et al. Clinical significance and therapeutic potential of the programmed death-1 ligand/programmed death-1 pathway in human pancreatic cancer. Clin Cancer Res 2007;13:2151-7 [DOI] [PubMed] [Google Scholar]
- 29.Hamanishi J, Mandai M, Iwasaki M, et al. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc Natl Acad Sci U S A 2007;104:3360-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakanishi J, Wada Y, Matsumoto K, et al. Overexpression of B7-H1 (PD-L1) significantly associates with tumor grade and postoperative prognosis in human urothelial cancers. Cancer Immunol Immunother 2007;56:1173-82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hino R, Kabashima K, Kato Y, et al. Tumor cell expression of programmed cell death-1 ligand 1 is a prognostic factor for malignant melanoma. Cancer 2010;116:1757-66 [DOI] [PubMed] [Google Scholar]
- 32.Ohigashi Y, Sho M, Yamada Y, et al. Clinical significance of programmed death-1 ligand-1 and programmed death-1 ligand-2 expression in human esophageal cancer. Clin Cancer Res 2005;11:2947-53 [DOI] [PubMed] [Google Scholar]
- 33.Gao Q, Wang XY, Qiu SJ, et al. Overexpression of PD-L1 significantly associates with tumor aggressiveness and postoperative recurrence in human hepatocellular carcinoma. Clin Cancer Res 2009;15:971-9 [DOI] [PubMed] [Google Scholar]
- 34.Wu C, Zhu Y, Jiang J, et al. Immunohistochemical localization of programmed death-1 ligand-1 (PD-L1) in gastric carcinoma and its clinical significance. Acta Histochem 2006;108:19-24 [DOI] [PubMed] [Google Scholar]
- 35.Duraker N, Naci CA, Gencler N. The prognostic significance of gastric juice CA 19-9 and CEA levels in gastric carcinoma patients. Eur J Surg Oncol 2002;28:844-9 [DOI] [PubMed] [Google Scholar]
- 36.Miki K, Morita M, Sasajima M, et al. Usefulness of gastric cancer screening using the serum pepsinogen test method. Am J Gastroenterol 2003;98:735-9 [DOI] [PubMed] [Google Scholar]
- 37.Miki K.Gastric cancer screening using the serum pepsinogen test method. Gastric Cancer 2006;9:245-53 [DOI] [PubMed] [Google Scholar]
- 38.Rabinovich GA, Gabrilovich D, Sotomayor EM. Immunosuppressive strategies that are mediated by tumor cells. Annu Rev Immunol 2007;25:267-96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol 2009;9:162-74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nishimura H, Okazaki T, Tanaka Y, et al. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science 2001;291:319-22 [DOI] [PubMed] [Google Scholar]
- 41.Keir ME, Freeman GJ, Sharpe AH. PD-1 regulates self-reactive CD8+ T cell responses to antigen in lymph nodes and tissues. J Immunol 2007;179:5064-70 [DOI] [PubMed] [Google Scholar]
- 42.Butte MJ, Keir ME, Phamduy TB, et al. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity 2007;27:111-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Park JJ, Omiya R, Matsumura Y, et al. B7-H1/CD80 interaction is required for the induction and maintenance of peripheral T cell tolerance. Blood 2010;116:1291-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Simon I, Zhuo S, Corral L, et al. B7-H4 is a novel membrane-bound protein and a candidate serum and tissue biomarker for ovarian cancer. Cancer Res 2006;66:1570-5 [DOI] [PubMed] [Google Scholar]
- 45.Oaks MK, Hallett KM. Cutting edge: a soluble form of CTLA-4 in patients with autoimmune thyroid disease. J Immunol 2000;164:5015-8 [DOI] [PubMed] [Google Scholar]
- 46.Xing YF, Zhang ZL, Shi MH, et al. The level of soluble programmed death-1 in peripheral blood of patients with lung cancer and its clinical implications. Zhonghua Jie He He Hu Xi Za Zhi (in Chinese)2012;35:102-6 [PubMed] [Google Scholar]
- 47.Wang BJ, Bao JJ, Wang JZ, et al. Immunostaining of PD-1/PD-Ls in liver tissues of patients with hepatitis and hepatocellular carcinoma. World J Gastroenterol 2011;17:3322-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zeng Z, Shi F, Zhou L, et al. Upregulation of circulating PD-L1/PD-1 is associated with poor post-cryoablation prognosis in patients with HBV-related hepatocellular carcinoma. PLoS One 2011;6:e23621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thompson RH, Kuntz SM, Leibovich BC, et al. Tumor B7-H1 is associated with poor prognosis in renal cell carcinoma patients with long-term follow-up. Cancer Res 2006,66:3381-5 [DOI] [PubMed] [Google Scholar]
- 50.Barber DL, Wherry EJ, Masopust D, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 2006;439:682-7 [DOI] [PubMed] [Google Scholar]
- 51.Kim PS, Ahmed R. Features of responding T cells in cancer and chronic infection. Curr Opin Immunol 2010;22:223-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shin H, Wherry EJ. CD8 T cell dysfunction during chronic viral infection. Curr Opin Immunol 2007;19:408-15 [DOI] [PubMed] [Google Scholar]
- 53.Gao Q, Wang XY, Qiu SJ, et al. Overexpression of PD-L1 significantly associates with tumor aggressiveness and postoperative recurrence in human hepatocellular carcinoma. Clin Cancer Res 2009;15:971-9 [DOI] [PubMed] [Google Scholar]
- 54.Kuang DM, Zhao Q, Peng C, et al. Activated monocytes in peritumoral stroma of hepatocellular carcinoma foster immune privilege and disease progression through PD-L1. J Exp Med 2009;206:1327-37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Blank C, Gajewski TF, Mackensen A. Interaction of PD-L1 on tumor cells with PD-1 on tumor-specific T cells as a mechanism of immune evasion: implications for tumor immunotherapy. Cancer Immunol Immunother 2005;54:307-14 [DOI] [PMC free article] [PubMed] [Google Scholar]