Abstract
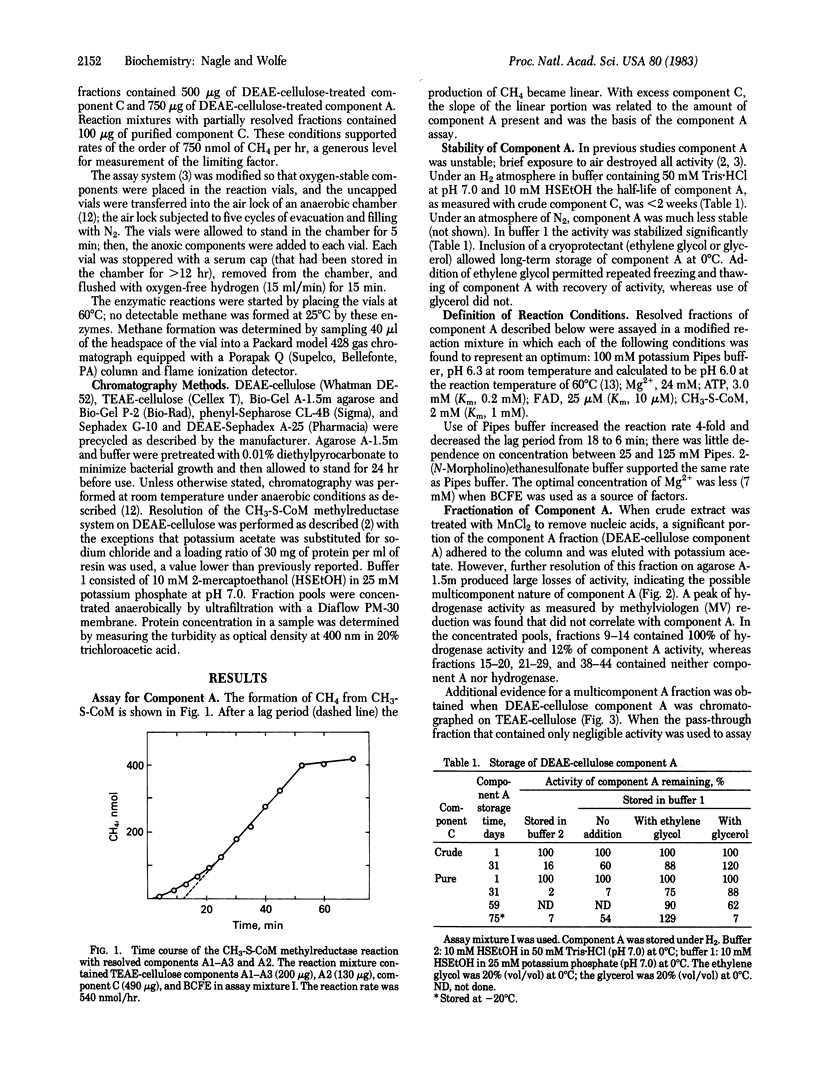
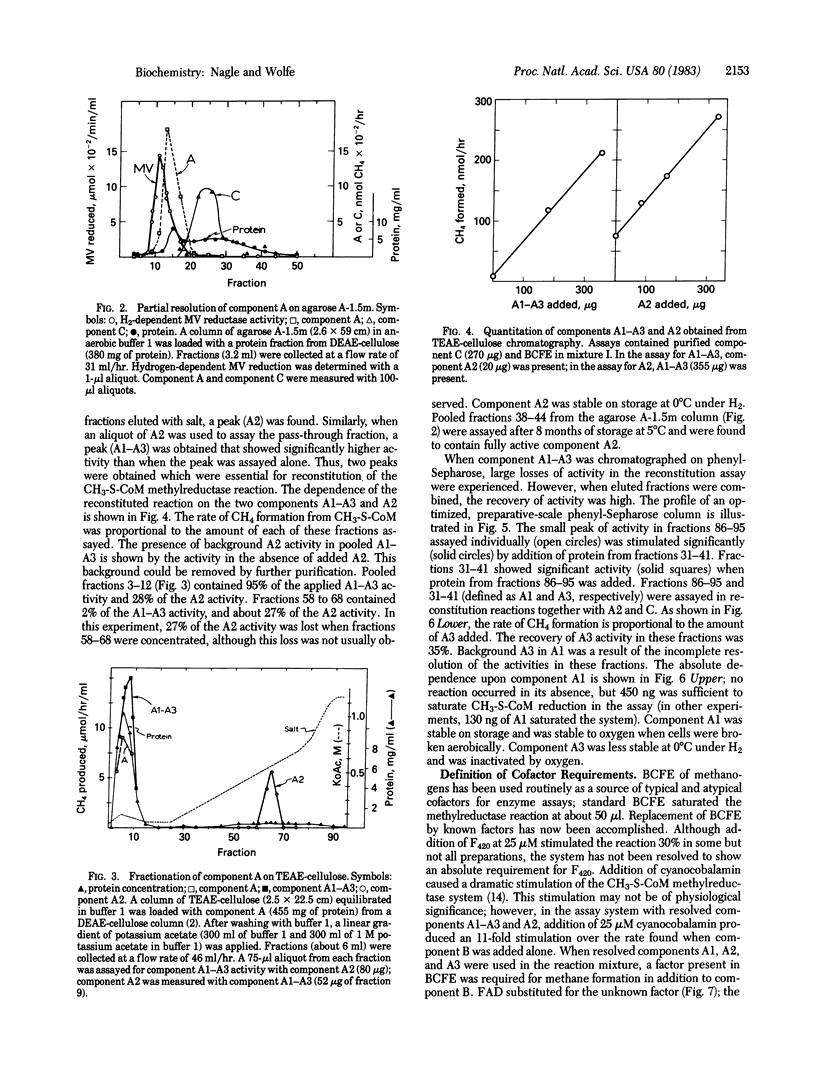
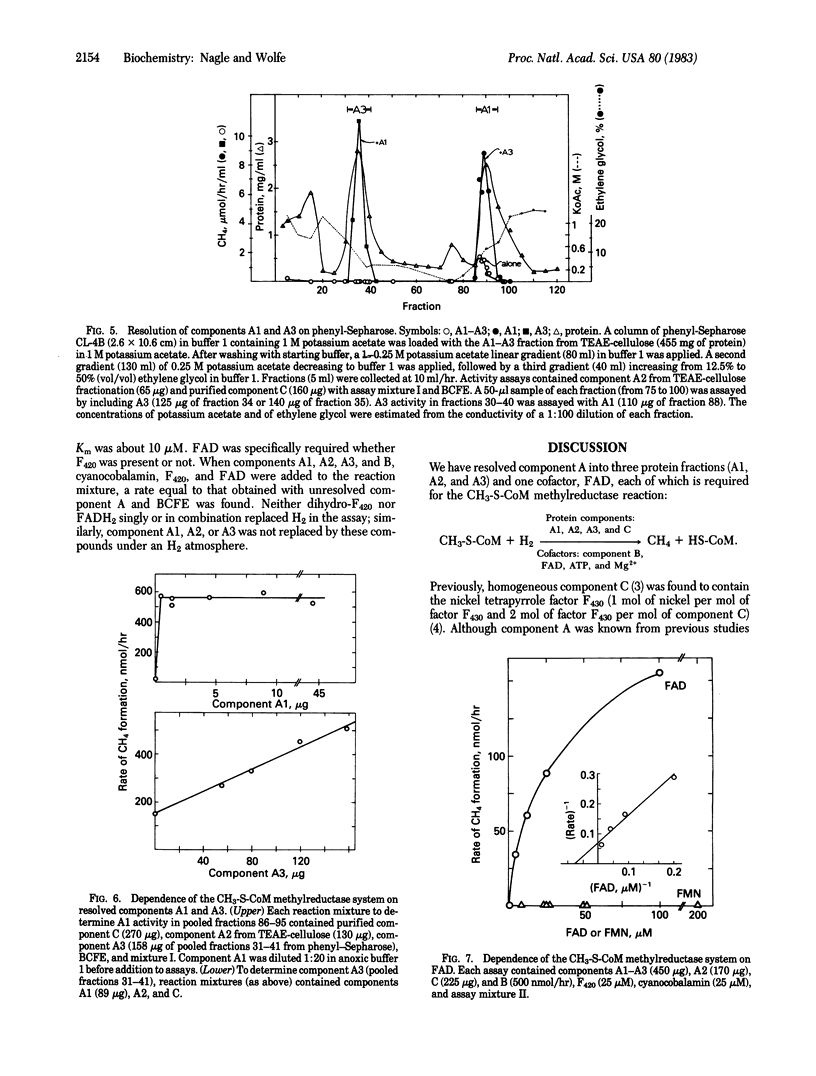
Component A, the oxygen-sensitive protein fraction of the methyl coenzyme M methylreductase system of Methanobacterium thermoautotrophicum, has been stabilized and resolved into three protein fractions and one cofactor that are required to reconstitute component A activity. Component A1 is oxygen-stable and contains hydrogen-dependent deazaflavin (coenzyme F420)-reducing activity. Component A2 is acidic; components A2 and A3 are oxygen sensitive. The specific functions of each component in methyl group reduction are unknown. Resolution of component A revealed a new cofactor requirement of the methylreductase system for FAD. Hydrogen-dependent reduction of methyl coenzyme M to methane and coenzyme M, the terminal step of CO2 reduction by methanogenic bacteria, requires protein components A1, A2, A3, and C in addition to component B, FAD, ATP, and Mg2+.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balch W. E., Fox G. E., Magrum L. J., Woese C. R., Wolfe R. S. Methanogens: reevaluation of a unique biological group. Microbiol Rev. 1979 Jun;43(2):260–296. doi: 10.1128/mr.43.2.260-296.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheeseman P., Toms-Wood A., Wolfe R. S. Isolation and properties of a fluorescent compound, factor 420 , from Methanobacterium strain M.o.H. J Bacteriol. 1972 Oct;112(1):527–531. doi: 10.1128/jb.112.1.527-531.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eirich L. D., Vogels G. D., Wolfe R. S. Distribution of coenzyme F420 and properties of its hydrolytic fragments. J Bacteriol. 1979 Oct;140(1):20–27. doi: 10.1128/jb.140.1.20-27.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellefson W. L., Whitman W. B., Wolfe R. S. Nickel-containing factor F430: chromophore of the methylreductase of Methanobacterium. Proc Natl Acad Sci U S A. 1982 Jun;79(12):3707–3710. doi: 10.1073/pnas.79.12.3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellefson W. L., Wolfe R. S. Component C of the methylreductase system of Methanobacterium. J Biol Chem. 1981 May 10;256(9):4259–4262. [PubMed] [Google Scholar]
- Ellefson W. L., Wolfe R. S. Role of component C in the methylreductase system of Methanobacterium. J Biol Chem. 1980 Sep 25;255(18):8388–8389. [PubMed] [Google Scholar]
- Good N. E., Winget G. D., Winter W., Connolly T. N., Izawa S., Singh R. M. Hydrogen ion buffers for biological research. Biochemistry. 1966 Feb;5(2):467–477. doi: 10.1021/bi00866a011. [DOI] [PubMed] [Google Scholar]
- Gunsalus R. P., Romesser J. A., Wolfe R. S. Preparation of coenzyme M analogues and their activity in the methyl coenzyme M reductase system of Methanobacterium thermoautotrophicum. Biochemistry. 1978 Jun 13;17(12):2374–2377. doi: 10.1021/bi00605a019. [DOI] [PubMed] [Google Scholar]
- Gunsalus R. P., Tandon S. M., Wolfe R. S. A procedure for anaerobic column chromatography employing an anaerobic Freter-type chamber. Anal Biochem. 1980 Jan 15;101(2):327–331. doi: 10.1016/0003-2697(80)90195-5. [DOI] [PubMed] [Google Scholar]
- Gunsalus R. P., Wolfe R. S. Methyl coenzyme M reductase from Methanobacterium thermoautotrophicum. Resolution and properties of the components. J Biol Chem. 1980 Mar 10;255(5):1891–1895. [PubMed] [Google Scholar]
- Jacobson F. S., Daniels L., Fox J. A., Walsh C. T., Orme-Johnson W. H. Purification and properties of an 8-hydroxy-5-deazaflavin-reducing hydrogenase from Methanobacterium thermoautotrophicum. J Biol Chem. 1982 Apr 10;257(7):3385–3388. [PubMed] [Google Scholar]
- Keltjens J. T., Whitman W. B., Caerteling C. G., van Kooten A. M., Wolfe R. S., Vogels G. D. Presence of coenzyme M derivatives in the prosthetic group (coenzyme MF430) of methylcoenzyme M reductase from Methanobacterium thermoautotrophicum. Biochem Biophys Res Commun. 1982 Sep 30;108(2):495–503. doi: 10.1016/0006-291x(82)90856-7. [DOI] [PubMed] [Google Scholar]
- Lancaster J. R., Jr Membrane-bound flavin adenine dinucleotide in Methanobacterium Bryantii. Biochem Biophys Res Commun. 1981 May 15;100(1):240–246. doi: 10.1016/s0006-291x(81)80088-5. [DOI] [PubMed] [Google Scholar]
- McKellar R. C., Sprott G. D. Solubilization and properties of a particulate hydrogenase from Methanobacterium strain G2R. J Bacteriol. 1979 Jul;139(1):231–238. doi: 10.1128/jb.139.1.231-238.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer R., Fisher J., Walsh C. Preparation, characterization, and chemical properties of the flavin coenzyme analogues 5-deazariboflavin, 5-deazariboflavin 5'-phosphate, and 5-deazariboflavin 5'-diphosphate, 5'leads to5'-adenosine ester. Biochemistry. 1976 Mar 9;15(5):1043–1053. doi: 10.1021/bi00650a015. [DOI] [PubMed] [Google Scholar]
- Yamazaki S. A selenium-containing hydrogenase from Methanococcus vannielii. Identification of the selenium moiety as a selenocysteine residue. J Biol Chem. 1982 Jul 25;257(14):7926–7929. [PubMed] [Google Scholar]



