Abstract
PURPOSE
The CAV1/CAV2 (caveolin 1 and 2) genomic region has been previously associated with primary open angle glaucoma (POAG), although replication among independent studies has been variable. The aim of this study is to assess the association between CAV1/CAV2 single nucleotide polymorphisms (SNPs) and POAG in a large case-control dataset and to further explore associations by gender and pattern of visual field (VF) loss.
DESIGN
case-control study
PARTICIPANTS
We analyzed two large POAG datasets, the Glaucoma Genes and Environment (GLAUGEN) study (976 cases, 1140 controls) and the National Eye Institute Glaucoma Human Genetics Collaboration (NEIGHBOR) consortium (2132 cases, 2290 controls).
METHODS
We studied the association between 70 SNPs located within the CAV1/CAV2 genomic region in GLAUGEN and NEIGHBOR, both genotyped on the Illumina Human 660WQuadv1C BeadChip array and imputed with MACH using the HapMap 3 reference panel. We used logistic regression models of POAG in the overall population and separated by gender, as well as by POAG subtypes defined by type of visual field defect (peripheral or paracentral). Results from GLAUGEN and NEIGHBOR were meta-analyzed and a Bonferroni corrected significance level of 7.7×10−4 was used to account for multiple comparisons.
MAIN OUTCOME MEASURES
Overall POAG, overall POAG by gender and POAG subtypes defined by pattern of initial visual field loss.
RESULTS
We found significant associations between ten CAV1/CAV2 SNPs and POAG (top SNP rs4236601, pooled p=2.61×10−7). Of these, nine were significant only in women (top SNP rs4236601, pooled p=1.59×10−5). Five of the ten CAV1/CAV2 SNPs were associated with POAG with paracentral VF loss only (top SNP rs17588172, pooled p=1.07×10−4), and none of the ten was associated with POAG with peripheral VF loss only or POAG among men.
CONCLUSIONS
CAV1/CAV2 SNPs were significantly associated with POAG overall, particularly among women. Furthermore, we found an association between CAV1/CAV2 SNPs and POAG with paracentral visual field defects. These data support a role for caveolins 1 and/or 2 in POAG and suggest that the caveolins may particularly affect POAG pathogenesis in women and in patients with initial paracentral visual field defects.
Introduction
Primary open-angle glaucoma (POAG) is a leading cause of blindness worldwide, affecting over 35 million people.1, 2 POAG is characterized by retinal ganglion cell death and defects in the visual field that ultimately cause functional visual loss.1 POAG has a genetic component, with contributions from both rare, highly penetrant alleles (MYOC, OPTN)3, 4 and common risk alleles with smaller effects (CAV1/CAV2, TMCO1, SIX1/SIX6, CDKN2BAS, and 8q22).5–8 The genomic region that includes CAV1 and CAV2 was initially identified in a genome-wide association study (GWAS) using cases and controls from Iceland.8 Significant associations in this region were also observed in the Glaucoma Genes and Environment (GLAUGEN) study using a sample consisting of 976 cases and 1140 controls.9 However, three other smaller studies including 545 cases and 297 controls from Iowa,10 220 cases and 405 controls from Saudi Arabia11 and 272 cases and 165 controls from Barbados,12 have not replicated the overall association between CAV1/CAV2 single nucleotide polymorphisms (SNPs) and POAG. This is likely due to modest associations that necessitate large sample sizes for detection.
CAV1 and CAV2 code for caveolin 1 and caveolin 2, which are members of the caveolin protein family. These proteins inhibit endothelial nitric oxide synthase (eNOS, coded by the gene NOS3, or nitric oxide synthase) activity within the caveolae, which are specialized invaginations of the plasma membrane that are especially prevalent in endothelial plasma membranes.13 This interaction alters nitric oxide generation and hence may lead to changes in vascular tone14, 15 and trabecular meshwork function,16 both of which have been implicated in POAG pathogenesis.17
Estrogen receptors are expressed in retinal ganglion cells,18 and estrogen is neuroprotective in animal models of POAG9, 19. Higher estrogen levels affect the expression of NOS320 leading to increased nitric oxide production, which may be protective against POAG.13, 21 Our group reported gene-environment interactions between NOS3 single nucleotide polymorphisms (SNPs) and post-menopausal hormone use with high tension POAG22 and between age at menarche and NOS3 SNPs with overall POAG.23 As endothelial nitric oxide synthase (NOS3) directly interacts with caveolin 1 (CAV1),24 there is a strong rationale to assess the impact of gender on the association of CAV1/CAV2 genomic variations with POAG.
The interaction between caveolin 1 and eNOS in the caveolae of the plasma membranes suggests that the CAV1/CAV2 genomic region SNPs may be associated with the POAG clinical subgroups that exhibit systemic vascular dysregulation. Several clinical parameters have been observed with higher frequency in POAG cases exhibiting systemic vascular dysregulation including paracentral visual field loss and disc hemorrhages.25 Furthermore, emerging evidence suggests that enzymes that influence vascular physiology, such as soluble guanylyl cyclase (sGC) are associated with initial paracentral loss in POAG patients. Buys et al. demonstrated that sGC knockout mice, which have defective nitric oxide signaling, develop open angle glaucoma and that variants in the genomic region containing genes for the α1 and β1 subunits of soluble guanylate cyclase are associated with paracentral visual field loss in women.26 Since the caveolins are an integral part of the nitric oxide signaling pathway, there is interest in whether the CAV1/CAV2 genomic region SNPs associated with POAG are also associated with the POAG subgroup that is defined by initial paracentral visual loss.
In this study, we investigate the association between SNPs located in the CAV1/CAV2 genomic region and overall POAG, as well as overall POAG separately by gender and by POAG subgroups defined by pattern of initial visual field loss.
Methods
Study populations
We used two POAG case-control groups in this study: the Glaucoma Genes and Environment (GLAUGEN) study, and the National Eye Institute Glaucoma Human Genetics Collaboration (NEIGHBOR) study. The GLAUGEN study (976 cases, 1140 controls) consists of two longitudinal cohort studies, the Nurses’ Health Study (NHS), and the Health Professionals Follow-Up Study (HPFS) and one clinic based study from the Massachusetts Eye and Ear Infirmary, the Genetic Etiologies of Primary Open-Angle Glaucoma study (GEP). The NEIGHBOR study (2132 cases, 2290 controls) consists of clinic-based case-control studies from twelve sites across the United States. Details of these studies, along with inclusion criteria, have been published previously.9, 27 The institutional review boards of the Massachusetts Eye and Ear Infirmary, Harvard School of Public Health, the Brigham and Women’s Hospital, University of Pittsburgh, Johns Hopkins University, Duke University, University of West Virginia, University of Miami, University of Michigan, Stanford University, Marshfield Clinic, and the University of California, San Diego approved this study. Informed consent was obtained from all participants.
Case and control definition
Definitions for POAG cases and controls have been previously described.27 Briefly, cases had visual field defects consistent with nerve fiber layer pathology occurring in the setting of a slit lamp biomicroscopic exam that did not reveal any significant findings (aside from possible media opacities) and open angles, regardless of intraocular pressure (IOP). Visual field (VF) loss was either reproduced on a subsequent test, with the same region of the visual field exhibiting VF loss on both visual field reports, and if it was not, there were signs suggestive of glaucomatous cupping as indicated by a cup to disc ratio (CDR)>0.7. Controls were under ophthalmic surveillance, with an eye exam within the last two years indicating CDR<0.6 and IOP<22.
Visual Field scoring
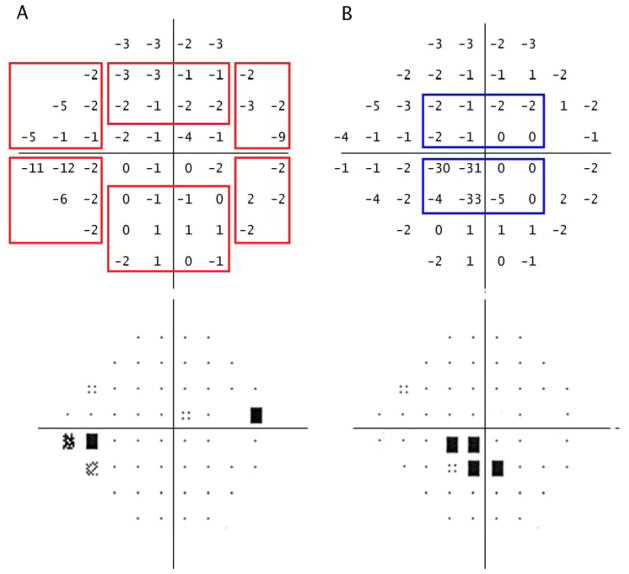
For each participant, we obtained the earliest available reliable VF that demonstrated defects consistent with nerve fiber layer pathology. Most VFs were performed with Humphrey Visual Field Analyzers (Carl Zeis, Dublin, CA) (>70%) although other types of parametric data derived from perimeters such as the Dicon Perimeter (Vision Systems, inc.; Taron Springs FL) or Octopus Perimeter (Haag-Streit; Bern, Switzerland) were used if no Humphrey VFs were available. Reliable VFs were defined based on having fixation loss ≤ 33%, false positive rates ≤ 20%, and false negative rates ≤ 20%. Regardless of VF type, each VF underwent systematic review whereby the pattern deviation plot (PD) was subdivided into paracentral, Bjerrum, nasal step and temporal wedge zones above and below the horizontal meridian (Figure 1). We examined these regions for clusters of three or more contiguous points with retinal sensitivity depression of one half log unit (−5 dB) relative to age-matched controls. Fields with isolated loss in the paracentral zone only without loss in other zones were labeled as paracentral loss cases. If the other zones were involved without loss in the paracentral zone, then the case was categorized as having only peripheral loss. Patients with both paracentral and peripheral VF loss were considered to have advanced functional deficits and were not included in secondary analysis based on type of visual field loss. Two reviewers assessed the VFs masked to genotype status and any differences were adjudicated to arrive at a consensus designation.
Figure 1.
Paracentral and peripheral visual field loss definitions.
Representative grey scale and pattern deviation plot for peripheral visual field loss (A) and paracentral visual field loss (B) in two right eyes.
A. The red boxes indicate each possible peripheral visual field loss region, including inferior and superior nasal steps, temporal wedge and Bjerrum regions. A cluster of three or more points with sensitivity of −5 decibels (dB) or greater in any of these regions represents peripheral visual field loss. In this case, there is visual field loss in the inferior nasal step zone.
B. The blue boxes indicate the superior and inferior paracentral visual field loss regions. A cluster of three or more points with sensitivity of −5 dB or greater in either of these regions represents paracentral visual field loss. In this case, there is visual field loss in the inferior paracentral region. There is overlap between the paracentral zone and Bjerrum areas consisting of the second row of points. In order for the Bjerrrum area to be considered to be involved there must be at least one point in the third row from the top or bottom that has a retinal sensitivity of −5 dB or more.
This figure illustrates the approach used for Humphrey visual fields. Subjects with other types of perimetric data (such as the Dicon or Octopus visual fields) were included and a similar strategy was used to grade the equivalent of the pattern deviation plot. Less than 1% of visual fields were kinetic tests and they were excluded from analyses related to pattern of field loss.
Genotyping and Imputation
We used the Illumina Human 660WQuadv1C BeadChip array (Illumina; San Diego, CA) to genotype all samples. Genotyping for GLAUGEN study participants occurred at the Broad Institute (Cambridge, MA), while genotyping for NEIGHBOR consortium participants was performed at the Center for Inherited Disease Research (Baltimore, MD). Details regarding quality control and data cleaning steps have been described previously.6 All data have been imputed with MACH (University of Michigan Center for Statistical Genetics, Available at: http://www.sph.umich.edu/csg/abecasis/MACH. Accessed Sept 9, 2012.) to the HapMap 3 reference panel.
SNP selection
All SNPs within 50kb upstream of CAV2, in and between CAV2 and CAV1, and within 50kb downstream of CAV1 were selected using the UCSC Genome Browser Table Browser tool (Feb 2009 CRCh37/hg19 assembly, Common SNPs(137) track; UCSC Genome Browser, Available at: www.genome.ucsc.edu. Accessed Sept 9, 2012). Subsequently we used the SNAP proxy search application (CEU population panel, distance limit of 500 bp, using a combination of 1000 Genomes Pilot 1, HapMap 22 and HapMap3 to maximize number of included SNPs; Broad Institute, Available at: http://www.broadinstitute.org/mpg/snap/ldsearch.php. Accessed Sept 9, 2012) to obtain a list of SNPs in strong linkage disequilibrium (LD) (R2≥0.8) with the selected SNPs. Of these, 70 SNPs were in both the GLAUGEN and NEIGHBOR datasets, and were evaluated in these analyses. The genomic locations of the SNPs included in this study are shown in Figure 2.
Figure 2. CAV1/CAV2 genomic region.
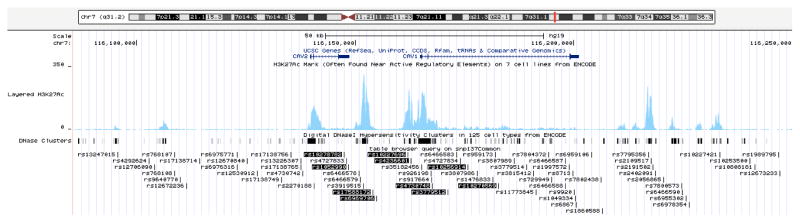
The CAV1/CAV2 genomic region including all single nucleotide polymorphisms (SNPs) examined in this study and the nine significantly associated SNPs are shown using the UCSC genome browser CAV1/CAV2 region (http://genome.ucsc.edu, Accessed April 2, 2013). SNPs significant overall are highlighted in black. H3K27Ac histone marks in human umbilical vascular endothelial cells (HUVEC) (typically found in genomic regions with regulatory activity) are indicated by the blue peaks. DNaseI hypersensitivity sites are represented by black rectangles.
Statistical analysis
Logistic regression was performed separately in GLAUGEN and NEIGHBOR (lambda inflation factor=1.009, 1.034, respectively)6 using ProbABEL (Erasmus University Medical Center, Available at: http://www.genabel.org/packages/ProbABEL. Accessed Sept 9, 2012). Subsequently, the results were pooled using the inverse weighted variance method based on regression coefficients and standard errors using the program METAL (University of Michigan Center for Statistical Genetics, Available at: http://www.sph.umich.edu/csg/abecasis/metal. Accessed Sept 9, 2012.) with the GENOMICCONTROL option on to correct for any residual population stratification or relatedness. In the GLAUGEN sample, we controlled for age, DNA source (blood or cheek), gender, site, method of extraction, and three principal components that adjust for population stratification. In the NEIGHBOR sample, we controlled for age, gender, site and two principal components. We performed an assessment for heterogeneity prior to combining data from the two studies. The analyses were first run using all participants, and then analyses were performed using men only and women only, as well as cases with only paracentral VF loss (no involvement of the temporal wedge region, Bjerrum areas and nasal step zones in either eye) and cases with only peripheral VF loss (only involvement of the temporal wedge region, Bjerrum areas and/or nasal step zones in either eye) vs. controls. We implemented Bonferroni correction to account for multiple comparisons based on number of LD blocks and number of analyses. Sixty-five of the 70 SNPs analyzed fell into one of 8 LD blocks. Five SNPs were not in LD with any other SNPs (Figure 3 available at http://aaojournal.org). We corrected for the 8 LD blocks, 5 independent SNPs and the five analyses outcomes (by POAG overall, among women only, among men only, by paracentral VF loss, and by peripheral VF loss) to obtain a significance level of 7.7×10−4 (13 LD blocks × 5 analyses=65; 0.05/65=7.7×10−4)28.
Results
The mean age of participants in GLAUGEN and NEIGHBOR was similar, although participants in NEIGHBOR were slightly older (Table 1). Cases in the NEIGHBOR consortium had lower IOP at study entry than cases in the GLAUGEN study (16.1 mm Hg vs. 18.0 mm Hg), higher CDR (0.76 vs. 0.67), higher pattern standard deviation on the earliest visual field (PSD) (6.67 dB vs. 5.62 dB) and more depressed mean defect on the earliest visual field (MD) (−8.38 dB vs. −5.83 dB). Females comprised 58% of GLAUGEN cases, and 52% of NEIGHBOR cases. For NEIGHBOR, 2% of cases had only paracentral initial visual field loss, while in GLAUGEN 18% of cases had only paracentral initial VF loss.
Table 1.
| GLAUGEN1 | NEIGHBOR1 | |||
|---|---|---|---|---|
| Variable1 | Cases | Controls | Cases | Controls |
| N | 976 | 1140 | 2132 | 2290 |
| Age (years), mean (SD) | 63.6 (9.8) | 65.5 (9.2) | 66.6 (13.7) | 68.9 (11.4) |
| IOP (mm Hg), mean (SD)2 | 18.0 (5.6) | n/a | 16.1 (6.0) | n/a |
| CDR, mean (SD)2 | 0.67 (0.19) | n/a | 0.76 (0.15) | n/a |
| MD, mean (SD)2, 3 | −5.83 (4.94) | n/a | −8.38 (6.72) | n/a |
| PSD, mean (SD)2, 3 | 5.62 (3.05) | n/a | 6.67 (3.50) | n/a |
| % Female | 58% | 60% | 52% | 55% |
| % Cases with only paracentral VF loss | 18% | n/a | 2% | n/a |
| % Cases with only peripheral VF loss | 52% | n/a | 23% | n/a |
Abbreviations: GLAUGEN=Glaucoma Genes and Environment, NEIGHBOR=National Eye Institute Glaucoma Human Genetics Collaboration, IOP=intraocular pressure, CDR=vertical cup to disc ratio, MD=mean deviation, PSD=pattern standard deviation, VF=visual field, SD=standard deviation, n/a=not available
Means are mean of both eyes.
These statistics are based on Humphrey Visual Field Analyzer data available for 859 GLAUGEN participants and 1369 NEIGHBOR consortium participants. Missing data reflects the fact that some participants had visual field tests other than Humphrey tests.
These statistics are based on Humphrey Visual Field Analyzer data available for 865 GLAUGEN participants and 1371 NEIGHBOR consortium participants. Since PSD spuriously declines as MD worsens, thus subjects with MD worse than −13dB were excluded.
Overall, ten SNPs were significant at a Bonferroni corrected p value of 7.7×10−4 (top SNP rs4236601: pooled p=2.61×10−7, OR=1.26, 95% confidence interval (CI)=1.16–1.38; Table 2). Four of the ten significant SNPs are located in a regulatory region between the CAV1 and CAV2 genes, two are located in the 3′ UTR (untranslated region) of CAV2 and four are in the second intron of CAV1 (Figure 2, Figure 4, available at http://aaojournal.org). The top SNP, rs4236601 is located within the binding site for the transcription factor c-FOS, and SNPs rs10256914, rs10270569, rs3779512, and rs4736740 are in DNaseI hypersensitivity sites (regions of DNA that are gene promoters or other regulatory sites) active in human vascular endothelial cells (Figure 4, available at http://aaojournal.org; regulatory regions determined from the ENCODE data in the UCSC Genome Browser, Available at www.genome.ucsc.edu. Accessed Sept 9, 2012).
Table 2.
Top 10 significant CAV1/CAV2 SNPs associated with POAG in meta-analysis of the combined GLAUGEN and NEIGHBOR dataset (N cases=3108, N controls=3430)1, 2.
| SNP | Position | Reference allele | Pooled OR (95% CI) | GLAUGEN P value | NEIGHBOR P value | Pooled P value3, 4 |
|---|---|---|---|---|---|---|
| rs4236601 | 49021016 | A | 1.26 (1.16,1.38) | 0.003 | 1.89×10−5 | 2.61×10−7 |
| rs6969706 | 49020340 | T | 1.26 (1.15,1.38) | 0.003 | 2.54×10−5 | 3.58×10−7 |
| rs10256914 | 49031338 | C | 1.24 (1.13,1.35) | 9.13×10−4 | 6.77×10−4 | 3.69×10−6 |
| rs17588172 | 49019406 | G | 1.22 (1.12,1.32) | 7.80×10−4 | 0.001 | 5.78×10−6 |
| rs10270569 | 49033664 | T | 1.23 (1.12,1.35) | 0.002 | 6. ×10−4 | 6.43×10−6 |
| rs1052990 | 49012056 | G | 1.21 (1.11,1.31) | 0.001 | 0.001 | 1.09×10−5 |
| rs10227696 | 49020430 | A | 1.24 (1.12,1.37) | 0.007 | 0.001 | 2.98×10−5 |
| rs4730748 | 49024676 | G | 1.23 (1.11,1.36) | 0.007 | 0.002 | 6.68×10−5 |
| rs10278782 | 49011162 | G | 1.22 (1.10,1.35) | 0.02 | 0.002 | 1.49×10−4 |
| rs3779512 | 49027534 | T | 1.15 (1.06,1.25) | 0.006 | 0.03 | 7.60×10−4 |
Abbreviations: CAV1=caveolin 1; CAV2=caveolin 2; SNPs=single nucleotide polymorphisms, POAG=primary open angle glaucoma, GLAUGEN=Glaucoma Genes and Environment, NEIGHBOR=National Eye Institute Glaucoma Human Genetics Collaboration, OR=odds ratio, CI=confidence interval
GLAUGEN N=2116 cases and controls NEIGHBOR N=4422 cases and controls
SNPs significant at Bonferroni corrected p<7.7×10 −4 (13 LD blocks × 5 analyses=65, 0.05/65=7.7×10−4) are in bold.
P for heterogeneity between GLAUGEN and NEIGHBOR is >0.33 for all SNPs.
When stratified by gender, nine of the ten SNPs showed significant associations among women (top SNP rs4236601: pooled p=1.59×10−5, OR=1.30, 95% CI=1.15–1.46; Table 3) but none were significant in men (top SNP rs17588172: pooled p=0.002). Tests of the SNP × gender interactions yielded no significant associations between CAV1/CAV2 SNPs and POAG (p≥0.18), but the slightly stronger odds ratios in women are suggestive of a differential effect.
Table 3.
Top 10 significant CAV1/CAV2 SNPs overall associated with POAG in women only and men only in meta-analysis of the combined GLAUGEN and NEIGHBOR dataset1.
| Women only (N cases=1682, N controls=1937) | Men only (N cases=1426, N controls=1493) | SNP X gender interaction P value | |||||
|---|---|---|---|---|---|---|---|
| SNP | Position | Reference allele | Pooled OR (95% CI) | Pooled P value2, 3 | Pooled OR (95% CI) | Pooled P value2, 3 | |
| rs4236601 | 49021016 | A | 1.30 (1.15,1.46) | 1.59×10−5 | 1.23 (1.07,1.40) | 0.003 | 0.57 |
| rs6969706 | 49020340 | T | 1.29 (1.15,1.46) | 2.07×10−5 | 1.22 (1.07,1.40) | 0.004 | 0.53 |
| rs10256914 | 49031338 | C | 1.29 (1.14,1.45) | 3.76×10−5 | 1.18 (1.03,1.35) | 0.02 | 0.39 |
| rs17588172 | 49019406 | G | 1.22 (1.09,1.36) | 6.24×10−4 | 1.23 (1.08,1.40) | 0.002 | 0.44 |
| rs10270569 | 49033664 | T | 1.28 (1.14,1.45) | 4.53×10−5 | 1.17 (1.02,1.34) | 0.02 | 0.28 |
| rs1052990 | 49012056 | G | 1.21 (1.08,1.35) | 8.38×10−4 | 1.22 (1.07,1.38) | 0.003 | 0.37 |
| rs10227696 | 49020430 | A | 1.35 (1.18,1.54) | 1.61×10−5 | 1.12 (0.96,1.31) | 0.14 | 0.27 |
| rs4730748 | 49024676 | G | 1.33 (1.17,1.53) | 3.07×10−5 | 1.11 (0.95,1.29) | 0.18 | 0.28 |
| rs10278782 | 49011162 | G | 1.32 (1.15,1.51) | 5.64×10−5 | 1.10 (0.94,1.28) | 0.22 | 0.18 |
| rs3779512 | 49027534 | T | 1.22 (1.09,1.36) | 4.33×10−4 | 1.07 (0.95,1.22) | 0.27 | 0.42 |
Abbreviations: CAV1=caveolin 1; CAV2=caveolin 2; SNPs=single nucleotide polymorphisms, POAG=primary open angle glaucoma, GLAUGEN=Glaucoma Genes and Environment, NEIGHBOR=National Eye Institute Glaucoma Human Genetics Collaboration, OR=odds ratio, CI=confidence interval
SNPs significant at Bonferroni corrected p<7.7×10 −4 (13 LD blocks × 5 analyses=65, 0.05/65=7.7×10−4) are in bold.
P for heterogeneity between GLAUGEN and NEIGHBOR is >0.20 for all SNPs.
Of the total 3108 cases, 224 had paracentral VF loss only, 993 had peripheral VF loss only, and the remaining 1891 cases were excluded from type of VF loss subanalyses because of advanced field loss making the paracentral only or peripheral only identification impossible. Analyses of POAG subgroups by type of VF loss identified five of the ten most significant CAV1/CAV2 POAG-overall SNPs associated with paracentral VF loss only (top SNP rs17588172: pooled p=1.07×10−4, OR=1.52, 95% CI=1.23–1.89; Table 4), while none of the ten were associated with POAG with peripheral VF loss only (top SNP rs4236601: pooled p=8.03×10−4). Odds ratios were stronger in the analysis of POAG with paracentral VF loss only compared to the overall analysis, even though this subset contained fewer cases.
Table 4.
Top 10 significant CAV1/CAV2 SNPs overall associated with two subtypes of POAG defined by location of visual field defects (paracentral versus peripheral) in meta-analysis of the combined GLAUGEN and NEIGHBOR dataset1.
| POAG with paracentral loss only (N cases=224, N controls=3430) | POAG with peripheral loss only (N cases=993, N controls=3430) | |||||
|---|---|---|---|---|---|---|
| SNP | Position | Reference allele | Pooled OR (95% CI) | Pooled P value2, 3 | Pooled OR (95% CI) | Pooled P value2, 3 |
| rs4236601 | 49021016 | A | 1.53 (1.23,1.91) | 1.45×10−4 | 1.24 (1.09,1.41) | 8.03×10−4 |
| rs6969706 | 49020340 | T | 1.53 (1.23,1.91) | 1.58×10−4 | 1.24 (1.09,1.41) | 0.001 |
| rs10256914 | 49031338 | C | 1.47 (1.18,1.84) | 5.49×10−4 | 1.21 (1.06,1.38) | 0.004 |
| rs17588172 | 49019406 | G | 1.52 (1.23,1.87) | 1.07×10−4 | 1.20 (1.07,1.36) | 0.003 |
| rs10270569 | 49033664 | T | 1.42 (1.14,1.77) | 0.002 | 1.20 (1.05,1.37) | 0.006 |
| rs1052990 | 49012056 | G | 1.48 (1.20,1.83) | 2.89×10−4 | 1.20 (1.06,1.35) | 0.003 |
| rs10227696 | 49020430 | A | 1.36 (1.06,1.74) | 0.02 | 1.25 (1.08,1.44) | 0.003 |
| rs4730748 | 49024676 | G | 1.33 (1.04,1.71) | 0.02 | 1.23 (1.06,1.42) | 0.006 |
| rs10278782 | 49011162 | G | 1.29 (1.01,1.66) | 0.04 | 1.23 (1.06,1.42) | 0.006 |
| rs3779512 | 49027534 | T | 1.17 (0.95,1.44) | 0.14 | 1.17 (1.03,1.31) | 0.01 |
Abbreviations: CAV1=caveolin 1; CAV2=caveolin 2; SNPs=single nucleotide polymorphisms, POAG=primary open angle glaucoma, GLAUGEN=Glaucoma Genes and Environment, NEIGHBOR=National Eye Institute Glaucoma Human Genetics Collaboration, OR=odds ratio, CI=confidence interval
SNPs significant at Bonferroni corrected p<7.7×10 −4 (13 LD blocks × 5 analyses=65, 0.05/65=7.7×10−4) are in bold.
P for heterogeneity between GLAUGEN and NEIGHBOR is >0.11 for all SNPs.
Risk allele odds ratios for the ten most significant SNPs overall were 1.15–1.26 in the overall analysis (3108 cases, 3430 controls), 1.21–1.35 in women only (1682 cases, 1937 controls) and 1.17–1.53 in cases with paracentral VF loss only (224 cases, 3430 controls), indicating the generally stronger associations in women, and in relation to POAG with paracentral VF loss only.
Discussion
In this study, we have confirmed the association between genetic variants in the CAV1/CAV2 genomic region and POAG overall and have shown that these associations may differ by gender and for subtypes of POAG defined by pattern of visual field loss. Our group initially replicated the CAV1/CAV2 findings in the GLAUGEN study alone,9 and here we have shown that meta-analyzing the results from the GLAUGEN study and with the NEIGHBOR study confirmed the association between CAV1/CAV2 genomic region SNPs and POAG overall. For example, for the CAV1/CAV2 SNP rs4236601, the strength of statistical association was enhanced in the combined dataset (pooled p=2.61×10−7) when compared to either the GLAUGEN (p=0.003) or NEIGHBOR dataset (p=1.89×10−5) alone. The differences in the statistical strength of the association in GLAUGEN and NEIGHBOR probably reflects the different case numbers in each cohort (Table 5, available at http://aaojournal.org)29. Our combined GLAUGEN-NEIGHBOR analysis is the largest POAG case-control sample currently available. It is possible that studies failing to replicate the POAG association with CAV1/CAV2 SNPs were underpowered due to smaller sample size. It is also interesting that the robust association initially observed in the Icelandic population8 included fewer cases and hence had lower power (n=1263 cases; power=61%; p=5.0×10−10) than the GLAUGEN-NEIGHBOR study (n=3108 cases; power=86%; p=2.61×10−7) yet the observed associations were more significant than in the GLAUGEN-NEIGHBOR combined dataset (Table 5, available at http://aaojournal.org).8,29 This could be due to a founder effect or a stronger allele effect in the Icelandic population
We found greater significance and stronger associations between the CAV1/CAV2 region SNPs and POAG in women than in men, supporting the impact of estrogen on the nitric oxide pathway. Previously, our group found that NOS3 interacts with reproductive factors such as age at menarche23 and postmenopausal hormone use.22 Similarly, Magalhães da Silva et al. found an association between NOS3 SNPs and POAG in women but not in men.30 Since the gene product of NOS3 (eNOS) directly interacts with caveolin 1, these genetic associations may reflect the altered protein interactions that influence the risk of POAG, especially in women.
We also found, despite a small number of cases, significant and stronger associations between the CAV1/CAV2 region and POAG cases with only initial paracentral VF loss. Manifest visual field loss in glaucoma commonly begins peripherally and proceeds toward the center of vision; however, visual loss can commence in the paracentral region. This type of visual field deficit can substantially decrease quality of life, making reading and driving more difficult.31,32 Some studies, although not all,33–35 have suggested that paracentral visual field loss is more likely to develop in patients with IOP levels in the normal range (<22 mm Hg), indicating that risk factors other than increased IOP may contribute to this POAG subtype.36,37 One such factor is p53 - a functional polymorphism in p53 was found to be associated with POAG and paracentral VF loss.38 A p53 SNP, which is thought to be functionally pro-apoptotic, may render the metabolically active maculopapillary nerve fiber bundles vulnerable to cell death resulting in paracentral VF loss seen in some POAG cases. Another major factor is systemic vascular dysregulation which has been associated with initial paracentral VF loss.25 The CAV1/CAV2-NOS3 pathway could contribute to abnormalities in systemic vascular tone and vasospastic phenomena. Recently, SNPs located in a genomic region near the GUCY1A3/GUCY1B3 genes coding for soluble guanylyl cyclase have also been associated with paracentral VF loss in POAG.26 Interestingly, the GUCY1A3/GUCY1B3 genomic region was associated with paracentral VF primarily in women, and soluble guanylyl cyclase serves as the intracellular receptor for nitric oxide, downstream of the interaction between eNOS and caveolin 1. CAV1 knockout mice have been studied in vascular related diseases such as atherosclerosis and pulmonary hypertension indicating a role for caveolin 1 in endothelial cell dysfunction, but the ocular phenotype of this mouse model has not been explored39,40. Thus, more research into the genetic factors that determine vascular dysregulation in relation to POAG with paracentral VF loss is warranted.
The intergenic region between CAV1/CAV2 contains several regulatory elements including H3K27Ac histone marks (indicating an active regulatory region), DNaseI hypersensitivity sites and transcription factor binding sites (Figure 2 and Figure 4, available at http://aaojournal.org). rs4236601 is the top SNP in our analysis and also in the Icelandic study.8 This SNP falls in the regulatory region 5′ of CAV1. Several transcription factors are predicted to bind in this region including c-FOS, a transcription factor known to be active in vascular endothelial cells, especially in response to shear stress.41,42 Additionally, the DNaseI sites in this region are active in human vascular endothelial cell lines, and several of the significantly associated SNPs are located in these active regulatory sites. Although preliminary, these results suggest that the associated SNPs may contribute to regulation of CAV1 and CAV2 gene expression in human vascular endothelial cells.
There are several limitations to our study. The NEIGHBOR study contained very few cases with initial paracentral only VF defects. The majority of NEIGHBOR participants with POAG were prevalent clinic cases, which made it difficult to obtain VFs at initial disease onset to determine the type of initial VF loss. Many of the GLAUGEN cases were incident cases identified during prospective follow-up of a population for several disease endpoints including glaucoma. Thus there was greater opportunity in GLAUGEN to access the initial VF that showed glaucomatous loss. Tests of the SNP × gender interactions were negative because they may have been underpowered or because the true interaction involves some other gender specific trait that remains unknown. It could also be argued that our subgroup analyses may be underpowered and hence the differential significance between women only and men only and between paracentral VF loss and peripheral VF loss could represent false negatives. Despite the smaller case numbers, however, the women only and men only analyses both were adequately powered to detect a significant association (power=93%, power=84%, respectively, Table 5, available at http://aaojournal.org)29. The paracentral VF loss analysis was underpowered, with power=31%, but still found significant associations. The peripheral VF loss analysis did have adequate power (power=99%). Thus we can conclude that the differences found in the subgroup analyses are most likely not spurious.
In this study we have confirmed the association of CAV1/CAV2 SNPs with POAG overall and have additional evidence that the relationship between CAV1/CAV2 and POAG may be stronger in women and for POAG with initial paracentral only VF defects. Additionally, this study contributes to the emerging evidence that the nitric oxide signaling pathway plays an important role in POAG pathogenesis. Further study of the impact of CAV1/CAV2 genetic variation on nitric oxide signaling could lead to new therapeutic targets for the treatment of POAG.
Supplementary Material
Acknowledgments
Financial Support: The sponsor or funding organization had no role in the design or conduct of this research.
This work was supported by the following: Genotyping services for the NEIGHBOR study were provided by the Center for Inherited Disease Research (CIDR) and were supported by the National Eye Institute through grant HG005259-01 (JL Wiggs). Additionally, CIDR is funded through a federal contract from the National Institutes of Health to The Johns Hopkins University, contract number HHSN268200782096C. Genotyping for the GLAUGEN dataset at the Broad Institute was supported by GENEVA project grant HG004728 (LR Pasquale) and U01-HG004424 (Broad Institute). Genotype data cleaning and analysis for the GLAUGEN study was supported by U01 HG004446 (C Laurie). Collecting and processing samples for the NEIGHBOR dataset was supported by the National Eye Institute through ARRA grants 3R01EY015872-05S1 (JL Wiggs) and 3R01EY019126-02S1 (MA Hauser). Funding for the collection of cases and controls was provided by NIH grants: EY015543 (RR Allingham), EY006827 (D Gaasterland); HL73042, HL073389, EY13315 (MA Hauser); CA87969 (JH Kang), CA49449 (JH Kang), UM1 CA167552 (JH Kang), EY009149 (PR Lichter), HG004608 (C McCarty), EY008208 (FA Medeiros), EY015473 (LR Pasquale), EY012118 (M Pericak-Vance), EY015682 (A Realini), EY011671 (JE Richards), EY09580 (JE Richards), EY013178 (JS Schuman), RR015574, EY015872 (JL Wiggs), EY010886 (JL Wiggs), EY009847 (JL Wiggs), EY022766 (JL Wiggs), EY011008, EY144428 (K Zhang), EY144448 (K Zhang), EY18660 (K Zhang). JL Wiggs and LR Pasquale are supported by the Harvard Glaucoma Center for Excellence and the Margolis Fund. JL Wiggs, LR Pasquale, and JE Richards are supported by Research to Prevent Blindness. YLiu is supported by the Glaucoma Research Foundation, The Glaucoma Foundation, and American Health Assistance Foundation.
Footnotes
Meeting presentation: This work has been presented as a poster at the Association for Research in Vision and Ophthalmology 2013 meeting.
Conflict of interest: No conflicting relationship exists for any author.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kwon YH, Fingert JH, Kuehn MH, Alward WL. Primary open-angle glaucoma. N Engl J Med. 2009;360:1113–24. doi: 10.1056/NEJMra0804630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90:262–7. doi: 10.1136/bjo.2005.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stone EM, Fingert JH, Alward WL, et al. Identification of a gene that causes primary open angle glaucoma. Science. 1997;275:668–70. doi: 10.1126/science.275.5300.668. [DOI] [PubMed] [Google Scholar]
- 4.Rezaie T, Child A, Hitchings R, et al. Adult-onset primary open-angle glaucoma caused by mutations in optineurin. Science. 2002;295:1077–9. doi: 10.1126/science.1066901. [DOI] [PubMed] [Google Scholar]
- 5.Burdon KP, Macgregor S, Hewitt AW, et al. Genome-wide association study identifies susceptibility loci for open angle glaucoma at TMCO1 and CDKN2B-AS1. Nat Genet. 2011;43:574–8. doi: 10.1038/ng.824. [DOI] [PubMed] [Google Scholar]
- 6.Wiggs JL, Yaspan BL, Hauser MA, et al. Common variants at 9p21 and 8q22 are associated with increased susceptibility to optic nerve degeneration in glaucoma. [Accessed August 18, 2013];PLoS Genet. 2012 8:e1002654. doi: 10.1371/journal.pgen.1002654. serial online. Available at: http://www.plosgenetics.org/article/info%3Adoi%2F10.1371%2Fjournal.pgen.1002654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ramdas WD, van Koolwijk LM, Cree AJ, et al. Clinical implications of old and new genes for open-angle glaucoma. Ophthalmology. 2011;118:2389–97. doi: 10.1016/j.ophtha.2011.05.040. [DOI] [PubMed] [Google Scholar]
- 8.Thorleifsson G, Walters GB, Hewitt AW, et al. Common variants near CAV1 and CAV2 are associated with primary open-angle glaucoma. Nat Genet. 2010;42:906–9. doi: 10.1038/ng.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wiggs JL, Kang JH, Yaspan BL, et al. Common variants near CAV1 and CAV2 are associated with primary open-angle glaucoma in Caucasians from the USA. Hum Mol Genet. 2011;20:4707–13. doi: 10.1093/hmg/ddr382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuehn MH, Wang K, Roos B, et al. Chromosome 7q31 POAG locus: ocular expression of caveolins and lack of association with POAG in a US cohort. [Accessed August 18, 2013];Mol Vis. 2011 17:430–5. serial online. Available at: http://www.molvis.org/molvis/v17/a48/ [PMC free article] [PubMed] [Google Scholar]
- 11.Abu-Amero KK, Kondkar AA, Mousa A, et al. Lack of association of SNP rs4236601 near CAV1 and CAV2 with POAG in a Saudi cohort. [Accessed August 18, 2013];Mol Vis. 2012 18:1960–5. serial online. Available at: http://www.molvis.org/molvis/v18/a205/ [PMC free article] [PubMed] [Google Scholar]
- 12.Cao D, Jiao X, Liu X, et al. CDKN2B polymorphism is associated with primary open-angle glaucoma (POAG) in the Afro-Caribbean population of Barbados, West Indies. [Accessed August 18, 2013];PLoS One. 2012 7:e39278. doi: 10.1371/journal.pone.0039278. serial online. Available at: http://www.plosone.org/article/info%3Adoi%2F10.1371%2Fjournal.pone.0039278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mineo C, Shaul PW. Regulation of eNOS in caveolae. Adv Exp Med Biol. 2012;729:51–62. doi: 10.1007/978-1-4614-1222-9_4. [DOI] [PubMed] [Google Scholar]
- 14.Ju H, Zou R, Venema VJ, Venema RC. Direct interaction of endothelial nitric-oxide synthase and caveolin-1 inhibits synthase activity. J Biol Chem. 1997;272:18522–5. doi: 10.1074/jbc.272.30.18522. [DOI] [PubMed] [Google Scholar]
- 15.Garcia-Cardena G, Martasek P, Masters BS, et al. Dissecting the interaction between nitric oxide synthase (NOS) and caveolin. Functional significance of the NOS caveolin binding domain in vivo. J Biol Chem. 1997;272:25437–40. doi: 10.1074/jbc.272.41.25437. [DOI] [PubMed] [Google Scholar]
- 16.Ellis DZ, Dismuke WM, Chokshi BM. Characterization of soluble guanylate cyclase in NO-induced increases in aqueous humor outflow facility and in the trabecular meshwork. Invest Ophthalmol Vis Sci. 2009;50:1808–13. doi: 10.1167/iovs.08-2750. [DOI] [PubMed] [Google Scholar]
- 17.Mroczkowska S, Benavente-Perez A, Negi A, et al. Primary open-angle glaucoma vs normal-tension glaucoma: the vascular perspective. JAMA Ophthalmol. 2013;131:36–43. doi: 10.1001/2013.jamaophthalmol.1. [DOI] [PubMed] [Google Scholar]
- 18.Munaut C, Lambert V, Noel A, et al. Presence of oestrogen receptor type beta in human retina. Br J Ophthalmol. 2001;85:877–82. doi: 10.1136/bjo.85.7.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou X, Li F, Ge J, et al. Retinal ganglion cell protection by 17-beta-estradiol in a mouse model of inherited glaucoma. Dev Neurobiol. 2007;67:603–16. doi: 10.1002/dneu.20373. [DOI] [PubMed] [Google Scholar]
- 20.Khorram O, Garthwaite M, Magness RR. Endometrial and myometrial expression of nitric oxide synthase isoforms in pre- and postmenopausal women. J Clin Endocrinol Metab. 1999;84:2226–32. doi: 10.1210/jcem.84.6.5759. [DOI] [PubMed] [Google Scholar]
- 21.Hisamoto K, Bender JR. Vascular cell signaling by membrane estrogen receptors. Steroids. 2005;70:382–7. doi: 10.1016/j.steroids.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 22.Kang JH, Wiggs JL, Rosner BA, et al. Endothelial nitric oxide synthase gene variants and primary open-angle glaucoma: interactions with sex and postmenopausal hormone use. Invest Ophthalmol Vis Sci. 2010;51:971–9. doi: 10.1167/iovs.09-4266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kang JH, Wiggs JL, Rosner BA, et al. Endothelial nitric oxide synthase gene variants and primary open-angle glaucoma: interactions with hypertension, alcohol intake, and cigarette smoking. Arch Ophthalmol. 2011;129:773–80. doi: 10.1001/archophthalmol.2011.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schwartz EA, Reaven E, Topper JN, Tsao PS. Transforming growth factor-beta receptors localize to caveolae and regulate endothelial nitric oxide synthase in normal human endothelial cells. Biochem J. 2005;390:199–206. doi: 10.1042/BJ20041182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park SC, De Moraes CG, Teng CC, et al. Initial parafoveal versus peripheral scotomas in glaucoma: risk factors and visual field characteristics. Ophthalmology. 2011;118:1782–9. doi: 10.1016/j.ophtha.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 26.Buys ES, Ko YC, Alt C, et al. Soluble guanylate cyclase alpha1-deficient mice: a novel murine model for primary open angle glaucoma. [Accessed August 18, 2013];PLoS One. 2013 8:e60156. doi: 10.1371/journal.pone.0060156. serial online. Available at: http://www.plosone.org/article/info%3Adoi%2F10.1371%2Fjournal.pone.0060156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wiggs JL, Hauser MA, Abdrabou W, et al. The NEIGHBOR Consortium Primary Open-Angle Glaucoma Genome-wide Association Study: rationale, study design, and clinical variables. J Glaucoma. doi: 10.1097/IJG.0b013e31824d4fd8. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tarone RE. A modified Bonferroni method for discrete data. Biometrics. 1990;46:515–22. [PubMed] [Google Scholar]
- 29.Skol AD, Scott LJ, Abecasis GR, Boehnke M. Joint analysis is more efficient than replication-based analysis for two-stage genome-wide association studies. Nat Genet. 2006;38:209–13. doi: 10.1038/ng1706. [DOI] [PubMed] [Google Scholar]
- 30.Magalhaes da Silva T, Rocha AV, Lacchini R, et al. Association of polymorphisms of endothelial nitric oxide synthase (eNOS) gene with the risk of primary open angle glaucoma in a Brazilian population. Gene. 2012;502:142–6. doi: 10.1016/j.gene.2012.04.047. [DOI] [PubMed] [Google Scholar]
- 31.Fujita K, Yasuda N, Oda K, Yuzawa M. Reading performance in patients with central visual field disturbance due to glaucoma [in Japanese] Nihon Ganka Gakkai Zasshi. 2006;110:914–8. [PubMed] [Google Scholar]
- 32.Coeckelbergh TR, Brouwer WH, Cornelissen FW, et al. The effect of visual field defects on driving performance: a driving simulator study. Arch Ophthalmol. 2002;120:1509– 16. doi: 10.1001/archopht.120.11.1509. [DOI] [PubMed] [Google Scholar]
- 33.Motolko M, Drance SM, Douglas GR. Visual field defects in low-tension glaucoma. Comparison of defects in low-tension glaucoma and chronic open angle glaucoma Arch Ophthalmol. 1982;100:1074–7. doi: 10.1001/archopht.1982.01030040052005. [DOI] [PubMed] [Google Scholar]
- 34.King D, Drance SM, Douglas G, et al. Comparison of visual field defects in normal-tension glaucoma and high579 tension glaucoma. Am J Ophthalmol. 1986;101:204–7. doi: 10.1016/0002-9394(86)90596-9. [DOI] [PubMed] [Google Scholar]
- 35.Iester M, De Feo F, Douglas GR. Visual field loss morphology in high- and normal-tension glaucoma. [Accessed August 18, 2013];J Ophthalmol. 2012 2012:327326. doi: 10.1155/2012/327326. serial online. Available at: http://www.hindawi.com/journals/jop/2012/327326/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hitchings RA, Anderton SA. A comparative study of visual field defects seen in patients with low-tension glaucoma and chronic simple glaucoma. Br J Ophthalmol. 1983;67:818–21. doi: 10.1136/bjo.67.12.818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Caprioli J, Spaeth GL. Comparison of visual field defects in the low-tension glaucomas with those in the high-tension glaucomas. Am J Ophthalmol. 1984;97:730–7. doi: 10.1016/0002-9394(84)90505-1. [DOI] [PubMed] [Google Scholar]
- 38.Wiggs JL, Hewitt AW, Fan BJ, et al. The p53 codon 72 PRO/PRO genotype may be associated with initial central visual field defects in Caucasians with primary open angle glaucoma. [Accessed August 18, 2013];PLoS One. 2012 7:e45613. doi: 10.1371/journal.pone.0045613. serial online. Available at: http://www.plosone.org/article/info%3Adoi%2F10.1371%2Fjournal.pone.0045613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fernandez-Hernando C, Yu J, Davalos A, et al. Endothelial-specific overexpression of caveolin-1 accelerates atherosclerosis in apolipoprotein E-deficient mice. Am J Pathol. 2010;177:998–1003. doi: 10.2353/ajpath.2010.091287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wunderlich C, Schmeisser A, Heerwagen C, et al. Chronic NOS inhibition prevents adverse lung remodeling and pulmonary arterial hypertension in caveolin-1 knockout mice. Pulm Pharmacol Ther. 2008;21:507–15. doi: 10.1016/j.pupt.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 41.Shiu YT, Li S, Yuan S, et al. Shear stress-induced c608 fos activation is mediated by Rho in a calcium-dependent manner. Biochem Biophys Res Commun. 2003;303:548–55. doi: 10.1016/s0006-291x(03)00388-7. [DOI] [PubMed] [Google Scholar]
- 42.Ballermann BJ, Dardik A, Eng E, Liu A. Shear stress and the endothelium. Kidney Int Suppl. 1998;67:S100–8. doi: 10.1046/j.1523-1755.1998.06720.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.