Abstract
With the failure of so many pre-clinical stroke studies to translate into the clinic, there is a need to find new therapeutics to minimize the extent of cellular damage and aid in functional recovery. Domain V (DV), the c-terminal protein fragment of the vascular basement membrane component, perlecan, was recently shown to afford significant protection in multiple transient middle cerebral artery occlusion stroke models. We sought here to determine whether DV might have similar therapeutic properties in a focal photothrombosis stroke model in both young and aged mice. Young (3-month old) and aged (24-month old) mice underwent photothrombotic stroke to the motor cortex and were then treated with DV or phosphate buffered saline vehicle at different initial time points up to 7 days. Stroke volume was analyzed histologically using cresyl violet and functional recovery assessed behaviorally on both the grid-walking and cylinder tasks. In young mice, DV administration resulted in a significant decrease in infarct volume when treatment started 3 or 6 h post-stroke. In aged mice, DV administration was only protective when started 3 h post-stroke. In addition to a decrease in the area of infarction, DV treatment was effective in significantly decreasing the number of foot-faults on the grid-walking task and improving use of the stroke-affected limb in the cylinder task in both young and aged. Previously, we have shown that DV can alter the expression profile of various astroglial markers. Consistent with our previous finding, treatment groups that showed therapeutic potential in both young and aged mice also showed an elevation in glial fibrillary acidic protein (GFAP) expression in peri-infarct regions. We conclude that DV is neuroprotective and affords significant improvements in functional recovery in both young and aged mice after focal ischemia. These data also highlight a therapeutic time-window shift that is narrower in aged compared with young mice and is associated with an elevation in GFAP expression and heightened astrogliosis.
Keywords: Cerebral infarct, Extracellular matrix, Behavioral recovery
Introduction
Despite decades of active research, stroke remains a significant cause of mortality and profound morbidity. The vast majority of strokes are ischemic, generating a core of rapid cell death surrounded by a region of tissue known as the “penumbral region” that remains at-risk [21, 22]. Within hours to days after an ischemic event, the brain begins to initiate processes associated with self-repair, including angiogenesis, neurogenesis, and axonal sprouting [8, 24]. However, the initiation of these self-repair mechanisms is limited and, without any form of intervention, are not enough to overcome the stroke-induced impairments and loss of functions. Others and we have previously hypothesized that changes in the neurovascular niche could be exploited therapeutically and in turn limited the extent of cell death and facilitate functional recovery [19]. However, such a neurorepair approach to stroke therapy is rife with difficulties, including the problem that many potential reparative therapies might also enhance injury. An example of this is vascular endothelial growth factor (VEGF), which may further destabilize the blood–brain barrier, promote brain edema, and enhance hemorrhagic transformation and brain infarct size when administered acutely [32] but is neuroprotective and enhances angiogenesis and neurogenesis when given chronically [17, 29]. One solution is to develop a stroke therapy that has both neuroprotective and neuroreparative properties.
We have recently demonstrated that domain V (DV), a proteolytic c-terminal fragment of the vascular basement membrane component, perlecan, is rapidly generated (within 24 h) and persistently elevated (for at least 15 days) after transient focal cerebral ischemia in both young mice and rats and, when deficient, results in larger ischemic infarcts. This suggested to us that it may play a role in post-stroke brain recovery that could be therapeutically exploited [19]. To that end, we have demonstrated that systemically administered DV following focal ischemia in young mice and rats homed to ischemic brain regions, crossed the blood–brain barrier and was neuroprotective and pro-angiogenic and lead to improved motor recovery [19]. We further demonstrated that DV accomplished this by binding to brain endothelial cell alpha5beta1 integrin resulting in the production of VEGF [19]. Additional studies have demonstrated further beneficial effects of DV after transient focal ischemia in young mice; it acutely (within the first several days after focal ischemia) increases peri-infarct glial activation to contain and limit the spread of the infarct and chronically decreases glial scar formation, which would otherwise serve as a barrier to post-stroke brain repair [1].
The present study was undertaken to further examine the protective effects of DV. As not all strokes result in reperfusion, we chose to assess histological and functional outcomes following DV administration in the mouse photothrombosis stroke model in both young and aged animals. We demonstrate that administration of DV following focal ischemia is protective and increases peri-infarct astrogliosis in both young and aged mice. Furthermore, this protection extends to improved recovery of motor functions in both age groups, however, with varying temporal windows to be able to afford protection. These data add further support for the potential therapeutic benefit of DV following an ischemic event.
Methods
Animals
Male C57BL/6 mice (28–32 g) were housed under a 12-h light/dark cycle with ad libitum access to food and water. The University of Otago Animal Research Committee approved all protocols.
DV Protein Production
Human DV was cloned, expressed, purified, and assessed for purity as previously described [19].
Photothrombosis
Focal stroke was induced by photothrombosis as previously described [12–14] using both young (2–3-month old) and aged (24-month old) C57BL/6 male mice. In brief, under isoflurane anesthesia (2–2.5 % in a 70 % N20/30 % O2 mixture), mice were placed in a stereotaxic apparatus, the skull exposed, and with a cold light source (KL1500 LCD, Zeiss) attached to a ×40 objective giving a 2 mm diameter illumination positioned 1.5 mm lateral from Bregma. Rose Bengal solution, 0.2 mL (Sigma; 10 g/L in normal saline), was administered intraperitoneally (IP). After 5 min, the brain was illuminated for 15 min. Animals returned to their normal housing conditions. Body temperature was maintained at 36.9±0.4 °C with a heating pad throughout the operation. After stroke, mice were treated with an initial IP administered dose of DV (2 mg/kg) or phosphate buffered saline (PBS) vehicle at various time points (3, 6, 12, or 24 h for young animals and 3 or 6 h for aged animals) and then on days 1, 2, 4, and 6 post-stroke.
Behavioral Assessment
Animals were tested once on both the grid-walking and cylinder tasks 1 week prior to surgery to establish baseline performance levels. For all of the studies, animals were tested 1 week post-stroke at approximately the same time each day at the end of their dark cycle prior to sacrificing the animals for histological assessment. Behaviors were scored by observers who were blind to the treatment group of the animals in the study as previously described [12–14]. An n=7 animals per group were assessed on all behavioral tasks.
Grid-Walking Task
Mice were run one trial per day at approximately the same time each day. The grid-walking apparatus was manufactured as previously described by Baskin [2], using 12 mm square wire mesh with a grid area 32 /20/50 cm (length/width/height). A mirror was placed beneath the apparatus to allow video footage in order to assess the animals’ stepping errors (i.e., ‘footfaults’). Each mouse was placed individually atop of the elevated wire grid and allowed to freely walk for a period of 5 min (measured in real time by stopwatch and confirmed afterward by reviewing videotape footage). During this 5-min period, the total number of footfaults for each limb along with the total number of non-footfault steps were counted and a ratio between footfaults and total steps taken calculated. Percent footfaults were calculated by: [#footfaults/(#footfaults+#non-footfault steps) × 100]. A ratio between footfaults and total steps taken was used to take into account differences in the degree of locomotion between animals and trials. A step was considered a footfault if it was not providing support and the foot went through the grid hole. Furthermore, if an animal was resting with the grid at the level of the wrist, this was also considered a fault. If the grid was anywhere forward of the wrist area, then this was considered a normal step.
Spontaneous Forelimb Task (Cylinder Task)
The spontaneous forelimb task encourages the use of forelimbs for vertical wall exploration/press in a cylinder [28]. When placed in a cylinder, the animal rears to a standing position, whilst supporting its weight with either one or both of its forelimbs on the side of the cylinder wall. As described previously by Schallert and colleagues (2000) [28], for rats, animals were placed inside a Plexiglas cylinder and videotaped for 5 min. To adjust for mice (from a rat model), a smaller cylinder 15 cm in height with a diameter of 10 cm was used [2]. Videotape footage of animals in the cylinder was evaluated quantitatively in order to determine forelimb preference during vertical exploratory movements. While the video footage was played in slow motion (1/5th real time speed), the time (seconds) during each rear that each animal spent on either the right forelimb, the left forelimb, or on both forelimbs were calculated. Only rears in which both forelimbs could be clearly seen were timed. From these three measures, the total amount of time spent on either limb independently as well as the time the animal spent rearing using both limbs was derived. The percentage of time spent on each limb was calculated, and these data were used to derive an SFL asymmetry index (% ipsilateral use/% contralateral use). The “contact time” method of examining the behavior was chosen over the “contact placement” method, as described by Schallert and colleagues (2000) [28], as it takes into account the slips that often occur during a bilateral wall press post-stroke.
Infarct Size
For the histological assessment of infarct size, brains were processed 1 week after stroke and sections stained using cresyl violet as previously described [12, 14]. An n=5 animals (for young) and n=7 animals (for aged) per group were used for histological assessments.
Immunohistochemical Assessment
Animals were anesthetized with sodium pentobarbital (3 mg/100 µl, IP) and transcardially perfused with 4 % paraformaldehyde in 0.1 m phosphate buffer (pH 7.6). The brains were removed, post-fixed in the same fixative for 60 min, and then transferred to a 30 % sucrose/Tris-buffered saline (TBS, 0.2 m Tris, 0.15 m sodium chloride) solution overnight. The next day, brains were frozen on the stage of a sliding microtome, and three sets of 40 µm thick coronal sections were cut (each section was separated by 120 µm).
Immunocytochemistry was performed for glial fibrillary acidic protein (GFAP) to assess the effects of DV on glial scar formation. Briefly, sections were washed thoroughly in TBS and incubated for 48 h at 4 °C in the polyclonal chicken anti-GFAP (dilution 1:2,000; Millipore, USA) in TBS containing 0.3 % Triton X-100 and 0.25 % bovine serum albumin (hereafter, referred to as incubation solution) containing 2 % normal donkey serum. Sections were then washed three times in TBS (10 min per wash) before being incubated in a donkey anti-chicken 549 secondary antibody (Jackson Immunoresearch, USA) at a dilution of 1:400 in incubation solution for 90 min at room temperature. After subsequent washing in TBS, sections were mounted onto gelatin-coated glass slides, air-dried, passed sequentially through alcohols (50 %, 70 %, 95 %, and 100 %) before being passed through xylene, and then coverslipped using DPX mounting solution. Images of the glial scar (400 um from the stroke border) encompassing what is known as the peri-infarct region were taken on an Olympus BX51 microscope and fluorescent intensity measures taken using ImageJ analysis. Fluorescent intensity measure of the scar were normalized to background reading on the contralateral hemisphere and an average fluorescence obtained from three sections from n=5 animals. An observer, blind as to the treatment group, took the images and fluorescent intensity measurements.
Statistics
All data are expressed as mean±standard error of the mean. Differences between treatment groups were analyzed using two-way ANOVA and Newman–Keuls' multiple pair-wise comparisons for post hoc comparisons. Fluorescent intensity measures were analyzed using Student ttests. The level of significance was set at <0.05.
Results
Perlecan DV Decreases Stroke Infarct Volume in Young and Aged Mice
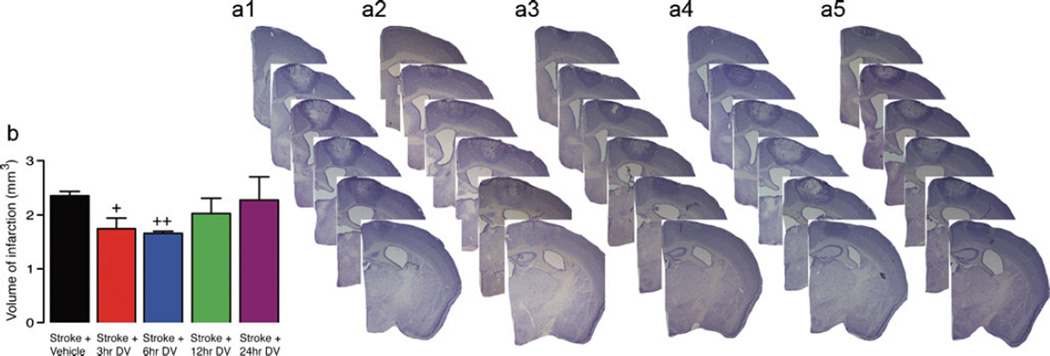
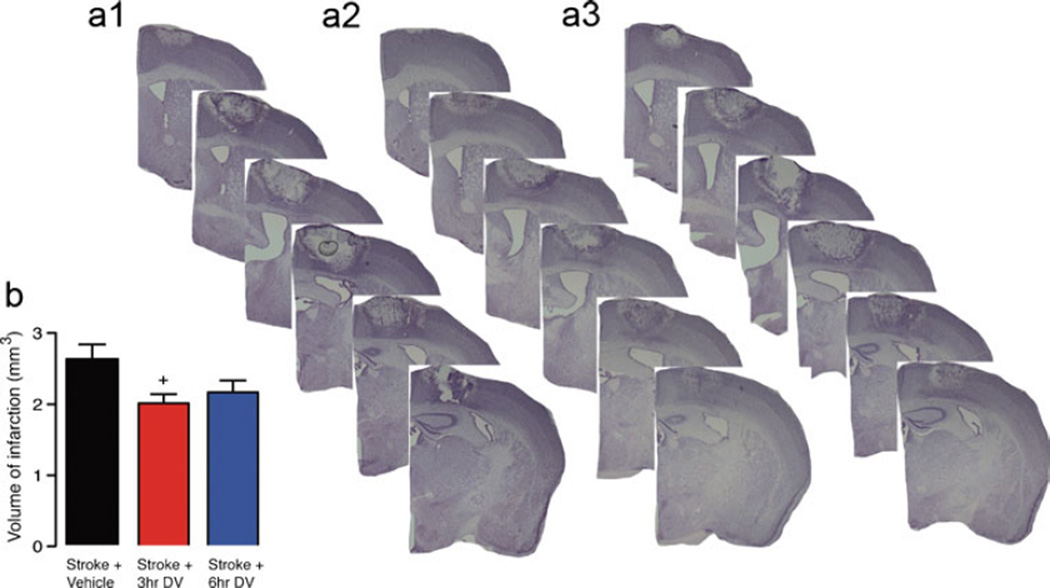
Previous studies have shown that perlecan DV can afford significant protection in multiple reperfusion models of stroke [19]. As not all strokes have a reperfusion component, we investigated the protective effects of perlecan DV in the focal photothrombosis model of stroke that has minimal reperfusion. Strokes were induced using a cold-light source positioned over the mouse motor cortex and shining the light through the intact skull for 15 min (Figs. 1 and 2). The area of damage affected by the stroke includes sensory forelimb and hindlimb as well as primary forelimb and hindlimb cortical areas [13]. After stroke, mice were treated with an initial IP administered dose of DV (2 mg/kg) or PBS vehicle at various time points (3, 6, 12, or 24 h for young animals and 3 or 6 h for aged animals) and then on days 1, 2, 4, and 6 post-stroke. Infarct volume was assessed in these animals 7 days after stroke (Figs. 1 and 2). For young animals, a significant decrease in infarct volume was observed in animals that received DV treatment at either 3 or 6 h after stroke (vehicle, 2.36±0.08 mm3 versus DV 3 h, 1.75±0.19 mm3, P=0.0209; DV 6 h 1.66±0.04 mm3, P<0.01, n=5 per group). For aged animals, a significant decrease in infarct volume was also observed in animals that received DV treatment at 3 h after stroke (vehicle, 2.61±0.21 mm3 versus DV 3 h, 1.99±0.12 mm3; P=0.0248, n=7 per group). Aged animals that received DV treatment at 6 h showed a marked decrease in infarct volume but not quite significantly (2.15±0.17 mm3, p=0.1124, n=7).
Fig. 1.
Histological assessment after stroke in young. Representative cresyl violet-stained sections were generated 7 days post-stroke from vehicle-treated young stroke control (a, 1) and Stroke+DV treatment starting from 3 h (a, 2), 6 h (a, 3), 12 h (a, 4), and 24 h (a, 5) after stroke. Daily IP administration of DV (2 mg/kg) resulted in a significant decrease in infarct volume when given from 3 or 6 h post-stroke. Tissue was collected and processed 7 days post-stroke to quantify lesion size as shown in panel b. A n=5 per group was used. +=P<0.05 and ++=P<0.01 compared with stroke+vehicle-treated controls
Fig. 2.
Histological assessment after stroke in aged. Representative cresyl violet-stained sections were generated 7 days post-stroke from vehicle-treated aged stroke control (a, 1) and stroke+DV treatment starting from 3 h (a, 2), and 6 h (a, 3) after stroke. Daily IP administration of DV (2 mg/kg) resulted in a significant decrease in infarct volume when given from 3 h post-stroke. Tissue was collected and processed 7 days post-stroke to quantify lesion size as shown in panel b. A n=5 per group were used. +=P<0.05 compared with stroke+vehicle-treated controls
Perlecan DV Improves Post-stroke Motor Function in Young and Aged Mice
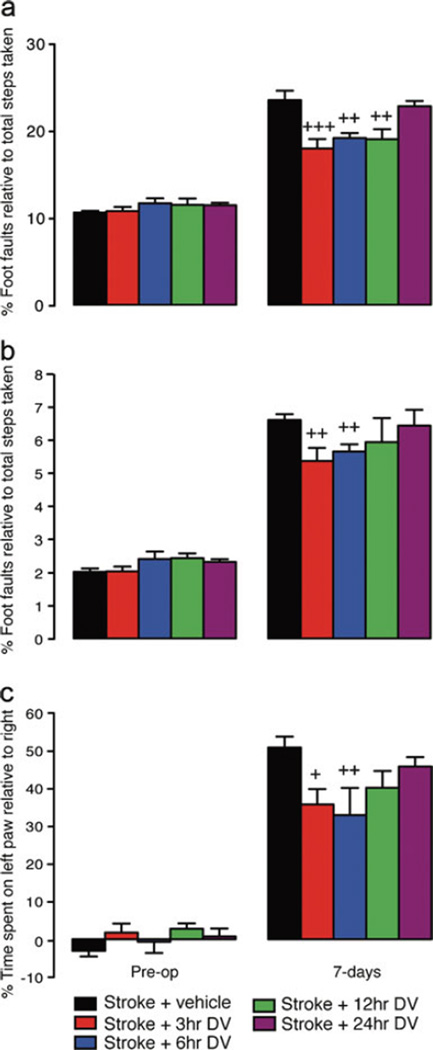
Behavioral assessment revealed an increase in the number of footfaults (both right forelimb and hindlimb) on the grid-walking test (Fig. 3a and b in young animals; Fig 4a and b in aged animals) and an increase in spontaneous forelimb asymmetry (decrease in relative time spent on the right forelimb) in the cylinder task (Figs. 3c and 4c, young and aged, respectively) 1 week post-stroke in both the young and aged animal groups. Young animals that were treated with DV showed a significant decrease in the number of forelimb footfaults when DV was given 3, 6 or 12 h after stroke (n=7 per group, P<0.001 for 3 h, P<0.01 for 6 h, P<0.01 for 12 h compared with vehicle-treated stroke controls). Assessment of hindlimb footfaults showed a significant decrease in the number of footfaults when DV treatment was initiated 3 and 6 h after stroke (n=7 per group, P<0.01 for both 3 and 6 h, compared with vehicle-treated stroke controls). Assessment of spontaneous forelimb asymmetry on the cylinder task revealed that DV treatment when started at either 3 or 6 h post-stroke resulted in improved use of the impaired right forelimb (n=7 per group, P<0.05 for 3, P<0.01 for 6 h, compared with vehicle-treated stroke controls).
Fig. 3.
Behavioral recovery after stroke in young. Behavioral recovery in young animals following photothrombosis stroke was assessed on grid-walking (panels a and b) and cylinder/forelimb asymmetry (panel c) tasks. Analysis of forelimb (a) and hindlimb (b) foot faults revealed a significant increase in the number of foot faults compared with baseline measurements 1 week post-stroke. Daily IP administration of DV (2 mg/kg) resulted in a significant decrease in the number of forelimb footfaults made when DV was given from 3, 6, or 12 h post-stroke and a significant decrease in the number of hindlimb footfaults made when DV was administered 3 or 6 h post-stroke. Assessment of forelimb asymmetry using the cylinder task (c) showed that the mice had a greater tendency to spend more time on their left forepaw post-stroke when pressing against the cylinder wall as revealed by an increase in the left/right ratio after stroke. Daily IP administration of DV (2 mg/kg) resulted in a significant improvement in the use of the impaired right forelimb when DV was given from 3 or 6 h post-stroke. An n=7 per group were used for these studies. +=P<0.05, ++=P<0.01 compared with stroke+vehicle-treated controls
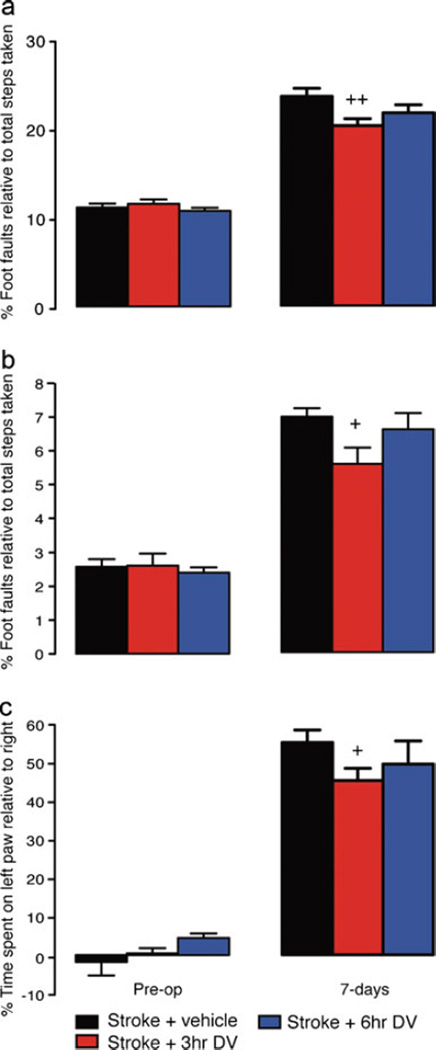
Fig. 4.
Behavioral recovery after stroke in aged. Behavioral recovery in aged animals following photothrombosis stroke was assessed on grid-walking (panels a and b) and cylinder/forelimb asymmetry (panel c) tasks. Analysis of forelimb (a) and hindlimb (b) footfaults revealed a significant increase in the number of foot faults compared with baseline measurements 1 week post-stroke. Daily IP administration of DV (2 mg/kg) resulted in a significant decrease in the number of forelimb and hindlimb footfaults made when DV was given 3 h post-stroke. Assessment of forelimb asymmetry using the cylinder task (c) showed that the mice had a greater tendency to spend more time on their left forepaw post-stroke when pressing against the cylinder wall as revealed by an increase in the left/right ratio after stroke. Daily IP administration of DV (2 mg/kg) resulted in a significant improvement in the use of the impaired right forelimb when DV was given 3 h post-stroke. An n=7 per group were used for these studies. +=P<0.05, ++=P<0.01 compared with stroke+vehicle-treated controls
Assessment of behavioral recovery in the aged animals revealed that treatment with DV afforded significant improvement in both forelimb and hindlimb function on the grid-walking task when treatment was started 3 h post-stroke (n=7 per group, P<0.01 for forelimb, P<0.05 for hindlimb, compared with vehicle-treated stroke controls). Assessment of spontaneous forelimb asymmetry in the aged mice showed that mice treated with DV 3 h after stroke had improved use of their right stroke-affected forelimb (n=7 per group, P<0.05 compared with vehicle-treated stroke controls).
DV Modulates GFAP Expression
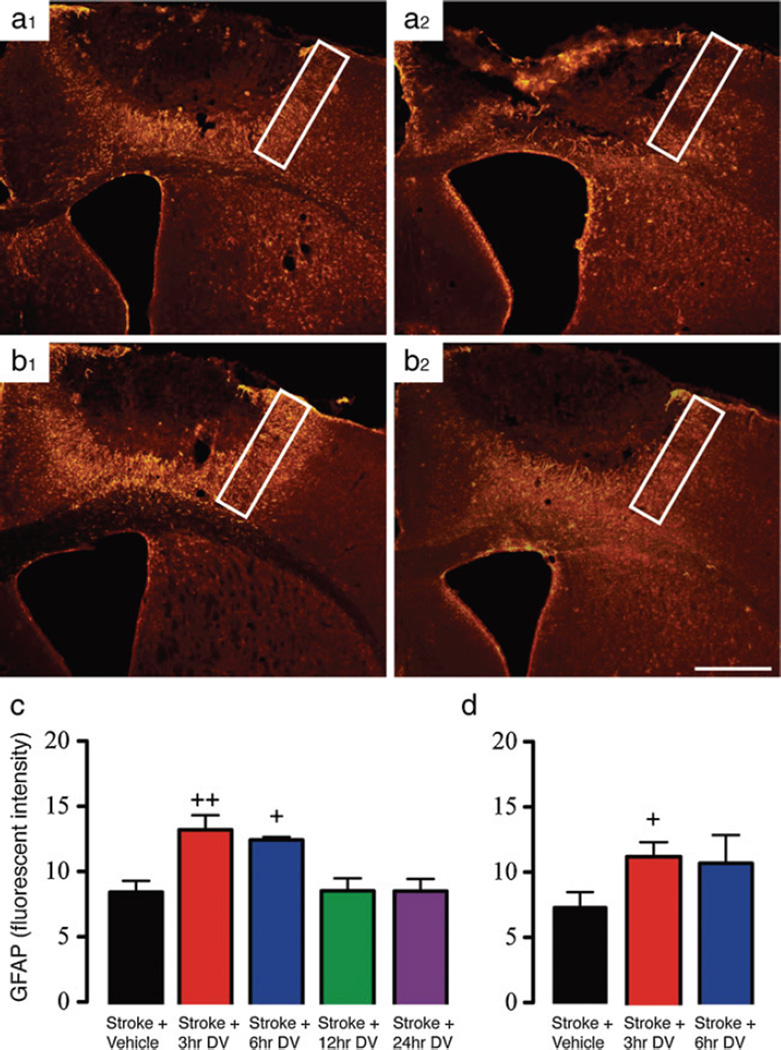
Previous studies have shown that DV can actively modulate the expression of GFAP and contribute to the formation of the glial scar [1]. To confirm that similar changes in astrogliosis occur across stroke models in the presence of DV, we investigated the changes in GFAP expression within the peri-infarct region following focal ischemia in both young and aged mice. Immunohistochemical assessment of GFAP was carried out 7 days post-stroke. In vehicle-treated stroke controls, there is an increase in the level of GFAP expression when values were normalized to the contralateral hemisphere. DV-treated animals showed a significant increase in GFAP expression compared with vehicle-treated controls when DV was administered from either 3 or 6 h post-stroke (P<0.01 and P<0.05 for 3 and 6 h, respectively; Fig. 5b, (1) and c). Administration of DV at either 12 or 24 h post-stroke failed to trigger an increase in GFAP expression compared with vehicle-treated controls. Assessment of GFAP expression in aged-stroke mice was also elevated in vehicle-treated controls. Of note, however, this increase was less than what was seen in vehicle-treated stroked young animals. DV-treatment similar to young resulted in a further increase in GFAP expression, however, this was only significant at the 3 h time point compared with vehicle treated stroke controls (P<0.05; Fig. 5b, (2) and d). These data suggest that early DV treatment (3–6 h post-stroke) can afford significant protection by promoting astrogliosis and post-stroke scar formation.
Fig. 5.
DV modulates peri-infarct GFAP expression. The level of GFAP expression was assessed in peri-infract regions of both young (panel a, 1 and b, 1) and aged (panel a, 2 and b, 2) mice treated with vehicle or DV starting from 3, 6, 12, or 24 h post-stroke. All peri-infarct GFAP measures were normalized to contralateral GFAP levels to standardize the levels across all groups. For young (panel c), we saw a significant increase in the level of GFAP expression in DV groups treated at either 3 or 6 h post-stroke. In mice treated at either 12 or 24 h post-stroke, GFAP measures were the same as vehicle-treated controls. In aged mice (panel d), there was an increase in the level of GFAP expression in both the 3 and 6 h groups, however, only the 3 h group showed any significant difference compared with vehicle-treated age-matched control. A n=5 per group was used for these studies. +=P<0.05, ++=P<0.01 compared with stroke + vehicle-treated controls. The white boxes in panels a and b represent the region where fluorescent intensity measures were taken. Scale bar in panel b, 2 is equal to 1 mm
Discussion
DV, an 85 kDa extracellular matrix protein fragment of the heparan sulfate proteoglycan, perlecan, has been demonstrated to modulate angiogenesis outside the brain [3, 4]. Angiogenic and astroglial responses play a critical role in mediating post-stroke brain repair. We previously investigated DV’s therapeutic potential in transient MCAo stroke models in young adult mice and rats [19] and highlighted that perlecan deficiency had a negative consequence for infarct sizes, astrogliosis, and angiogenic response. We also demonstrated that systemic DV treatment was neuroprotective, pro-astrogliotic, pro-angiogenic and afforded substantial motor recovery [19]. In these studies, intraperitoneal administered DV was shown to be home to the area of brain injury and cross the stroke-disrupted blood–brain barrier (BBB) [19]. DV then induces neuroprotection by binding to the brain endothelial cell alpha5beta1 integrin receptor and via ERK-dependent intracellular signaling and triggers the production and release of VEGF from endothelial cells in stroked brain tissues [11, 19]. The mechanisms and involvement of VEGF in mediating neuroprotection associated with DV administration were confirmed by using the alpha5beta1 integrin blocking antibody or following intravenous injection of the VEGF receptor blocker PTK787/ZK 222584, prior to administration of DV [19]. Additionally, DV treatment after experimental stroke results in diminished apoptosis as determined by decreased TUNEL stain, caspase-3 immunohistochemistry, and improved cresyl violet neuronal morphology in the stroke-affected brains [19].
However, nearly 90 % of all strokes happen in humans aged >65 years [30]. In addition, the elderly have a reduced capacity to recover from their impairments compared with younger stroke survivors [18]. Given these facts, most experimental stroke research is still carried out using young animals, despite the recommendations by the Stroke Therapy Academic Industry Round and other stroke committees to use aged animals in preclinical studies in addition to using various stroke models [27]. Many human stroke survivors have small infarcts [7], with the most common functional deficit following stroke being motor impairments of the contralateral upper limb with approximately 60 % of stroke patients having lasting deficits [16]. For these reasons, in the present study, we asked whether DV administration could also be neuroprotective and improve motor recovery in a focal photothrombosis motor cortex stroke model, in both young and aged mice.
The photothrombosis model of stroke affords very little ischemic penumbra to neuroprotect and, in many ways, resembles traumatic brain injury more than stroke due to the larger than normal edema response [9]. Furthermore, the permanent nature of this stroke model, with little local collateral flow, affords no conduit for administered DV to directly reach the stroke affected tissue, limiting its potential neuroprotective impact to this small ischemic penumbra. Importantly, the relative protection that was observed following treatment with DV in the current study was time-dependent, inasmuch as, the sooner DV was administered, the more neuroprotection (and functionally beneficial) the DV treatment was. This time dependence is likely due to several important factors.
In the photothrombotic stroke model, the infarct volume rapidly evolves such that it has essentially reached its maximal volume by 24 h post stroke [6, 10]. However, others have reported that apoptosis is maximal between 1–3 days after the stroke with secondary apoptosis continuing for up to 4 weeks [6]. As it takes IP administered DV approximately 4 h to reach the stroked brain [19], DV doses administered at 3, 6, 12, and 24 h post-stroke are effectively reaching the stroked brain at 7, 10, 16, and 28 h. Interestingly, the relative difference in therapeutic time window for DV to afford protection between young and aged mice (broader for younger mice) could suggest that the stroke infarct volume evolves more rapidly in aged mice, as has been suggested for various other stroke models in rats [25].
Alternatively, increased BBB susceptibility with age and ischemic injury could also be playing a significant role in regulating therapeutic time windows [15, 20, 31]. Recent studies have shown that the aged brain is more susceptible to ischemic injury, with aged suffering larger strokes, having reduced functional recovery with a twofold increase in BBB disruption that precedes cell death [15]. This is likely to alter the time frame for which DV is likely to reach the brain and have any protective effects, an area of active investigation in our laboratories. Nevertheless, this work is the first to demonstrate that DV is effective in aged stroked animals, thereby further supporting its potential therapeutic benefit in ischemic stroke.
The formation of the glial scar/astrogliosis plays an essential role after stroke. While it can acutely serve to confine the stroke and limit the spread of cellular damage into the penumbra and or regions of healthy tissue acutely, it can also chronically impede recovery by serving as a physical barrier to neurorepair [26]. We have previously documented that DV treatment can enhance acute (3 days post-stroke) peri-infarct astrogliosis after transient MCAo in young mice through an elevation in GFAP expression, while decreasing it and the chondroitin sulfate proteoglycan glial scar components neurocan and phosphacan chronically (14 days post-stroke) [1]. Consistent with these findings, we also saw an elevation in peri-infarct GFAP expression in DV-treated animals 7 days after photothrombosis in young and old animals. However, this elevation only occurred in groups where we also saw significant protection and improvement in functional recovery. Future longer-term studies (14–21-days after photothrombosis) will determine whether DV treatment also suppresses chronic astrogliosis, as suggested by our previous work.
DV appears to be acting on post-stroke astrogliosis like a double-edged sword; that is by increasing astrogliosis acutely but suppressing astrogliosis chronically. While the mechanisms underlying this dual function of DV in astrogliosis after stroke remain to be determined, our previous work suggests that it could be due, at least in part, to DV’s variable interactions with three distinct receptors on astrocytes, the alpha2beta1 integrin, the alpha5beta1 integrin, and alphadystroglycan [1]. Indeed, DV had multiple effects on astrocytes in vitro, depending on which receptor DV interacted with. Specifically, DV interaction with alpha5beta1 integrin or alpha-dystroglycan increased astrocyte migration in vitro, while interaction with alpha2beta1 integrin decreased it [1]. The stimulating effects of DV on alpha5beta1 integrin (and subsequent release of pro-angiogenic VEGF from brain endothelial cells) also is responsible for DV’s increase in post-stroke angiogenesis as we have previously reported [19]. This is also consistent with recent publications showing that chronic cerebral hypoxia can induce angiogenic remodeling of vessels through the switch in beta1 integrins from alpha6 to alpha5 [5]. Perlecan and DV may contribute to this switch as perlecan increases alpha5beta1 integrin expression on brain endothelial cells significantly more than other extracellular matrix components (e.g., collagen I, collagen IV, and fibronectin), and DV has a similar effect after transient MCAo [19, 23]. In addition, we have previously shown that the DV-induced increase in post-stroke angiogenesis could be inhibited following administration of the alpha5beta1 integrin function-blocking antibody [19]. Therefore, it is intriguing to speculate that DV’s varying acute and chronic effects on astrogliosis after stroke could be due to varying astrocyte receptor expression at different time points after stroke. This is an ongoing area of investigation in our lab.
Summary/Conclusions
In this study, we have demonstrated that perlecan DV is neuroprotective and functionally beneficial in a permanent stroke model in both young and aged mice. This result lends further support to the potential therapeutic benefit of DV in ischemic stroke. Of important note, however, is the potential shift in the therapeutic time window to being shorter in aged compared with young.
Acknowledgments
We would like to acknowledge the help of Lisa Auckland. This work was supported by a Sir Charles Hercus Fellowship from the Health Research Council of New Zealand (A.N.C.) and the National Institutes of Health, NINDS grant R01NS065842-01A01 (G.J.B.).
Footnotes
Compliance with Ethics Dr. Gregory Bix declares that he has no conflict of interest. Ms. Emma Gowing declares that she has no conflict of interest. Dr Andrew Clarkson declares that he has no conflict of interest. All institutional and national guidelines for the care and use of laboratory animals were followed.
Contributor Information
Gregory J. Bix, Anatomy and Neurobiology, University of Kentucky College of Medicine, Sanders Brown Center for Aging, Lexington, KY 40536, USA Neurology, University of Kentucky College of Medicine, Sanders Brown Center for Aging, Lexington, KY 40536, USA.
Emma K. Gowing, Anatomy, University of Otago, P.O. Box 913, Dunedin 9054, New Zealand Brain Health Research Center, University of Otago, P.O. Box 913, Dunedin 9054, New Zealand.
Andrew N. Clarkson, Email: andrew.clarkson@anatomy.otago.ac.nz, Anatomy, University of Otago, P.O. Box 913, Dunedin 9054, New Zealand; Psychology, University of Otago, P.O. Box 913, Dunedin 9054, New Zealand; Brain Health Research Center, University of Otago, P.O. Box 913, Dunedin 9054, New Zealand.
References
- 1.Al-Ahmad AJ, Lee B, Saini M, Bix GJ. Perlecan domain V modulates astrogliosis in vitro and after focal cerebral ischemia through multiple receptors and increased nerve growth factor release. Glia. 2011;59:1822–1840. doi: 10.1002/glia.21227. [DOI] [PubMed] [Google Scholar]
- 2.Baskin YK, Dietrich WD, Green EJ. Two effective behavioral tasks for evaluating sensorimotor dysfunction following traumatic brain injury in mice. J Neurosci Methods. 2003;129:87–93. doi: 10.1016/s0165-0270(03)00212-7. [DOI] [PubMed] [Google Scholar]
- 3.Bix G, Castello R, Burrows M, Zoeller JJ, Weech M, Iozzo RA, et al. Endorepellin in vivo: targeting the tumor vasculature and retarding cancer growth and metabolism. J Natl Cancer Inst. 2006;98:1634–1646. doi: 10.1093/jnci/djj441. [DOI] [PubMed] [Google Scholar]
- 4.Bix G, Fu J, Gonzalez EM, Macro L, Barker A, Campbell S, et al. Endorepellin causes endothelial cell disassembly of actin cytoskeleton and focal adhesions through alpha2beta1 integrin. J Cell Biol. 2004;166:97–109. doi: 10.1083/jcb.200401150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boroujerdi A, Welser-Alves JV, Tigges U, Milner R. Chronic cerebral hypoxia promotes arteriogenic remodeling events that can be identified by reduced endoglin (CD105) expression and a switch in beta1 integrins. J Cereb Blood Flow Metab. 2012;32:1820–1830. doi: 10.1038/jcbfm.2012.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Braun JS, Jander S, Schroeter M, Witte OW, Stoll G. Spatiotemporal relationship of apoptotic cell death to lymphomonocytic infiltration in photochemically induced focal ischemia of the rat cerebral cortex. Acta Neuropathol. 1996;92:255–263. doi: 10.1007/s004010050516. [DOI] [PubMed] [Google Scholar]
- 7.Brott T, Marler JR, Olinger CP, Adams HP, Jr, Tomsick T, Barsan WG, et al. Measurements of acute cerebral infarction: lesion size by computed tomography. Stroke. 1989;20:871–875. doi: 10.1161/01.str.20.7.871. [DOI] [PubMed] [Google Scholar]
- 8.Carmichael ST. Plasticity of cortical projections after stroke. Neuroscientist. 2003;9:64–75. doi: 10.1177/1073858402239592. [DOI] [PubMed] [Google Scholar]
- 9.Carmichael ST. Rodent models of focal stroke: size, mechanism, and purpose. NeuroRx. 2005;2:396–409. doi: 10.1602/neurorx.2.3.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen F, Suzuki Y, Nagai N, Jin L, Yu J, Wang H, et al. Rodent stroke induced by photochemical occlusion of proximal middle cerebral artery: evolution monitored with MR imaging and histopathology. Eur J Radiol. 2007;63:68–75. doi: 10.1016/j.ejrad.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 11.Clarke DN, Al Ahmad A, Lee B, Parham C, Auckland L, Fertala A, et al. Perlecan domain V induces VEGf secretion in brain endothelial cells through integrin alpha5beta1 and ERK-dependent signaling pathways. PLoS One. 2012;7:e45257. doi: 10.1371/journal.pone.0045257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clarkson AN, Huang BS, Macisaac SE, Mody I, Carmichael ST. Reducing excessive GABA-mediated tonic inhibition promotes functional recovery after stroke. Nature. 2010;468:305–309. doi: 10.1038/nature09511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clarkson AN, Lopez-Valdes HE, Overman JJ, Charles AC, Brennan KC, Thomas Carmichael S. Multimodal examination of structural and functional remapping in the mouse photothrombotic stroke model. J Cereb Blood Flow Metab. 2013;33:716–723. doi: 10.1038/jcbfm.2013.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clarkson AN, Overman JJ, Zhong S, Mueller R, Lynch G, Carmichael ST. AMPA receptor-induced local brain-derived neurotrophic factor signaling mediates motor recovery after stroke. J Neurosci. 2011;31:3766–3775. doi: 10.1523/JNEUROSCI.5780-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dinapoli VA, Huber JD, Houser K, Li X, Rosen CL. Early disruptions of the blood–brain barrier may contribute to exacerbated neuronal damage and prolonged functional recovery following stroke in aged rats. Neurobiol Aging. 2008;29:753–764. doi: 10.1016/j.neurobiolaging.2006.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dobkin BH. Training and exercise to drive poststroke recovery. Nat Clin Pract Neurol. 2008;4:76–85. doi: 10.1038/ncpneuro0709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hansen TM, Moss AJ, Brindle NP. Vascular endothelial growth factor and angiopoietins in neurovascular regeneration and protection following stroke. Curr Neurovasc Res. 2008;5:236–245. doi: 10.2174/156720208786413433. [DOI] [PubMed] [Google Scholar]
- 18.Kelly-Hayes M, Beiser A, Kase CS, Scaramucci A, D'agostino RB, Wolf PA. The influence of gender and age on disability following ischemic stroke: the Framingham study. J Stroke Cerebrovasc Dis. 2003;12:119–126. doi: 10.1016/S1052-3057(03)00042-9. [DOI] [PubMed] [Google Scholar]
- 19.Lee B, Clarke D, Al Ahmad A, Kahle M, Parham C, Auckland L, et al. Perlecan domain V is neuroprotective and proangiogenic following ischemic stroke in rodents. J Clin Invest. 2011;121:3005–3023. doi: 10.1172/JCI46358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu F, Yuan R, Benashski SE, Mccullough LD. Changes in experimental stroke outcome across the life span. J Cereb Blood Flow Metab. 2009;29:792–802. doi: 10.1038/jcbfm.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lo EH. A new penumbra: transitioning from injury into repair after stroke. Nat Med. 2008;14:497–500. doi: 10.1038/nm1735. [DOI] [PubMed] [Google Scholar]
- 22.Lo EH, Dalkara T, Moskowitz MA. Mechanisms, challenges and opportunities in stroke. Nat Rev Neurosci. 2003;4:399–415. doi: 10.1038/nrn1106. [DOI] [PubMed] [Google Scholar]
- 23.Milner R, Hung S, Wang X, Berg GI, Spatz M, Del Zoppo GJ. Responses of endothelial cell and astrocyte matrix-integrin receptors to ischemia mimic those observed in the neurovascular unit. Stroke. 2008;39:191–197. doi: 10.1161/STROKEAHA.107.486134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ohab JJ, Fleming S, Blesch A, Carmichael ST. A neurovascular niche for neurogenesis after stroke. J Neurosci. 2006;26:13007–13016. doi: 10.1523/JNEUROSCI.4323-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Popa-Wagner A, Buga AM, Kokaia Z. Perturbed cellular response to brain injury during aging. Ageing Res Rev. 2011;10:71–79. doi: 10.1016/j.arr.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 26.Rolls A, Shechter R, Schwartz M. The bright side of the glial scar in CNS repair. Nat Rev Neurosci. 2009;10:235–241. doi: 10.1038/nrn2591. [DOI] [PubMed] [Google Scholar]
- 27.Saver JL, Albers GW, Dunn B, Johnston KC, Fisher M. Stroke Therapy Academic Industry Roundtable (STAIR) recommendations for extended window acute stroke therapy trials. Stroke. 2009;40:2594–2600. doi: 10.1161/STROKEAHA.109.552554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schallert T, Fleming SM, Leasure JL, Tillerson JL, Bland ST. CNS plasticity and assessment of forelimb sensorimotor outcome in unilateral rat models of stroke, cortical ablation, parkinsonism and spinal cord injury. Neuropharmacol. 2000;39:777–787. doi: 10.1016/s0028-3908(00)00005-8. [DOI] [PubMed] [Google Scholar]
- 29.Sun Y, Jin K, Xie L, Childs J, Mao XO, Logvinova A, et al. VEGF-induced neuroprotection, neurogenesis, and angiogenesis after focal cerebral ischemia. J Clin Invest. 2003;111:1843–1851. doi: 10.1172/JCI17977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Truelsen T, Piechowski-Jozwiak B, Bonita R, Mathers C, Bogousslavsky J, Boysen G. Stroke incidence and prevalence in Europe: a review of available data. Eur J Neurol. 2006;13:581–598. doi: 10.1111/j.1468-1331.2006.01138.x. [DOI] [PubMed] [Google Scholar]
- 31.Yao H, Yoshii N, Akira T, Nakahara T. Reperfusion-induced temporary appearance of therapeutic window in penumbra after 2 h of photothrombotic middle cerebral artery occlusion in rats. J Cereb Blood Flow Metab. 2009;29:565–574. doi: 10.1038/jcbfm.2008.147. [DOI] [PubMed] [Google Scholar]
- 32.Zhang ZG, Zhang L, Jiang Q, Zhang R, Davies K, Powers C, et al. VEGF enhances angiogenesis and promotes blood–brain barrier leakage in the ischemic brain. J Clin Invest. 2000;106:829–838. doi: 10.1172/JCI9369. [DOI] [PMC free article] [PubMed] [Google Scholar]