Abstract
Rationale
Autophagy is an essential survival mechanism during energy stress in the heart. Oxidative stress is activated by energy stress, but its role in mediating autophagy is poorly understood. Nox4 is an enzyme that generates reactive oxygen species (ROS) at intracellular membranes. Whether Nox4 acts as a sensor of energy stress to mediate activation of autophagy is unknown.
Objective
We investigated whether Nox4 is involved in the regulation of autophagy and cell survival during energy stress in cardiomyocytes (CMs).
Methods and Results
Production of ROS in CMs was increased during glucose deprivation (GD) in a Nox4-dependent manner. Protein levels and the ROS-producing activity of Nox4 were increased in the endoplasmic reticulum (ER), but not in mitochondria, in response to GD. Selective knockdown of Nox4, but not Nox2, or selective reduction of ROS in the ER with ER-targeted catalase, but not mitochondria-targeted perioxiredoxin3, abrogated GD-induced autophagy. Nox4 promoted autophagy during GD through activation of the PKR-like ER kinase (PERK) pathway by suppression of prolyl hydroxylase4 (PHD4). The decrease in cell survival during GD in the presence of Nox4 knockdown was rescued by reactivation of autophagy by Atg7 overexpression, indicating that the effect of Nox4 upon cell survival is critically mediated through regulation of autophagy. Nox4 was activated during fasting and prolonged ischemia in the mouse heart, where Nox4 is also required for autophagy activation and cardioprotection.
Conclusions
Nox4 critically mediates autophagy in response to energy stress in CMs by eliciting ROS in the ER and stimulating the PERK signaling pathway.
Keywords: Nox4, autophagy, fasting, ROS, energy stress
INTRODUCTION
Autophagy is an evolutionarily conserved intracellular bulk degradation process for the elimination of long-lived proteins and damaged organelles1. Autophagy plays a critical role in mediating cell adaptation to energy stress1–4. Autophagy is activated in energy-deprived cells, where it promotes cell survival by providing energetic substrates for the production of ATP and by favoring the turnover of damaged proteins and dysfunctional organelles1. The beneficial role of autophagy during energy stress is highly relevant to human cardiac disease. Autophagy is activated in the heart during myocardial ischemia, a common and highly morbid clinical condition characterized by nutrient deprivation and severe energy stress, in which activation of autophagy is essential for protection of cardiomyocytes (CMs) and limitation of myocardial injury2, 5. Autophagy is a complex and dynamic multi-step process which requires tight regulation and coordination by multiple signaling pathways. The signaling mechanisms that promote autophagy activation and cell survival during nutrient deprivation and energy stress have yet to be fully clarified.
Nox2 and Nox4 are main NADPH oxidase (Nox) isoforms in CMs6. The Nox family proteins are the only cellular enzymes that are devoted to the purposeful production of reactive oxygen species (ROS), including O2− and H2O2. Nox2 is mainly localized on the plasma membrane, whereas Nox4 is localized on intracellular membranes, particularly in mitochondria and the endoplasmic reticulum (ER)6. Nox2 is regulated through interaction with cytosolic factors, including p40phox, p47phox, p67phox and Rac1, whereas Nox4 is primarily regulated through transcriptional mechanisms, although Nox4 activity has been shown to also be regulated by Poldip2 6. Although chronic and high activation of Nox4 is detrimental in CMs during severe stress, such as severe pressure overload and reperfusion injury7–9, a physiological and moderate activation of Nox4 promotes adaptive and physiological functions in response to cellular stress6. Under hypoxia, Nox4 was suggested to promote erythropoietin secretion in renal cells10. Nox4 also protects kidneys in diabetes11, mediates the cellular response against proteotoxic stress through local activation of the Ras/ERK pathway12, and promotes angiogenesis in response to inflammatory stress and chronic limb ischemia13, 14. In the heart, although single KO of Nox2 or Nox4 reduces reperfusion injury by preventing excessive accumulation of ROS, combined KO of Nox2 and Nox4 exacerbates myocardial reperfusion injury through PPARα activation9. Pressure overload exacerbated left ventricular dysfunction in systemic Nox4 KO mice15 but not in cardiac specific Nox4 KO mice7. Thus, it appears that Nox2 and Nox4 have cell protective functions in some cardiac pathological conditions, activating physiological signaling mechanisms, perhaps in a cell type-specific manner. However, the involvement of Nox isoforms in the regulation of cardiomyocyte autophagy and survival in response to energy deprivation has not been established.
Autophagy is either positively or negatively regulated by oxidative stress16. Intracellular ROS levels increase in response to energy deprivation17, 18. However, exactly where and how ROS are produced and how ROS affect autophagy and survival of CMs during energy deprivation remains to be elucidated. Here we tested the hypothesis that Nox4, that produces ROS at intracellular compartments, plays a critical role in promoting autophagy and cell survival in response to energy deprivation in CMs. Thus, the goals in this study were: 1) to test whether Nox4 is activated in energy-deprived CMs and, if so, 2) to elucidate how Nox4 regulates autophagy in CMs during energy deprivation, and 3) to test whether Nox4 regulates survival of CMs during energy deprivation through autophagy, both in vitro and in vivo.
METHODS
An expanded Methods section is available in the Online Data Supplement.
ROS measurements
Detection of NADPH-dependent O2− production, and detection of O2− and H2O2 production by using dihydroethidium, 5-(and-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate, acetyl ester and the Amplex Red kit (Invitrogen) have been described7, 19. Mitochondrial and ER-specific H2O2 production were evaluated through the expression of compartment-specific HyPer protein9, 12.
Mouse models
Cardiac-specific Nox4 knockout mice, fasting and prolonged ischemia models have been described2, 3, 7, 19. Adenovirus (1x109 pfu) was administered by direct injection into the LV free wall (two sites, 25 μl/site).
RESULTS
Nox4 upregulation in the ER is required for energy-deprivation-induced ROS production in CMs
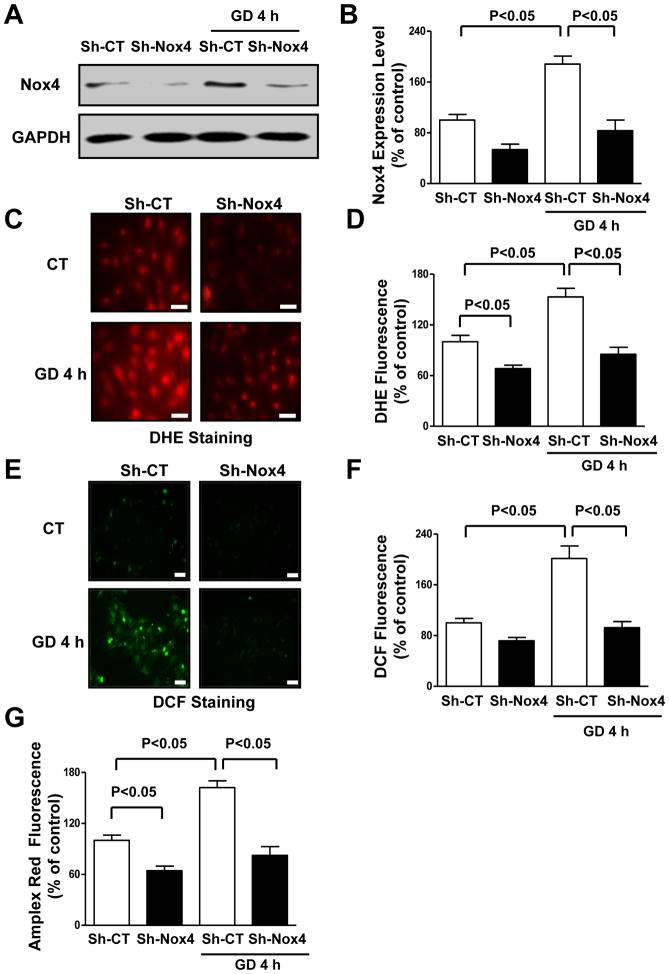
We evaluated whether Nox4 is modulated during the early CM response to energy deprivation. mRNA levels of Nox4 were time-dependently increased in CMs subjected to glucose deprivation (GD) (Online Figure IA). Nox4 protein levels were also increased during GD (Figure 1A–B). Nox4 can be regulated by NF-κB, which is activated during energy stress19, 20. NF-κB was activated in CMs during GD and GD-induced Nox4 activation was abrogated when NF-κB was inhibited by overexpression of constitutively active IκB protein19 (Online Figure IB–D). This suggests that Nox4 upregulation during GD is NF-κB-dependent. ROS levels, as assessed by dihydroethidium (DHE) staining for O2− detection and 2′,7′-dichlorofluorescein diacetate (DCF) and Amplex Red assays for H2O2 detection, also time-dependently increased in glucose-deprived CMs, paralleling the increase in Nox4 levels (Figure 1C–G; Online Figure IE). sh-RNA-mediated Nox4 knockdown (Figure 1A–B) significantly reduced ROS production in CMs during GD (Figure 1C–G), indicating that Nox4 is required for ROS production during energy deprivation in CMs.
Figure 1. Nox4 levels are increased in CMs during GD and promote ROS production.
A–B. CMs were transduced with adenoviruses expressing a short hairpin sequence targeting Nox4 (Sh-Nox4) or short hairpin scramble (Sh-CT) for 96 hours and then subjected to GD. Densitometric analysis of Nox4 expression levels was performed. N=5. C–G. CMs with and without Nox4 knockdown were subjected to GD. DHE fluorescence (C–D), DCF fluorescence (E–F) and Amplex Red assay (G) were then performed to evaluate ROS levels. Data are presented as a percentage of control (Sh-CT). N=4–8. Bar=50 μm.
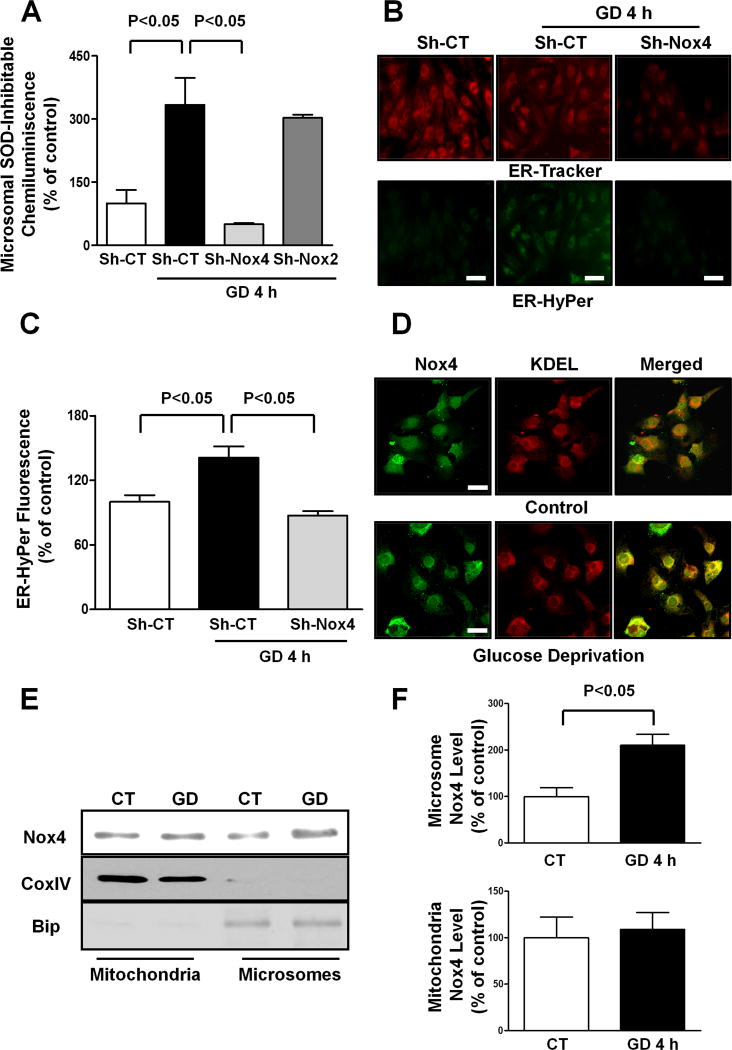
Nox4 is localized on intracellular membranes, mainly in mitochondria and the ER. We investigated in which subcellular compartment Nox4 activity increases during the early response to energy deprivation in CMs. NADPH-dependent generation of O2−, evaluated as the superoxide dismutase (SOD) inhibitable component of lucigenin chemiluminescence, was not increased in the mitochondrial fraction of CMs subjected to GD compared to at baseline (Online Figure IIA). Conversely, the SOD-inhibitable component of lucigenin chemiluminescence was significantly increased in the microsomal fraction of glucose-deprived CMs, an effect which was abolished by Nox4 knockdown (Figure 2A). In contrast, knockdown of Nox2, which is mainly located on the plasma membrane, did not affect the GD-induced increase in the SOD-inhibitable component of lucigenin chemiluminescence (Figure 2A). These results suggest that Nox4, but not Nox2, plays a critical role in mediating the GD-induced ROS production in the ER.
Figure 2. Nox4 is upregulated in the ER of glucose-deprived CMs.
A. CMs were transduced with Sh-CT, Sh-Nox4 or Sh-Nox2 for 96 hours, and then CMs were cultured with normal or glucose-free medium for 4 hours. The microsomal fraction was isolated and a lucigenin assay to evaluate NADPH-dependent O2− production was performed. N=3–5. B–C. CMs were transduced with Sh-CT or Sh-Nox4 for 96 hours together with adenovirus expressing HyPer protein targeted to the ER (Ad-ER-HyPer) for the last 48 hours. CMs were cultured with normal or glucose-free medium for 4 hours. CMs were stained with ER-tracker red. Representative images of ER-Hyper and ER-tracker fluorescence are shown (B) together with quantification (C). Bar=50 μm. D. CMs were cultured with normal or glucose-free medium for 4 hours. An immunocytofluorescence assay of Nox4 and KDEL was performed and cells were observed with a confocal microscope. Bar=20 μm. E–F. In the same conditions, mitochondrial and microsomal fractions were isolated and Nox4 protein levels were assessed in each fraction. Representative immunoblots (E) together with densitometric quantification are shown (F). N=3. All values in the bar graphs are expressed as a percentage of the control.
In order to further support this hypothesis, we expressed HyPer selectively in either mitochondria or the ER in CMs, through adenoviral transduction. HyPer allows sensitive measurement of H2O2 in cells12, 19. In CMs expressing mitochondrial-targeted HyPer, mitochondrial green fluorescence was not increased in response to GD, further excluding an increase in mitochondrial ROS production during the early response to energy deprivation (Online Figure IIB–C). Conversely, in CMs expressing ER-targeted HyPer, ER green fluorescence was significantly increased in response to GD (Figure 2B–C). Nox4 knockdown abrogated GD-induced increases in the ER HyPer signal, suggesting that Nox4 promotes ROS production in the ER of CMs in response to GD.
Co-immunostaining with ER markers, including KDEL and calreticulin, showed that Nox4 co-localization with the ER is enhanced during GD in CMs (Figure 2D; Online Figure IID). Parallel immunoblot analyses showed that GD-induced upregulation of Nox4 protein occurs in microsomes but not in mitochondria, confirming that Nox4 upregulation in response to GD occurs in the ER (Figure 2E–F). This is consistent with the fact that membrane proteins are mainly synthesized in the ER and with the evidence that the N-terminal portion of Nox4 contains multiple ER-specific signal sequences21, 22.
Nox4 upregulation in the ER promotes autophagy in energy-deprived CMs
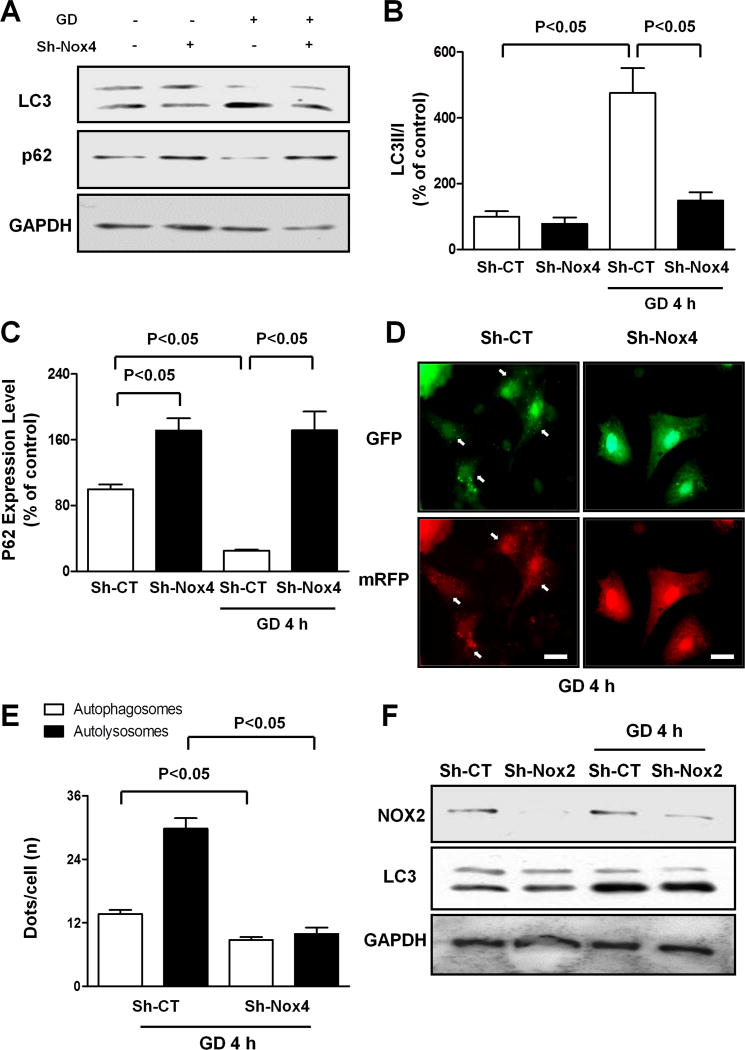
We then investigated whether Nox4 regulates autophagy in CMs subjected to energy deprivation. As shown previously, autophagy was upregulated in CMs subjected to GD, as indicated by increases in LC3II and decreases in p62 and by a significant increase in both mRFP-LC3 and GFP-LC3 puncta in Ad-tf-LC3-transduced CMs. The GD-induced increase in autophagy was abolished in Nox4-depleted CMs (Figure 3A–E), as indicated by reversal of these parameters, even in the presence of bafilomycin A1, a lysosome inhibitor (Online Figure IIIA). This suggests that Nox4 is required for GD-induced upregulation of autophagy and promotion of autophagic flux. In addition, Nox4 overexpression was sufficient to stimulate autophagy in CMs under basal conditions (Online Figure IIIB–D). Conversely, Nox2 levels were not changed during GD, and downregulation of Nox2 did not affect GD-induced autophagy in CMs (Figure 3F).
Figure 3. Nox4 is required for autophagy activation in CMs during GD.
A–C. CMs were transduced with Sh-CT or Sh-Nox4 for 96 hours, and then CMs were cultured with normal or glucose-free medium. LC3 and p62 protein levels were evaluated. Representative immunoblots (A) and densitometric quantification (B–C) are shown. N=4. Data are presented as a percentage of Sh-CT. D–E. CMs were transduced with Sh-CT or Sh-Nox4 for 96 hours together with Ad-mRFP-GFP-LC3 for the last 48 hours. CMs were cultured with glucose-free medium. Representative images of mRFP and GFP dots are shown (D), together with quantification of autophagosomes and autolysosomes (E). N=5. Bar=10 μm. F. CMs were transduced with Sh-CT or Sh-Nox2 for 96 hours, and then CMs were cultured with normal or glucose-free medium. LC3II and Nox2 levels were evaluated.
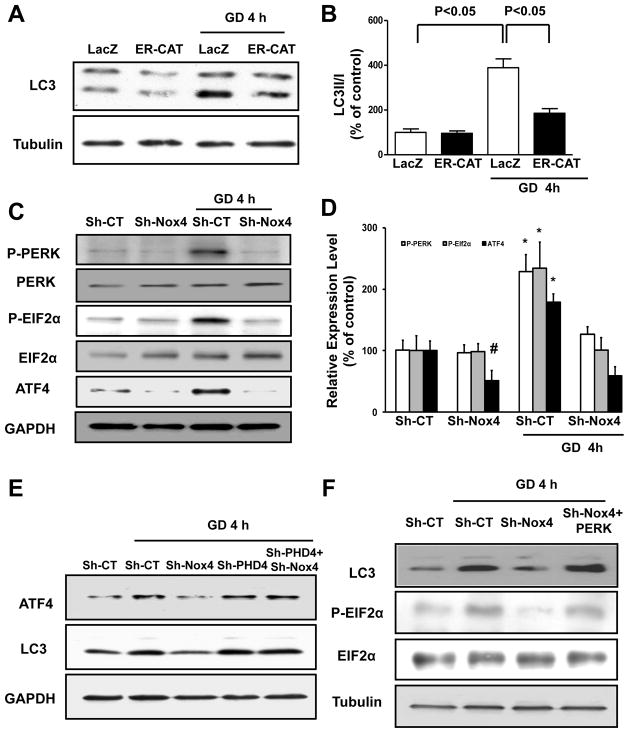
We then studied the biological relevance of Nox4-dependent ROS production in the ER of energy-deprived CMs. Specific overexpression of catalase in the ER, using adenovirus encoding the catalase protein fused to an N-terminal ER-targeting motif12, significantly reduced ROS in the ER and blunted autophagy during GD, suggesting that increases in ER ROS levels by Nox4 play a critical role in the promotion of GD-induced autophagy (Figure 4A–B; Online Figure IVA–B). Conversely, overexpression of peroxiredoxin-3, which scavenges H2O2 selectively in mitochondria19, did not affect autophagy during GD (Online Figure IVC).
Figure 4. Nox4 activation in the ER promotes autophagy in energy-deprived CMs through activation of the PERK/eIF-2α/ATF4 pathway.
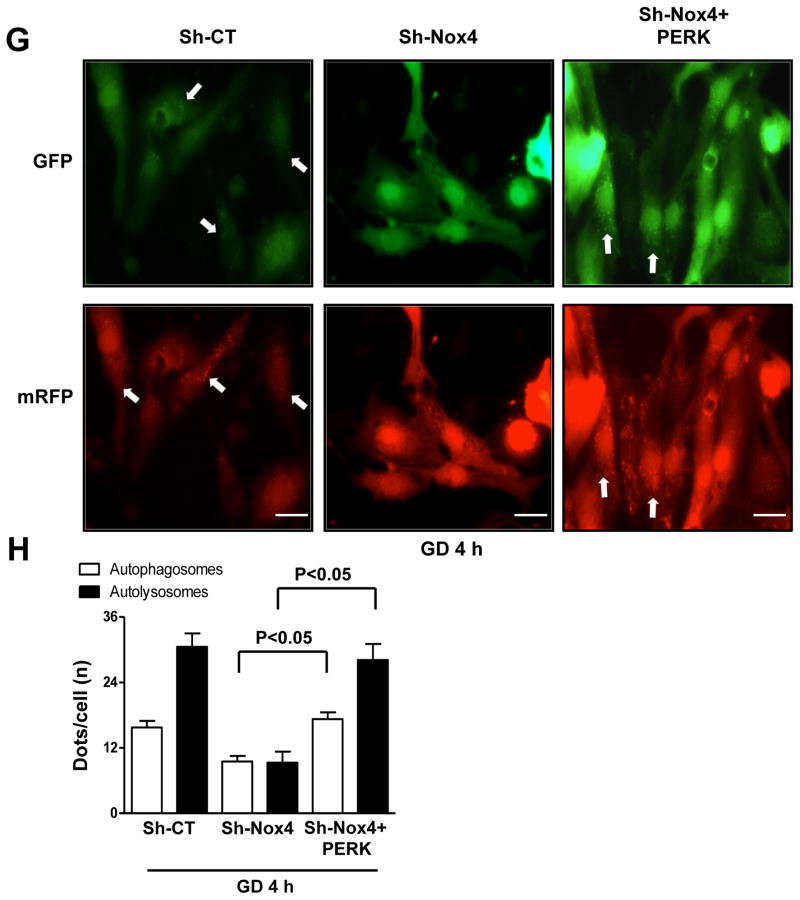
A–B. CMs were transduced with Ad-LacZ or Ad-ER-catalase for 48 hours, and then CMs were cultured with normal or glucose-free medium for 4 hours. LC3II levels were evaluated. Representative immunoblots (A) and densitometric quantification (B) are shown. C–D. CMs were transduced with Sh-CT or Sh-Nox4 for 96 hours, and then CMs were cultured with normal or glucosefree medium. Phospho-PERK (Thr980), phospho-eIF-2α (Ser51) and ATF4 protein levels were evaluated. Representative immunoblots (C) and densitometric quantification (D) are shown. N=4. # p<0.05 vs. Sh-CT; * p<0.05 vs. Sh-CT and Sh-Nox4 GD. E. CMs were transduced with Sh-CT or Sh-Nox4 for 96 hours, with and without Sh-PHD4. CMs were cultured with normal or glucose-free medium. ATF4 and LC3 levels were evaluated. F. CMs were transduced with Sh-CT or Sh-Nox4 for 96 hours, together with Ad-LacZ or Ad-PERK for the last 48 hours. CMs were cultured with normal or glucose-free medium. LC3II and phospho-eIF-2α levels were evaluated. G–H. CMs were transduced with Sh-CT or Sh-Nox4 for 96 hours, together with Ad-LacZ or Ad-PERK and with Ad-mRFP-GFP-LC3 for the last 48 hours. CMs were cultured with glucose-free medium. Representative images of mRFP and GFP dots are shown (G), together with quantification of autophagosomes and autolysosomes (H). N=4. Bar=10 μm.
Then, we investigated whether Nox4-derived ROS production in the ER regulates autophagy through modulation of ER signaling. Energy stress was previously shown to promote upregulation of the PERK/eIF-2α/ATF4 pathway17. We hypothesized that Nox4-derived ROS in the ER of energy-depleted CMs upregulate the PERK/eIF-2α/ATF4 pathway, which can promote autophagy23, 24. The PERK/eIF-2α/ATF4 pathway was significantly activated in CMs subjected to GD, as indicated by increased phosphorylation of PERK (Thr980) and eIF-2α (Ser51) and increased expression levels of ATF4. GD-induced activation of this pathway was abolished when Nox4 was downregulated, suggesting that Nox4 activation is required for activation of the PERK/eIF-2α/ATF4 pathway during CM energy deprivation (Figure 4C–D). ROS can inactivate prolyl hydroxylase enzymes (PHDs)9, 15, 25 and PHD inhibition was shown to activate PERK and ATF426, 27. We found that knockdown of the ER-specific PHD, namely PHD428, rescues ATF4 expression and autophagy in Nox4-depleted CMs during GD. This result suggests that Nox4-derived ROS in the ER promote activation of the PERK/eIF-2α/ATF4 pathway through inactivation of PHD4 (Figure 4E; Online Figure IVD). Of note, Nox4 knockdown did not inhibit the ERK pathway at baseline and during GD, as it does in response to ER stress12. Remarkably, neither 4 hours of GD nor Nox4 knockdown affected the activity of ATF6 or XBP-1 (Online Figure IVE–K). Of note, although Nox4 knockdown still inhibited ATF4 upregulation, it did not inhibit ATF6 or XBP-1 activity even after prolonged GD, when these molecules are strongly activated (Online Figure IVL). Rather, ATF6 and XBP-1 tended to be even more active in Nox4-depleted CMs after prolonged GD, very likely as a secondary compensatory/death-limiting response to ER stress caused by cellular damage. Finally, we found that pharmacological inhibition of PERK, but not of ATF6 or XBP-1 suppresses autophagy in the early phase of GD (Online Figure IVM–N).
In order to test the hypothesis that inhibition of GD-induced autophagy by Nox4 knockdown is mediated by suppression of the PERK/eIF-2α/ATF4 pathway, we reactivated the pathway through adenovirus-mediated overexpression of PERK. PERK overexpression in Nox4-depleted CMs under GD restored eIF-2α phosphorylation and completely rescued autophagy activation (Figure 4F–H). This indicates that Nox4 promotes autophagy in energy-deprived CMs through activation of the PERK/eIF-2α/ATF4 pathway. Overexpression of ER-catalase, but not peroxiredoxin-3, diminished Nox4-induced activation of PERK and autophagy (Online Figure IVO–P) suggesting that Nox4 promotes PERK activation through ROS production in the ER, but not in mitochondria.
Nox4 activation during CM energy deprivation promotes survival through autophagy activation
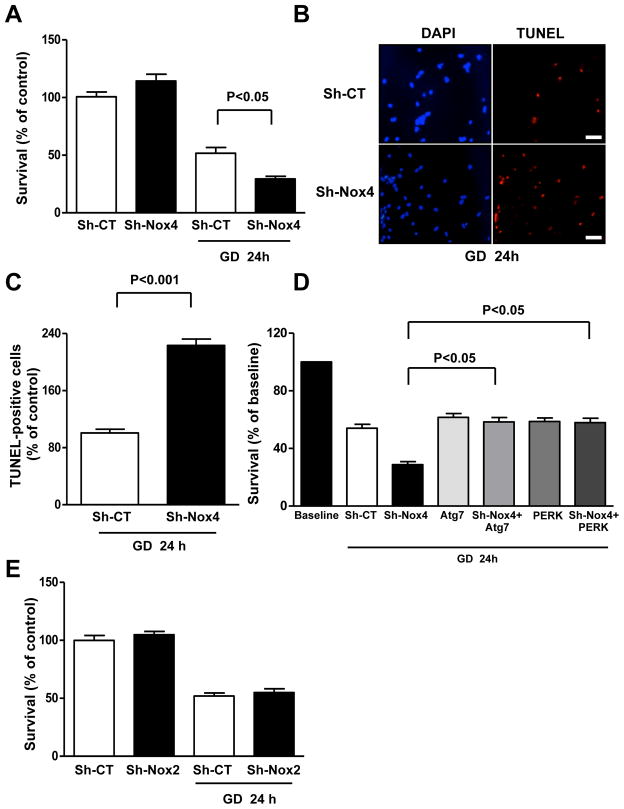
We tested the physiological relevance of Nox4 activation during CM energy deprivation. The survival of CMs during GD was significantly reduced when Nox4 was downregulated (Figure 5A). The percentage of TUNEL-positive cells was also higher in Nox4-depleted CMs than in control CMs (Figure 5B–C). Both overexpression of Atg7, which strongly activates autophagy (Online Figure V) and represents a validated method to rescue autophagic activity in a specific manner2, 29, 30, and overexpression of PERK completely reversed the enhancement of GD-induced cell death in Nox4-downregulated CMs (Figure 5D). These results suggest that Nox4 inhibition reduces CM survival through suppression of PERK activation and inhibition of autophagy. Conversely, Nox2 knockdown did not affect CM survival during GD (Figure 5E).
Figure 5. Nox4 disruption reduces CM survival and promotes apoptosis in CMs during GD.
A–C. CMs were transduced with Sh-CT or Sh-Nox4 for 96 hours, and then CMs were cultured with normal or glucose-free medium for 24 hours. Cell survival was assessed by Cell Titer Blue assay (CTB, A). N=4. Percentage of TUNEL-positive cells was also calculated. Representative pictures (B), together with quantification (C) are presented. N=3. Bar=50 μm D. CMs were transduced with Sh-CT or Sh-Nox4 for 96 hours, together with Ad-LacZ or Ad-Atg7 or Ad-PERK for 48 hours. CMs were then cultured with normal or glucose-free medium. Cell survival was assessed by CTB. N=4. Data are expressed as percentage of baseline (cells cultured with regular medium). E. CMs were transduced with Sh-CT or Sh-Nox2 for 96 hours, and then CMs were cultured with normal or glucose-free medium. Cell survival was then assessed by CTB. N=3.
Nox4 is activated in the energy-deprived heart in vivo, thereby promoting autophagy and cardioprotection
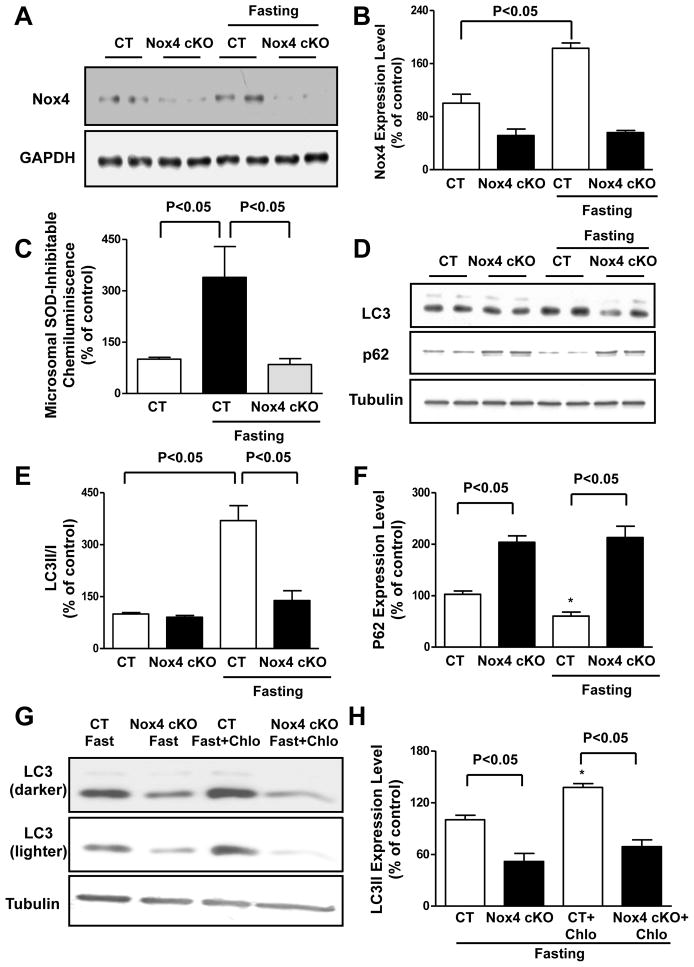
We then tested our hypothesis in vivo by subjecting mice to fasting for 48 hours. The level of Nox4 in the heart was increased significantly in response to fasting (Figure 6A–B). The Nox4 protein levels and the NADPH-dependent generation of O2− were not increased in the cardiac mitochondrial fraction during fasting (Online Figure VIA–B), whereas they were significantly increased in the microsomal fraction (Figure 6C). The fasting-induced increase in the microsomal generation of O2− was totally abolished in the heart of cardiac-specific Nox4 knockout (Nox4 cKO) mice.
Figure 6. Nox4 is activated and promotes autophagy in the heart during fasting in vivo.
A–B. Control and Nox4 cKO mice were subjected to 48 hours of fasting. Cardiac Nox4 protein abundance was evaluated. Representative immunoblot (A) and densitometric analyses are shown (B). N=4. C. Control and Nox4 cKO mice were subjected to 48 hours of fasting. Cardiac microsomes were isolated and NADPH-dependent O2− production was assessed by lucigenin assay. N=4–6. D–F. Control and Nox4 cKO mice were subjected to 48 hours of fasting. Cardiac LC3 and p62 protein levels were evaluated. Representative immunoblots (D) and densitometric analyses are shown (E–F). N=4–5. G–H. Control and Nox4 cKO mice were subjected to 48 hours of fasting, and some received chloroquine (10 mg/kg i.p) 4 hours before they were sacrificed. Representative immunoblot of cardiac LC3 is shown, together with quantification analysis. N=3. * p<0.05 vs. CT. Data are expressed as a percentage of the control.
Fasting-induced increases in autophagy in the heart were abolished in Nox4 cKO mice, as indicated by decreases in the LC3II/I ratio and abolished degradation of p62 (Figure 6D–F). Cardiac LC3II levels after fasting were significantly lower in Nox4 cKO mice even after chloroquine treatment, which inhibits lysosomal activity and autolysosome formation (Figure 6G–H), indicating that there is reduced autophagosome formation and/or autophagic flux in the heart of Nox4 cKO mice during energy deprivation. Nox4 levels were also increased in the heart during prolonged ischemia, and disruption of Nox4 resulted in autophagy inhibition (Online Figure VIC–E). Of note, Nox4 disruption also modestly affected autophagic flux at baseline, as indicated by higher levels of p62 and a slight reduction in LC3II accumulation after lysosome inhibition (Figure 6D; Online Figure VIF).
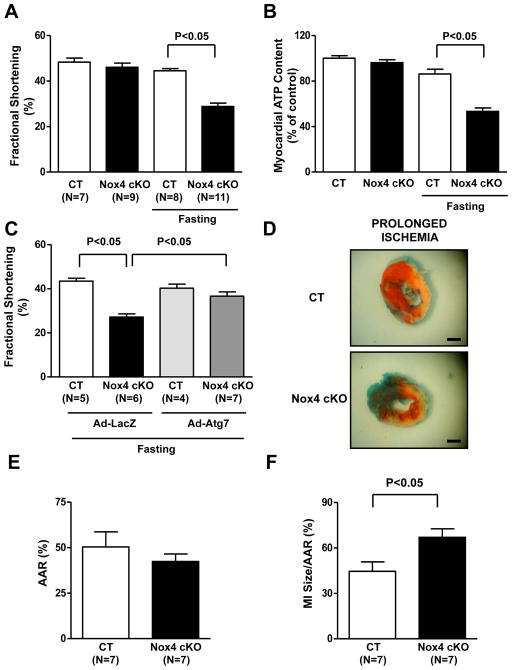
We then evaluated whether cardiac Nox4 activation during energy deprivation is an adaptive response to energy stress. Echocardiographic and hemodynamic analyses showed that Nox4 cKO mice exhibited a significant reduction in left ventricular (LV) systolic and diastolic function after 48 hours of fasting, whereas LV function was preserved in control mice, indicating that Nox4 activation is required for maintaining cardiac function during energy deprivation (Figure 7A; Online Figure VIIA–B). Although myocardial ATP content during fasting was only minimally decreased in control mice, it was reduced significantly in Nox4 cKO mice (Figure 7B). This would suggest that reduction of ATP levels may contribute to the impairment of cardiac function in Nox4 cKO mice, since a normal myocardial energy status is critical for maintenance of cardiac function31. In order to test the hypothesis that the detrimental effect of Nox4 disruption during fasting was due to inhibition of autophagy, we reactivated autophagy in the hearts of Nox4 cKO mice through cardiac injection of adenovirus harboring Atg7. To test the efficacy of the gene transfer, we injected Ad-LacZ and conducted whole-heart X-gal staining. β-galactosidase staining was found to be positive in the majority of the heart in mice injected with Ad-LacZ, in contrast with uninjected mice (Online Figure VIIC). Atg7 overexpression significantly promoted autophagy in the hearts of Nox4 cKO animals, as indicated by increases in LC3II accumulation (Online Figure VIID). We found that, as compared with Ad-LacZ injection, Ad-Atg7 injection significantly improved the systolic function and restored the myocardial ATP content of Nox4 cKO mice subjected to fasting (Figure 7C; Online Figure VIIE). This suggests that Nox4 activation preserves cardiac function during fasting through promotion of autophagy, which is important for preservation of energy status, recycle of damage proteins and organelles and regeneration of important proteins, as also previously shown1, 3, 4, 32.
Figure 7. Nox4 disruption in the heart is detrimental during energy deprivation in vivo.
A. Control and Nox4 cKO mice were subjected to 48 hours of fasting and then underwent echocardiographic evaluation. Fractional shortening was calculated. B. Control and Nox4 cKO mice were subjected to 48 hours of fasting. Myocardial ATP content was evaluated. N=4. Data are expressed as a percentage of the control. C. Control and Nox4 cKO mice received cardiac injection of Ad-LacZ or Ad-Atg7. After 48 hours, they were subjected to 48 hours of fasting. Fractional shortening was calculated. D–F. Control and Nox4 cKO mice were subjected to 3 hours of ischemia. Representative pictures of 2,3,5-triphenyltetrazolium chloride staining are shown (D), together with quantification of the area at risk (AAR, E) and myocardial infarct size (MI/AAR, F). Bar= 1 mm.
Finally, we observed that Nox4 cKO mice display an increased infarct size and necrosis with respect to control mice after prolonged ischemia (3 hours) without reperfusion2, 5, 33 (Figure 7D–F; Online Figure VIIF–G). This model is characterized by high energy stress and is clinically relevant, since patients with myocardial infarction usually experience prolonged coronary occlusion. In addition, this model allows us to selectively investigate the effect of Nox4 disruption on ischemic injury. This is in contrast to ischemia/reperfusion models, in which the ischemic period is very short and it is impossible to distinguish the ischemic and reperfusion phase-specific effects of an intervention, even though these phases are characterized by completely different pathophysiological mechanisms of damage (i.e., energy stress during ischemia and oxidative stress during reperfusion). Thus, this evidence further corroborates our observations regarding the physiological significance of Nox4 in the energy-deprived heart.
DISCUSSION
Autophagy upregulation is a critical survival mechanism in cells subjected to energy deprivation that preserves the cellular energy status and degrades damaged proteins and organelles1, 2. However, the mechanisms promoting autophagy in energy-depleted cells are not fully understood. We here elucidate a novel mechanism promoting autophagy during energy stress by demonstrating for the first time that Nox4 is a critical mediator of autophagy upregulation in CMs during energy deprivation. Nox4 is upregulated in an NF-κB-dependent manner during GD and Nox4 is selectively upregulated in the ER of energy-deprived CMs. This increase in Nox4-derived ROS in the ER is a crucial event which promotes autophagy through activation of the PERK/eIF-2α/ATF4 pathway. Nox4 activation during energy deprivation is cardioprotective both in vitro and in vivo.
Nox2 and Nox4 are the main isoforms of NADPH oxidase in cardiomyocytes. These two isoforms share conserved trans-membrane domains, FAD- and NADPH-binding domains in their C-terminal regions, and two heme groups. In addition, they both form a functional complex with p22phox, a membrane-integrated protein, which is required for their activity6. We found that the function of Nox4 regarding autophagy and CM survival in response to energy deprivation is not shared by Nox2; Nox2 was not involved in ROS production, autophagy regulation and survival in energy-depleted CMs. This observation is consistent with previous evidence indicating that Nox4 promotes several adaptive cellular responses to stresses such as hypoxia, proteotoxic stress and metabolic disorders6. This unique physiological role of Nox4 in the regulation of cellular processes may be dependent upon its intracellular localization and/or its ability to produce H2O2, which can be a stable and diffusible signaling molecule, through its E-loop portion6, although it is not clear whether Nox4 can directly produce H2O2 in CMs. We observed that both mRNA and protein levels of Nox4 are increased in CMs during energy deprivation. Nox4 is localized mainly in the ER and mitochondria, depending on the cell type6. In CMs, Nox4 is localized primarily to mitochondria and the ER, but it is also present in the nucleus7, 8, 19. The localization of Nox4 in intracellular membranes is dependent upon the presence of signal sequences specific to the ER22, to mitochondria8 and to the nucleus19 in the primary sequence. The increase in Nox4 protein levels observed in energy-depleted CMs occurs selectively in the ER but not in mitochondria. This may be explained by the fact that Nox4 is a membrane-bound protein and, therefore, it is mainly translated in the ER, through a co-translational translocation mechanism21. However, it is very likely that the preferential cellular localization of Nox4 is stimulus-dependent. Recent evidence suggests that alternative splicing of Nox4 mRNA may drive Nox4 synthesis in different subcellular compartments34. Alternatively, post-translational modifications35 of Nox4 nascent protein or other unknown mechanisms may be involved in this selective activation of Nox4 in the ER during GD.
We here demonstrate that this early upregulation of Nox4 in the ER is biologically relevant and critical for the cellular response to energy deprivation. An increase in Nox4-derived ROS levels in the ER during GD is required for autophagy activation. Normalization of ROS levels by catalase targeted to the ER in glucose-deprived CMs significantly blunted autophagy activation. Conversely, selective inhibition of ROS in mitochondria by peroxiredoxin-3 failed to inhibit autophagy, making the involvement of mitochondrial ROS in the regulation of autophagy during energy deprivation less likely.
We found for the first time that Nox4 activation in the ER is required for autophagy upregulation during energy deprivation, because it promotes activation of the PERK/eIF-2α/ATF4 pathway, which stimulates autophagy through the activation of the Atg5-Atg12 complex23, 24. In fact, reactivation of PERK in Nox4-depleted CMs rescued autophagy during GD. We have shown previously that activation of AMPK and inhibition of Rheb/mTORC1, both of which regulate Ulk1 and Atg7, are also required for autophagy activation during CM energy deprivation2, 5. In addition, ROS promote autophagy during amino acid deprivation through oxidation of Atg4b in ovary and cancer cells18. This clearly indicates that activation of autophagy during energy deprivation requires coordination of multiple signaling pathways, through tight regulation of the proteins involved in the autophagic machinery.
Our results suggest that Nox4-dependent accumulation of ROS in the ER is responsible for activation of the PERK/eIF-2α/ATF4 pathway in energy-deprived CMs. Nox4-derived ROS may directly oxidize PERK, thereby regulating its dimerization and activation. However, previous studies showed that the cysteine residues contained in the luminal domain of PERK, which is important for PERK dimerization and activation, are not involved in the regulation of PERK activity/signaling36. We found that inhibition of PHD4 in the ER rescues ATF4 levels in Nox4-deprived CMs during GD. This suggests that activation of Nox4 in the ER activates the PERK/eIF-2α/ATF4 pathway through ROS-induced inhibition of PHD4. This is consistent with the evidence that inhibition of PHDs activates PERK and ATF426, 27, and that Nox4-derived ROS can strongly inhibit PHDs9, 15, 25. Of note, Nox4-dependent activation of the PERK pathway during energy deprivation does not appear to be a part of the unfolded protein response, because activation of the PERK signaling pathway is activated by GD long before the accumulation of misfolded proteins is induced. In addition, PERK activation was not paralleled by a concomitant activation of the ATF6 and IRE1-α pathways in the early phase of GD. Remarkably, Nox4 does not seem to regulate the ATF6 and IRE-1 pathways even in later stages of GD, when these signals are strongly activated to limit cell death37. PERK is activated during nutrient deprivation via the integrated stress response independently of the other branches of the unfolded protein response38. Given that autophagy plays a major role in the integrated stress response, it is possible that the Nox4-mediated activation of PERK and autophagy in CMs during energy deprivation happens in the context of a cardiomyocyte integrated stress response. Nox4 was previously shown to promote cell survival in response to ER stress through a focal activation of the RAS/ERK pathway12. However, our results show that Nox4 does not activate ERK in response to energy deprivation in CMs.
We show that activation of endogenous Nox4 in CMs subjected to energy deprivation is cardioprotective. Nox4 knockdown in vitro reduces CM survival, whereas disruption of Nox4 in vivo deteriorates cardiac function during fasting and increases ischemic injury after prolonged ischemia. Importantly, Nox4 activation is protective through activation of autophagy, since restoration of autophagy in Nox4-depleted CMs through Atg7 overexpression, which would reactivate the Atg5/12 complex, improved survival in vitro and both cardiac function and ATP content in vivo during energy deprivation. However, the activation of Nox4 during cardiac stress may not always be protective. Inhibition of Nox4 improves cardiac function and reduces cardiac hypertrophy during severe pressure overload7, and reduces reperfusion damage9. Thus, it is important to understand why the function of Nox4 is stimulus-dependent. It is possible that activation of Nox4 during energy deprivation is moderate and physiological, whereas during pressure overload and reperfusion it is exaggerated and detrimental. During energy deprivation, Nox4 activation is important for autophagy activation, which is cardioprotective. On the other hand, during pressure overload or reperfusion damage, mechanisms activated by physiological ROS are easily overridden by the pathological response activated by excessive ROS. During acute energy deprivation, Nox4 is increased in the ER but it does not accumulate in mitochondria. Conversely, Nox4 markedly accumulates in the mitochondria during chronic stress, such as pressure overload or late reperfusion injury7. This suggests that localization of Nox4 in the ER may be cardioprotective whereas localization in the mitochondria detrimental, although further investigations are needed to verify this hypothesis. Finally, autophagy is protective during fasting and prolonged ischemia, but it was reported to be detrimental during pressure overload when it promoted maladaptive hypertrophy39, and during reperfusion damage5, 33. Therefore, it is possible that Nox4 activation is both protective during energy deprivation and detrimental during pressure overload and reperfusion injury due to its ability to promote autophagy, which is protective in the former condition but detrimental in the latter. We have shown recently that, although single Nox4 or Nox2 disruption reduces reperfusion injury, double knockout of Nox4 and Nox2 in the adult heart is associated with increased reperfusion damage through HIF-1α inhibition and PPARα activation. This indicates that Nox4 and Nox2 play redundant functions in the CM response to severe oxidative stress, which occurs during reperfusion damage. In contrast, our current study demonstrates that during energy stress Nox4 activation in the ER is required for activating autophagy, thereby promoting survival. Of note, this property of Nox4 is not shared by Nox2 and is apparently HIF-1α-independent, since we previously showed that HIF-1α levels are unchanged in the hearts of Nox4 cKO mice as compared to controls9.
In conclusion, our study demonstrates for the first time that Nox4 plays a critical role in the regulation of the CM response to energy deprivation. Nox4 protein levels and activity are increased in the ER of CMs during energy deprivation. Nox4-dependent accumulation of ROS in the ER promotes autophagy through activation of the PERK/eIF-2α/ATF4 pathway. Nox4 activation is a physiological response that protects the heart during nutrient deprivation and ischemia through activation of autophagy (Online Figure VIIH). We propose that the Nox4/PERK/eIF-2α/ATF4 signaling pathway is a novel intracellular cascade that promotes activation of autophagy and CM survival during energy deprivation.
Supplementary Material
Novelty and Significance.
What is known?
Autophagy is activated and promotes cell survival in cardiomyocytes during energy deprivation.
NADPH oxidase 4 (Nox4) is a major source of reactive oxygen species (ROS) in cardiomyocytes during stress, purposefully generating O2− and H2O2 at intracellular membranes.
Although high activation of Nox4 is maladaptive, activation of Nox4 at physiological levels is involved in some cellular adaptive mechanisms, such as hypoxic adaptation.
What New Information Does This Article Contribute?
Nox4 is activated in cardiomyocytes during energy deprivation and promotes ROS production in the endoplasmic reticulum (ER).
Nox4-dependent ROS production in the ER of energy deprived-cardiomyocytes is required for autophagy activation through activation of the PERK/eIF-2α/ATF4 pathway.
Nox4 activation promotes cardiomyocyte survival during energy deprivation through autophagy activation both in vitro and in vivo.
Autophagy is an evolutionarily conserved intracellular bulk degradation process. Autophagy activation is an essential survival mechanism in cardiomyocytes during energy deprivation. However, the mechanisms activating autophagy in energy-depleted cells are not fully understood. Nox4 is an enzyme that purposefully generates ROS at intracellular membranes and is an important source of ROS in cardiomyocytes during stress. However, the physiological role of Nox4 in cardiomyocytes during stress remains unclear. In addition, whether Nox4 regulates cellular autophagy and survival in response to energy deprivation is unknown. We here demonstrate that Nox4 is a critical mediator of autophagy in cardiomyocytes during energy deprivation. Nox4 protein abundance and activity are selectively increased in the ER of energy-deprived cardiomyocytes. Nox4-dependent ROS production in the ER is a crucial event that promotes autophagy through activation of the PERK/eIF-2α/ATF4 pathway. Remarkably, Nox4 promotes survival in energy-deprived cardiomyocytes both in vitro and in vivo through activation of autophagy. Our study demonstrates for the first time that the Nox4/PERK/eIF-2α/ATF4 signaling pathway is a critical intracellular cascade activating autophagy during energy deprivation; it clarifies the biological significance of ROS production in the ER in energy-deprived cardiomyocytes; and it elucidates a crucial physiological function of Nox4 in cardiomyocytes during stress.
Acknowledgments
The authors wish to thank Christopher D. Brady for critical reading of the manuscript.
SOURCES OF FUNDING
This work was supported in part by U.S. Public Health Service Grants HL102738, HL67724, HL69020, HL91469, AG23039, and AG27211. This work was also supported by the Fondation Leducq Transatlantic Networks of Excellence. SS has been supported by a Postdoctoral Fellowship from the Founders Affiliate, American Heart Association, and partially by a grant from the Italian Society of Cardiology and Italian Society of Hypertension.
Abbreviation list
- CMs
cardiomyocytes
- GD
glucose deprivation
- ER
endoplasmic reticulum
- PHDs
prolyl hydroxylase enzymes
- PERK
PKR-like endoplasmic reticulum kinase
- eIF-2α
eukaryotic initiation factor 2α
- ATF4
activating transcription factor 4
- DCF
5-(and-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate, acetyl ester
- DHE
dihydroethidium
- O2−
superoxide anion
- H2O2
hydrogen peroxide
Footnotes
DISCLOSURES
None.
References
- 1.Singh R, Cuervo AM. Autophagy in the cellular energetic balance. Cell Metab. 2011;13:495–504. doi: 10.1016/j.cmet.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sciarretta S, Zhai P, Shao D, Maejima Y, Robbins J, Volpe M, Condorelli G, Sadoshima J. Rheb is a critical regulator of autophagy during myocardial ischemia: pathophysiological implications in obesity and metabolic syndrome. Circulation. 2012;125:1134–1146. doi: 10.1161/CIRCULATIONAHA.111.078212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hariharan N, Maejima Y, Nakae J, Paik J, Depinho RA, Sadoshima J. Deacetylation of FoxO by Sirt1 Plays an Essential Role in Mediating Starvation-Induced Autophagy in Cardiac Myocytes. Circ Res. 2010;107:1470–1482. doi: 10.1161/CIRCRESAHA.110.227371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuma A, Hatano M, Matsui M, Yamamoto A, Nakaya H, Yoshimori T, Ohsumi Y, Tokuhisa T, Mizushima N. The role of autophagy during the early neonatal starvation period. Nature. 2004;432:1032–1036. doi: 10.1038/nature03029. [DOI] [PubMed] [Google Scholar]
- 5.Matsui Y, Takagi H, Qu X, Abdellatif M, Sakoda H, Asano T, Levine B, Sadoshima J. Distinct roles of autophagy in the heart during ischemia and reperfusion: roles of AMP-activated protein kinase and Beclin 1 in mediating autophagy. Circ Res. 2007;100:914–922. doi: 10.1161/01.RES.0000261924.76669.36. [DOI] [PubMed] [Google Scholar]
- 6.Lassegue B, San Martin A, Griendling KK. Biochemistry, physiology, and pathophysiology of NADPH oxidases in the cardiovascular system. Circ Res. 2012;110:1364–1390. doi: 10.1161/CIRCRESAHA.111.243972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuroda J, Ago T, Matsushima S, Zhai P, Schneider MD, Sadoshima J. NADPH oxidase 4 (Nox4) is a major source of oxidative stress in the failing heart. Proc Natl Acad Sci U S A. 2010;107:15565–15570. doi: 10.1073/pnas.1002178107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ago T, Kuroda J, Pain J, Fu C, Li H, Sadoshima J. Upregulation of Nox4 by hypertrophic stimuli promotes apoptosis and mitochondrial dysfunction in cardiac myocytes. Circ Res. 2010;106:1253–1264. doi: 10.1161/CIRCRESAHA.109.213116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matsushima S, Kuroda J, Ago T, Zhai P, Ikeda Y, Oka S, Fong GH, Tian R, Sadoshima J. Broad Suppression of NADPH Oxidase Activity Exacerbates Ischemia/Reperfusion Injury Through Inadvertent Downregulation of Hypoxia-inducible Factor-1alpha and Upregulation of Peroxisome Proliferator-activated Receptor-alpha. Circ Res. 2013;112:1135–1149. doi: 10.1161/CIRCRESAHA.111.300171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shiose A, Kuroda J, Tsuruya K, Hirai M, Hirakata H, Naito S, Hattori M, Sakaki Y, Sumimoto H. A novel superoxide-producing NAD(P)H oxidase in kidney. J Biol Chem. 2001;276:1417–1423. doi: 10.1074/jbc.M007597200. [DOI] [PubMed] [Google Scholar]
- 11.Babelova A, Avaniadi D, Jung O, Fork C, Beckmann J, Kosowski J, Weissmann N, Anilkumar N, Shah AM, Schaefer L, Schroder K, Brandes RP. Role of Nox4 in murine models of kidney disease. Free Radic Biol Med. 2012;53:842–853. doi: 10.1016/j.freeradbiomed.2012.06.027. [DOI] [PubMed] [Google Scholar]
- 12.Wu RF, Ma Z, Liu Z, Terada LS. Nox4-derived H2O2 mediates endoplasmic reticulum signaling through local Ras activation. Mol Cell Biol. 2010;30:3553–3568. doi: 10.1128/MCB.01445-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schroder K, Zhang M, Benkhoff S, Mieth A, Pliquett R, Kosowski J, Kruse C, Luedike P, Michaelis UR, Weissmann N, Dimmeler S, Shah AM, Brandes RP. Nox4 is a protective reactive oxygen species generating vascular NADPH oxidase. Circ Res. 2012;110:1217–1225. doi: 10.1161/CIRCRESAHA.112.267054. [DOI] [PubMed] [Google Scholar]
- 14.Craige SM, Chen K, Pei Y, Li C, Huang X, Chen C, Shibata R, Sato K, Walsh K, Keaney JF., Jr NADPH oxidase 4 promotes endothelial angiogenesis through endothelial nitric oxide synthase activation. Circulation. 2011;124:731–740. doi: 10.1161/CIRCULATIONAHA.111.030775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang M, Brewer AC, Schroder K, Santos CX, Grieve DJ, Wang M, Anilkumar N, Yu B, Dong X, Walker SJ, Brandes RP, Shah AM. NADPH oxidase-4 mediates protection against chronic load-induced stress in mouse hearts by enhancing angiogenesis. Proc Natl Acad Sci U S A. 2010;107:18121–18126. doi: 10.1073/pnas.1009700107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kubli DA, Gustafsson AB. Mitochondria and mitophagy: the yin and yang of cell death control. Circ Res. 2012;111:1208–1221. doi: 10.1161/CIRCRESAHA.112.265819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu L, Wise DR, Diehl JA, Simon MC. Hypoxic reactive oxygen species regulate the integrated stress response and cell survival. J Biol Chem. 2008;283:31153–31162. doi: 10.1074/jbc.M805056200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scherz-Shouval R, Shvets E, Fass E, Shorer H, Gil L, Elazar Z. Reactive oxygen species are essential for autophagy and specifically regulate the activity of Atg4. EMBO J. 2007;26:1749–1760. doi: 10.1038/sj.emboj.7601623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matsushima S, Kuroda J, Ago T, Zhai P, Park JY, Xie LH, Tian B, Sadoshima J. Increased oxidative stress in the nucleus caused by Nox4 mediates oxidation of HDAC4 and cardiac hypertrophy. Circ Res. 2013;112:651–663. doi: 10.1161/CIRCRESAHA.112.279760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu C, Liang B, Wang Q, Wu J, Zou MH. Activation of AMP-activated protein kinase alpha1 alleviates endothelial cell apoptosis by increasing the expression of anti-apoptotic proteins Bcl-2 and survivin. J Biol Chem. 2010;285:15346–15355. doi: 10.1074/jbc.M110.102491. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 21.Stephens SB, Nicchitta CV. Divergent regulation of protein synthesis in the cytosol and endoplasmic reticulum compartments of mammalian cells. Mol Biol Cell. 2008;19:623–632. doi: 10.1091/mbc.E07-07-0677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen K, Kirber MT, Xiao H, Yang Y, Keaney JF., Jr Regulation of ROS signal transduction by NADPH oxidase 4 localization. J Cell Biol. 2008;181:1129–1139. doi: 10.1083/jcb.200709049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rzymski T, Milani M, Pike L, Buffa F, Mellor HR, Winchester L, Pires I, Hammond E, Ragoussis I, Harris AL. Regulation of autophagy by ATF4 in response to severe hypoxia. Oncogene. 2010;29:4424–4435. doi: 10.1038/onc.2010.191. [DOI] [PubMed] [Google Scholar]
- 24.Kouroku Y, Fujita E, Tanida I, Ueno T, Isoai A, Kumagai H, Ogawa S, Kaufman RJ, Kominami E, Momoi T. ER stress (PERK/eIF2alpha phosphorylation) mediates the polyglutamine-induced LC3 conversion, an essential step for autophagy formation. Cell Death Differ. 2007;14:230–239. doi: 10.1038/sj.cdd.4401984. [DOI] [PubMed] [Google Scholar]
- 25.Diebold I, Flugel D, Becht S, Belaiba RS, Bonello S, Hess J, Kietzmann T, Gorlach A. The hypoxia-inducible factor-2alpha is stabilized by oxidative stress involving NOX4. Antioxid Redox Signal. 2010;13:425–436. doi: 10.1089/ars.2009.3014. [DOI] [PubMed] [Google Scholar]
- 26.Natarajan R, Salloum FN, Fisher BJ, Smithson L, Almenara J, Fowler AA., 3rd Prolyl hydroxylase inhibition attenuates post-ischemic cardiac injury via induction of endoplasmic reticulum stress genes. Vascul Pharmacol. 2009;51:110–118. doi: 10.1016/j.vph.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 27.Koditz J, Nesper J, Wottawa M, Stiehl DP, Camenisch G, Franke C, Myllyharju J, Wenger RH, Katschinski DM. Oxygen-dependent ATF-4 stability is mediated by the PHD3 oxygen sensor. Blood. 2007;110:3610–3617. doi: 10.1182/blood-2007-06-094441. [DOI] [PubMed] [Google Scholar]
- 28.Koivunen P, Tiainen P, Hyvarinen J, Williams KE, Sormunen R, Klaus SJ, Kivirikko KI, Myllyharju J. An endoplasmic reticulum transmembrane prolyl 4-hydroxylase is induced by hypoxia and acts on hypoxia-inducible factor alpha. J Biol Chem. 2007;282:30544–30552. doi: 10.1074/jbc.M704988200. [DOI] [PubMed] [Google Scholar]
- 29.Pattison JS, Osinska H, Robbins J. Atg7 induces basal autophagy and rescues autophagic deficiency in CryABR120G cardiomyocytes. Circ Res. 2011;109:151–160. doi: 10.1161/CIRCRESAHA.110.237339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang L, Li P, Fu S, Calay ES, Hotamisligil GS. Defective hepatic autophagy in obesity promotes ER stress and causes insulin resistance. Cell Metab. 2010;11:467–478. doi: 10.1016/j.cmet.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gupta A, Akki A, Wang Y, Leppo MK, Chacko VP, Foster DB, Caceres V, Shi S, Kirk JA, Su J, Lai S, Paolocci N, Steenbergen C, Gerstenblith G, Weiss RG. Creatine kinase-mediated improvement of function in failing mouse hearts provides causal evidence the failing heart is energy starved. J Clin Invest. 2012;122:291–302. doi: 10.1172/JCI57426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takemura G, Kanamori H, Goto K, Maruyama R, Tsujimoto A, Fujiwara H, Seishima M, Minatoguchi S. Autophagy maintains cardiac function in the starved adult. Autophagy. 2009;5:1034–1036. doi: 10.4161/auto.5.7.9297. [DOI] [PubMed] [Google Scholar]
- 33.Zhai P, Sciarretta S, Galeotti J, Volpe M, Sadoshima J. Differential Roles of GSK-3{beta} During Myocardial Ischemia and Ischemia/Reperfusion. Circ Res. 2011;109:502–511. doi: 10.1161/CIRCRESAHA.111.249532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anilkumar N, Jose GS, Sawyer I, Santos CX, Sand C, Brewer AC, Warren D, Shah AM. A 28-kDa splice variant of NADPH oxidase-4 is nuclear-localized and involved in redox signaling in vascular cells. Arterioscler Thromb Vasc Biol. 2013;33:e104–112. doi: 10.1161/ATVBAHA.112.300956. [DOI] [PubMed] [Google Scholar]
- 35.Colombo S, Longhi R, Alcaro S, Ortuso F, Sprocati T, Flora A, Borgese N. N-myristoylation determines dual targeting of mammalian NADH-cytochrome b5 reductase to ER and mitochondrial outer membranes by a mechanism of kinetic partitioning. J Cell Biol. 2005;168:735–745. doi: 10.1083/jcb.200407082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ma K, Vattem KM, Wek RC. Dimerization and release of molecular chaperone inhibition facilitate activation of eukaryotic initiation factor-2 kinase in response to endoplasmic reticulum stress. J Biol Chem. 2002;277:18728–18735. doi: 10.1074/jbc.M200903200. [DOI] [PubMed] [Google Scholar]
- 37.Doroudgar S, Thuerauf DJ, Marcinko MC, Belmont PJ, Glembotski CC. Ischemia activates the ATF6 branch of the endoplasmic reticulum stress response. J Biol Chem. 2009;284:29735–29745. doi: 10.1074/jbc.M109.018036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harding HP, Zhang Y, Zeng H, Novoa I, Lu PD, Calfon M, Sadri N, Yun C, Popko B, Paules R, Stojdl DF, Bell JC, Hettmann T, Leiden JM, Ron D. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol Cell. 2003;11:619–633. doi: 10.1016/s1097-2765(03)00105-9. [DOI] [PubMed] [Google Scholar]
- 39.Zhu H, Tannous P, Johnstone JL, Kong Y, Shelton JM, Richardson JA, Le V, Levine B, Rothermel BA, Hill JA. Cardiac autophagy is a maladaptive response to hemodynamic stress. J Clin Invest. 2007;117:1782–1793. doi: 10.1172/JCI27523. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.