Abstract
Chagas disease is caused by an intracellular parasitic protist, Trypanosoma cruzi. As there are no highly effective drugs against this agent that also demonstrate low toxicity, there is an urgent need for development of new drugs to treat Chagas disease. We have previously demonstrated that the parasite inositol 1,4,5-trisphosphate receptor (TcIP3R) is crucial for invasion of the mammalian host cell by T. cruzi. Here, we report that TcIP3R is a short-lived protein and that its expression is significantly suppressed in trypomastigotes. Treatment of trypomastigotes, an infective stage of T. cruzi, with antisense oligonucleotides specific to TcIP3R deceased TcIP3R protein levels and impaired trypomastigote invasion of host cells. Due to the resulting instability and very low expression level of TcIP3R in trypomastigotes indicates that TcIP3R is a promising target for antisense therapy in Chagas disease.
The parasitic protist, Trypanosoma cruzi, is an etiologic agent of Chagas disease1. Human infection is initiated by invasion of infective, metacyclic trypomastigotes in the urine of blood-sucking reduviid bugs2. After invasion, the parasite transforms into amastigotes inside the host cells and begins to proliferate. Parasite proliferation is often accompanied by destruction of the vital tissues, such as heart muscle. Chemotherapy of Chagas disease relies exclusively on two drugs, benznidazole and nifurtimox, but their effects are limited and often evoke severe side effects. Therefore, development of new therapeutic measures are urgently needed.
Ca2+ serves as an second messenger of cellular signaling and its concentration is strictly maintained in the cytosol at 0.1 μM order3. Transient increase of intracellular Ca2+ concentration plays a crucial role for its functions and is mediated in response to both external and internal stimuli. D-myo-inositol 1,4,5-trisphosphate (IP3) is a second messenger generated by phosphoinositide phospholipase C (PI-PLC) upon external stimuli via cell-surface receptors4 and provokes activation of its receptor, IP3R. IP3R is a Ca2+ channel located on the endoplasmic reticulum (ER) and is activated by the binding of IP3, which initiates Ca2+ release from ER as a Ca2+ pool5.
Recently, we reported that a homologue of IP3R in T. cruzi (TcIP3R) is an essential protein and participates in the growth and transformation of the parasite and its ability to infect the host cell. Furthermore, we demonstrated that TcIP3R is a determinant of the virulence of the parasite in vivo6. Combined with the fact that the primary structure of TcIP3R has low similarity to that of human IP3Rs, TcIP3R is a promising drug target for Chagas disease.
Antisense oligonucleotides are a new generation of therapeutic agents that work by silencing the genes responsible for the diseases. One example is fomivirsen (marketed as Vitravene®), which has been approved by the US Food and Drug Administration (FDA) in 1998 for the treatment of cytomegalovirus retinitis7. In parasitic infections, use of antisense oligonucleotides to inhibit specific mRNA synthesis and translation may represent a good chemotherapeutic strategy8. There are several reports showing that treatment of T. cruzi in vitro with antisense oligonucleotides decreased expression levels of the target proteins9,10,11. Thus, an antisense strategy against pivotal proteins of T. cruzi holds promise for a new treatment for Chagas disease.
In the present study, we examined whether TcIP3R is a potential target for antisense oligonucleotide treatment against T. cruzi by phenotypic analysis of trypomastigotes in an in vitro culture system. We show considerably reduced levels of parasite invasion of host cells, implying that antisense oligonucleotide chemotherapy against TcIP3R may be a viable approach to treatment in Chagas disease.
Results
TcIP3R is a short-lived protein in epimastigotes
Since antisense oligonucleotides specifically interfere with both mRNA stability and its translation into protein, short-lived proteins are desirable targets to ensure effective, functional knock-down by antisense oligonucleotides. In order to establish whether TcIP3R is suitable as a target for antisense strategy, we treated T. cruzi epimastigotes with cycloheximide (CHX), an authentic inhibitor of protein synthesis, for 0.5–10 h, and monitored degradation of TcIP3R by western blot analysis (Fig. 1A). Expression levels of TcIP3R declined after CHX treatment, whereas it was difficult to estimate its half-life, due exclusively to its low levels of expression.
Figure 1. Expression levels of TcIP3R protein in Trypanosoma cruzi.
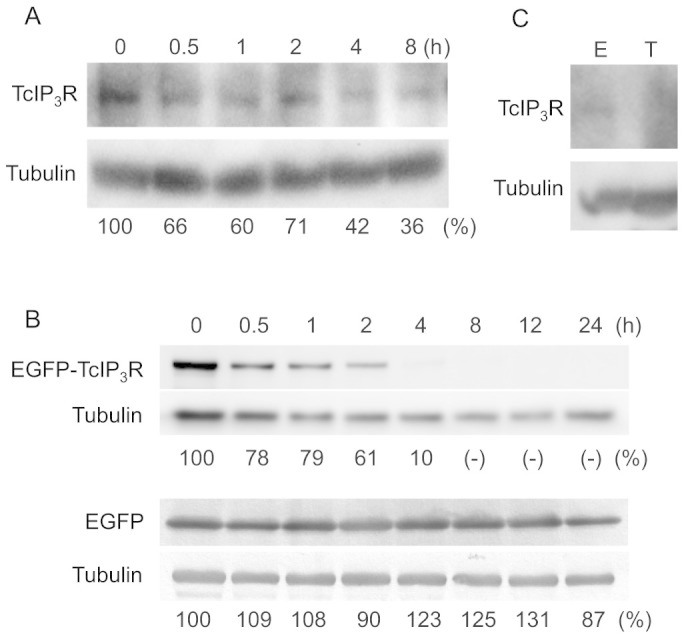
(A) Life-span of TcIP3R in epimastigotes. T. cruzi epimastigotes were incubated with 200 μg/mL cycloheximide (CHX) for the indicated time, and expression of TcIP3R was analyzed by western blotting using anti-TcIP3R antibody. The expression levels of TcIP3R were normalized to the levels of tubulin and the relative ratio (%) was indicated. (B) Life-span of recombinant TcIP3R in epimastigotes. Epimastigotes overexpressing EGFP-TcIP3R fusion protein were incubated with 200 μg/mL CHX for the indicated time, and the expression of EGFP-TcIP3R was analyzed by western blotting using anti-TcIP3R antibody. Epimastigotes expressing EGFP was used as control, and western blots were probed with anti-EGFP antibody. Tubulin was used as loading control. Parallel images were processed from the same gel. The levels of EGFP-TcIP3R were normalized to tubulin expression level using a freeware, ImageJ Ver. 1.47 and the relative ratio (%) was indicated. (C) Suppressed expression of TcIP3R in trypomastigotes. Cell extracts from epimastigotes (E) or trypomastigotes (T) were resolved by SDS-PAGE, and western blots were probed with anti-TcIP3R antibody or anti-tubulin antibody as an internal control. Note that TcIP3R was undetectable in trypomastigotes. Full-length blots of panels A, B, and C are presented in Supplementary Figure S1, S2, and S3, respectively.
We have recently established T. cruzi that overexpress recombinant TcIP3R fused to enhanced green fluorescent protein (EGFP) at its N-terminal (EGFP-TcIP3R), which is physiologically functional in the parasite6. We examined the inhibitory effect of CHX treatment on expression of EGFP-TcIP3R to ascertain whether TcIP3R domain-specific protein degradation occurs. Western blots showed that the protein signals of EGFP-TcIP3R decreased rapidly and became undetectable by 8 h after CHX treatment, whereas the band for EGFP remained almost intact (Fig. 1B). These results clearly indicated that the degradation of EGFP-TcIP3R is specific to the TcIP3R domain. The half-life of EGFP-TcIP3R was estimated to be about 3 h, while the half-life of mammalian IP3Rs in unstimulated cultured cells is 10–12 h12, suggesting that TcIP3R is more unstable than mammalian IP3Rs. We concluded that TcIP3R is a short-lived protein at least in epimastigotes, and possibly other forms of T. cruzi.
Protein level of TcIP3R is very low in trypomastigotes
We have recently shown that transcription of TcIP3R mRNA occurs throughout the parasite life cycle, but that its transcription level was much lower in trypomastigotes than in epimastigotes6. In the present study, the protein levels of TcIP3R were examined by western blotting using an anti-TcIP3R monoclonal antibody, and were compared between epimastigotes and trypomastigotes. TcIP3R was detected in epimastigotes, but was undetectable in trypomastigotes, while the levels of β-tubulin, a control protein, were consistent between the 2 parasite forms (Fig. 1C). These results indicated that the protein level of TcIP3R is very low in trypomastigotes.
Because the native TcIP3R protein is undetectable in trypomastigotes, we tested whether EGFP-TcIP3R was detectable in trypomastigotes of the transgenic T. cruzi. We could detect EGFP-TcIP3R by western blots using an EGFP-specific antibody, confirming that these EGFP-TcIP3R-expressing T. cruzi were suitable for further analysis (Fig. 2). Notably, expression levels of EGFP-TcIP3R in trypomastigotes (Fig. 2A, untreated) was very low and was reduced to less than 10% of that in epimastigotes (Fig. 2, epimastigotes), consistent with the results found in the wild-type parasite.
Figure 2. Phosphorothioate antisense TcIP3R oligonucleotide inhibits EGFP-TcIP3R expression in trypomastigotes.
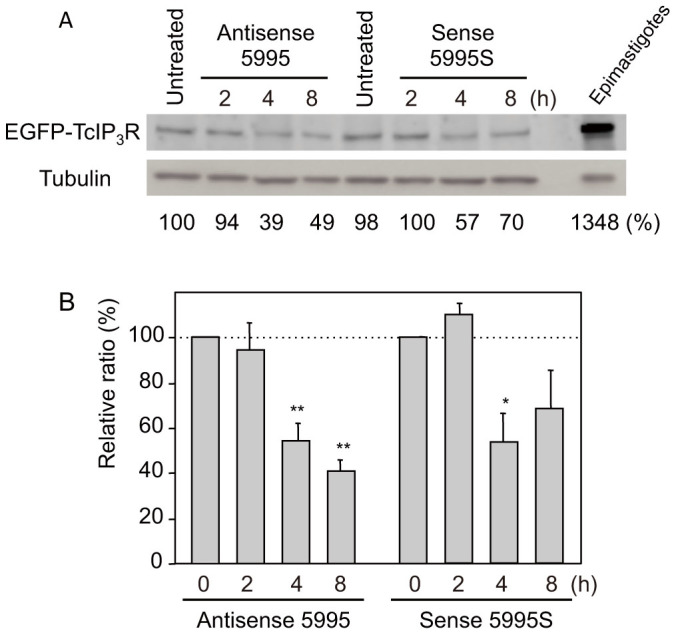
(A) Western blots of trypomastigotes overexpressing EGFP-TcIP3R, treated for 8 h with 40 μM of either Antisense 5995 or the complementary Sense 5995S phosphorothioate oligonucleotide, were probed with anti-EGFP antibody or an anti-tubulin antibody as internal control. The lysate of EGFP-TcIP3R-expressing epimastigotes without treatment was loaded as expression control. Parallel images were processed from the same gel. The expression levels of TcIP3R were normalized to the levels of tubulin and the relative ratio (%) is indicated. Representative data of three independent experiments are shown. Full-length blots are presented in Supplementary Figure S4. (B) Decrease of the expression levels of EGFP-TcIP3R in trypomastigotes treated with Antisense 5995 or Sense 5995S. Intensity of the bands was measured densitometrically using a freeware, ImageJ version 1.47. Bars indicate the mean ± S.E. (n = 3). Statistical differences (*p < 0.05, **p < 0.01) are given as a comparison between untreated (0) and treated (2, 4, and 8 hours) groups.
Expression of TcIP3R is blocked by the antisense oligonucleotide treatment
It has been reported that antisense oligonucleotides can be incorporated into non-dividing, infective trypomastigotes by co-incubation in medium, without specific treatment, leading to an effective knock-down of target protein expression10. Given this, and the fact that trypomastigotes are the exclusive invasive form in non-phagocytic cells, we selected trypomastigotes as the target stage of the parasite for antisense treatment.
Because of the difficulty to determine the stability of native TcIP3R, we addressed whether treatment with antisense oligonucleotides inhibits expression of EGFP-TcIP3R in trypomastigotes (Fig. 2). Expression levels of EGFP-TcIP3R in trypomastigotes treated with the antisense oligonucleotide (Antisense 5995) was significantly reduced to 54% (vs. untreated; p = 0.003) and 41% (p = 0.0006) after 4 h and 8 h treatment, respectively (Fig. 2B). Although the stability of TcIP3R in trypomastigotes is unclear, reduction of the protein levels of EGFP-TcIP3R is only attributable to the degradation of premade proteins under the conditions that protein synthesis is suppressed by antisense oligonucleotide treatment, as well as by CHX treatment (see also Fig. 1). This is also supported by the fact that the reduction of EGFP-TcIP3R levels is time-dependent. Therefore, it is likely that EGFP-TcIP3R protein, and possibly native TcIP3R, are a short-lived protein in trypomastigotes.
Treatment with the complementary sense oligonucleotide (Sense 5995S) also showed 54% reduction of EGFP-TcIP3R expression after 4 h treatment (vs. untreated; p = 0.017), whereas the effect was rather limited. This was probably due to association of the sense oligonucleotide with the antisense DNA strand, which may interfere with transcription and lead to the inhibition of transcription.
Infectivity of trypomastigotes is decreased by treatment with antisense oligonucleotides targeted against TcIP3R mRNA
To establish whether treatment with oligonucleotides led to impairment of trypomastigote invasion, we compared the inhibitory effects between Antisense 5995 and Sense 5995S on trypomastigotes. By 8 h after treatment of trypomastigotes with Antisense 5995, invasion by parasites was significantly inhibited (vs. control; p < 0.001, vs. Sense 5995S; p < 0.05; Fig. 3A). These results strongly suggested that loss of infectivity by trypomastigotes is mediated by treatment with antisense oligonucleotides that result in knock-down of TcIP3R.
Figure 3. Inhibitory effect of antisense TcIP3R oligonucleotides on trypomastigote cell invasion.
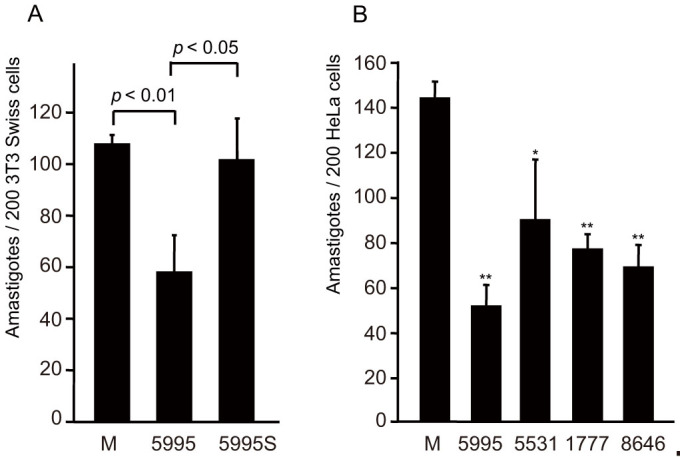
(A) Trypomastigotes (2 × 105) were treated for 8 h with 40 μM of either Antisense 5995 or its complementary Sense 5995S and were then incubated with 2 × 104 3T3-Swiss albino cells for 12 h at 37°C at a multiplicity of infection (MOI) of 10. (B) Trypomastigotes (4 × 105) were treated with 40 μM of phosphorothioate oligonucleotides (5995, 5531, 1777, and 8646, see Materials and Methods) for 8 h and were then incubated with 4 × 104 HeLa cells for 12 h at 37°C (10 MOI). For calculation of trypomastigote infectivity, the number of intracellular parasites in a total of 200 cells was counted after Giemsa staining. Data shown are the mean ± S.D. of 3 independent experiments. Statistical differences (*p < 0.05, **p < 0.01) are given as a comparison between the mock (M) and experimental groups (n = 3).
We further tested the inhibitory effects of additional 3 antisense oligonucleotides (Antisense 5531, 1777, and 8646) on invasion of HeLa cells by trypomastigotes. Treatment of trypomastigotes with each of these antisense oligonucleotides resulted in impaired invasion of HeLa cells by the parasite (Fig. 3B). These results indicated that suppression of expression of TcIP3R is dependent on the antisense oligonucleotide sequence, and can result in impaired trypomastigote infectivity.
Discussion
Chemotherapy of Chagas disease currently relies essentially on 2 old compounds, benznidazole and nifurtimox, both of which elicit harmful side effects. Therefore, development of novel drugs for treatment of this disease is crucial. New therapeutic measures against infectious diseases include antisense oligonucleotides, which aim to knock down an essential component in the responsible pathogens. We recently reported that when the expression level of TcIP3R decreased to only two-thirds of that of the wild-type, invasion of T. cruzi trypomastigotes was significantly impaired, indicating that TcIP3R is a potential therapeutic target6. In light of the fact that a canonical RNA interference pathway is absent in T. cruzi13, we evaluated knock-down of TcIP3R using antisense oligonucleotides as a practical measure.
We found that TcIP3R is a short-lived protein at least in epimastigotes. Consistently, the recombinant EGFP-TcIP3R is also a short-lived protein in epimastigotes, due to TcIP3R domain-specific protein degradation. In trypomastigotes, we found significant reduction of the protein levels of EGFP-TcIP3R by antisense oligonucleotide treatment in a time-dependent manner, suggesting that EGFP-TcIP3R is rapidly degraded in trypomastigotes (54% reduction after 4 h treatment, Fig. 2). Therefore, it is likely that TcIP3R is a short-lived protein in both epimastigotes and trypomastigotes. Notably, mammalian IP3Rs are post-translationally regulated by degradation via the ubiquitin-proteasome pathway14. Thus, these findings have important implications for development of antisense therapy against TcIP3R.
Upon attachment to the host cell, IP3R-dependent Ca2+ release is evident in trypomastigotes6, indicating the presence and a physiological role of TcIP3R in trypomastigotes. Notably, mRNA and protein levels of TcIP3R were found to be very low in trypomastigotes, when compared with those in epimastigotes. However, it was difficult to detect and even quantify the turnover of TcIP3R protein in trypomastigotes by western blots, most likely due to the very low transcription/expression levels of TcIP3R. In contrast, EGFP-TcIP3R was detectable in trypomastigotes of the recombinant parasites and, importantly, its expression level in trypomastigotes was less than 10% of that in epimastigotes.
In the present study, EGFP-TcIP3R was expressed using pTREX expression vector via the ribosomal RNA promoter that facilitates constitutive, high-level transcription15. Therefore, it is likely that TcIP3R-specific protein degradation is more active in trypomastigotes than in epimastigotes, while we cannot exclude the possibility that the transcription level of EGFP-TcIP3R, as well as its protein level, in trypomastigotes is very low.
Reduction of infectivity of trypomastigotes after treatment with TcIP3R-specific antisense oligonucleotides strongly suggested that the suppression of transcription of this gene led to reduced levels of TcIP3R protein, which occurred in conjunction with rapid turnover at the protein level in trypomastigotes. Thus, expression levels of TcIP3R is tightly regulated in trypomastigotes at both transcription and protein levels.
Low expression levels of TcIP3R in trypomastigotes, as well as its rapid turnover, are advantageous for further development of antisense therapy against TcIP3R. Firstly, trypomastigotes are the only invasive stage of T. cruzi, and are responsible for the virulence of the parasite. Therefore, efficient targeting of the relatively small copy number of TcIP3R transcripts by introduction of antisense oligonucleotides followed by suppression of infectivity of trypomastigotes should be possible. Secondly, trypomastigotes represent the non-dividing stage, so that the intracellular concentration of antisense oligonucleotides can be stabilized. It is worth noting that when antisense nucleotides are incorporated in the dividing stage of the parasites (e.g. epimastigotes or amastigotes), the concentration of the oligonucleotide in the parasites may become diluted as the parasite replicates. Thirdly, antisense oligonucleotides can be incorporated into trypomastigotes without artificial treatment10, which facilitates the strategy.
In terms of Chagas disease, the antisense approach is suitable particularly in the acute phase, in which the blood-circulating trypomastigotes predominate, whereas they are often undetectable in other phases, such as the indeterminate and chronic stages of infection. In addition, it is important to know whether this therapeutic approach is effective not only in trypomastigotes, but also in amastigotes. We are now planning to investigate this using an in vitro infection system and an experimental animal model. In conjunction with the fact that TcIP3R shares far less similarity with mammalian IP3Rs6, TcIP3R holds great promise as a target for antisense treatment with reduced side-effects.
It has been reported that PI-PLC of T. cruzi (TcPI-PLC) is essential for the parasite, and TcPI-PLC has been shown to be related to trypomastigote-to-amastigote differentiation by experiments using antisense oligonucleotides9. Since PI-PLC synthesizes IP3, followed by stimulation of TcIP3R by the generated IP3, the antisense oligonucleotides against TcPI-PLC may also inhibit trypomastigote invasion of the host cell. Interestingly, the mRNA levels of TcPI-PLC are lower in trypomastigotes than in epimastigotes and amastigotes16, similar to that of TcIP3R. This suggests the particular physiological importance of IP3-mediated Ca2+ signaling during the mammalian stage of infection.
We could significantly inhibit parasite invasion of the host cells by antisense oligonucleotide treatment, but this effect was incomplete. This is probably due to inefficient knock-down by the phosphorothioate oligonucleotides used in the present study. It is important to note that T. cruzi with TcIP3R-knock-down (in which 1 of 2 gene loci was disrupted), rather than null-mutants, became thoroughly avirulent in the experimental murine model, whereas in trypomastigotes with this knock-down showed only a 40% reduction in infectivity of the host cell6. In the present study, antisense treatment showed comparable levels of reduction in infectivity. Therefore, it is possible that in vivo antisense treatment can be effective for preventing development of the disease.
Knock-down efficacy may be improved by using antisense oligonucleotides with 2′-O,4′-C-Ethylene-bridged nucleic acid species (ENAs)17. ENAs have higher binding affinity for the complementary RNA strand and are more resistant to nucleases than are phosphorothioate nucleic acids. Therefore, ENA antisense oligonucleotides are more favorable from a therapeutic viewpoint. Further analysis using ENA-based antisense oligonucleotides and an experimental animal model is necessary to optimize the conditions for this therapeutic strategy against Chagas disease.
Methods
Parasite and host cells
Epimastigotes of the T. cruzi Tulahuen strain were cultured as described18. Metacyclic development was induced as previously described19. Mammalian stages of the parasites were maintained in in vitro culture using 3T3-Swiss albino cells (Health Science Research Bank, Tokyo, Japan) and tissue culture-grown trypomastigotes were collected from the culture supernatants by centrifugation, essentially as previously described20. For in vitro experimental infection, 3T3-Swiss albino cells and human-derived HeLa cells were used.
Antibodies and reagents
The anti-TcIP3R monoclonal antibody was prepared as described previously6. The anti-EGFP and anti-tubulin antibodies were purchased from Molecular Probes, Inc. (Eugene, OR) and Thermo Fisher Scientific, Inc. (Rockford, IL), respectively. Cycloheximide was purchased from Wako Pure Chemicals Industries, Ltd. (Osaka, Japan). Quick-CBB PLUS (Wako) was used for CBB staining. Western blotting was performed as described21.
Oligonucleotides
The following phosphorothioate oligonucleotides were designed and purchased from Integrated DNA Technologies, Inc. (Diego, CA). Antisense 1777, 5531, 5995 and 8649 correspond to the complementary sequence of TcIP3R gene, 5′-TTCCAAGCCTCCACCATCCC-3′, 5′-TCTCTTCCCAGCCACCACCT-3′, 5′-GTCCTCCCTTTCCGTGCTGT-3′, and 5′-TCCTCCTCCCTTCCGCCATT-3′, respectively. Sense Oligonucleotide, 5995S (5′-ACAGCACGGAAAGGGAGGAC-3′), is complementary to Antisense 5995.
Statistical analysis
Statistical analysis between the groups was performed using one-way ANOVA and Fisher's PLSD post hoc test.
Author Contributions
M.H., T.N. and K.M. designed the study. M.H., H.H. and T.N. did the experiments. M.H. and T.N. wrote the manuscript. M.H., M.E., J.M. and K.M. interpreted the data. All authors reviewed the manuscript.
Supplementary Material
Supplementary information
Acknowledgments
This work was supported in part by grants-in-aid for scientific research Nos. 24390102 (to T. Nara); and 25221002 (to K. Mikoshiba) and by the Foundation of Strategic Research Projects in Private Universities (S1201013; to T. Nara) from the Ministry of Education, Culture, Sport, Science, and Technology, Japan (MEXT).
References
- Rassi A. Jr, Rassi A. & Marin-Neto J. A. Chagas disease. Lancet. 375, 1388–1402 (2010). [DOI] [PubMed] [Google Scholar]
- Brener Z. Biology of Trypanosoma cruzi. Annu Rev Microbiol. 27, 347–382 (1973). [DOI] [PubMed] [Google Scholar]
- Bootman M. D., Lipp P. & Berridge M. J. The organisation and functions of local Ca2+ signals. J Cell Sci. 114, 2213–2222 (2001). [DOI] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and calcium signalling. Nature. 361, 315–325 (1993). [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Bootman M. D. & Roderick H. L. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 4, 517–529 (2003). [DOI] [PubMed] [Google Scholar]
- Hashimoto M. et al. Inositol 1,4,5-trisphosphate receptor regulates replication, differentiation, infectivity and virulence of the parasitic protist Trypanosoma cruzi. Mol Microbiol. (2013). [DOI] [PubMed] [Google Scholar]
- Razonable R. R. Antiviral drugs for viruses other than human immunodeficiency virus. Mayo Clin Proc. 86, 1009–1026 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker R. H. Jr, Metelev V., Rapaport E. & Zamecnik P. Inhibition of Plasmodium falciparum malaria using antisense oligodeoxynucleotides. Proc Natl Acad Sci U S A. 93, 514–518 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okura M. et al. A lipid-modified phosphoinositide-specific phospholipase C (TcPI-PLC) is involved in differentiation of trypomastigotes to amastigotes of Trypanosoma cruzi. J Biol Chem. 280, 16235–16243 (2005). [DOI] [PubMed] [Google Scholar]
- Málaga S. & Yoshida N. Targeted reduction in expression of Trypanosoma cruzi surface glycoprotein gp90 increases parasite infectivity. Infect Immun. 69, 353–359 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araya J. E. et al. Calcineurin B of the human protozoan parasite Trypanosoma cruzi is involved in cell invasion. Microbes Infect. 10, 892–900 (2008). [DOI] [PubMed] [Google Scholar]
- Wojcikiewicz R. J. H. Inositol 1,4,5-trisphosphate receptor degradation pathways. WIREs Membr Transp Signal. 1, 126–135 (2012). [Google Scholar]
- Ullu E., Tschudi C. & Chakraborty T. RNA interference in protozoan parasites. Cell Microbiol. 6, 509–519 (2004). [DOI] [PubMed] [Google Scholar]
- Lu J. P., Wang Y., Sliter D. A., Pearce M. M. & Wojcikiewicz R. J. RNF170 protein, an endoplasmic reticulum membrane ubiquitin ligase, mediates inositol 1,4,5-trisphosphate receptor ubiquitination and degradation. J Biol Chem. 286, 24426–24433 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzi H. A., Vazquez M. P. & Levin M. J. Integration of expression vectors into the ribosomal locus of Trypanosoma cruzi. Gene. 310, 91–99 (2003). [DOI] [PubMed] [Google Scholar]
- Furuya T., Kashuba C., Docampo R. & Moreno S. N. A novel phosphatidylinositol-phospholipase C of Trypanosoma cruzi that is lipid modified and activated during trypomastigote to amastigote differentiation. J Biol Chem. 275, 6428–6438 (2000). [DOI] [PubMed] [Google Scholar]
- Takagi M. et al. Enhancement of the inhibitory activity of oatp antisense oligonucleotides by incorporation of 2′-O,4′-C-ethylene-bridged nucleic acids (ENA) without a loss of subtype selectivity. Biochemistry. 43, 4501–4510 (2004). [DOI] [PubMed] [Google Scholar]
- Iizumi K. et al. Molecular cloning and characterization of ouabain-insensitive Na(+)-ATPase in the parasitic protist, Trypanosoma cruzi. Biochim Biophys Acta. 1758, 738–746 (2006). [DOI] [PubMed] [Google Scholar]
- Gluenz E., Taylor M. C. & Kelly J. M. The Trypanosoma cruzi metacyclic-specific protein Met-III associates with the nucleolus and contains independent amino and carboxyl terminal targeting elements. Int J Parasitol. 37, 617–625 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima-Shimada J., Hirota Y. & Aoki T. Inhibition of Trypanosoma cruzi growth in mammalian cells by purine and pyrimidine analogs. Antimicrob Agents Chemother. 40, 2455–2458 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata E., Hashimoto M. & Aoki T. Interaction between cFLIP and Itch, a ubiquitin ligase, is obstructed in Trypanosoma cruzi-infected human cells. Microbiol Immunol. 52, 539–543 (2008). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information


