Abstract
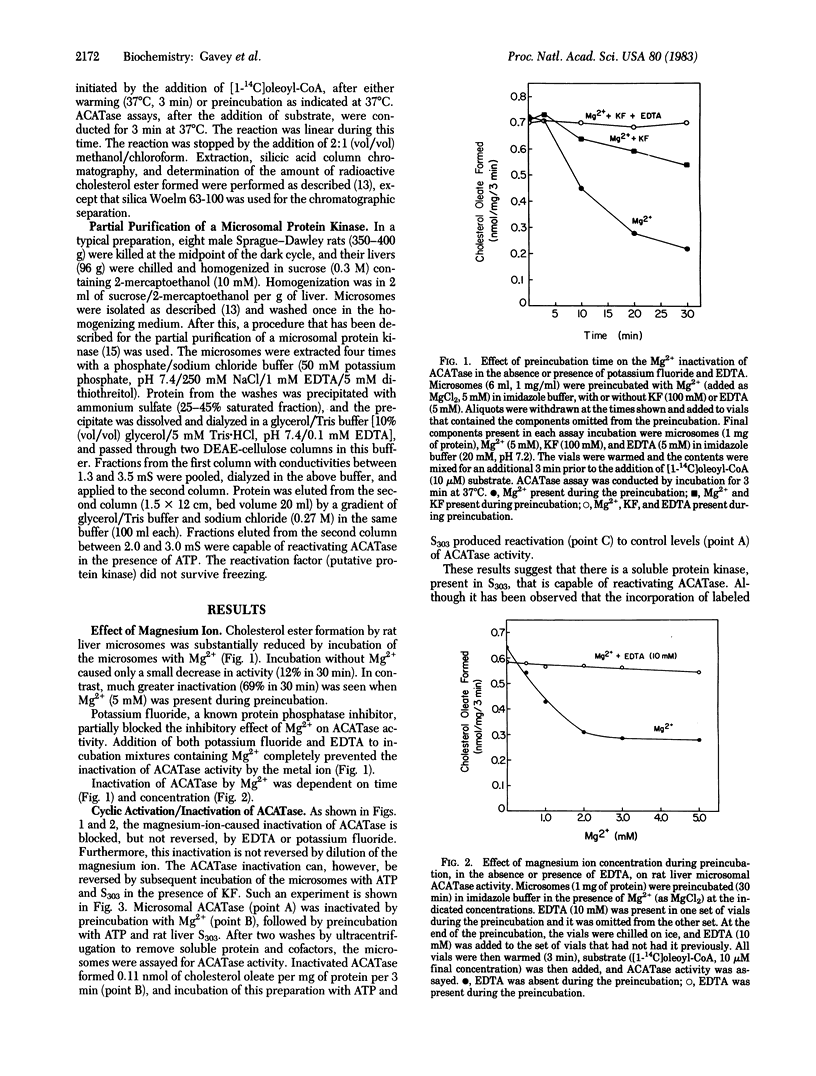
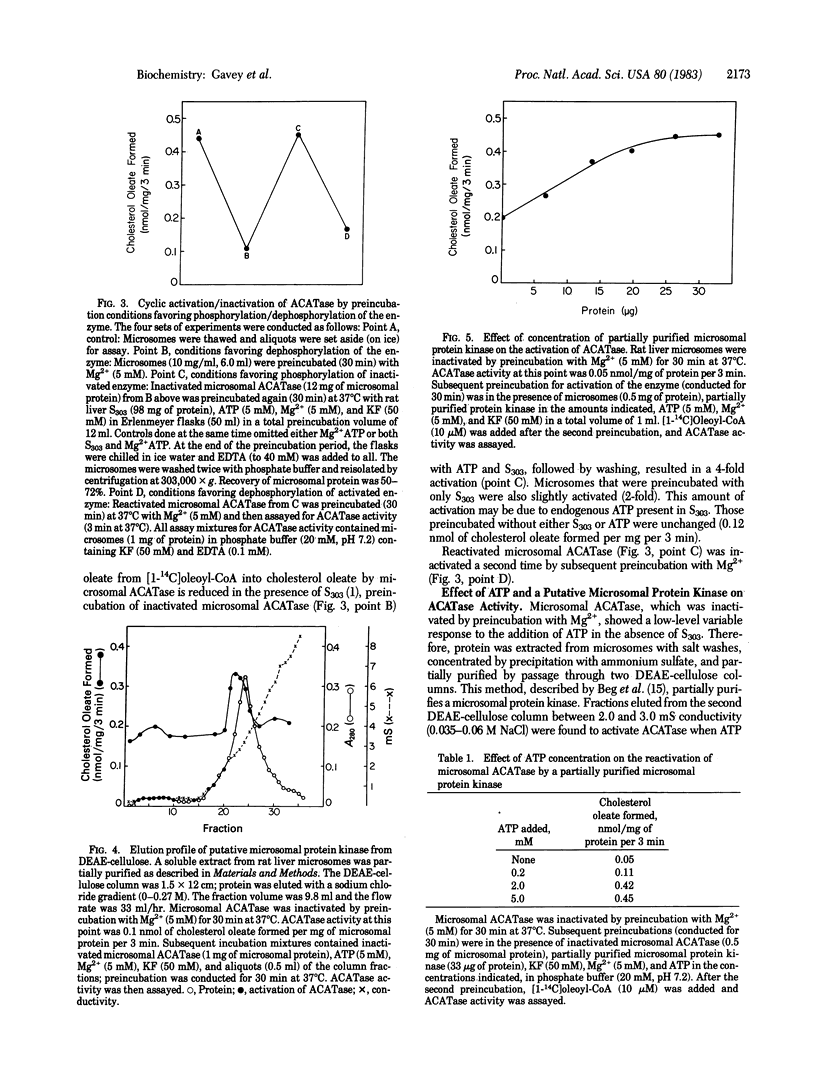
Acyl-coenzyme A:cholesterol O-acyltransferase (ACATase; EC 2.3.1.26) is a membrane-bound microsomal enzyme that catalyzes the formation of long-chain fatty-acyl cholesterol esters in rat liver and other tissues. This enzyme is important in regulating the concentration of unesterified cholesterol in the cell. Having recently demonstrated that rat liver 3-hydroxy-3-methylglutaryl-coenzyme A reductase (HMG-CoA reductase; EC 1.1.1.34), the major regulatory enzyme in cholesterol biosynthesis, undergoes in vivo phosphorylation and inactivation after a single cholesterol meal, we decided to test the hypothesis that the enzyme ACATase, important in cholesterol utilization and storage, is also subject to regulation by phosphorylation/dephosphorylation. The results show that rat liver ACATase can be reversibly inactivated/activated, in vitro, by incubation conditions that favor dephosphorylation/phosphorylation. Activation was also achieved by using a partially purified protein kinase extracted from microsomes. It is significant that HMG-CoA reductase is inactivated by phosphorylation whereas ACATase is activated by phosphorylation. ACATase is, therefore, regulated by phosphorylation in a manner exactly opposite to that of HMG-CoA reductase. We propose that the coordinate regulation of ACATase and HMG-CoA reductase by phosphorylation/dephosphorylation provides a mechanism for short-term intracellular cholesterol homeostasis.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arebalo R. E., Hardgrave J. E., Scallen T. J. The in vivo regulation of rat liver 3-hydroxy-3-methylglutaryl coenzyme A reductase. Phosphorylation of the enzyme as an early regulatory response following cholesterol feeding. J Biol Chem. 1981 Jan 25;256(2):571–574. [PubMed] [Google Scholar]
- Beg Z. H., Stonik J. A., Brewer H. B., Jr Characterization and regulation of reductase kinase, a protein kinase that modulates the enzymic activity of 3-hydroxy-3-methylglutaryl-coenzyme A reductase. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4375–4379. doi: 10.1073/pnas.76.9.4375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Davis R. A., McNeal M. M., Moses R. L. Intrahepatic assembly of very low density lipoprotein. Competition by cholesterol esters for the hydrophobic core. J Biol Chem. 1982 Mar 10;257(5):2634–2640. [PubMed] [Google Scholar]
- Drevon C. A., Engelhorn S. C., Steinberg D. Secretion of very low density lipoproteins enriched in cholesteryl esters by cultured rat hepatocytes during simulation of intracellular cholesterol esterification. J Lipid Res. 1980 Nov;21(8):1065–1071. [PubMed] [Google Scholar]
- Drevon C. A., Weinstein D. B., Steinberg D. Regulation of cholesterol esterification and biosynthesis in monolayer cultures of normal adult rat hepatocytes. J Biol Chem. 1980 Oct 10;255(19):9128–9137. [PubMed] [Google Scholar]
- Erickson S. K., Shrewsbury M. A., Brooks C., Meyer D. J. Rat liver acyl-coenzyme A:cholesterol acyltransferase: its regulation in vivo and some of its properties in vitro. J Lipid Res. 1980 Sep;21(7):930–941. [PubMed] [Google Scholar]
- Gavey K. L., Noland B. J., Scallen T. J. The participation of sterol carrier protein2 in the conversion of cholesterol to cholesterol ester by rat liver microsomes. J Biol Chem. 1981 Mar 25;256(6):2993–2999. [PubMed] [Google Scholar]
- Harry D. S., Dini M., McIntyre N. Effect of cholesterol feeding and biliary obstruction on hepatic cholesterol biosynthesis in the rat. Biochim Biophys Acta. 1973 Jan 19;296(1):209–220. doi: 10.1016/0005-2760(73)90061-1. [DOI] [PubMed] [Google Scholar]
- Havel C., Hansbury E., Scallen T. J., Watson J. A. Regulation of cholesterol synthesis in primary rat hepatocyte culture cells. Possible regulatory site at sterol demethylation. J Biol Chem. 1979 Oct 10;254(19):9573–9582. [PubMed] [Google Scholar]
- Kempen H. J. Lipoprotein secretion by isolated rat hepatocytes: characterization of the lipid-carrying particles and modulation of their release. J Lipid Res. 1980 Aug;21(6):671–680. [PubMed] [Google Scholar]
- Mitropoulos K. A., Balasubramaniam S., Venkatesan S., Reeves B. E. On the mechanism for the regulation of 3-hydroxy-3-methylglutaryl-coenzyme A reductase, of cholesterol 7alpha-hydroxylase and of acyl-coenzyme A:cholesterol acyltransferase by free cholesterol. Biochim Biophys Acta. 1978 Jul 25;530(1):99–111. doi: 10.1016/0005-2760(78)90130-3. [DOI] [PubMed] [Google Scholar]
- Sanghvi A., Grassi E., Diven W. Loss of cholesterol 7 alpha-hydroxylase activity in vitro in the presence of bivalent metal ions and by dialysis of rat liver microsomes. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2175–2178. doi: 10.1073/pnas.80.8.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanghvi A., Grassi E., Warty V., Diven W., Wight C., Lester R. Reversible activation-inactivation of cholesterol 7alpha-hydroxylase possibly due to phosphorylation-dephosphorylation. Biochem Biophys Res Commun. 1981 Dec 15;103(3):886–892. doi: 10.1016/0006-291x(81)90893-7. [DOI] [PubMed] [Google Scholar]
- Spector A. A., Mathur S. N., Kaduce T. L. Role of acylcoenzyme A: cholesterol o-acyltransferase in cholesterol metabolism. Prog Lipid Res. 1979;18(1):31–53. doi: 10.1016/0163-7827(79)90003-1. [DOI] [PubMed] [Google Scholar]
- Suckling K. E., Boyd G. S., Smellie C. G. Properties of a solubilised and reconstituted preparation of acyl-CoA:cholesterol acyltransferase from rat liver. Biochim Biophys Acta. 1982 Feb 15;710(2):154–163. doi: 10.1016/0005-2760(82)90145-x. [DOI] [PubMed] [Google Scholar]



