Summary
In the apicomplexan protozoans motility and cell invasion are mediated by the TRAP/MIC2 family of transmembrane proteins, members of which link extracellular adhesion to the intracellular actomyosin motor complex. Here we characterize a new member of the TRAP/MIC2 family, named TRAP-Like Protein (TLP), that is highly conserved within the Plasmodium genus. Similar to the Plasmodium sporozoite protein, TRAP, and the ookinete protein, CTRP, TLP possesses an extracellular domain architecture that is comprised of von Willebrand factor A (vWA) and thrombospondin type 1 (TSP1) domains, plus a short cytoplasmic domain. Comparison of the vWA domain of TLP genes from multiple Plasmodium falciparum isolates showed relative low sequence diversity, suggesting that the protein is not under selective pressures of the host immune system. Analysis of transcript levels by quantitative reverse transcription polymerase chain reaction (RT-PCR) showed that TLP is predominantly expressed in salivary gland sporozoites of P. falciparum and P. berghei. Targeted disruption of P. berghei TLP resulted in a decreased capacity for cell traversal by sporozoites, and reduced infectivity of sporozoites in vivo, whereas in vitro sporozoite motility and hepatocyte invasion were unaffected. These results indicate a role of TLP in cell traversal by sporozoites.
Introduction
Sporozoites of the malaria parasite, Plasmodium, develop in oocysts within the midgut wall of infected mosquitoes and, when mature, exit the oocyst and invade salivary glands. Following inoculation into the dermis during feeding by the infected mosquito, sporozoites glide randomly until they contact an endothelial cell, which they breach to enter the blood circulation and rapidly arrest in the liver. Within the liver sporozoites cross the sinusoidal barrier and enter the liver parenchyma, where they invade hepatocytes and develop into mature exoerythrocytic stages, thus establishing malaria infection in the mammalian host (reviewed in Sinnis and Coppi, 2007). Studies have shown that sporozoites can interact with target cells in one of two ways: either they can migrate through the cell, wounding it in the process, or they can productively invade the cell with the concomitant formation of a parasitophorous vacuole (Mota et al., 2001). As sporozoites must cross several cell barriers in order to reach hepatocytes, the ability to traverse cells is critical for sporozoite infectivity in vivo (Ishino et al., 2004; 2005; Bhanot et al., 2005; Kariu et al., 2006). Recently it has been shown that the sulfation level of heparan sulfate proteoglycans (HSPGs) on the surface of a target cell determines whether sporozoites will traverse or invade a cell, and the highly sulfated HSPGs of hepatocytes trigger a signalling cascade in sporozoites that results in productive invasion (Coppi et al., 2007).
Plasmodium sporozoites move via gliding motility, similar to all motile invasive stages of apicomplexan parasites (Keeley and Soldati, 2004). Gliding motility is not dependent on flagella or a change in cell shape, but rather is propelled by a subpellicular actomyosin motor that powers the posterior translocation of adhesive proteins, resulting in the forward movement of the parasite. Unlike bacterial and viral pathogens which are passively taken up by host cells, the ‘zoite’ stages of apicomplexan parasites actively invade cells, and parasites that are defective in gliding motility are unable to enter cells (Sultan et al., 1997). Cell invasion is aided by the sequential and tightly controlled secretion from specialized apical organelles called micronemes and rhoptries, of proteins which are involved in recognition and interaction events (reviewed in Carruthers, 1999). This repertoire of proteins includes members of the TRAP/MIC2 family that have been shown to participate in motility and cell invasion. TRAP/MIC2 family members are unified by a type I transmembrane architecture that couples host cell recognition, via putative adhesive domains within the extracellular region, to motility via a short charged cytoplasmic domain that interacts with an intracellular actomyosin motor network (reviewed in Keeley and Soldati, 2004; Brossier and Sibley, 2005).
TRAP/MIC2-like proteins are thus far specific to Apicomplexa, but are not highly conserved as orthologues between genera and show a great plasticity in their architectures of extracellular ‘mix and match’ adhesive modules. Moreover, TRAP/MIC2-like proteins are amplified in a lineage-specific manner within the genomes of different members of the Apicomplexa. In the genome of Plasmodium species at least six members are present, including the characterized proteins, TRAP, CTRP, MTRAP and PTRAMP (Robson et al., 1988; Trottein et al., 1995; Thompson et al., 2004; Baum et al., 2006), as well as two uncharacterized proteins, the S6 sporozoite-induced protein (Kaiser et al., 2004) and ‘TRAP-Like Protein’ (TLP) which is the subject of the present study. Toxoplasma also has multiple predicted TRAP/MIC2 proteins, including MIC2 (Wan et al., 1997; Brossier and Sibley, 2005), and Cryptosporidium has at least one family member, TRAP-C1 (Spano et al., 1998). The general architecture of TRAP/MIC2 family proteins is comprised of one or multiple thrombospondin type 1 (TSP1) domains in the extracellular region; a transmembrane domain; plus a short, charged cytoplasmic domain that has a canonical microneme trafficking motif and a conserved tryptophan near the carboxy-terminus (Templeton and Kaslow, 1997; Brossier and Sibley, 2005). Several TRAP/MIC2 proteins, such as TRAP, CTRP and MIC2, additionally possess one or more von Willebrand factor A (vWA) domains in their extracellular region. The biological functions of the vWA and TSP1 adhesive domains are not well understood, but they are predicted to serve as adhesive domains which recognize host tissues and target cells (Wengelnik et al., 1999). TRAP has a sporozoite-specific expression and essential function in sporozoite motility and invasion (Sultan et al., 1997), whereas CTRP is expressed in ookinetes and has an essential role in ookinete motility and invasion of the mosquito midgut epithelium (Dessens et al., 1999; Yuda et al., 1999; Templeton et al., 2000). Two recent studies have implicated a role for the TRAP/MIC2-like proteins MTRAP and PTRAMP (Thompson et al., 2004; Baum et al., 2006) in merozoite invasion. Here we describe a new member of the Plasmodium TRAP/MIC2 family, TLP, which is conserved between the human malaria parasites, Plasmodium falciparum and P. vivax; the primate malaria parasite, P. knowlesi; and the rodent parasites, P. berghei and P. yoelii. The widespread orthologous conservation of TLP in Plasmodium suggests that it performs a function that is critical to all Plasmodium species. We present evidence that TLP is expressed in the salivary gland sporozoite stage and plays a role in cell traversal of the sporozoite.
Results
Isolation of the TLP gene and predicted protein architecture
Plasmodium falciparum TLP (PfTLP) was identified as a DNA fragment in the course of a microarray screen that compared expression of asexual and gametocyte stage cDNAs against a panel of 3648 random inserts from a P. falciparum mung bean nuclease genomic library (Hayward et al., 2000). At that time the PfTLP gene was incomplete in the 3D7 isolate genome nucleotide sequence database, likely due to assembly problems within repeats in the PfTLP coding region, and therefore the full-length gene was isolated by 5′and 3′RACE methodologies (data not shown). The subsequent resolution of gaps in the 3D7 isolate genome project confirmed the complete PfTLP sequence within a single exon gene (PFF0800w). TLP orthologues were identified in the genome nucleotide sequence databases for P. vivax (PVX_113965) and for the rodent malaria parasites, P. yoelii (PY01499) and P. berghei (AY484471). No putative orthologue was found in other apicomplexan genera, including Theileria spp., Cryptosporidium parvum and Toxoplasma gondii.
The TRAP/MIC2-like domain architecture is conserved between the orthologues of TLP in the different Plasmodium species; namely, an N-terminal signal peptide sequence, two vWA domains that flank a single TSP1 domain, a transmembrane domain, and a TRAP/MIC2-like short cytoplasmic (~40 aa) domain that has a conserved carboxy-terminal penultimate tryptophan residue (Fig. 1). The first vWA domain of TLP is cryptic and was identified only via sensitive reiterative BLAST analysis and HMMER screens, and by scanning for diagnostic signatures of vWA domains such as the putative magnesium ion-binding MIDAS motif, DxSxS/N (Lee et al., 1995). Adjacent to the second vWA domain is an extended low complexity region that contains two charged repeats. A micronemal targeting signal motif is predicted to lie at the intracellular side of the transmembrane domain, similar to a motif that was identified in T. gondii MIC2 (Di Cristina et al., 2000). A second motif located at the extracellular side of the transmembrane region is highly conserved between the TLP proteins of rodent and human parasites and this motif is predicted to be the site of cleavage by a specific protease (Opitz et al., 2002; Baker et al., 2006). Proteolytic cleavage is a common theme of micronemal proteins, resulting in the release of adhesins from the parasite cell surface during gliding motility and cell invasion (Brydges et al., 2000; Carruthers et al., 2000; Brecht et al., 2001; Howell et al., 2001; Miller et al., 2001; Harris et al., 2005; Baker et al., 2006).
Figure 1.
Schematic showing the domain architecture of the Plasmodium TRAP/MIC2 family proteins. The open boxes represent signal peptide sequences and the grey boxes represent predicted transmembrane domains. The vWA domains are represented by hexagons that are labelled ‘A’; and TSP type 1 repeat domains are represented by the label ‘T’. Relative protein lengths are drawn to scale (CTRP equals 2098 aa), with the exception of PF14_0404 (3504 aa); domain lengths and other architectural features are not drawn to scale. The gene identifiers for the 3D7 isolate versions are as follows: TLP, PFF0800w; CTRP, PFC0640w; TRAP, PF13_0201; PTRAMP, PFL0870w; and MTRAP, PF10_0281. PF14_0404 is the P. falciparum orthologue of P. berghei S6 sporozoite-induced protein (Kaiser et al., 2004).
TLP exhibits limited diversity in P. falciparum isolates
To estimate the extent of sequence diversity of PfTLP between different isolates of P. falciparum, we compared the sequence diversity of the second vWA domain of TLP with the diversity in the vWA domain of PfTRAP and in the second vWA domain of PfCTRP. These regions were PCR-amplified from genomic DNA that was purified from a panel of different P. falciparum isolates using specific primers. Additional sequences were obtained from genome sequence projects of P. falciparum isolates (nucleotide sequence data accessed at the Sanger Center and the Broad Institute). Alignments of the different sequences showed that few polymorphisms are present in the vWA domains of CTRP and PfTLP compared with the vWA domain of TRAP (Fig. S1). A low level of diversity of CTRP might be anticipated as CTRP is expressed in the ookinete stage in the mosquito and thus does not encounter antibody-mediated immune pressure of the mammalian host. In contrast, TRAP plays a role in sporozoite motility and invasion in the mammalian host, and has been shown to possess diversity across isolates in P. falciparum (Robson et al., 1990). The low level of diversity of PfTLP is somewhat surprising because TLP plays a role in traversal of mammalian host cells (see below) and therefore might encounter immune pressure.
Transcription of PfTLP and P. berghei TLP in different life cycle stages
Initial microarray screening and confirmatory quantitative reverse transcription polymerase chain reaction (RT-PCR) studies indicated that PfTLP transcripts are markedly upregulated in gametocytes in comparison with asexual blood stage parasites (data not shown). To determine the transcript levels of TLP throughout the parasite life cycle, real-time RT-PCR was performed using cDNA that was prepared from different asexual and mosquito stage parasites from both P. falciparum and P. berghei (Fig. 2). PfTLP and P. berghei TLP (PbTLP) are predominantly transcribed in salivary gland sporozoites, although significant transcript levels are also present in gametocytes and in midgut sporozoites. Transcripts were not detected during the asexual intraerythrocytic cycle. Translational repression of mRNA transcripts in the gametocyte stage has been noted for a large number of genes (Paton et al., 1993; Hall et al., 2005; Mair et al., 2006; Braks et al., 2008), and thus it is possible that gametocyte TLP transcripts do not result in protein production. The PfTLP and PbTLP transcript levels in salivary gland sporozoites were comparable to the levels of P. falciparum and P. berghei ama-1 (Pfama-1 and Pbama-1) transcripts (PF11-0344 and PB000821.01.0 respectively), which were used as controls for sporozoite transcription in addition to the housekeeping genes, P. falciparum arginyl-tRNA synthetase and P. berghei hsp70 (data not shown).
Figure 2.
Life cycle stage-specific expression of the TLP gene. Gene-specific transcript levels were analysed by quantitative PCR using cDNA prepared from asexual and mosquito stage parasites of P. falciparum (A) and P. berghei (B). P. falciparum values were normalized to the expression of the control gene, arginyl-tRNA synthetase (PFL0900c); and P. berghei values were normalized to the control gene, Pbhsp70 (PB001074.01.0). The abbreviations used to describe the parasite stages are as follows: M, merozoites; R, rings; ER, early rings; LR, late rings; ET, early trophozoites; LT, late trophozoites; Sc, schizonts; G, gametocytes; 5, 5-day oocysts; 8, 8-day oocysts; 10, 10-day oocysts; 15, 15-day oocysts; Sp, salivary gland sporozoites.
TLP is not essential for intraerythrocytic propagation or mosquito stage development
The abundance of TLP transcripts in salivary gland sporozoites in comparison with midgut sporozoites indicates that TLP might participate in sporozoite infectivity to the mammalian host, rather then playing a role in sporozoite maturation within the mosquito. To address the function of TLP, the TLP gene was disrupted in P. falciparum and in P. berghei (Figs S2 and S3 respectively). The PfTLP disruption construct was designed using the pHTK vector for double-cross-over homologous recombination. Correct disruption of PfTLP was verified in several clones by diagnostic PCR (Fig. S2B) and in all clones a pseudodiploid locus was generated by the integration of the plasmid via a single homologous recombination event and we were unable to detect double-cross-over recombination (Fig. S2A). The first gene copy in the pseudo-diploid locus is truncated at the 3′end, whereas the second copy is truncated at the 5′end and lacks a start methionine, a signal peptide sequence and the start of the coding region. Southern blot analysis of HindIII- and NdeI-digested genomic DNA from wild-type (wt) and mutant (PfTLP−) parasites showed correct disruption of PfTLP locus, and confirmed that a single recombination event had generated a pseudo-diploid locus (Fig. S2C). PfTLP− clones were capable of propagation of the intraerythrocytic stages and production of gametocytes comparable to wt parasites, indicating that PfTLP is not essential for intraerythrocytic development (data not shown). Moreover, gamete emergence and exflagellation of male gametes appeared normal in in vitro assays (data not shown). The lack of a clear phenotype in asexual blood stages and gametocytes of PfTLP− parasites, combined with the observed upregulation of transcript levels in sporozoites, points to a role for TLP in the mosquito stages.
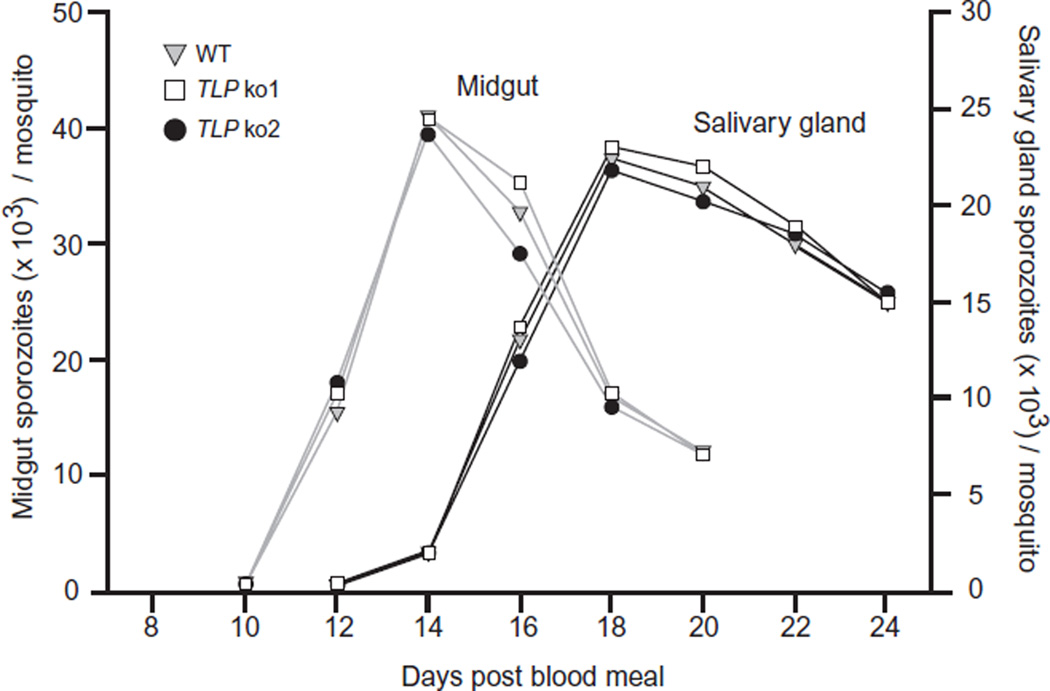
Due to limitations in using P. falciparum to study mosquito stages and the initial events of infection in the mammalian host, we decided to analyse the phenotype of mutant parasites deficient in TLP expression using the P. berghei rodent model. The high degree of homology between TLP of P. falciparum and P. berghei (43% identity within the TSP1 and vWA domains) indicates that TLP likely serves a function that is conserved between these two species. PbTLP disruption was achieved via double-cross-over homologous recombination using the vector pDEF-hDHFR (Fig. S3A). Correct disruption of PbTLP was verified by diagnostic PCR (Fig. S3B), and confirmed by Southern blot analysis of EcoRI-digested genomic DNA from wt and PbTLP− parasites (Fig. S3C). Two PbTLP− clones were selected for further characterization. Propagation of asexual intraerythrocytic stages and gametocyte production was similar in PbTLP− and wt parasites, and thus the gene is not essential for intraerythrocytic development (data not shown). To characterize the phenotype in the mosquito, wt and PbTLP− gametocytes were fed to Anopheles stephensi mosquitoes, and the number of sporozoites in oocysts and salivary glands was determined at different time points after feeding. Deletion of PbTLP had no effect on sporozoite development in oocysts; and PbTLP− sporozoites were able to invade salivary glands as efficiently as their wt counterparts (Fig. 3). PbTLP transcripts were not detected by RT-PCR in salivary gland sporozoites of PbTLP− parasites (Fig. S3D).
Figure 3.
Development of wt and PbTLP− sporozoites in the mosquito. Mosquitoes were allowed to feed on mice that were infected with either wt or PbTLP− sporozoites, and 10–24 days after blood-feeding the midguts and salivary glands from groups of 20 mosquitoes were harvested and total sporozoites counted. Shown are the numbers of sporozoites associated with midguts and salivary glands over time. Results are shown from wt (upside-down grey triangle) and two PbTLP− clones, ko1 (open square) and ko2 (black circle).
Gliding motility and hepatocyte invasion of PbTLP−parasites
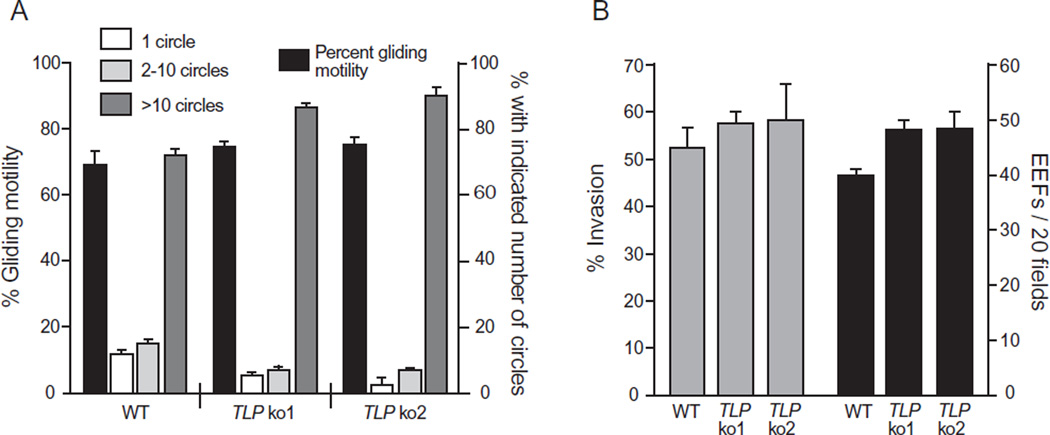
Motility plays a central role in the ability of Plasmodium sporozoites to locate and invade target cells. TRAP has been shown to be essential for gliding motility and host cell invasion (Sultan et al., 1997), and it was therefore of interest to determine if PbTLP also functions in sporozoite motility. Gliding motility assays take advantage of the typical circular movement of sporozoites that are placed on glass, and the deposition of trails of circumsporozoite (CS) protein behind the parasite. The quality and number of trails left by the sporozoites is an indication of their motility. In this assay, PbTLP− sporozoites exhibited similar motility as their wt counterparts, as measured by the capacity for gliding motility and the numbers of trailing circles per sporozoite (Fig. 4A). Therefore, TLP does not appear to be essential for sporozoite motility on a glass substrate.
Figure 4. Gliding motility and infectivity of PbTLP− sporozoites in vitro.
A. Motility; wt and PbTLP− sporozoites were allowed to glide for 1 h at 37°C on wells and then sporozoites and trails were visualized and counted. Shown is the percentage of sporozoites that exhibited gliding motility (black bars, left axis), and the percentage of these sporozoites that are associated with 1 (white bars), 2–10 (light grey bars) or >10 (dark grey bars) circles per trail (right axis). Over 100 sporozoites per well were counted and shown are the means ± SD of quadruplicates. This experiment was performed twice and shown is a representative experiment.
B. Infectivity to hepatocytes in vitro. P. berghei wt and two clones of PbTLP− sporozoites (ko1 and ko2) were added to Hepa 1–6 cells for 1 h and either fixed for an invasion assay (grey bars) or grown for an additional 2 days before fixing and staining for exoerythrocytic stages (EEFs; black bars). For each assay at least 50 fields per well were counted and shown are the means ± SD of quadruplicates. The experiment was repeated twice with similar results.
To determine if TLP is involved in sporozoite invasion of hepatocytes, sporozoites were incubated with hepatocytes and after 1 h the number of intracellular and extracellular sporozoites were counted. The level of hepatocyte invasion with PbTLP− sporozoites was similar to wt sporozoites (Fig. 4B). Moreover, following hepatocyte invasion PbTLP− sporozoites were able to efficiently develop into exoerythrocytic forms (EEFs) and both the numbers of EEFs formed and the size of the EEFs were similar to wt parasites (Fig. 4B and data not shown). Taken together, these data suggest that PbTLP does not play a role in either sporozoite invasion of target cells or subsequent development of the parasite in the liver.
In vitro migration through cells
Contact of host cells by Plasmodium sporozoites results in either the productive invasion of the host cell with the formation of a parasitophorous vacuole, or migration through the cell with concomitant rupture of the host cell membrane (Mota et al., 2001). While invasion is predominantly associated with hepatocytes, cell traversal has been observed using a broad range of cell types, thus implying universal recognition by sporozoites (Coppi et al., 2007). Recent studies using P. berghei have identified four proteins that are specifically involved in cell traversal, namely SPECT1, SPECT2, CelTOS and phospholipase (PL) (Ishino et al., 2004; 2005; Bhanot et al., 2005; Kariu et al., 2006). Sporozoites that lack any one of these proteins will invade and develop normally in vitro if placed directly on hepatocytes, but in vivo they have diminished infectivity as they are not able to cross cell barriers in the skin or the liver sinusoid to reach their target, the hepatocyte. In order to determine if TLP is similarly involved in migration, we performed in vitro migration assays using Hepa 1–6 cells and mouse dermal fibroblasts (MDFs) that were loaded with a fluorescent dye (Coppi et al., 2007). As sporozoites migrate through cells, they rupture the plasma membrane leading to the release of the intracellular fluorescent dye into the supernatant, which can then be quantified using a fluorimeter. As shown in Fig. 5, PbTLP− sporozoites exhibited a twofold reduction in their migratory activity compared with wt sporozoites when the assay was performed with either hepatocytes or MDFs. Treatment with the cysteine protease inhibitor E-64d, which prevents invasion and results in maximally migratory sporozoites (Coppi et al., 2007), increased the cell traversal activity of PbTLP− sporozoites but not to the level that was observed with wt sporozoites. These data strongly suggest that TLP plays a role in Plasmodium sporozoite passage through cells.
Figure 5.
Cell traversal of wt and PbTLP− sporozoites. Sporozoites were added to calcein green-loaded Hepa 1–6 (grey and white bars) or MDF cells (black bars), in the absence (grey and black bars) or the presence (white bars) of E-64d. After incubation for 1 h at 37°C the fluorescence released into the supernatant was measured. Migratory activities of wt and two clones of PbTLP− sporozoites (ko1 and ko2) were measured as arbitrary units (a.u.) of fluorescence and are shown as the means ± SD of triplicate values. This experiment was performed three times and shown is a representative experiment.
In vivo infectivity of PbTLP− sporozoites
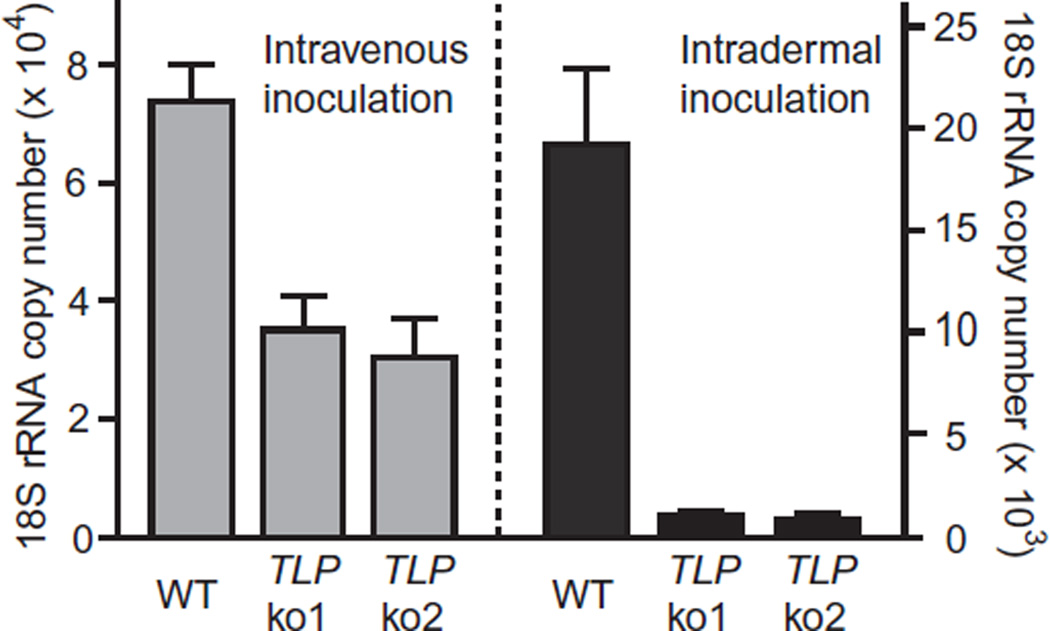
To determine if the diminished cell traversal activity of PbTLP− sporozoites manifests as a phenotype in vivo, where sporozoites must cross cell barriers to infect hepatocytes, PbTLP− or wt sporozoites were inoculated either intravenously or intradermally into Swiss Webster mice and 40 h later the liver parasite burden was assayed by quantitative PCR. This method will only detect infection if sporozoites have invaded hepatocytes and undergone several rounds of replication, as sporozoites alone are below the level of detection of this assay (Briones et al., 1996).
When PbTLP− sporozoites were administered intravenously, there was a twofold decrease in infectivity compared with wt (Fig. 6). Intradermal inoculation of PbTLP− sporozoites, which mimics mosquito injection with the advantage of controlling the number of sporozoites that are introduced into the mammalian host, showed a 20-fold decrease in infectivity compared with wt (Fig. 6). These results together with the in vitro infectivity and cell traversal data (Figs 4B and 5) suggest that TLP participates in traversing cell barriers or tissues that are encountered by the sporozoite as it travels to its target, the hepatocyte.
Figure 6.
Infectivity of wt and PbTLP− parasites in vivo. Mice were injected intravenously (left) or intradermally (right) with 104 wt or two clones of PbTLP− sporozoites (ko1 and ko2) and were sacrificed 40 h later. Total liver RNA was extracted and the liver parasite burden was measured by quantitative PCR using cDNA as template. Infection is expressed as the number of copies of P. berghei 18S rRNA. There are five mice per group and shown are the means ± SD. This experiment was performed three times and shown is a representative experiment.
Discussion
The domain architecture of TLP places it within the TRAP/MIC2 family of transmembrane adhesive proteins that are involved in parasite motility and invasion of host cells. The conservation of TLP as orthologues across the Plasmodium genus suggests that TLP serves a function that is important for parasite development during one or more life cycle stages. TLP shares conserved features within the short (~40 aa) cytoplasmic domain that are typical of TRAP/MIC2 family members, including a putative micronemal targeting motif and a conserved tryptophan residue near the carboxy-terminus. TLP and the other Plasmodium TRAP/MIC2 family members, except for PTRAMP, have a putative rhomboid cleavage site at the extracellular side of the transmembrane region (see fig. 1 in Baker et al., 2006) and, indeed, P. falciparum rhomboid 4 (PfROM4) and T. gondii rhomboid 5 (TgROM5) have been recently shown to cleave recombinant PfTLP in vitro (Baker et al., 2006). Interestingly, PfROM4 also cleaves Plasmodium TRAP and MTRAP in vitro, while TgROM5 is the protease that cleaves the Toxoplasma TRAP homologue, MIC2. As rhomboid enzymes are highly specific, the above observations suggest that PfROM4 is the major ‘sheddase’ that cleaves the Plasmodium TRAP/MIC2 family members.
PfTLP was initially identified in an asexual versus gametocyte transcript microarray screen, and transcription in gametocytes was confirmed by quantitative RT-PCR for both the P. falciparum and P. berghei genes, albeit at lower expression levels than the predominant expression that is observed in salivary gland sporozoites. The gametocyte and gamete stages do not possess a capacity for gliding motility or cellular invasion and do not possess microneme organelles, and thus it is not apparent what function TLP would serve in the sexual stages. It is possible that TLP expression is regulated post-transcriptionally in gametocytes and is not translated, similar to what has been observed for the proteins P25 and P28 (Mair et al., 2006). Analyses of mutant parasites that are deficient in expressing TRAP or CTRP indicate that these proteins have essential roles in the sporozoite and ookinete stages respectively. TRAP-deficient sporozoites are not motile and are unable to invade salivary glands (Sultan et al., 1997), whereas parasites lacking CTRP are unable to traverse the epithelial cells of the mosquito midgut to form oocysts (Dessens et al., 1999; Yuda et al., 1999; Templeton et al., 2000). MTRAP is a putative merozoite-stage TRAP/MIC2 family member and repeated attempts to disrupt MTRAP were unsuccessful, implying that the protein is essential for intraerythrocytic development (Baum et al., 2006). To begin to address the function of TLP, the gene was disrupted in P. falciparum and P. berghei. In both species of malaria parasites the targeted disruption of TLP did not affect intraerythrocytic propagation, gametocytogenesis or gametogenesis. Mosquito transmission studies with PbTLP− parasite lines showed that oocyst production and levels of salivary gland sporozoites were similar to wt. PbTLP− sporozoites were shown to be defective in cell traversal in vitro and sporozoite infectivity in vivo, most markedly after intradermal inoculation.
The deletion of the sporozoite stage genes, SPECT1, SPECT2, CelTOS or PL, also result in decreased or absent cell sporozoite traversal activity in vitro and marked decrease in sporozoite infectivity in vivo but not in vitro (Ishino et al., 2004; 2005; Bhanot et al., 2005; Kariu et al., 2006). How sporozoites traverse cells and precisely how each of these proteins functions in this process are not known; however, the clear phenotype of each individual gene knockout suggests that these proteins do not overlap in function and that cell traversal is likely a complex process requiring many different proteins. PL possesses lipase activity and SPECT2 contains a perforin-like membrane attack complex (MACPF) domain which may function in rupturing the membrane of the host cell during entry and/or exit of the sporozoite. SPECT1 and CelTOS possess no recognizable domains or similarity to other proteins and their role in cell traversal remains unknown. TLP is the first TRAP/MIC2 family protein to be implicated in cell traversal and its TRAP/MIC2-like domain architecture suggests that TLP may function in gliding motility specific to cell traversal. Gliding motility assays determine whether sporozoites are capable of gliding on glass slides, a substrate that offers little resistance to movement and therefore requires less motive force than motility through matrix or cells. It is possible that greater force would be required of the sporozoite to move through extracellular matrix or host cells, and we postulate that additional transmembrane motor molecules, such as TLP, are involved in this process.
Experimental procedures
Parasites and mosquitoes
The P. falciparum NF54 isolate (Ponnudurai et al., 1981) was cultivated in vitro using RPMI 1640 medium supplemented with 10% heat-inactivated human serum and human erythrocytes at a 5% haematocrit as described (Ifediba and Vanderberg, 1981). Cultures were synchronized by the magnetic isolation of schizonts using a MACS depletion column (Milteny Biotec) in conjunction with a magnetic separator, and placed back into culture (Taylor et al., 2002). To collect parasites throughout an intraerythrocytic developmental cycle, parasites were harvested at different time points after erythrocyte invasion, pelleted by centrifugation and lysed with 0.1% saponin in PBS. Thin smears of blood were made at each time point and stained with Giemsa reagent for microscopy. Harvested parasites consisted largely (>90%) of merozoites at 4–6 h post synchronization (hps), ring stage at 16–20 hps, early trophozoite stage at 22–26 hps, late trophozoite stage at 36–40 hps and schizonts at 42–46 hps. Gametocytes were prepared by extended in vitro culturing of asexual stage parasites and purified by magnetic isolation at two 24 h interval time points. Gametocyte preparations consisted largely of stage IV and V parasites with minor contamination of mixed asexual stage parasites. Mosquito stages were obtained after membrane feeds of 3- to 5-day-old An. stephensi mosquitoes on human erythrocytes that contained wt P. falciparum stage V gametocytes as described (Bishop and Gilchrist, 1946). Midgut and salivary gland sporozoites were obtained by dissection of mosquitoes at 8 and 18 days after infection respectively.
The gametocyte-producing reference clone, cl15cy1 (HP), of the ANKA strain of P. berghei, was maintained by cyclic passages in Swiss Webster mice and An. stephensi mosquitoes, and was used between the first and fifth direct mouse passage. In addition, the non-gametocyte-producing clone (HPE) of the ANKA strain was used in some experiments (Janse et al., 1989). To collect parasites from different stages of the intraerythrocytic developmental cycle, synchronized blood stage infections of P. berghei HPE clone were performed in vivo as described by Janse and Waters (1995). Thin smears of blood were made at each time point and stained with Giemsa reagent for microscopy. Harvested parasites consisted largely (>90%) of early ring stage at 5 hps, of late ring stage at 12 hps, of early trophozoite stage at 16 hps, of late trophozoite stage at 20 hps and of schizonts at 24 hps. Gametocytes from the HP clone were obtained by adding 0.07 mg ml−1 pyrimethamine to the drinking water of mice that harboured infections of ~3% parasitaemia. No asexual stages were observed in Giemsa-stained tail blood smears after 48 h of drug treatment. Mosquito stages were obtained after feeding 3-to 5-day-old An. stephensi mosquitoes on Swiss Webster mice that were infected with either P. berghei ANKA HP or the PbTLP− clones. Oocyst samples for RT-PCR consisted of infected midguts that were removed 5, 10 and 15 days post infection. To determine parasite development in the mosquito, oocyst sporozoites were counted from midguts removed at days 10 through 20 post infection, and salivary gland sporozoites were quantified from dissected salivary glands on days 12 through 24 post infection. In order to obtain live salivary gland sporozoites for in vitro and in vivo assays, on days 18–20 post infective blood meal mosquitoes were anaesthetized on ice, rinsed in 70% ethanol, washed in Dulbecco’s modified Eagle’s medium (DMEM), and the salivary glands were removed. Tissue was gently ground in a tissue homogenizer, centrifuged at 80 g for 3 min to remove mosquito debris, and sporozoites were counted in a haemocytometer and kept on ice until use.
Anopheles stephensi mosquitoes were reared at 27°C and 80% humidity under a 12/12 h light/dark cycle, and adults were fed on 10% sucrose solution. P. berghei-infected mosquitoes were maintained at 19°C, whereas P. falciparum-infected mosquitoes were kept at 28°C.
Apicomplexan genome sequence analysis
The domain architecture of TLP was determined via HMMER searches of domains using the web-based sites, SMART (Shultz et al., 1997; http://smart.embl-heidelberg.de) and NCBI Conserved Domains (http://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi); via reiterative BLAST screens of GenBank (nr); and by visual inspection for sequence motifs. Multiple alignments were generated using a web-based version of T-Coffee (Notredame et al., 2000; http://tcoffee.vital-it.ch/cgi-bin/Tcoffee/tcoffee_cgi/index.cgi). Signal peptides were predicted using the SignalP program (Emanuelsson et al., 2007; http://www.cbs.dtu.dk/services/SignalP) and transmembrane domains were predicted using the TMHMM2 package (Krogh et al., 2001). TLP, TRAP and CTRP genes were identified in P. falciparum isolates via local standalone BLAST screening of genome nucleotide databases that were downloaded from the Broad Institute (http://www.broad.mit.edu), namely HB3, 7G8, D10, D6, FCC-2/Hainan, RO-33, SL, K1, Senegal_V34.04 and VS/1. Additional sequences were isolated using gene-specific primers and genomic DNA template from P. falciparum isolates (DD2, Peru, MONT-S1, ECP, Santa Lucia and Genieve) that were the generous gift of Karen Hayton (NIAID/NIH).
Cells and antibodies
Hepa 1–6 cells (CRL-1830; ATCC, Rockville, MD) and MDF (Coppi et al., 2007) were maintained in DMEM supplemented with 10% fetal calf serum (FCS) and 1 mM glutamine (DMEM/FCS). In addition, the following monoclonal antibodies were used: monoclonal antibody (mAb) 3D11, directed against the repeat region of P. berghei CSP (Yoshida et al., 1980); and mAb 2E6, directed against P. berghei Hsp70 (Tsuji et al., 1994).
RNA isolation and transcript expression analysis by real-time RT-PCR
RNA was isolated with Trizol (Invitrogen) according to the manufacturer’s instructions, treated with DNase I (Invitrogen), and purified on RNeasy MinElute Cleanup columns (Qiagen). RNA was reverse-transcribed using Superscript II that was primed with random hexanucleotides (Invitrogen). Real-time PCR was performed using an ABI Prism 7900HT sequence detector (Applied Biosystems). Reactions were prepared in 20 µl volumes using a SYBR Green PCR master mix (Applied Biosystems) and 1 µM gene-specific primers. The absence of genomic DNA contamination was confirmed by PCR amplification on sham-treated RNA samples that lacked reverse transcriptase. The specificity of amplification for each PCR product was confirmed by dissociation curve analysis. The efficiency of amplification was verified by using different concentrations of genomic DNA as templates in order to calculate the median cycle threshold (CT) value for each primer pair. All primer pairs included in this study displayed the same median CT value. Relative quantification of cDNA was performed using the standard curve method (User Bulletin 2, ABI, http://www.appliedbiosystems.com) to determine the efficiency of the target and reference amplification and to quantify cDNA in each sample. The normalized expression for each gene was determined as the ratio: (relative amount of target gene cDNA/ relative amount of control gene cDNA). Triplicate PCR reactions were analysed for each sample. Transcript expression of PfTLP was normalized to the expression of the control gene, arginyl-tRNA synthetase (PFL0900c), while the transcript expression of PbTLP was normalized to the expression of the control gene, Pbhsp70 (PB001074.01.0). Gene-specific primers were empirically designed for PfTLP (sense 5′-AGATGATGAGCCGGTTGGAC, antisense 5′-CCCTTATTTCTATTTGTTGG); PbTLP (sense 5′-AAGATGATGATTGGAAAACCAAA, antisense 5′-GC ATAAAATGCAGATGCTCCT); Pfama-1 (sense 5′-TCCAATCAA CGAACATAGGG, antisense 5′-TTTTGTTCTTGTGGTGCGGG); Pbama-1 (sense 5′-CAGCCCAAGAAAATATGGG, antisense 5′-TTTACAATAACCATCAACCC); Pfarginyl-tRNA synthetase (sense 5′-AAGAGATGCATGTTGGTC, antisense 5′-GTACCCCAATCACCTACA); and Pbhsp70 (sense 5′-AGAGAAGCAGCTGAAACAGC, antisense 5′-TCCCTTTAATAAATCATGGC).
Targeted disruption of PfTLP and PbTLP
Two ~1100 bp fragments of the PfTLP gene were amplified by PCR from P. falciparum NF54 genomic DNA using the following specific primer pairs: TLP5′SpeI (5′-CCACTAGTTCGGTTTT GTTGATGATTCTG) plus TLP3′BglII (5′-GAAGATCTTTAAGTATGACTAGAGTATGTAG); and TLP5′ClaI (5′-CCCATCGATGCTCAGGAGTGGAAACAAG) plus TLP3′NcoI (5′-GGGCCATGGAAAGTCCATAAGGGAGACACC). To derive the disruption plasmid these PCR fragments were sequentially cloned into the pHTK plasmid (Duraisingh et al., 2002) using the SpeI/BglII sites, followed by insertion into the ClaI/NcoI sites. The gene targeting disruption plasmid was introduced into the P. falciparum isolate NF54 via the erythrocyte-loading method (Deitsch et al., 2001), and stable transfected parasites were selected with pyrimethamine and ganciclovir to drive plasmid integration by double-cross-over homologous recombination (Duraisingh et al., 2002). As described in Results, the ganciclovir step was unsuccessful and the resulting PfTLP− lines were via single integration events of the disruption plasmid. Pyrimethamine-resistant polyclonal lines were established, and clonal lines were isolated by limiting dilution. Correct integration of the targeting plasmid into the genomic locus was verified by PCR and Southern blot analyses diagnostic of a disrupted locus. PCR was performed with 100 ng of genomic DNA from wt or PfTLP− parasites for 35 cycles of amplification and with an annealing temperature of 50°C, using the primers a (5′-CTCAGGAGTGGAAACAAG); b (5′-GGAAAATATGGAAGCAGACGTAGGAGG); and a’ (5′-GTTTTGTAATTTATGGGATAGCG) (shown in Fig. S2A). Southern blotting was performed with 10 µg of genomic DNA from wt or PfTLP− parasites, digested with HindIII and NdeI, separated by 0.8% agarose gel electrophoresis and transferred to Hybond N+ (Amersham Biosciences). A 1100 bp hybridization probe corresponding to PfTLP was amplified with the primers TLP5′ClaI and TLP3′NcoI. The probe was labelled with digoxigenin and hybridization was performed at 48°C using DIG High Prime DNA Labelling and Detection Starter Kit II (Roche Diagnostics), according to the manufacturer’s instructions.
PbTLP− parasites were generated by double homologous recombination using the targeting vector pDEF-hDHFR (available from MR4; http://www.malaria.mr4.org) and P. berghei ANKA erythrocytic stages. Two fragments of PbTLP, of 758 bp and 791 bp, were amplified by PCR using as template genomic DNA from P. berghei ANKA parasites and the primer pairs: PbTLP5S-HindIII (5′-CATAAATGGCAACCCAAGCC) plus PbTLP5AS-PstI (5′-GACAGAGTAACAACAAAAGGAGA); and PbTLP3S-KpnI (5′-CTACAAAATTGTTCGGCTTTAAG) plus PbTLP3AS-EcoRI (5′-GATAATATCGATACAGACCC). These PCR fragments were sequentially cloned into the restriction sites HindIII/PstI and KpnI/EcoRI of the plasmid pDEF-hDHFR. The gene targeting disruption plasmid was digested with HindIII and EcoRI to release the fragment for transfection. P. berghei schizonts were collected from Wistar rats and electroporated with 5 µg of DNA using the Amaxa Nucleofector (program U33) as described (Janse et al., 2006). Selection and cloning by limiting dilution were performed in mice as described (Menard et al., 1997). Integration of the transfected DNA at the correct location was verified by both diagnostic PCR and Southern blot analysis. PCR was performed with 100 ng of genomic DNA from wt or PbTLP− parasites for 35 cycles and annealing temperature of 50°C using the primers a (5′-GATATAATAGTCATTTTTTCCGCG); b (5′-CCTTTTGTTGTTACTCTGTCAATTAATG); b’ ‘(5′-GCTCACCCCTCAAAGCACCAG); c (same as PbTLP3S-KpnI); c’ (5′-CAATGATTCATAAATAGTTGGACTTG); and d (5′-GATGAGTTAGACAAAATGAACAAATC) (shown in Fig. S3A). Southern blot analysis was performed with 5 µg of genomic DNA from wt or PbTLP− parasites, digested with EcoRI. A 758 bp hybridization probe corresponding to PbTLP was amplified with the primers PbTLP5SHindIII and PbTLP5AS-PstI. Probe labelling, hybridization and detection were performed as described for PfTLP− parasites. Lack of TLP transcripts in the PbTLP− clones was confirmed by RT-PCR, using RNA that was isolated from salivary gland sporozoites. cDNA was synthesized as described in the previous section, and PCR was performed for 35 cycles of amplification using the oligonucleotides ‘e’ (5′-AATTCCGAACGTTTTAGAGG) and ‘f’ (5′-CCAAATGAGCTTATACAAAGT).
Sporozoite invasion and development assays
Hepa 1–6 cells were seeded in Permanox eight-chambered Laboratory-Tek wells (2.5 × 105 per well) and allowed to grow overnight until subconfluent. On the day of the experiment, 5 × 105 P. berghei wt or TLP− sporozoites were added per well and centrifuged at 300 g for 3 min. After 1 h at 37°C, the cells were washed, fixed, and sporozoites were stained with a double-staining assay that distinguishes intracellular from extracellular sporozoites (Renia et al., 1988; Pinzon-Ortiz et al., 2001). To quantify EEF development, 8 × 105 P. berghei sporozoites were added to the cells as described above, and cells with sporozoites were grown for an additional 2 days. At this time the cells were fixed with methanol and stained with mAb 2E6 followed by goat anti-mouse IgG conjugated to FITC. In all assays, at least 50 fields per well were counted and each point was performed in triplicate.
Gliding motility assay
Gliding motility assays were performed as described (Coppi et al., 2006). Briefly, sporozoites were added to Laboratory-Tek wells pre-coated with mAb 3D11 for 1 h at 37°C, after which time the medium was removed and the wells were fixed and stained with biotinylated mAb 3D11 followed by streptavidin-FITC (1:100 dilution; GE Bioscience) in order to visualize the CS protein-containing trails. Gliding motility was quantified by counting the number of sporozoites associated with trails and, for those sporozoites with trails, counting the number of circles in each trail. Over 200 sporozoites were counted per well and triplicate wells were counted for each parasite line.
Calcein migration assay
Hepa 1–6 cells and MDF were plated in 96-well plates and allowed to grow until subconfluent. They were then washed with DMEM without phenol red, loaded with 10 µM calcein green AM (Invitrogen) for 1 h at 37°C, and washed three times to remove unincorporated dye. Approximately 8.5 × 105 sporozoites were centrifuged (300 g) onto each well of calcein-loaded cells and incubated for 1 h at 37°C. Supernatants containing the released calcein were then transferred to a ThermoElectron Microfluor 96-well plate and fluorescence was read in a Labsystems Fluoroscan II using excitation and emission wavelengths of 485 nm and 538 nm respectively. Additional experimental details are outlined in Coppi et al. (2007).
In vivo quantitative analysis of liver infection
Female Swiss Webster mice 4–5 weeks old were injected intravenously or intradermally with 104 P. berghei wt or TLP− sporozoites. Livers were harvested 40 h later and total RNA was isolated and cDNA was generated via reverse transcription. The liver parasite burden was quantified by real-time PCR using cDNA template as outlined in Bruna-Romero et al. (2001), using primers that recognize sequences specific to P. berghei genomic DNA within 18S rRNA as described (Kumar et al., 2004). A standard curve was created using 10-fold dilutions of a plasmid construct containing the P. berghei 18S rRNA gene.
Supplementary Material
Acknowledgements
T.J.T. would like to acknowledge the generous support of the William Randolph Hearst Foundation. P.S. was supported by NIH Grant R01 AI056840. The authors would like to thank Sandra Gonzalez and Jean Nonon for their assistance with the rearing and infection of the mosquitoes.
Footnotes
Supplementary material
The following supplementary material is available for this article online:
Sequence diversity of PfTRAP (top), PfCTRP (middle) and PfTLP (bottom) in various isolates of P. falciparum. Select regions of the genes were either amplified by PCR and sequenced, or obtained by BLAST analyses of genome databases that are available at the Sanger Center and Broad Institutes. The amplified regions were specific to the single vWA of PfTRAP (aa positions 52–239 of the 3D7 version, PF13_0201); the second vWA domain of CTRP (aa positions 308–493 of the 3D7 version, PFC0640w); and the second vWA domain of PfTLP (aa positions 341–526 of the 3D7 version, PFF0800w). The locations of variations are indicated by stars above the alignment and variant amino acids are indicated by grey boxes.
Targeted gene disruption of PfTLP via homologous recombination in the P. falciparum isolate, NF54.
A. Schematic of the PfTLP locus and the locations of PCR primers that yield products diagnostic of wt and disrupted loci. The shaded boxes indicate the regions within PfTLP that were used to target homologous recombination of an insert within the disruption plasmid. H, HindIII; C, ClaI; N, NdeI.
B. EtBr-stained agarose gel electrophoresis of PCR products diagnostic of integration events, using primer pairs indicated above the respective lanes. Results from two PfTLP− clones are shown, ko1 and ko2.
C. Southern blot analysis of HindIII- plus NdeI-digested genomic DNA from wt and PfTLP− lines showing complete disruption of the PfTLP gene locus. The probe used to screen the Southern blot is indicated in (A) as a solid horizontal bar above the 5′-coding region of the PfTLP gene. The ~1.5 kb band corresponds to the disruption plasmid. The intensity of this band is higher than the high-molecular-weight bands because the disruption plasmid forms concatemers and several copies of the plasmid become integrated in tandem into the PfTLP locus.
Targeted gene disruption of TLP in P. berghei ANKA via double homologous recombination.
A. Schematic of the PbTLP locus and the locations of PCR primers that yield products diagnostic of wt and disrupted loci.
B. EtBr-stained agarose gel electrophoresis of PCR products amplified from primer pairs diagnostic of wt locus and integration events. Results from two PbTLP− clones are shown, ko1 and ko2.
C. Southern blot analysis of EcoRI-digested genomic DNAfrom wt and PbTLP− lines showing complete disruption of the TLP gene locus. The probe used to screen the Southern blot is indicated in (A) as a solid horizontal bar above the coding region of the PbTLP gene.
D. RT-PCR of PbTLP transcripts with primer set ‘ef’ shows loss of expression in TLP−. Reverse transcriptase (RT) and sham controls are indicated above the lanes by ‘+’ and ‘−’ respectively. The P. berghei gene, Pbhsp70 (PB001074.01.0), was used as a control.
This material is available as part of the online article from: http://www.blackwell-synergy.com/doi/abs/10.1111/j.1462-5822.2008.01143.x
Please note: Blackwell Publishing is not responsible for the content or functionality of any supplementary materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Baker RP, Wijetilaka R, Urban S. Two Plasmodium rhomboid proteases preferentially cleave different adhesins implicated in all invasive stage of malaria. PLoS Pathog. 2006;2:e113. doi: 10.1371/journal.ppat.0020113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum J, Richard D, Healer J, Rug M, Krnajski Z, Gilberger TW, et al. A conserved molecular motor drives cell invasion and gliding motility across malaria life cycle stages and other apicomplexan parasites. J Biol Chem. 2006;281:5197–5208. doi: 10.1074/jbc.M509807200. [DOI] [PubMed] [Google Scholar]
- Bhanot P, Schauer K, Coppens I, Nussenzweig V. A surface phospholipase is involved in the migration of Plasmodium sporozoites through cells. J Biol Chem. 2005;280:6752–6760. doi: 10.1074/jbc.M411465200. [DOI] [PubMed] [Google Scholar]
- Bishop A, Gilchrist BM. Experiments upon the feeding of Aedes aegypti through animal membranes with a view to applying this method to the chemotherapy of malaria. Parasitology. 1946;37:85–100. doi: 10.1017/s0031182000013202. [DOI] [PubMed] [Google Scholar]
- Braks JA, Mair GR, Franke-Fayard B, Janse CJ, Waters AP. A conserved U-rich RNA region implicated in regulation of translation in Plasmodium female gametocytes. Nucleic Acids Res. 2008;36:1176–1186. doi: 10.1093/nar/gkm1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brecht S, Carruthers VB, Ferguson DJ, Giddings OK, Wang G, Jakle U, et al. The Toxoplasma micronemal protein MIC4 is an adhesin composed of six conserved apple domains. J Biol Chem. 2001;276:4119–4127. doi: 10.1074/jbc.M008294200. [DOI] [PubMed] [Google Scholar]
- Briones MR, Tsuji M, Nussenzweig V. The large difference in infectivity for mice of Plasmodium berghei and Plasmodium yoelii sporozoites cannot be correlated with their ability to enter into hepatocytes. Mol Biochem Parasitol. 1996;77:7–17. doi: 10.1016/0166-6851(96)02574-1. [DOI] [PubMed] [Google Scholar]
- Brossier F, Sibley DL. Toxoplasma gondii: microneme protein MIC2. Int J Biochem Cell Biol. 2005;37:2266–2272. doi: 10.1016/j.biocel.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Bruna-Romero O, Hafalla JC, González-Aseguinolaza G, Sano G, Tsuji M, Zavala F. Detection of malaria liver-stages in mice infected through the bite of a single Anopheles mosquito using a highly sensitive real-time PCR. Int J Parasitol. 2001;31:1499–1502. doi: 10.1016/s0020-7519(01)00265-x. [DOI] [PubMed] [Google Scholar]
- Brydges SD, Sherman GD, Nockemann S, Loyens A, Daubener W, Dubremetz JF, Carruthers VB. Molecular characterization of TgMIC5, a proteolytically processed antigen secreted from the micronemes of Toxoplasma gondii. Mol Biochem Parasitol. 2000;111:51–66. doi: 10.1016/s0166-6851(00)00296-6. [DOI] [PubMed] [Google Scholar]
- Carruthers VB. Armed and dangerous: Toxoplasma gondii uses an arsenal of secretory proteins to infect host cells. Parasitol Int. 1999;48:1–10. doi: 10.1016/s1383-5769(98)00042-7. [DOI] [PubMed] [Google Scholar]
- Carruthers VB, Sherman GD, Sibley LD. The Toxoplasma adhesive protein MIC2 is proteolytically processed at multiple sites by two parasite-derived proteases. J Biol Chem. 2000;275:14346–14353. doi: 10.1074/jbc.275.19.14346. [DOI] [PubMed] [Google Scholar]
- Coppi A, Cabinian M, Mirelman D, Sinnis P. Antimalarial activity of allicin, a biologically active compound from garlic cloves. Antimicrob Agents Chemother. 2006;50:1731–1737. doi: 10.1128/AAC.50.5.1731-1737.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppi A, Tewari R, Bishop JR, Bennett BL, Lawrence R, Esko JD, et al. Heparan sulfate proteoglycans provide a signal to Plasmodium sporozoites to stop migrating and productively invade cells. Cell Host Microbe. 2007;2:316–327. doi: 10.1016/j.chom.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deitsch KW, Driskill CL, Wellems TE. Transformation of malaria parasites by the spontaneous uptake and expression of DNA from human erythrocytes. Nucleic Acids Res. 2001;29:850–853. doi: 10.1093/nar/29.3.850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dessens JT, Beetsma AL, Dimopoulos G, Wengelnik K, Crisanti A, Kafatos FC, Sinden RE. CTRP is essential for mosquito infection by malaria ookinetes. EMBO J. 1999;18:6221–6227. doi: 10.1093/emboj/18.22.6221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Cristina M, Spaccapelo R, Soldati D, Bistoni F, Crisanti A. Two conserved amino acid motifs mediate protein targeting to the micronemes of the apicomplexan parasite Toxoplasma gondii. Mol Cell Biol. 2000;20:7332–7341. doi: 10.1128/mcb.20.19.7332-7341.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duraisingh MT, Triglia T, Cowman AF. Negative selection of Plasmodium falciparum reveals targeted gene deletion by double crossover recombination. Int J Parasitol. 2002;32:81–89. doi: 10.1016/s0020-7519(01)00345-9. [DOI] [PubMed] [Google Scholar]
- Emanuelsson O, Brunak S, von Heijne G, Nielsen H. Locating proteins in the cell using TargetP, SignalP, and related tools. Nat Protoc. 2007;2:953–997. doi: 10.1038/nprot.2007.131. [DOI] [PubMed] [Google Scholar]
- Hall N, Karras M, Raine JD, Carlton JM, Kooij TW, Berriman M, et al. A comprehensive survey of the Plasmodium life cycle by genomic, transcriptomic, and proteomic analyses. Science. 2005;307:82–86. doi: 10.1126/science.1103717. [DOI] [PubMed] [Google Scholar]
- Harris PK, Yeoh S, Dluzewski AR, O’Donnell RA, Withers-Martinez C, Hackett F, et al. Molecular identification of a malaria merozoite surface sheddase. PLoS Pathog. 2005;1:241–251. doi: 10.1371/journal.ppat.0010029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward RE, Derisi JL, Alfadhli S, Kaslow DC, Brown PO, Rathod PK. Shotgun DNA microarrays and stage-specific gene expression in Plasmodium falciparum malaria. Mol Microbiol. 2000;35:6–14. doi: 10.1046/j.1365-2958.2000.01730.x. [DOI] [PubMed] [Google Scholar]
- Howell SA, Withers-Martinez C, Kocken CH, Thomas AW, Blackman MJ. Proteolytic processing and primary structure of Plasmodium falciparum apical membrane antigen-1. J Biol Chem. 2001;276:31311–31320. doi: 10.1074/jbc.M103076200. [DOI] [PubMed] [Google Scholar]
- Ifediba T, Vanderberg JP. Complete in vitro maturation of Plasmodium falciparum gametocytes. Nature. 1981;294:364–366. doi: 10.1038/294364a0. [DOI] [PubMed] [Google Scholar]
- Ishino T, Yano K, Chinzei Y, Yuda M. Cell-passage activity is required for the malarial parasite to cross the liver sinusoidal cell layer. PLoS Biol. 2004;2:77–84. doi: 10.1371/journal.pbio.0020004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishino T, Chinzei Y, Yuda M. A Plasmodium sporozoite protein with a membrane attack complex domain is required for breaching the liver sinusoidal cell layer prior to hepatocyte infection. Cell Microbiol. 2005;7:199–208. doi: 10.1111/j.1462-5822.2004.00447.x. [DOI] [PubMed] [Google Scholar]
- Janse CJ, Waters AP. Plasmodium berghei: the application of cultivation and purification techniques to molecular studies of malaria parasites. Parasitol Today. 1995;11:138–143. doi: 10.1016/0169-4758(95)80133-2. [DOI] [PubMed] [Google Scholar]
- Janse CJ, Boorsma EG, Ramesar J, van Vianen P, van der Meer R, Zenobi P, et al. Plasmodium berghei: gametocyte production, DNA content, and chromosome-size polymorphisms during asexual multiplication in vivo. Exp Parasitol. 1989;68:274–282. doi: 10.1016/0014-4894(89)90109-4. [DOI] [PubMed] [Google Scholar]
- Janse CJ, Ramesar J, Waters AP. High-efficiency transfection and drug selection of genetically transformed blood stages of the rodent malaria parasite Plasmodium berghei. Nat Protoc. 2006;1:346–356. doi: 10.1038/nprot.2006.53. [DOI] [PubMed] [Google Scholar]
- Kaiser K, Matuschewski K, Camargo N, Ross J, Kappe SH. Differential transcriptome profiling identifies Plasmodium genes encoding pre-erythrocytic stage-specific proteins. Mol Microbiol. 2004;51:1221–1232. doi: 10.1046/j.1365-2958.2003.03909.x. [DOI] [PubMed] [Google Scholar]
- Kariu T, Ishino T, Yano K, Chinzei Y, Yuda M. CelTOS, a novel malarial protein that mediates transmission to mosquito and vertebrate hosts. Mol Microbiol. 2006;59:1369–1379. doi: 10.1111/j.1365-2958.2005.05024.x. [DOI] [PubMed] [Google Scholar]
- Keeley A, Soldati D. The glideosome: a molecular machine powering motility and host-cell invasion by Apicomplexa. Trends Cell Biol. 2004;14:528–532. doi: 10.1016/j.tcb.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Krogh A, Larsson B, von Heijne G, Sonnhammer EL. Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J Mol Biol. 2001;305:567–580. doi: 10.1006/jmbi.2000.4315. [DOI] [PubMed] [Google Scholar]
- Kumar KA, Oliveira GA, Edelman R, Nardin E, Nussenzweig V. Quantitative Plasmodium sporozoite neutralization assay. J Immunol Methods. 2004;292:157–164. doi: 10.1016/j.jim.2004.06.017. [DOI] [PubMed] [Google Scholar]
- Lee JO, Rieu P, Arnaout MA, Liddington R. Crystal structure of the A domain from the alpha subunit of integrin CR3 (CD11b/CD18) Cell. 1995;80:631–638. doi: 10.1016/0092-8674(95)90517-0. [DOI] [PubMed] [Google Scholar]
- Mair GR, Braks JA, Garver LS, Wiegant JC, Hall N, Dirks RW, et al. Regulation of sexual development of Plasmodium by translational repression. Science. 2006;313:667–669. doi: 10.1126/science.1125129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menard R, Sultan AA, Cortes C, Altszuler R, van Dijk MR, Janse CJ, et al. Circumsporozoite protein is required for development of malaria sporozoites in mosquitoes. Nature. 1997;385:336–340. doi: 10.1038/385336a0. [DOI] [PubMed] [Google Scholar]
- Miller SA, Binder EM, Blackman MJ, Carruthers VB, Kim K. A conserved subtilisin-like protein TgSUB1 in microneme organelles of Toxoplasma gondii. J Biol Chem. 2001;276:45341–45348. doi: 10.1074/jbc.M106665200. [DOI] [PubMed] [Google Scholar]
- Mota M, Pradel G, Vanderberg JP, Hafalla JCR, Frevert U, Nussenzweig RS, et al. Migration of Plasmodium sporozoites through cells before infection. Science. 2001;291:141–144. doi: 10.1126/science.291.5501.141. [DOI] [PubMed] [Google Scholar]
- Notredame C, Higgins DG, Heringa J. T-Coffee: a novel method for fast and accurate multiple sequence alignment. J Mol Biol. 2000;302:205–217. doi: 10.1006/jmbi.2000.4042. [DOI] [PubMed] [Google Scholar]
- Opitz C, Di Cristina M, Reiss M, Ruppert T, Crisanti A, Soldati D. Intramembrane cleavage of microneme proteins at the surface of the apicomplexan parasite Toxoplasma gondii. EMBO J. 2002;21:1577–1585. doi: 10.1093/emboj/21.7.1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton MG, Barker GC, Matsuoka H, Ramesar J, Janse CJ, Waters AP, Sinden RE. Structure and expression of a post-transcriptionally regulated malaria gene encoding a surface protein from the sexual stages Plasmodium berghei. Mol Biochem Parasitol. 1993;59:263–275. doi: 10.1016/0166-6851(93)90224-l. [DOI] [PubMed] [Google Scholar]
- Pinzon-Ortiz C, Friedman J, Esko J, Sinnis P. The binding of the circumsporozoite protein to cell surface heparan sulfate proteoglycans is required for Plasmodium sporozoite attachment to target cells. J Biol Chem. 2001;276:26784–26791. doi: 10.1074/jbc.M104038200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponnudurai T, Leeuwenberg AD, Meuwissen JH. Chloroquine sensitivity of isolates of Plasmodium falciparum adapted to in vitro culture. Trop Geogr Med. 1981;33:50–54. [PubMed] [Google Scholar]
- Renia L, Miltgen F, Charoenvit Y, Ponnudurai T, Verhave JP, Collins WD, Mazier D. Malaria sporozoite penetration: a new approach by double staining. J Immunol Methods. 1988;112:201–205. doi: 10.1016/0022-1759(88)90358-4. [DOI] [PubMed] [Google Scholar]
- Robson KJ, Hall JR, Jennings MW, Harris TJ, Marsh K, Newbold CI, et al. A highly conserved amino-acid sequence in thrombospondin, properdin and in proteins from sporozoites and blood stages of a human malaria parasite. Nature. 1988;335:79–82. doi: 10.1038/335079a0. [DOI] [PubMed] [Google Scholar]
- Robson KJ, Hall JR, Davies LC, Crisanti A, Hill AV, Wellems TE. Polymorphism of the TRAP gene of Plasmodium falciparum. Proc Biol Sci. 1990;242:205–216. doi: 10.1098/rspb.1990.0126. [DOI] [PubMed] [Google Scholar]
- Shultz J, Milpetz F, Bork P, Ponting CP. SMART, a simple modular architecture research tool: identification of signaling domains. Proc Natl Acad Sci USA. 1997;95:5857–5864. doi: 10.1073/pnas.95.11.5857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinnis P, Coppi A. A long and winding road: the Plasmodium sporozoite’s journey in the mammalian host. Parasitol Int. 2007;56:171–178. doi: 10.1016/j.parint.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spano F, Putignani L, Naitza S, Puri C, Wright S, Crisanti A. Molecular cloning and expression analysis of a Cryptosporidium parvum gene encoding a new member of the thrombospondin family. Mol Biochem Parasitol. 1998;92:147–162. doi: 10.1016/s0166-6851(97)00243-0. [DOI] [PubMed] [Google Scholar]
- Sultan AA, Thathy V, Frevert U, Robson KJ, Crisanti A, Nussenzweig V, et al. TRAP is necessary for gliding motility and infectivity of Plasmodium sporozoites. Cell. 1997;90:511–522. doi: 10.1016/s0092-8674(00)80511-5. [DOI] [PubMed] [Google Scholar]
- Taylor HM, Grainger M, Holder AA. Variation in the expression of a Plasmodium falciparum protein family implicated in erythrocyte invasion. Infect Immun. 2002;70:5779–5789. doi: 10.1128/IAI.70.10.5779-5789.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Templeton TJ, Kaslow DC. Cloning and cross-species comparison of the thrombospondin-related anonymous protein (TRAP) gene from Plasmodium knowlesi, Plasmodium vivax and Plasmodium gallinaceum. Mol Biochem Parasitol. 1997;84:13–24. doi: 10.1016/s0166-6851(96)02775-2. [DOI] [PubMed] [Google Scholar]
- Templeton TJ, Kaslow DC, Fidock DA. Developmental arrest of the human malaria parasite Plasmodium falciparum within the mosquito midgut via CTRP gene disruption. Mol Microbiol. 2000;36:1–9. doi: 10.1046/j.1365-2958.2000.01821.x. [DOI] [PubMed] [Google Scholar]
- Thompson J, Cooke RE, Moore S, Anderson LF, Janse CJ, Waters AP. PTRAMP; a conserved Plasmodium thrombospondin-related apical merozoite protein. Mol Biochem Parasitol. 2004;134:225–232. doi: 10.1016/j.molbiopara.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Trottein F, Triglia T, Cowman AF. Molecular cloning of a gene from Plasmodium falciparum that codes for a protein sharing motifs found in adhesive molecules from mammals and plasmodia. Mol Biochem Parasitol. 1995;74:129–141. doi: 10.1016/0166-6851(95)02489-1. [DOI] [PubMed] [Google Scholar]
- Tsuji M, Mattei D, Nussenzweig RS, Eichinger D, Zavala F. Demonstration of heat-shock protein 70 in the sporozoite stage of malaria parasites. Parasitol Res. 1994;80:16–21. doi: 10.1007/BF00932618. [DOI] [PubMed] [Google Scholar]
- Wan KL, Carruthers VB, Sibley LD, Ajioka JW. Molecular characterisation of an expressed sequence tag locus of Toxoplasma gondii encoding the micronemal protein MIC2. Mol Biochem Parasitol. 1997;84:203–214. doi: 10.1016/s0166-6851(96)02796-x. [DOI] [PubMed] [Google Scholar]
- Wengelnik K, Spaccapelo R, Naitza S, Robson KJ, Janse CJ, Bistoni F, et al. The A-domain and the thrombospondin-related motif of Plasmodium falciparum TRAP are implicated in the invasion process of mosquito salivary glands. EMBO J. 1999;18:5195–5204. doi: 10.1093/emboj/18.19.5195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida N, Nussenzweig RS, Potocnjak P, Nussenzweig V, Aikawa M. Hybridoma produces protective antibodies directed against the sporozoite stage of malaria parasite. Science. 1980;207:71–73. doi: 10.1126/science.6985745. [DOI] [PubMed] [Google Scholar]
- Yuda M, Sakaida H, Chinzei Y. Targeted disruption of the Plasmodium berghei CTRP gene reveals its essential role in malaria infection of the vector mosquito. J Exp Med. 1999;190:1711–1716. doi: 10.1084/jem.190.11.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.