Abstract
Objectives
Suppressor of cytokine signalling (SOCS) proteins are inhibitors of cytokine signalling that function via the JAK/STAT pathway (Janus kinase/signal transducers and activators of transcription). Eight SOCS proteins, SOCS1–SOCS7 and CIS-1 (cytokine-inducible SH2-domain, with similar structure to the other SOCS proteins) have been identified, of which SOCS1, 2, and 3 and CIS-1 are the best characterised. A characteristic feature of osteoarthritis (OA) is increased production by articular chondrocytes of proinflammatory cytokines, such as interleukin-1 beta (IL-1β) and tumor necrosis factor alpha (TNFα), which may be induced by mechanotransduction and contribute to cartilage destruction. In this study, we have compared the gene expression of SOCS1, 2, 3 and CIS-1 in healthy and OA human chondrocytes, and also analyzed the effects of IL-1β and TNFα on the levels of mRNA encoding these SOCS family members. In addition, SOCS2 protein production was assessed and the CpG methylation status of the SOCS2 promoter was analyzed to determine the role of epigenetics in its regulation.
Methods
Femoral heads were obtained after joint replacement surgery for late stage OA and hemiarthroplasty following a fracture of the neck of femur (#NOF). Chondrocytes from the superficial layer of OA cartilage and the deep zone of #NOF cartilage were isolated by sequential treatment with trypsin, hyaluronidase and collagenase B. Total DNA and RNA were extracted from the same chondrocytes, and the levels of SOCS1, 2, 3 and CIS-1 mRNA were determined by qRT-PCR. The percentage of methylation in the CpG sites of the SOCS2 proximal promoter was quantified by pyrosequencing. Alternatively, healthy chondrocytes were isolated from #NOF cartilage and cultured with and without a mixture of IL-1β and oncostatin M (OSM, both 2.5 ng/ml) or TNFα (10 ng/ml). The short-term cultures with single cytokine treatment were harvested 24 and 72 h after treatment, and the long-term cultures were maintained for 4–5 weeks until confluent with periodical cytokine stimulation. Total RNA was extracted and mRNA levels were determined by qRT-PCR.
Results
The SOCS2 and CIS-1 mRNA levels were reduced by approximately 10-fold in OA samples compared to control samples, while SOCS1 and SOCS3 showed similar expression patterns in OA and control chondrocytes. The SOCS2 and CIS-1 mRNA levels declined by 6-fold and 3-fold with long-term treatment with IL-1β and OSM in combination and TNFα, respectively. There was no significant difference in the CpG methylation status of the SOCS2 promoter between healthy and OA chondrocytes. Similarly, cytokine stimulation did not change the CpG methylation status of the SOCS2 promoter.
Conclusions
This study demonstrates the reduced expression of SOCS2 and CIS-1 in OA, while SOCS1 and SOCS3 were unaffected. The observation that long-term treatment with inflammatory cytokines attenuated the expression of SOCS2 and CIS-1 suggests a potential positive feedback mechanism, and a role of SOCS in the pathology of OA.
Keywords: Osteoarthritis (OA), Chondrocytes, Suppressors of cytokine signalling (SOCS), Cytokine-inducible SH2 protein (CIS-1), IL-1β, TNFα
1. Introduction
Osteoarthritis (OA), the most common and disabling form of arthritic disease, is characterized by a slow but progressive degeneration of articular cartilage. The etiology of the disease remains unclear; however, there are several known risk factors, including genetic predisposition, obesity, hypermobility, diabetes, hypertension, hyperuricaemia, previous trauma to the joint, and aging. Moreover, synovial membrane and subchondral bone also participate in the progression of the disease actively [1]. Although OA is not a classical inflammatory arthritis, chondrocytes in OA cartilage produce pro-inflammatory cytokines, such as interleukin 1 beta (IL-1β) and tumor necrosis factor alpha (TNFα). Recent reports suggest that the development and progression of OA may involve inflammation even in the early stages of the disease [2], although the gene expression levels of pro-inflammatory cytokines are lower than those observed in rheumatoid arthritis (RA) [3].
Our previous studies showed that DNA demethylation at specific CpG sites is related with the aberrant expression of matrix metalloproteinases (MMP) 3, 9 and 13, ADAMTS4 and IL1B in human articular chondrocytes [4–7], indicating that DNA hypermethylation can also be playing a key role in the loss of expression of some genes during the OA process. Thus, understanding changes in DNA methylation, together with the roles of cytokines, growth factors, and changes in matrix composition, is important in determining the complex gene expression patterns observed in OA chondrocytes [8].
Suppressor of cytokine signalling (SOCS) proteins are inhibitors of cytokine signalling that function via the JAK/STAT pathway (Janus kinase/signal transducers and activators of transcription). Eight SOCS proteins, SOCS1-SOCS7 and CIS-1 (cytokine-inducible SH2-domain-1) with similar structure, have been identified so far. Of these, SOCS1, 2, and 3 and CIS-1 are the best characterised. These proteins have a conserved C-terminal motif named the SOCS box, a central SH2 domain, and a variable N-terminal domain [9,10]. SOCS1 and SOCS3 inhibit the tyrosine kinase activity of JAK directly, as they contain a kinase inhibitory region (KIR) immediately upstream of the central SH2 domain, which has been proposed to function as a pseudo-substrate that plays an important role in the suppression of cytokine signals [11]. Typically, SOCS proteins block cytokine signalling by acting as (i) kinase inhibitors of JAK proteins (SOCS1 and SOCS3), (ii) binding competitors against STATs (SOCS3 and CIS), and (iii) ubiquitin ligases, thereby promoting the degradation of their partners (SOCS1, SOCS3 and CIS). Recently, aberrant methylation patterns of specific SOCS genes were reported in relation to some types of cancers [12,13], but a potential role in degenerative diseases such as OA remain to be clarified. This study has examined the gene expression of SOCS1, 2 and 3 and CIS-1 in healthy and OA human chondrocytes, and the effect of the cytokines IL-1β and TNFα on SOCS expression.
2. Material and methods
2.1. Cartilage dissection and chondrocyte isolation
Human articular cartilage was obtained after hemiarthroplasty following femoral neck fracture (#NOF) or total hip arthroplasty for OA, respectively, with full patient consent and approval from the local ethics committee. The cartilage from #NOF patients is widely used as a suitable non-OA control [14]. Cartilage was dissected within 6 h after surgery. Only chondrocytes from the super-ficial layer of OA or the deep zone of #NOF cartilage were isolated, as justified in previous studies [14]. After cutting the cartilage in small pieces, the tissue was digested by sequential treatment with 10% trypsin (Lonza, Wokingham, UK) in phosphate buffer saline (PBS) for 30 min; 1 mg/ml of hyaluronidase (Sigma–Aldrich, Gillingham, UK) in PBS for 15 min and, finally, collagenase B (Roche, Lewes, UK) in Dulbecco's Modified Eagle's Medium: Nutrient Mixture F-12 (DMEM/F12; Invitrogen, Paisley, UK) for 12–15 h at 37 °C. Additional slices of cartilage were fixed in freshly prepared paraformaldehyde overnight and processed into paraffin wax for further immunohistochemistry studies.
2.2. Chondrocyte culture
Control chondrocytes were isolated from #NOF cartilage and cultured until confluence at a density of 2–4 × 105 cells in a 25 cm2 flask in DMEM/F12 supplemented with 5% fetal calf serum (FCS; Invitrogen, Paisley, UK), 1% insulin-transferrin-selenium (Sigma–Aldrich), 100 units/ml of penicillin and 100 μg/ml of streptomycin (Lonza), and 100 μg/ml of ascorbic acid (Sigma–Aldrich) in 5% CO2 at 37 °C. Chondrocytes were passaged once and, cultured without treatment (control culture) or with a mixture of 2.5 ng/ml IL-1β and 2.5 ng/ml oncostatin M (OSM), or 10 ng/ml TNFα (Sigma–Aldrich). In the short-term cultures, chondrocytes were incubated once with each cytokine and, were harvested after 24 and 72 h of treatment, and the long-term culture was maintained for 4 to 5 weeks until confluent with cytokine stimulation at every medium change (twice a week).
2.3. DNA and RNA extraction
Total RNA and genomic DNA were extracted simultaneously from harvested samples using the AllPrep DNA/RNA Mini Kit (Qiagen, Crawley, UK), according to the manufacturer's instructions. RNA was immediately reverse-transcribed with avian myeloblastosis virus reverse transcriptase and both oligo(dT)15 and random primers [15].
2.4. Quantitative reverse transcription-polymerase chain reaction (qRT-PCR)
Relative quantification of gene expression was performed with an ABI Prism 7500 detection system (Applied Biosystems, Warrington, UK). Primer Express 3.0 software (Applied Biosystems) was used to design primers bracketing exon-exon boundaries. The 20 μl reaction mixture was prepared in triplicate, containing 1 μl of complementary DNA, 10 μl of Power SYBR Green PCR Master Mix (Applied Biosystems), and 250 nM of each primer. Thermal cycler conditions consisted of an initial activation step at 95 °C for 10 min, followed by a 2-step PCR program of 95 °C for 15 s and 60 °C for 60 s for 40 cycles. A dissociation curve was obtained for each run. The 2–ΔΔCt method was employed for relative quantification of gene expression and the data were normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) expression. The primers used for qRT-PCR are shown in Table 1a.
Table 1.
(a) Primers used for qRT-PCR (F: forward; R:reverse), (b) Primers used for pyrosequecing (F: forward; R:reverse; S: sequencing).
| Amplicon ID (length; bp) | Primer sequence (5′–3′) |
|---|---|
| (a) | |
| GAPDH (108) | F (CCAGGTGGTCTCCTCTGACTTC) |
| R (TCATACCAGGAAATGAGCTTGACA) | |
| SOCS1 (244) | F (CTGGGATGCCGTGTTATTTT) |
| R (TAGGAGGTGCGAGTTCAGGT) | |
| SOCS2 (162) | F (CAGGGAATGGCAGAGACACT) |
| R (TGGCAGAGAGAGAAGGGATG) | |
| SOCS3 (162) | F (GCCACCTACTGAACCCTCCT) |
| R (ACGGTCTTCCGACAGAGATG) | |
| CIS-1 (204) | F (AGCCCAGACAGAGAGTGAGC) |
| R (TGACAGCGTGAACAGGTAGC) | |
| (b) | |
| SOCS2_1 (126) | F (GTATAAAAATGTTAGGGTTAGGAGGG) |
| R (CAAATTTCCCCCTATTAATCAAACTAATCTC) | |
| S (TAGGGTTAGGAGGGG) | |
| SOCS2_2 (130) | F (AGGATAGGTTGAATTTAGGAGTT) |
| R (CTCAACCTCCCCAAAAACTAAAATTACAA) | |
| S (GTTAGAGATTAGTTTGATTAATAGG) | |
| SOCS2_3 (128) | F (AGGATAGGTTGAATTTAGGAGTT) |
| R (CAACCTCCCCAAAAACTAAAATTACAA) | |
| S (CTCCCCAAAAACTAAAATTACAA) | |
| SOCS2_4 (63) | F (GTGGTGGGAGTTTGTAATTTTAGTTT) |
| R (CCAAATTCAAACAATTCTCCTACCT) | |
| S (GTGGGAGTTTGTAATTTTAGTTTT) | |
| SOCS2_5 (105) | F (GGTTGAGGTAGGAGAATTGT) |
| R (AACATAACCAATCTCCCTCTA) | |
| S (GGTAGGAGAATTGTTTGAA) | |
| SOCS2_6 (119) | F (GGTTTTTTATTTTTTGATGGAGAA) |
| R (AAAACTTAATCATACTCCCTCCC) | |
| S (AAAGGTATTTATTTTTAAAATTG) | |
| SOCS2_7 (107) | F (AGGTTTTATATGGAATTTGATTTGTTT) |
| R (ACTTACCCTCTTTAAACCTCTAAC) | |
| S (ATTTTAGTTTTTTATTTAGAAT) | |
| SOCS2_8 (250) | F (TGGTTTTTTTAATAATTTTTTTGT) |
| R (AAATCCAAAAAAAATAAATACCTT) | |
| S (AATTTTATGTAGATGATAAGT) | |
| SOCS2_9 (107) | F (TTTTGTAAATTTTGTTTGGTGTTT) |
| R (AATTATTAAAAAAACCATTAATCCC) | |
| S (TGTAAATTTTGTTTGGTGT) | |
| SOCS2_10 (113) | F (TTTGAAAGGTTAATGGTTATTTTTAG) |
| R (AAAAAAACACCAAACAAAATTTAC) | |
| S (GGTTATGGGAAGTTGG) |
2.5. Immunohistochemistry
Formalin-fixed paraffin-embedded cartilage sections were deparaffinized, and the antigen epitopes were revealed by boiling in 10 mM citrate buffer (pH 6.0) for 10 min followed by cooling at room temperature for 20 min. Any endogenous peroxidase activity was blocked by incubating the slides in 3% H2O2 for 5 min. The sections were stained with a rabbit polyclonal antibody anti-SOCS2 ab74533 (Abcam, Cambridge, UK) in 1:100 concentration overnight at 4 °C in blocking solution (1% BSA in PBS). The antibody was visualized using the appropriate biotinylated secondary antibody, followed by treatment with avidin-peroxidase and 3-amino-9-ethyl-carbazole. Sections were counterstained with 1% alcian blue, viewed with a Zeiss Universal light microscope (Zeiss, Welwyn Garden City, UK), followed by image capture with a digital camera.
2.6. Analysis of DNA methylation
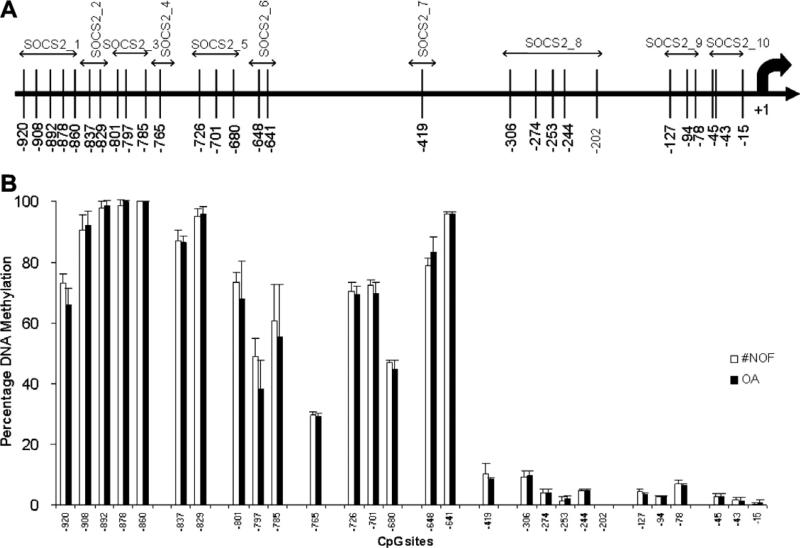
Bisulfite modification was performed with 500 ng of each genomic DNA using the EZ DNA Methylation-Gold™ Kit (Zymo Research Corporation, CA 92867, USA) according to the manufacturer's instructions. After bisulfite modification, a 40 μl PCR was carried out in 3.2 μl bisulfite modified DNA (30 ng), 36 μl Platinum® PCR Supermix or Supermix High Fidelity (Invitrogen), and 200 nM of each primer. Thermal cycling conditions consisted of an initial activation step at 95 °C for 5 min, followed by a 3-step PCR program of 95 °C for 15 s, annealing for 30 s (52 °C for SOCS2_6; 54 °C for SOCS2_8, 9, 10; 56 °C for SOCS2_1, 5, 7; 58 °C for SOCS2_2, 3, 4) and 72 °C for 30 s for 50 cycles. PCR products were checked by 1% agarose-gel electrophoresis using 10 μl of each product. The percent DNA methylation in the SOCS2 promoter was quantified using PyroMark™ MD (Qiagen) according to the manufacturer's instructions. The primers used for pyrosequencing (Table 1b) were designed with Pyrosequencing™ Assay Design Software Ver 2.0 (Qiagen). Ten primer sets were designed to cover all 28 CpG sites within 1100 bps of the SOCS2 promoter (GenBank accession No. AF132441) (Fig. 3A).
Fig. 3.
Methylation status of SOCS2 promoter. (A) Locations of CpG sites in SOCS2 proximal promoter are represented by vertical bars. The sequences used for PCR primers for pyrosequencing are indicated over the CpG sites with double-headed arrows. (B) Percentage methylation at each CpG site in the SOCS2 proximal promoter is compared in healthy (#NOF) and OA human articular chondrocytes.
2.7. Statistical analysis
All the statistical analyses were performed with SPSS software, version 17.0 (SPSS, Chicago, IL). Student's t-test or Welch's t-test were used for comparison of two groups. P values less than 0.05 were considered significant. All the data are expressed as the mean ± SD.
3. Results
3.1. The expression profiles of SOCS1, SOCS2, SOCS3 and CIS-1 in articular chondrocytes
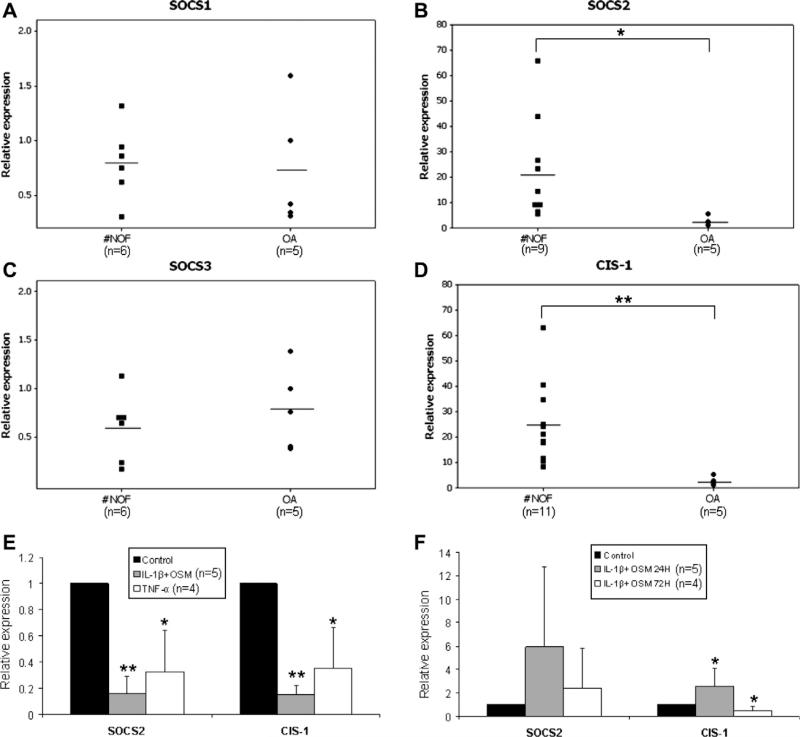
In all patients, the expression of SOCS2 (Fig. 1B) was reduced by 10-fold in OA samples (2.5 ± 1.9) compared to control samples (22.3 ± 20.8). Similarly, CIS-1 (Fig. 1D) mRNA was reduced in OA samples (2.5 ± 1.8) compared to control (25.0 ± 15.9). In contrast, SOCS1 and SOCS3 mRNA (Fig. 1A and C) showed no difference between OA and #NOF chondrocytes.
Fig. 1.
(A–D) Expression of SOCS1, 2, and 3 and CIS-1 mRNA in articular chondrocytes. Total RNA was extracted from human #NOF and OA articular chondrocytes and analyzed by qRT-PCR. The horizontal line is the mean in each group. (E) SOCS2 and CIS-1 expression in articular chondrocytes after long-term cytokine stimulation. SOCS2 and CIS-1 expression levels were analyzed in human articular chondrocytes after stimulation with a mixture of 2.5 ng/ml IL-1β and 2.5 ng/ml OSM or with 10 ng/ml TNFα for 4–5 weeks. (F) SOCS2 and CIS-1 expression in articular chondrocytes after short-term culture. The SOCS2 and CIS-1 expression levels were analyzed after 24 and 72 h of incubation with a mixture of 2.5 ng/ml IL-1β and 2.5 ng/ml OSM. All data were normalized to GAPDH and shown as the mean ± SD. The treated samples were compared with corresponding control samples (*P ≤ 0.05; **P ≤ 0.01).
3.2. The regulation of SOCS expression by cytokine stimulation
We examined the expression of SOCS family members after long-term and short-term (single cytokine addition) stimulation with inflammatory cytokines in human chondrocyte cultures. Both SOCS2 and CIS-1 mRNAs were reduced by 6-fold in IL-1β and OSM-treated cultures and by 3-fold in TNFα-treated cultures after 4–5 weeks of stimulation (Fig. 1E). In contrast, short-term culture groups treated with mixture of IL-1β and OSM formed peaks in both SOCS2 and CIS-1 expression after 24 h, followed by falls at 72 h (Fig. 1F). CIS-1 expression was increased almost 3-fold at 24 h (2.6 ± 0.8 versus 1.0 ± 0.0) but dropped significantly after 72 h (0.5 ± 0.2) (Fig. 1F).
3.3. Production of SOCS2 protein in the articular cartilage
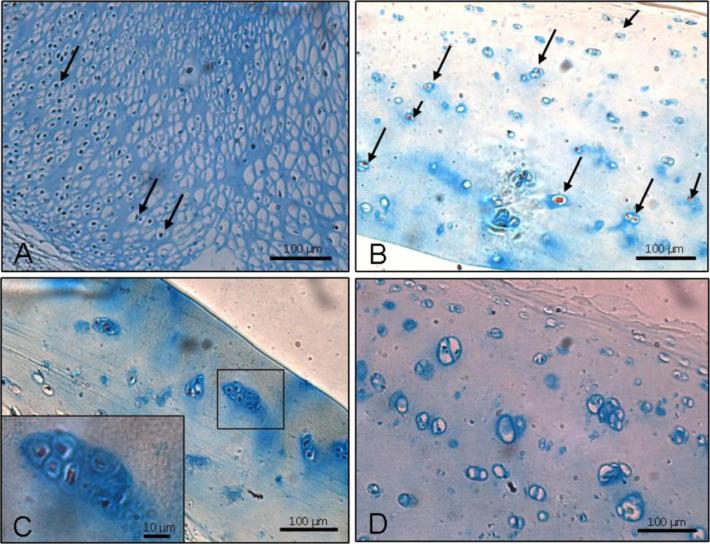
Immunohistochemistry of SOCS2 demonstrated the ubiquitous existence of SOCS2 protein in human articular cartilage with strong localization in the growth plate. The proliferative chondrocytes of the chick femur, a positive control, also showed intense staining for SOCS2 (Fig. 2A). In #NOF cartilage (Mankin score 1–2), SOCS2-positive chondrocytes were found throughout the cartilage (Fig. 2B). On the other hand, the cartilage from OA patients (Mankin score 4–8) showed variable (Fig. 2C and D) SOCS2-positive chondrocytes. Furthermore, clonal chondrocytes in high-grade OA patients (Fig. 2C magnified) demonstrated SOCS2 protein production.
Fig. 2.
Localization of SOCS2 in chick bone and human articular chondrocytes by immunohistochemistry. (A) Strong staining is observed within proliferating chondrocytes (arrows) in chick femur. (B) Positive staining for SOCS2 is evident in chondrocytes from #NOF cartilage (Mankin score 1–2) in whole thickness (arrows). (C) Staining of SOCS2 is negligible in OA cartilage (Mankin score 4–8), but can be observed in chondrocyte clusters (see inset at higher magnification). (D) Staining of SOCS2 completely imperceptible in some sections of OA cartilage (Mankin score 4–8). Scale bars represent 10 μm and 100 μm.
3.4. Methylation status of the SOCS2 promoter in healthy and OA chondrocytes
The methylation status of 28 CpG sites within 1100 bp upstream of the SOCS2 transcription start site was quantified by pyrosequencing. The position of each CpG site is shown in Fig. 3A. Sixteen CpG sites located between –920 and –641 bp were observed to be mostly hypermethylated (75.5 ± 22%), while the proximal thirteen CpG sites between –419 and –15 bp were mostly demethylated (4 ± 3%) in all the samples. There was no statistical difference in methylation status between #NOF and OA chondrocytes (Fig. 3B).
3.5. Methylation status of the SOCS2 promoter in cytokine-treated chondrocyte cultures
The methylation status of the 28 CpG sites in the SOCS2 proximal promoter was analyzed in chondrocyte cultures after cytokine stimulation, as described above. The methylation status was unaffected by cytokine treatment and the percentage of methylation remained at the same level as in control cultures (data not shown).
4. Discussion
OA is a common disorder with a complex etiology comprising genetic, mechanical and environmental factors, and there is an urgent unmet need for strategies to address this debilitating condition. Excessive cytokine signalling is known to be regulated by a number of inhibitors, including protein inhibitor of activated STAT (PIAS), protein tyrosine phosphatases, and SOCS [16,17]. In this study, we demonstrate for the first time that the expression of SOCS proteins, specifically SOCS2 and CIS-1, is reduced in OA. SOCS proteins are attenuators of cytokine-induced processes mediated via the JAK/STAT pathway, and their reduced expression can result in increased responsiveness of cells to various cytokines. Interestingly, the consequences of SOCS down-regulation in OA would not appear to be related to modulation of tyrosine kinase activity, since SOCS1 and SOCS3 are the only members of the SOCS family that possess the kinase inhibitory region (KIR) [11]. In this study, we observed no modulation of SOCS1 or SOCS3 in any OA patients examined.
Previous in vitro and in vivo studies have shown the importance of IL-1β and TNFα in the development and progression of OA [18–20]. Moreover, IL-1β and TNFα were reported to have important roles in regulating apoptosis in a human OA chondrocyte culture model [21]. On the other hand, a recent study has demonstrated the effectiveness of intra-articular injection of IL-1 receptor antagonist (IL-1Ra) in the modulation of the inflammatory response and suppression of cartilage destruction in OA animal models [22]. Thus, neutralization of these inflammatory mediators appears to be promising as a therapeutic modality against OA [23]. Our observation that IL-1β and TNFα reduce the expression of their suppressors of signalling in long-term cultures suggests a potential positive feedback mechanism of inflammatory cytokines in OA pathology. Although short-term, single cytokine treatment models showed increased expression of SOCS proteins, this is not thought to mimic the OA condition according to the literature [5]. Interestingly, NF-κB is thought to regulate SOCS expression and to modulate STAT activity, indicating an exquisite regulation of cytokine activity. Comparable results were found in peripheral blood mononuclear cells, which showed that short-term exposure to inflammatory synovial fluids and various cytokines such as IL-1β, IL-6, IL-10, TNFα and IFN-γ clearly resulted in upregulation of SOCS expression, while prolonged exposure resulted in downregulation [24].
The alteration of subchondral bone is another significant aspect in the pathology of OA. In regards of relationship between SOCS and bone, Lorentzon and collaborators [25] reported that the absence of SOCS2 induces losses in the trabecular and cortical bone mineral densities. Furthermore, Ouyang and colleagues [26] suggested that an absence of SOCS2 could induce severe defects in bone mineralization via acceleration of osteoblast differentiation, as observed in mice lacking SOCS2. In contrast, Macrae et al. [27] indicate that physiological levels of SOCS2 inhibit signalling through the GH/IGF-I axis and thus negatively regulate bone formation and endochondral growth. Thus further studies are needed to elucidate the precise role of SOCS2 in bone physiology and subsequent precise involvement in the pathology of OA.
Immunohistochemistry and qRT-pCR analyses, demonstrated ubiquitous production of SOCS2 in #NOF (control) and some OA patient samples. It is known that in normal development, a low level of inflammatory cytokines is required with a concomitant expression of SOCS proteins to regulate cytokine signalling. Thus, the ubiquitous expression of SOCS2 is not totally unexpected.
Our previous studies demonstrated that DNA demethylation at specific CpG sites in promoters with sparse CpG sites accounts for the aberrant expression of catabolic genes in OA. The SOCS2 promoter possesses relatively concentrated CpG sites and the CIS-1 promoter has so-called CpG islands. Although some recent reports have indicated a reduction in the expression of SOCS genes in association with DNA hypermethylation in cancers [28,29,9,30,12,13,31], we found no differences in the methylation status of SOCS2 promoter between healthy and OA chondrocytes. Furthermore, no change in methylation status was found in the human chondrocyte culture models following cytokine stimulation. Similar findings were recently reported on the cell cycle progression inhibitor p21 (WAF1/CIP1) which is downregulated in OA chondrocytes regardless of its promoter methylation status [32].
In summary, we have demonstrated that SOCS2 and CIS-1 expression are reduced in OA. These results indicate a potential positive feedback mechanism in OA chondrocytes, with significant implications for OA pathology. Further understanding of the role of SOCS proteins in chondrocytes will be needed to determine their therapeutic potential for OA.
Acknowledgments
The authors thank the Orthopaedic Surgeons at Southampton General Hospital for provision of femoral heads from patients undergoing total hip replacement surgery. Grant support from National Institutes of Health grants R21-AR054887 (MBG and HIR) and R01-AG022021 (MBG), Wessex Medical Research (M19; HIR and RO) and BBSRC G006970/1 (RO) is gratefully acknowledged. M.C.A. was supported by Programa Sara Borrell, Instituto de Salud Carlos III. This paper is dedicated to the memory of Dr. Helmtrud I. (Trudy) Roach.
References
- 1.Kapoor M, Martel-Pelletier J, Lajeunesse D, Pelletier JP, Fahmi H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat. Rev. Rheumatol. 2011;7:33–42. doi: 10.1038/nrrheum.2010.196. [DOI] [PubMed] [Google Scholar]
- 2.Felson DT. Clinical practice. Osteoarthritis of the knee. N. Engl. Med. 2006;354:841–848. doi: 10.1056/NEJMcp051726. [DOI] [PubMed] [Google Scholar]
- 3.Sakkas LI, Scanzello C, Johanson N, Burkholder J, Mitra A, Salgame P, Katsetos CD, Platsoucas CD. T cells and T-cell cytokine transcripts in the synovial membrane in patients with osteoarthritis. Clin. Diagn. Lab Immunol. 1998;5:430–437. doi: 10.1128/cdli.5.4.430-437.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheung KS, Hashimoto K, Yamada N, Roach HI. Expression of ADAMTS-4 by chondrocytes in the surface zone of human osteoarthritic cartilage is regulated by epigenetic DNA de-methylation. Rheumatol. Int. 2009;29:525–534. doi: 10.1007/s00296-008-0744-z. [DOI] [PubMed] [Google Scholar]
- 5.Hashimoto K, Oreffo RO, Gibson MB, Goldring MB, Roach HI. DNA demethylation at specific CpG sites in the IL1B promoter in response to inflammatory cytokines in human articular chondrocytes. Arthritis Rheum. 2009;60:3303–3313. doi: 10.1002/art.24882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Imagawa K, de Andrés MC, Hashimoto K, Pitt D, Itoi E, Goldring MB, Roach HI, Oreffo RO. The epigenetic effect of glucosamine and a nuclear factor-kappa B (NF-kB) inhibitor on primary human chondrocytes - implications for osteoarthritis. Biochem. Biophys. Res. Commun. 2011;405:362–367. doi: 10.1016/j.bbrc.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roach HI, Yamada N, Cheung KS, Tilley S, Clarke NM, Oreffo RO, Kokubun S, Bronner F. Association between the abnormal expression of matrix-degrading enzymes by human osteoarthritic chondrocytes and demethylation of specific CpG sites in the promoter regions. Arthritis Rheum. 2005;52:3110–3124. doi: 10.1002/art.21300. [DOI] [PubMed] [Google Scholar]
- 8.Roach HI, Aigner T. DNA methylation in osteoarthritic chondrocytes: a new molecular target. Osteoarthritis. Cartilage. 2007;15:128–137. doi: 10.1016/j.joca.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 9.Rico-Bautista E, Flores-Morales A, Fernandez-Perez L. Suppressor of cytokine signaling (SOCS) 2, a protein with multiple functions. Cytokine Growth Factor Rev. 2006;17:431–439. doi: 10.1016/j.cytogfr.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 10.Tollet-Egnell P, Flores-Morales A, Stavreus-Evers A, Sahlin L, Norstedt G. Growth hormone regulation of SOCS-2, SOCS-3, and CIS messenger ribonucleic acid expression in the rat. Endocrinology. 1999;140:3693–3704. doi: 10.1210/endo.140.8.6878. [DOI] [PubMed] [Google Scholar]
- 11.Yasukawa H, Misawa H, Sakamoto H, Masuhara M, Sasaki A, Wakioka T, Ohtsuka S, Imaizumi T, Matsuda T, Ihle JN, Yoshimura A. The JAK-binding protein JAB inhibits Janus tyrosine kinase activity through binding in the activation loop. EMBO J. 1999;18:1309–1320. doi: 10.1093/emboj/18.5.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Naka T, Fujimoto M, Tsutsui H, Yoshimura A. Negative regulation of cytokine and TLR signalings by SOCS and others. Adv. Immunol. 2005;87:61–122. doi: 10.1016/S0065-2776(05)87003-8. [DOI] [PubMed] [Google Scholar]
- 13.Sutherland KD, Lindeman GJ, Choong DY, Wittlin S, Brentzell L, Phillips W, Campbell IG, Visvader JE. Differential hypermethylation of SOCS genes in ovarian and breast carcinomas. Oncogene. 2004;23:7726–7733. doi: 10.1038/sj.onc.1207787. [DOI] [PubMed] [Google Scholar]
- 14.da Silva MA, Yamada N, Clarke NM, Roach HI. Cellular and epigenetic features of a young healthy and a young osteoarthritic cartilage compared with aged control and OA cartilage. J. Orthop. Res. 2009;27:593–601. doi: 10.1002/jor.20799. [DOI] [PubMed] [Google Scholar]
- 15.Hashimoto K, Kokubun S, Itoi E, Roach HI. Improved quantification of DNA methylation using methylation-sensitive restriction enzymes and real-time PCR. Epigenetics. 2007;2:86–91. doi: 10.4161/epi.2.2.4203. [DOI] [PubMed] [Google Scholar]
- 16.Zadjali F, Pike AC, Vesterlund M, Sun J, Wu C, Li SS, Ronnstrand L, Knapp S, Bullock AN, Flores-Morales A. Structural basis for c-KIT inhibition by the suppressor of cytokine signaling 6 (SOCS6) ubiquitin ligase. J. Biol. Chem. 2011;286:480–490. doi: 10.1074/jbc.M110.173526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rakesh K, Agrawal DK. Controlling cytokine signaling by constitutive inhibitors. Biochem. Pharmacol. 2005;70:649–657. doi: 10.1016/j.bcp.2005.04.042. [DOI] [PubMed] [Google Scholar]
- 18.Fernandes JC, Martel-Pelletier J, Pelletier JP. The role of cytokines in osteoarthritis pathophysiology. Biorheology. 2002;39:237–246. [PubMed] [Google Scholar]
- 19.Goldring MB. Osteoarthritis and cartilage: the role of cytokines. Curr. Rheumatol. Rep. 2000;2:459–465. doi: 10.1007/s11926-000-0021-y. [DOI] [PubMed] [Google Scholar]
- 20.Lotz M, Blanco FJ, von KJ, Dudler J, Maier R, Villiger PM, Geng Y. Cytokine regulation of chondrocyte functions. J. Rheumatol. Suppl. 1995;43:104–108. [PubMed] [Google Scholar]
- 21.Lopez-Armada MJ, Carames B, Lires-Dean M, Cillero-Pastor B, Ruiz-Romero C, Galdo F, Blanco FJ. Cytokines, tumor necrosis factor-alpha and interleukin-1beta, differentially regulate apoptosis in osteoarthritis cultured human chondrocytes. Osteoarthritis. Cartilage. 2006;14:660–669. doi: 10.1016/j.joca.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 22.Fox BA, Stephens MM. Treatment of knee osteoarthritis with Orthokine-derived autologous conditioned serum. Expert. Rev. Clin. Immunol. 2010;6:335–345. doi: 10.1586/eci.10.17. [DOI] [PubMed] [Google Scholar]
- 23.Calich AL, Domiciano DS, Fuller R. Osteoarthritis: can anti-cytokine therapy play a role in treatment? Clin Rheumatol. 2010;29:451–455. doi: 10.1007/s10067-009-1352-3. [DOI] [PubMed] [Google Scholar]
- 24.Isomaki P, Alanara T, Isohanni P, Lagerstedt A, Korpela M, Moilanen T, Visakorpi T, Silvennoinen O. The expression of SOCS is altered in rheumatoid arthritis. Rheumatology (Oxford) 2007;46:1538–1546. doi: 10.1093/rheumatology/kem198. [DOI] [PubMed] [Google Scholar]
- 25.Lorentzon M, Greenhalgh CJ, Mohan S, Alexander WS, Ohlsson C. Reduced bone mineral density in SOCS-2-deficient mice. Pediatr. Res. 2005;57:223–226. doi: 10.1203/01.PDR.0000148735.21084.D3. [DOI] [PubMed] [Google Scholar]
- 26.Ouyang X, Fujimoto M, Nakagawa R, Serada S, Tanaka T, Nomura S, Kawase I, Kishimoto T, Naka T. SOCS-2 interferes with myotube formation and potentiates osteoblast differentiation through upregulation of JunB in C2C12 cells. J. Cell Physiol. 2006;207:428–436. doi: 10.1002/jcp.20579. [DOI] [PubMed] [Google Scholar]
- 27.Macrae VE, Horvat S, Pells SC, Dale H, Collinson RS, Pitsillides AA, Ahmed SF, Farquharson C. Increased bone mass, altered trabecular architecture and modified growth plate organization in the growing skeleton of SOCS2 deficient mice. J. Cell Physiol. 2009;218:276–284. doi: 10.1002/jcp.21593. [DOI] [PubMed] [Google Scholar]
- 28.Hendriksen PJ, Dits NF, Kokame K, Veldhoven A, van Weerden WM, Bangma CH, Trapman J, Jenster G. Evolution of the androgen receptor pathway during progression of prostate cancer. Cancer Res. 2006;66:5012–5020. doi: 10.1158/0008-5472.CAN-05-3082. [DOI] [PubMed] [Google Scholar]
- 29.Marini A, Mirmohammadsadegh A, Nambiar S, Gustrau A, Ruzicka T, Hengge UR. Epigenetic inactivation of tumor suppressor genes in serum of patients with cutaneous melanoma. J. Invest. Dermatol. 2006;126:422–431. doi: 10.1038/sj.jid.5700073. [DOI] [PubMed] [Google Scholar]
- 30.Wikman H, Kettunen E, Seppanen JK, Karjalainen A, Hollmen J, Anttila S, Knuutila S. Identification of differentially expressed genes in pulmonary adenocarcinoma by using cDNA array. Oncogene. 2002;21:5804–5813. doi: 10.1038/sj.onc.1205726. [DOI] [PubMed] [Google Scholar]
- 31.Yoshikawa H, Matsubara K, Qian GS, Jackson P, Groopman JD, Manning JE, Harris CC, Herman JG. SOCS-1, a negative regulator of the JAK/STAT pathway, is silenced by methylation in human hepatocellular carcinoma and shows growth-suppression activity. Nat. Genet. 2001;28:29–35. doi: 10.1038/ng0501-29. [DOI] [PubMed] [Google Scholar]
- 32.Sesselmann S, Soder S, Voigt R, Haag J, Grogan SP, Aigner T. DNA methylation is not responsible for p21WAF1/CIP1 down-regulation in osteoarthritic chondrocytes. Osteoarthritis. Cartilage. 2009;17:507–512. doi: 10.1016/j.joca.2008.09.006. [DOI] [PubMed] [Google Scholar]