Abstract
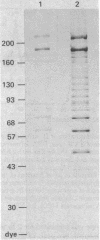
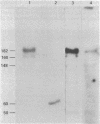
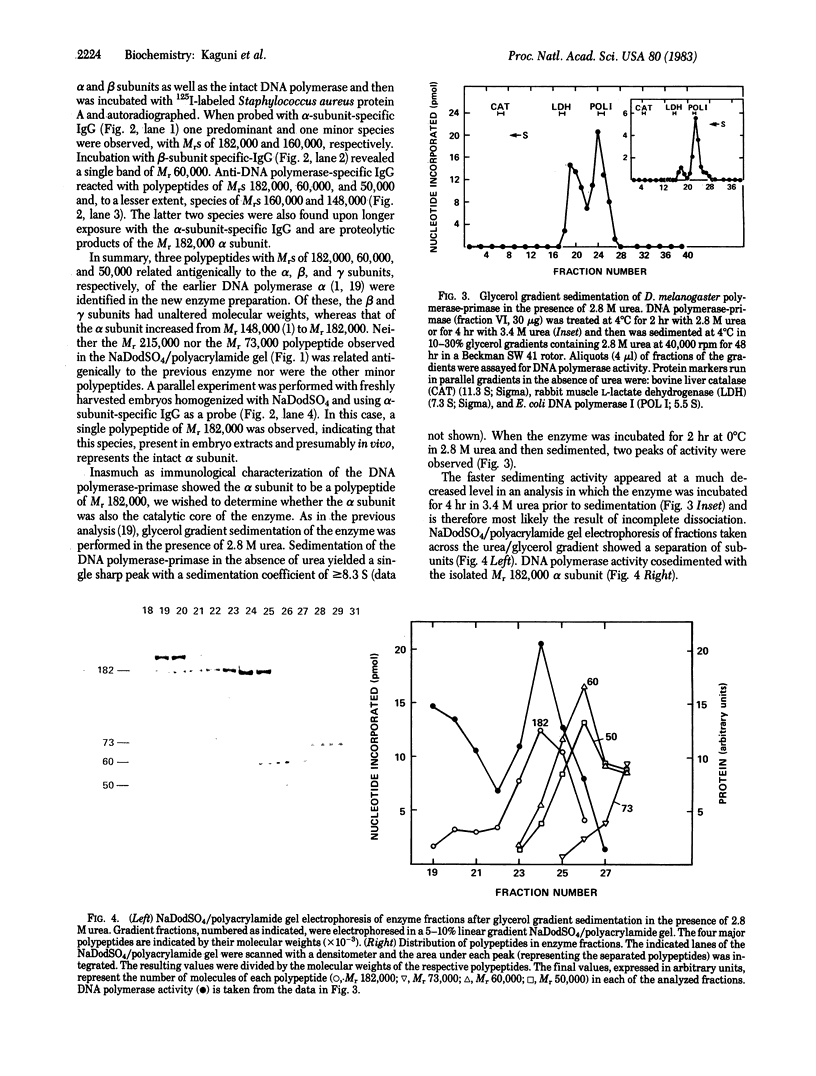
A procedure has been devised for the purification of intact DNA polymerase alpha from early embryos of Drosophila melanogaster. The purified enzyme consists of at least three polypeptides with Mrs of 182,000, 60,000, and 50,000. These are related antigenically to the alpha (Mr 148,000), beta (Mr 58,000), and gamma (Mr 46,000) subunits, respectively, of the DNA polymerase described previously [Banks, G. R., Boezi, J. A. & Lehman, I. R. (1979) J. Biol. Chem. 254, 9886-9892]. The alpha subunit (Mr 182,000) has a molecular weight indistinguishable from that observed in extracts of freshly harvested embryos and presumably present in vivo. As in the previous preparation, the alpha subunit is required for DNA polymerase activity and is very likely the catalytic subunit of the enzyme. The ratio of primase to polymerase remains constant throughout the purification. Thus, the primase is very likely an integral component of the Drosophila DNA polymerase alpha. The purified DNA polymerase-primase contains no detectable endo- or exodeoxyribonuclease and has pH, MgCl2, (NH4)2SO4, and NaCl optima identical to those reported previously. In contrast, the Km for dTTP is 3.7 microM as compared with 17.5 microM for the previous enzyme. Sensitivities to aphidicolin and N-ethylmaleiimide and resistance to dideoxy TTP are unchanged.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albert W., Grummt F., Hübscher U., Wilson S. H. Structural homology among calf thymus alpha-polymerase polypeptides. Nucleic Acids Res. 1982 Feb 11;10(3):935–946. doi: 10.1093/nar/10.3.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks G. R., Boezi J. A., Lehman I. R. A high molecular weight DNA polymerase from Drosophila melanogaster embryos. Purification, structure, and partial characterization. J Biol Chem. 1979 Oct 10;254(19):9886–9892. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brakel C. L., Blumenthal A. B. Multiple forms of Drosophila embryo DNA polymerase: evidence for proteolytic conversion. Biochemistry. 1977 Jul 12;16(14):3137–3143. doi: 10.1021/bi00633a016. [DOI] [PubMed] [Google Scholar]
- Chen Y. C., Bohn E. W., Planck S. R., Wilson S. H. Mouse DNA polymerase alpha. Subunit structure and identification of a species with associated exonuclease. J Biol Chem. 1979 Nov 25;254(22):11678–11687. [PubMed] [Google Scholar]
- Conaway R. C., Lehman I. R. A DNA primase activity associated with DNA polymerase alpha from Drosophila melanogaster embryos. Proc Natl Acad Sci U S A. 1982 Apr;79(8):2523–2527. doi: 10.1073/pnas.79.8.2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fansler B. S., Loeb L. A. Sea urchin nuclear DNA polymerase. Methods Enzymol. 1974;29:53–70. doi: 10.1016/0076-6879(74)29009-8. [DOI] [PubMed] [Google Scholar]
- Filpula D., Fisher P. A., Korn D. DNA polymerase-alpha. Common polypeptide core structure of three enzyme forms from human KB cells. J Biol Chem. 1982 Feb 25;257(4):2029–2040. [PubMed] [Google Scholar]
- Grosse F., Krauss G. Purification and partial characterization of a DNA polymerase alpha species from calf thymus. Nucleic Acids Res. 1980 Dec 11;8(23):5703–5714. doi: 10.1093/nar/8.23.5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosse F., Krauss G. Purification of a 9S DNA polymerase alpha species from calf thymus. Biochemistry. 1981 Sep 15;20(19):5470–5475. doi: 10.1021/bi00522a019. [DOI] [PubMed] [Google Scholar]
- Hübscher U., Spanos A., Albert W., Grummt F., Banks G. R. Evidence that a high molecular weight replicative DNA polymerase is conserved during evolution. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6771–6775. doi: 10.1073/pnas.78.11.6771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVY H. B., SOBER H. A. A simple chromatographic method for preparation of gamma globulin. Proc Soc Exp Biol Med. 1960 Jan;103:250–252. doi: 10.3181/00379727-103-25476. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Masaki S., Koiwai O., Yoshida S. 10 S DNA polymerase alpha of calf thymus shows a microheterogeneity in its large polypeptide component. J Biol Chem. 1982 Jun 25;257(12):7172–7177. [PubMed] [Google Scholar]
- McKune K., Holmes A. M. Further studies on partially purified calf thymus DNA polymerase a. Nucleic Acids Res. 1979 Jul 25;6(10):3341–3352. doi: 10.1093/nar/6.10.3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechali M., Abadiedebat J., de Recondo A. M. Eukaryotic DNA polymerase alpha. Structural analysis of the enzyme from regenerating rat liver. J Biol Chem. 1980 Mar 10;255(5):2114–2122. [PubMed] [Google Scholar]
- RICHARDSON C. C., SCHILDKRAUT C. L., APOSHIAN H. V., KORNBERG A. ENZYMATIC SYNTHESIS OF DEOXYRIBONUCLEIC ACID. XIV. FURTHER PURIFICATION AND PROPERTIES OF DEOXYRIBONUCLEIC ACID POLYMERASE OF ESCHERICHIA COLI. J Biol Chem. 1964 Jan;239:222–232. [PubMed] [Google Scholar]
- Riedel H. D., König H., Stahl H., Knippers R. Circular single stranded phage M13-DNA as a template for DNA synthesis in protein extracts from Xenopus laevis eggs: evidence for a eukaryotic DNA priming activity. Nucleic Acids Res. 1982 Sep 25;10(18):5621–5635. doi: 10.1093/nar/10.18.5621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer B., Lehman I. R. Immunological comparison of purified DNA polymerase alpha from embryos of Drosophila melanogaster with forms of the enzyme present in vivo. J Biol Chem. 1982 Oct 25;257(20):12394–12398. [PubMed] [Google Scholar]
- Shioda M., Nelson E. M., Bayne M. L., Benbow R. M. DNA primase activity associated with DNA polymerase alpha from Xenopus laevis ovaries. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7209–7213. doi: 10.1073/pnas.79.23.7209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng B. Y., Ahlem C. N. DNA primase activity from human lymphocytes. Synthesis of oligoribonucleotides that prime DNA synthesis. J Biol Chem. 1982 Jul 10;257(13):7280–7283. [PubMed] [Google Scholar]
- Villani G., Sauer B., Lehman I. R. DNA polymerase alpha from Drosophila melanogaster embryos. Subunit structure. J Biol Chem. 1980 Oct 10;255(19):9479–9483. [PubMed] [Google Scholar]
- Yagura T., Kozu T., Seno T. Mouse DNA polymerase accompanied by a novel RNA polymerase activity: purification and partial characterization. J Biochem. 1982 Feb;91(2):607–618. doi: 10.1093/oxfordjournals.jbchem.a133732. [DOI] [PubMed] [Google Scholar]
- Yamaguchi M., Tanabe K., Takahashi T., Matsukage A. Chick embryo DNA polymerase alpha. Polypeptide components and their microheterogeneity. J Biol Chem. 1982 Apr 25;257(8):4484–4489. [PubMed] [Google Scholar]