Abstract
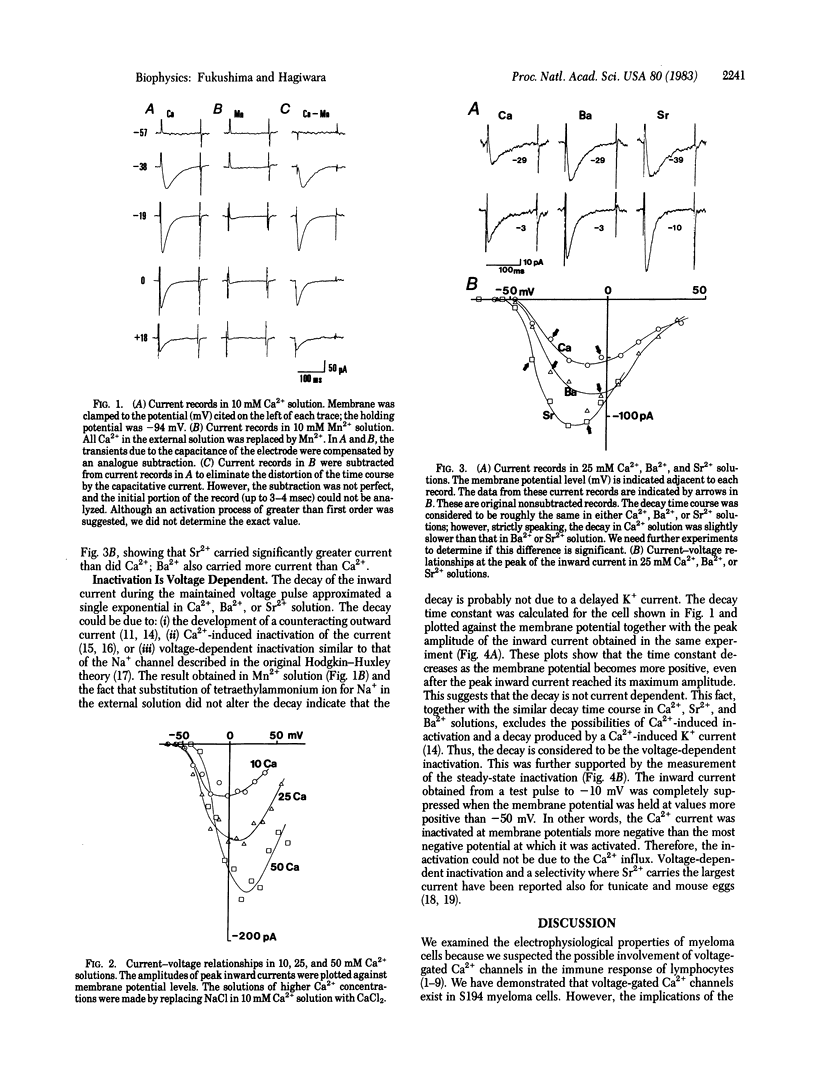
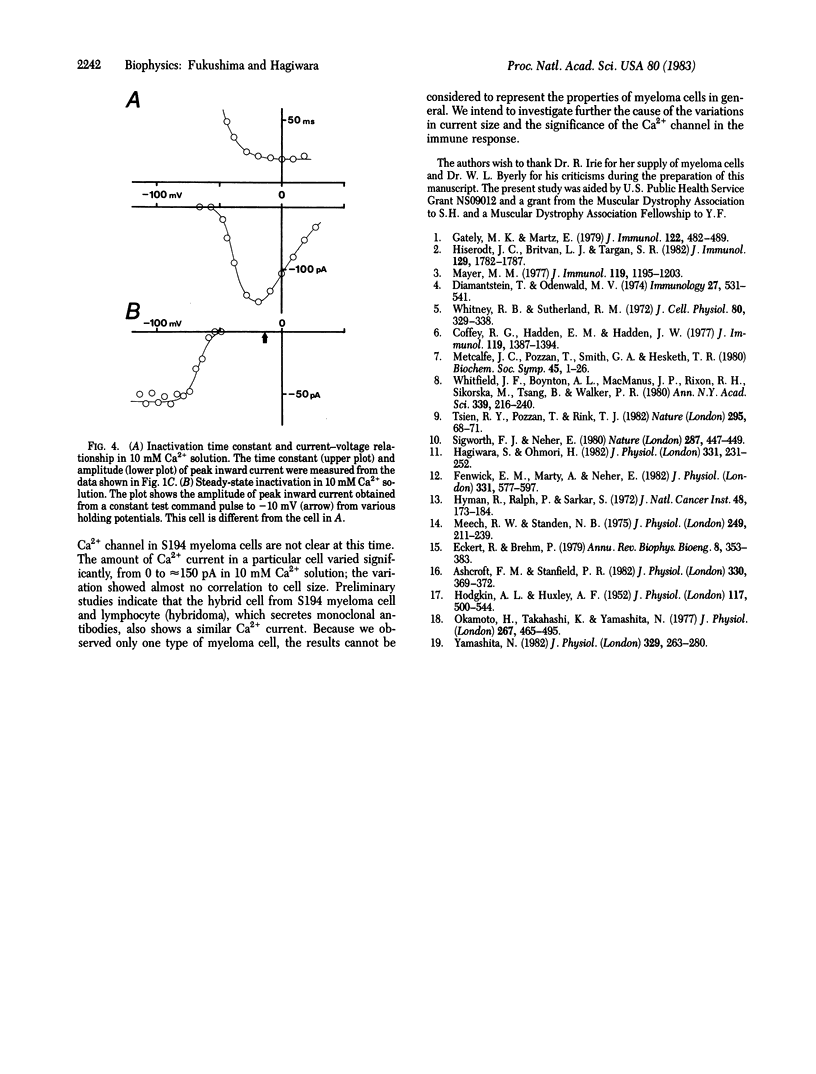
Electrical properties of the cell membrane were studied in the neoplastic lymphocyte, mouse myeloma cell line S194, by using the whole-cell patch clamp technique. Inward Ca2+ currents due to voltage-gated Ca2+ channels were found. The current, which decayed exponentially after reaching a peak, was first activated at about -50 mV and attained its maximum peak amplitude at about -20 mV in a 10 mM Ca2+ solution. Outward current was negligible for the potential range more negative than +30 mV. The channel was permeable to Sr2+ and Ba2+ in addition to Ca2+. Among these species, Sr2+ carried the greatest current. The time constants of the decay of the current depended neither on the species nor on the concentration of charge carrier. The steady-state inactivation was observed at potentials more negative than those at which the inward Ca2+ current was activated. Thus, we concluded that the inactivation of the channel was mainly voltage dependent. For reasons that are not yet understood, the amplitude of the Ca2+ current varied greatly among cells.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashcroft F. M., Stanfield P. R. Calcium inactivation in skeletal muscle fibres of the stick insect, Carausius morosus. J Physiol. 1982 Sep;330:349–372. doi: 10.1113/jphysiol.1982.sp014345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffey R. G., Hadden E. M., Hadden J. W. Evidence for cyclic GMP and calcium mediation of lymphocyte activation by mitogens. J Immunol. 1977 Oct;119(4):1387–1394. [PubMed] [Google Scholar]
- Diamantstein T., Odenwald M. V. Control of the immune response in vitro by calcium ions. I. The antagonistic action of calcium ions on cell proliferation and on cell differentiation. Immunology. 1974 Oct;27(4):531–541. [PMC free article] [PubMed] [Google Scholar]
- Eckert R., Brehm P. Ionic mechanisms of excitation in Paramecium. Annu Rev Biophys Bioeng. 1979;8:353–383. doi: 10.1146/annurev.bb.08.060179.002033. [DOI] [PubMed] [Google Scholar]
- Fenwick E. M., Marty A., Neher E. A patch-clamp study of bovine chromaffin cells and of their sensitivity to acetylcholine. J Physiol. 1982 Oct;331:577–597. doi: 10.1113/jphysiol.1982.sp014393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gately M. K., Martz E. Early steps in specific tumor cell lysis by sensitized mouse T lymphocytes. III. Resolution of two distinct roles for calcium in the cytolytic process. J Immunol. 1979 Feb;122(2):482–489. [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Ohmori H. Studies of calcium channels in rat clonal pituitary cells with patch electrode voltage clamp. J Physiol. 1982 Oct;331:231–252. doi: 10.1113/jphysiol.1982.sp014371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiserodt J. C., Britvan L. J., Targan S. R. Characterization of the cytolytic reaction mechanism of the human natural killer (NK) lymphocyte: resolution into binding, programming, and killer cell-independent steps. J Immunol. 1982 Oct;129(4):1782–1787. [PubMed] [Google Scholar]
- Hyman R., Ralph P., Sarkar S. Cell-specific antigens and immunoglobulin synthesis of murine myeloma cells and their variants. J Natl Cancer Inst. 1972 Jan;48(1):173–184. [PubMed] [Google Scholar]
- Mayer M. M. Presidential address to the American Association of Immunologists, delivered in Chicago, Illinois, April 6, 1977. Mechanism of cytolysis by lymphocytes: A comparison with complement. J Immunol. 1977 Oct;119(4):1195–1203. [PubMed] [Google Scholar]
- Meech R. W., Standen N. B. Potassium activation in Helix aspersa neurones under voltage clamp: a component mediated by calcium influx. J Physiol. 1975 Jul;249(2):211–239. doi: 10.1113/jphysiol.1975.sp011012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalfe J. C., Pozzan T., Smith G. A., Hesketh T. R. A calcium hypothesis for the control of cell growth. Biochem Soc Symp. 1980;45:1–26. [PubMed] [Google Scholar]
- Okamoto H., Takahashi K., Yamashita N. Ionic currents through the membrane of the mammalian oocyte and their comparison with those in the tunicate and sea urchin. J Physiol. 1977 May;267(2):465–495. doi: 10.1113/jphysiol.1977.sp011822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigworth F. J., Neher E. Single Na+ channel currents observed in cultured rat muscle cells. Nature. 1980 Oct 2;287(5781):447–449. doi: 10.1038/287447a0. [DOI] [PubMed] [Google Scholar]
- Tsien R. Y., Pozzan T., Rink T. J. T-cell mitogens cause early changes in cytoplasmic free Ca2+ and membrane potential in lymphocytes. Nature. 1982 Jan 7;295(5844):68–71. doi: 10.1038/295068a0. [DOI] [PubMed] [Google Scholar]
- Whitfield J. F., Boynton A. L., MacManus J. P., Rixon R. H., Sikorska M., Tsang B., Walker P. R., Swierenga S. H. The roles of calcium and cyclic AMP in cell proliferation. Ann N Y Acad Sci. 1980;339:216–240. doi: 10.1111/j.1749-6632.1980.tb15980.x. [DOI] [PubMed] [Google Scholar]
- Whitney R. B., Sutherland R. M. Requirement for calcium ions in lymphocyte transformation stimulated by phytohemagglutinin. J Cell Physiol. 1972 Dec;80(3):329–337. doi: 10.1002/jcp.1040800303. [DOI] [PubMed] [Google Scholar]
- Yamashita N. Enhancement of ionic currents through voltage-gated channels in the mouse oocyte after fertilization. J Physiol. 1982 Aug;329:263–280. doi: 10.1113/jphysiol.1982.sp014302. [DOI] [PMC free article] [PubMed] [Google Scholar]


