SUMMARY
Sex hormones and insulin have been implicated in articular cartilage metabolism. To supplement previous findings on the regulation of matrix synthesis with 17β-estradiol and insulin and to find a possible model to study cartilage metabolism in vitro, we evaluated the expression of estrogen receptors α and α (ERα, ERβ), androgen receptor (AR) and insulin receptor (IR), in immortalized C-28/I2 and T/C-28a2 chondrocytes and in human primary articular cartilage cells. Chondrocytes were treated with increasing concentrations of 17β-estradiol, dihydrotestosterone or insulin and analyzed by means of RT-PCR and Western blotting. Both cell lines as well as human articular chondrocytes expressed ER αand β, AR and IR at mRNA and protein levels. In immortalized C-28/I2 chondrocytes, we showed that increasing concentrations of 17β-estradiol diminished the 95 kDa band of IR. Since 17β-estradiol suppresses insulin-induced proline incorporation and type II collagen synthesis, as we have previously demonstrated, our findings give the first clue that 17β-estradiol may have negative effects on cartilage anabolism triggered by insulin during hormonal imbalance. Compared to chondrocytes cultured without hormones, immunostaining for ERα/β, AR and IR was decreased in both cell lines after incubation of cells with the receptor-specific hormones. It can be assumed that C-28/I2 and T/C-28a2 chondrocytes interact with the respective hormones. Our findings provide a reproducible model for investigating sex hormone and insulin receptors, which are present in low concentrations in articular chondrocytes, in the tissue-specific context of cartilage metabolism.
Keywords: Chondrocyte cell lines C-28/I2 and T/C-28a2, Human primary articular chondrocytes, 17β-Estradiol, insulin, Sex hormone receptors, Insulin receptor, Gene expression
1. Introduction
Receptors for estrogens (Chen et al., 2004) and androgens (Abu et al., 1997) have been described in osteoblasts. The beneficial action of sex hormones on bone maintenance is well established (Manolagas et al., 2002; Jin and Tian, 2002; Liang and Liao, 2004; Zallone, 2006). This is carried by receptors for estrogens and androgens in osteoblasts. The presence of sex hormone receptors has also been well established in chondrocytes of growth plate (Egerbacher et al., 2002; Nilsson et al., 2003; Bonnelye et al., 2007), laryngeal (Holt et al., 1986; Sassoon et al., 1986; Claassen et al., 2006a) and costal cartilage (Itagane et al., 1991). However, the action of sex hormones on cartilage has hardly been elucidated and the clinical impact of sex hormones in articular cartilage is completely unclear to date.
The incidence of osteoarthritis (OA) increases with age in both men and women. However, women seem to be protected against OA until menopause (Spector and Champion, 1989; Spector et al., 1991, 1997; Wluka et al., 2000). Estrogens have been considered possible factors in the predisposition to postmenopausal OA, and estrogen depletion or altered metabolism has been regarded as among the risk factors for OA (For a review see Gokhale et al., 2004). By contrast, men seem to lack protection against OA beginning at the age of 30. Very early reports in mice revealed a detrimental effect of testosterone on articular cartilage (Silberberg and Silberberg, 1961, 1963). On the other hand, recent clinical studies have shown a positive correlation between testosterone and tibial cartilage volume (Cicuttini et al., 2003; Hanna et al., 2005). Due to hormonal changes in mid-life and the so-called metabolic syndrome, insulin may also play a role in modifying articular cartilage metabolism (Athanasiou et al., 1999). Recently, we showed that 17β-estradiol suppresses the anabolic effects of insulin in female bovine articular chondrocytes (Claassen et al., 2006b).
To gain further insights into a possible involvement of sex hormones and insulin in articular cartilage metabolism, we evaluated the expression of receptors of the above-mentioned hormones at the mRNA and protein levels. Because previous studies to investigate hormonal influences on human articular cartilage metabolism have provided variable results due to the uncontrolled sources of cartilage and insufficient numbers of cells obtained from random procedures, we used und studied the immortalized chondrocyte cell lines, C-28/I2 and T/C-28a2, which have already been shown to be a reproducible cell culture model of human origin (Goldring et al., 1994; Loeser et al., 2000; Goldring, 2004). Furthermore, we compared our results with the respective ones in human primary articular chondrocytes. Due to our recent finding that 17β-estradiol influenced the anabolic insulin effect (Claassen et al., 2006b), we incubated immortalized chondrocytes C-28/I2 and T/C-28a2 with physiological and supraphysiological doses of 17β-estradiol and with insulin followed by examination of insulin receptor (estrogen receptor) expresssion.
2. Materials and methods
2.1. Cell culture
The immortalized chondrocyte cell lines, C-28/I2 and T/C-28a2, originated from cells isolated from rib cartilage of a 15-year-old female and were transduced with simian virus 40 (SV 40) containing the large T-antigen (Goldring et al., 1994). Chondrocytes of both cell lines were seeded at a density of 100,000 cells/cm2 in 25 cm2 flasks or on glass coverslips and cultured in DMEM/F-12 medium (Gibco 11039) containing 10% fetal calf serum (Seromed S0115), 100 U/ml penicillin/streptomycin (Seromed A2210), 2.5 μg/ml amphotericin B (Seromed A2610), 50 μg/ml ascorbic acid (Sigma A-8960) and 50 μM alpha-tocopherol (Sigma T-1157) for 5–7 days at 37 °C and 5% O2. The medium was changed every third day. Thereafter, cells were washed with Hank’s buffered saline solution (HBSS, Gibco 14025-050) and cultured in medium without serum containing DMEM/F-12, 100 U/ml penicillin/streptomycin, 2.5 μg/ml amphotericin B, 50 μg/ml ascorbic acid, 50 μM alpha-tocopherol, 100 mM sodium pyruvate (Gibco 11360) and 0.1576 mg/100 ml cysteine (Sigma C-1276) for 2 days by addition of 17β-estradiol, dihydrotestosterone or insulin. During the total culture period of 7 or 9 days cells became confluent but did not dedifferentiate and expressed mRNAs of type II-collagen and aggrecan as cartilage specific proteins.
For culturing human articular chondrocytes, articular cartilage was obtained with institutional review board approval from three female patients (aged 65, 70 and 71 years) who underwent joint replacement surgery in the Department of Orthopaedics of the Martin-Luther-University of Halle-Wittenberg. The patients had given their informed consent to the project before operation. Experiments were performed according to the Helsinki Declaration. Immediately after resection of one hip joint, in each case specimens were transferred into sterile HBSS and transported to the Department of Anatomy and Cell Biology, where all the following procedures took place. In laboratory, hip joint specimens were washed three times with a sterile Hank’s buffered salt solution (HBSS, Seromed L2035) and dissected under aseptic conditions. Specimens from joint cartilage were excised from unfibrillated zones of cartilage which did not show OA lesions macroscopically.
The harvested cartilage slices were washed three times in Hank’s buffered salt solution (HBSS, Seromed L2035) containing 100 U/ml penicillin/streptomycin (Seromed A2210) and 2.5 μg/ml amphotericin b (Seromed A2610). Slices of cartilage were incubated in 0.35% pronase (Boehringer 1459643)/HBSS for 60 min at 37 °C, washed, and transferred overnight into a 0.05% collage-nase solution (Sigma C-1889) in Hams F12 without phenol red (Seromed F0723). After centrifugation (400 × g, 10 min) the cell pellet was resuspended in 10 ml medium containing serum (Hams F12, 10% fetal calf serum (FCS, Seromed S0115), 100 U/ml penicillin/streptomycin, 2.5 μg/ml amphotericin b, 50 μg/ml ascorbic acid, 50 μM alpha-tocopherol) and filtered through a 20 μm nylon mesh. Vital cells in the final cell suspension were counted by the trypan blue exclusion method with a hemocytometer. For culture in monolayers, chondrocytes were seeded at a density of 400,000 cells/ml medium/well in 12 well culture plates (Costar). Cells were incubated for 5–7 days with medium containing serum (see above) at 37 °C and 5% O2. Medium was changed every third day.
2.2. Incubation with 17β-estradiol, dihydrotestosterone and insulin
After the cultures of C-28/I2 and T/C-28a2 chondrocytes were changed to serum-free medium, cells were incubated alone or with a range of concentrations from 2.72 pg/ml (10−11 M) to 27.2 μg/ml (10−4 M) 17β-estradiol (Sigma E-8875), 2.99 ng/ml (10−8 M) and 29.9 ng/ml (10−7 M) dihydrotestosterone (Sigma A-8380) or 5 μg/ml insulin (Sigma I-1882) during the 2 serum-free days (days 6–7 or 8–9, respectively). For this purpose, stock solutions of 10−1 M 17β-estradiol, 10−1 M dihydrotestosterone in 100% ethanol or 1 mg/ml insulin in PBS/NaOH were prepared and dissolved stepwise. Controls were incubated with 0.1% ethanol or PBS/NaOH instead of the hormones.
Incubations were performed with a broad range of 17β-estradiol concentrations for the following reasons: The physiological concentrations of 17β-estradiol range from 5 pg/ml (approximately 10−11 M) to 300 pg/ml (approximately 10−9 M) during the menstrual cycle. In pregnancy the concentrations of 17β-estradiol range from 30 ng/ml (10−7 M) to 450 ng/ml (1.6 × 10−6 M). Since the adequate dose for chondrocytes in culture is unknown, cells additionally were incubated with supraphysiological 17β-estradiol doses of 2.77 βg/ml (10−5 M) and 27.2 βg/ml (10−4 M).
2.3. Human tissues
Positive control specimens were obtained from one woman and from two men undergoing uterine, prostate or liver surgery as well as from a placenta.
2.4. Molecular analysis by RT-PCR
Total RNA from cultured C-28/I2 and T/C-28a2 chondrocytes, from human primary articular cartilage cells, from human gran-ulosa cell line COV434 as positive control for ERα (Zhang et al., 2000a,b) and from control tissues was isolated with Trizol reagent (Invitrogen 15596-018) and quantified by spectrophotometry at 260 nm. RNA with a 260/280 nm ratio in the range 1.8–2.0 was considered high quality. Total RNA was reverse transcribed in 12 μl of reaction mixture containing 2 μg of RNA, 1 μl (0.5 μg/ml) of oligo dT-primers, and DEPC water to volume. This mixture was heated at 70 °C for 10 min and afterwards cooled on ice. The following reagents were added: 4 μl reaction buffer (5×), 2 μl (0.1 M) dithiothreitol (DTT), 1 μl (10 mM) desoxynucle-oside triphosphates (dNTPs) and 1 μl (200 U) reverse transcriptase (Superscript II Reverse Transcriptase, Invitrogen 18064-014). The mixture was incubated for 60 min at 37 °C. PCR was carried out using a Biozym MultiCycler PTC 200. The PCR of 20 μl consisted of 2 μl cDNA (corresponding to 330 ng RNA), 2 μl dNTPs, 1 μl of each of the sequence specific primers (10 pmol), 2 μl PCR buffer (10×), 2 μl MgCl2, 0.2 Taq polymerase (Invitrogen 18064-014) filled ad 30 μl with PCR water. Each cycle consisted of 2 min denaturation at 95 °C, annealing at specific temperatures and 1 min extension at 72 °C. The final extension was at 72 °C for 10 min at the completion of the last cycle. The nucleotide sequences, annealing temperatures, reaction cycles and predicted sizes of the cDNA products of the primers used and names were as follows.
ERα PCR: forward 5′-CCTTTGGCCAAGCCCGCTCA-3′; reverse 5′-TGGCACCCTCTTCGCCCAGT-3′(65 °C, 40 cycles, 483 bp; according to Hombach-Klonisch et al., 2005). ERβ PCR: forward 5′-CGATGCT-TTGGTTTGGGTGAT-3′; reverse 5′-CTTTAGGCCACCGAGTTGATT-3′(65 °C, 40 cycles, 295 bp; according to Hombach-Klonisch et al., 2005). AR PCR: forward 5′-CCTGGCTTCCGCAACTTA-CAC-3′; reverse 5′-GGACTTGTGCATGCGGTACTCA-3′ (62 °C, 40 cycles, 168 bp; according to Latil et al., 2001). IR PCR: forward 5′-CTGGGAGTGGA-GCAAACACAAC-3′; reverse 5′-TGGTCTTCAGGGCAATGTCG-3′ (58 °C, 40 cycles, 152 bp).
All PCR products were resolved in 2% agarose gel by electrophoresis in Tris–acetate–EDTA buffer in the presence of 0.5 μg/ml ethidium bromide. The DNA bands were visualized in the presence of ultraviolet illumination.
2.5. Protein preparation
Frozen articular cartilage from autopsy cases (two 70-year-old female persons) at the Institute of Legal Medicine, Charité University Medicine, Berlin, Germany and from control samples (uterine, placenta, prostate and liver) were ground with mortar and pestle under liquid nitrogen, added to RIPA buffer (PBS, 1% NP-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate with a protease inhibitor cocktail) and homogenized on ice with an Ultra-Turrax T25 homogenizer (Janke & Kunkel, Staufen i. Br., Germany). After centrifugation for 30 min at 16,000 × g, the supernatant was transferred to Eppendorf tubes. The concentration of protein was determined using a protein assay kit (Biorad 500–0006) and a spectrophotometer at 595 nm.
Chondrocytes were lysed by the addition of Triton reagent supplemented with a protease inhibitor cocktail and centrifuged at 3000 × g for 10 min at room temperature. Concentration of total protein was determined as described above.
2.6. Specific detection of hormone receptors
Tissue or cell homogenates equivalent to 20 μg protein/lane were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) on 10% acrylamide separating gels, with equivalent protein loading onto each gel lane. The resolved proteins were transferred to Hybond-ECL nitrocellulose membranes (Amersham Life Technologies, IL, USA). Membranes were blocked for 1 h with 5% bovine serum albumin (BSA) or 5% skim milk powder (SMP) and then probed with specific antibodies at 4 °C overnight (Table 1).
Table 1.
Molecular weight of estrogen receptors α and β, androgen receptor and insulin receptor proteins and the specific antibodies used for their detection in Western blot analysis and immunocytochemistry.
| Protein | Molecular weight | Method | Antibody | Concentration | Company |
|---|---|---|---|---|---|
| ERα | 66 | WB | Mouse monoclonal anti-ERα | 1:500 in 1% BSA | Chemicon, MAB 461 |
| ERα | 66 | IC | Rabbit polyclonal anti-ERα | 1:75 in 1% PBS | Santa Cruz, HC-20, sc-543 |
| ERβ | 57 | WB | Rabbit polyclonal anti-ERβ | 1:500 in 1% BSA | Chemicon, MAB 1410 |
| ERβ | 57 | WB | Mouse monoclonal anti-ERβ | 1:100 in 1% BSA | DAKO, clone 8D5, Dr. J. Askaa |
| ERβ | 57 | IC | Rabbit polyclonal anti-ERβ | 1:75 in 1% PBS | Bio Reagents, PA1-311 |
| AR | 110 | WB | Mouse monoclonal anti-AR | 1:250 in 1% BSA | DAKO, M3562, clone AR 441a |
| AR | 110 | WB | Rabbit polyclonal anti-AR | 1:250 in 1% BSA | Santa Cruz, AR N-20, sc-816b |
| AR | 110 | IC | Mouse monoclonal anti-AR | 1:50 in 1% PBS | Lab Vision, AB-1 |
| IR | 95 | WB | Mouse monoclonal anti-IR | 1:200 in 5% SMP | Calbiochem, GR 36, β-subunit |
| IR | 95 | IC | Rabbit polyclonal anti-IR | 1:50 in 1% PBS | Santa Cruz, C-19, sc-711 |
Molecular weight is given in kDa. BSA = bovine serum albumin, SMP = skim milk powder, PBS = phosphate buffered saline.
The antibody AR 441 is a monoclonal mouse antibody raised to amino acids 299–315 of AR of human origin.
The antibody N-20 is a rabbit polyclonal antibody raised to a peptide mapping at the N-terminus of AR of human origin.
Blots were washed and incubated with secondary goat anti-mouse (Santa Cruz, CO 104: sc-2005) or goat anti-rabbit (Santa Cruz, C2304: sc-2004) antibodies conjugated with horse radish peroxidase dissolved 1:10,000 for 2 h at room temperature. After washing, the blots were incubated with ECL chemiluminiscence substrate (ECL Western blotting detection kit, Amersham RPN 2106) for 3 min and the immunoreactivity was visualized by exposing a Hyperfilm ECL (Amersham RPN 3103K) for 5 min at room temperature.
C-28/I2 and T/C-28a2 chondrocytes cultured on glass coverslips were washed three times in TBS (0.14 M NaCl in 20 mM TRIS/NaCl buffer, ph 7.4) and fixed in −20 °C cold methanol for 5 min. Following washing three times in TBS, chondrocytes were incubated with 5 mg/ml hyaluronidase for 30 min at 37 °C in a humid chamber. Afterwards, cells were incubated over night with the respective antibodies to ERα/α, AR and IR (see Table 1) at 4 °C. Cryostat sections of uterine, prostate and liver were used for positive control. For preparation of a negative control, the primary antibody was replaced by nonimmune serum. As secondary antibodies, respective fluorescein-isothiocyanate (FITC)-conjugated antibodies (Alexa 488) diluted 1:200 with TBS were used. Cells were embedded with DAPI-Glycerol (PBS-Glycerol 1:1, by adding 10 μl of 2 mg/ml DAPI stock solution) on glass slides. Immunoreaction was evaluated using a laser scanning microscope (Zeiss LSM 510).
3. Results
Since our previous experiments, in which ethanol was used as a solvent for 17β-estradiol, showed responses in cartilage cells, we used two controls in the present experiments. In the first control, C1, cells were incubated with serum-free medium. In the second control, C2, cells were incubated with serum-free medium containing ethanol or PBS/NaOH, the solvent or vehicle for sex hormones or insulin, respectively.
3.1. Expression of sex hormone and insulin receptors at mRNA level
To characterize the expression of sex hormone receptors in C28/I2 and T/C-28a2 chondrocytes and to determine whether these cells are similar to adult human cartilage chondrocytes, we analyzed the samples by RT-PCR. With primers specific for human ERα and ERβ, PCR products of the expected sizes, 483 and 295 bp, occurred in C-28/I2 and T/C-28a2 chondrocytes and in human primary articular chondrocytes (Fig. 1A). As positive control for ERα and β, human uterine tissue showed a PCR signal at 483 and 285 bp, respectively (Fig. 1A). Compared to control cells, incubations with 5 μg/ml insulin diminished the PCR signal for ERα and ERβ in C-28/I2 cells (Fig. 1B). As positive control for ERα expression, the human granulosa cell line COV434 showed a PCR signal at 483 bp (Fig. 1B).
Fig. 1.
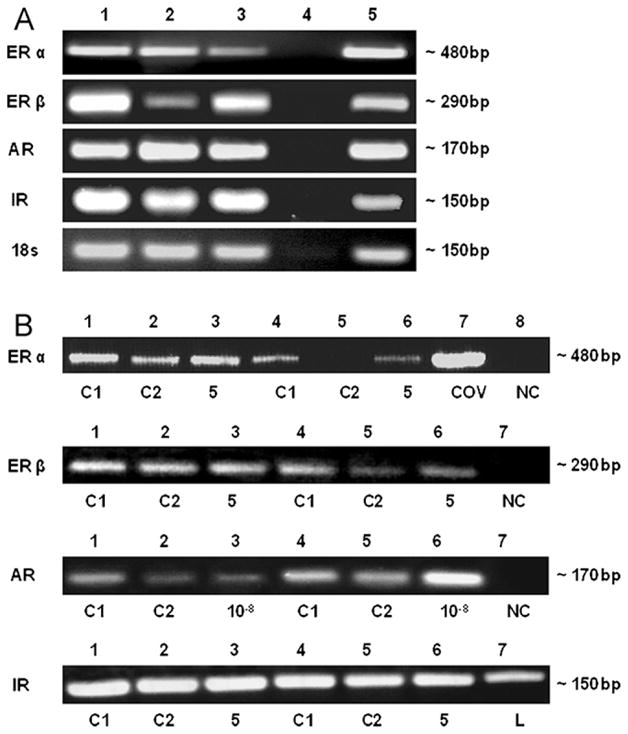
Detection of estrogen receptors (ER) α and β, androgen receptor (AR) and insulin receptor (IR) transcripts in chondrocyte cell lines C-28/I2 and T/C-28a2 and in human primary articular chondrocytes cultured without hormones (A) and with hormones (B). (A) ER alpha (483 bp) and beta (295 bp), AR (168 bp) and IR (152 bp) mRNAs were detected in human primary articular cartilage cells (lane 1), C-28/I2 and T/C-28a2 chondrocytes (lanes 2, 3) and in uterine cells used as positive control (lane 5). DEPC-water served as negative control (lane 4). As further control, RT-PCR analysis of the household gene 18s was performed. PCR bands were shown by means of agarose gel electrophoresis. (B) Untreated controls were C1 (serum-free medium) and C2 (serum-free medium with solvent, PBS/NaOH). DEPC-water as negative control (NC) is indicated. ERβ mRNA (483 bp) levels were analyzed by RT-PCR in C-28/I2 cells incubated without (lanes 1, 2, 4, 5) or with 5 μg/ml insulin (lanes 5, 6), compared to total RNA extracted from human granulosa cells (COV434) as positive control (lane 7). ERβ mRNA (295 bp) levels were analyzed in C-28/I2 cells incubated without (lanes 1, 2, 4, 5) or with 5 μg/ml insulin (lanes 3, 6). AR mRNA (168 bp) was analyzed in T/C-28a2 cells incubated without (lanes 1, 2) or with 10−8 M dihydrotestosterone (lane 3) and in C28/I2 cells incubated without (lanes 4, 5) or with 10−8 M dihydrotestosterone (lane 6). IR mRNA (152 bp) was analyzed in C-28/I2 cells incubated without (lanes 1, 2, 4, 5) or with 5 μg/ml insulin (lanes 3, 6), compared to total RNA extract from liver (L). PCR bands were shown by means of agarose gel electrophoresis.
AR mRNAs were present in both C-28/I2 and T/C-28a2 chondrocytes and in human primary articular chondrocytes (Fig. 1A). Using primers recognizing sequences within regions of the human AR gene, a product of 168 bp (AR) was detected. In comparison to control cells, the incubations with 10−8 M dihydrotestosterone diminished the PCR signal in T/C-28a2 cells but increased it in C-28/I2 cells (Fig. 1B).
With primers specific for human insulin receptor, a PCR product of the expected size, 152 bp, occurred in both C-28/I2 and T/C-28a2 chondrocytes and in human primary articular chondrocytes (Fig. 1A). Note that the PCR signal was of approximately the same intensity in incubations with 5 μg/ml insulin and in control incubations (Fig. 1B). Human liver tissue used as a positive control for IR expression showed a PCR signal at 152 bp (Fig. 1B).
3.2. Expression of sex hormone and insulin receptors at the protein level
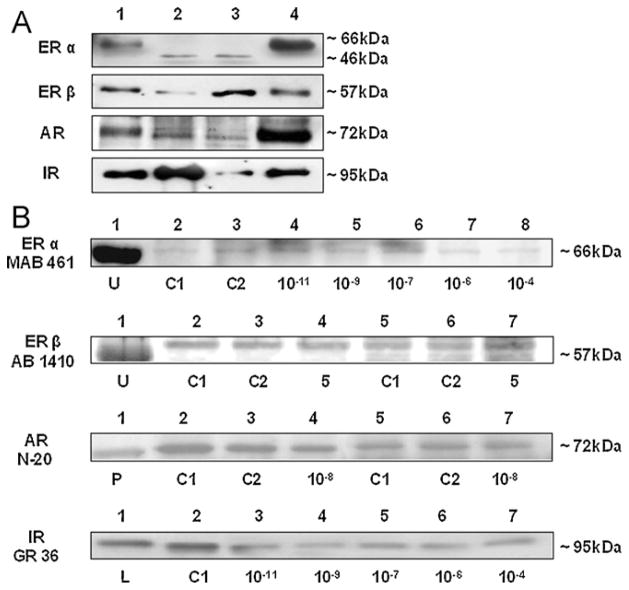
Western blots for ERα and ERβ revealed that both cell lines and human primary articular chondrocytes expressed the two estrogen receptors of the expected molecular weight at 66 or 46 kDa and 57 kDa, respectively (Fig. 2A). As positive controls for ERα and β, placentar tissue showed 66 and 57 kDa bands, respectively (Fig. 2A). Compared to uterine tissue, where the antibody MAB 461 detected a strong 66 kDa band, the respective bands in C-28/I2 chondrocytes incubated with different concentrations of 17β-estradiol were weaker (Fig. 2B). Western blot analysis of ERβ with antibody AB 1410 identified a predominant ERβ product of 57 kDa in C-28/I2 chondrocytes (Fig. 2B). To confirm this result, we performed Western blotting with another ERβ antibody, 8D5, which detected a 57 kDa band in T/C-28a2 cells and in C-28/I2 cells (not shown). In comparison to C-28/I2 cells incubated without hormones, cells incubated with 5 μg/ml of insulin showed a 57 kDa band of approximately the same intensity (Fig. 2B).
Fig. 2.
Detection by Western blotting of estrogen receptors alpha and beta (ERα, ERβ), androgen receptor (AR) and insulin receptor (IR) proteins in chondrocyte cell lines C-28/I2 and T/C-28a2 and in human articular cartilage chondrocytes cultured without (A) and with hormones (B). (A) ER alpha (66 or 46 kDa) and beta (57 kDa), AR (72 kDa) and IR (95 kDa) proteins were detected in C-28/I2 and T/C-28a2 chon-drocytes (lanes 2, 3), in human articular cartilage chondrocytes (lane 4) and in placentar tissue used as positive control (lane 1). (B) Positive controls were protein extracts from uterine (U), prostate (P) and liver (L) tissues. Untreated controls were C1 (serum-free medium) and C2 (serum-free medium with solvents, ethanol or PBS/NaOH). ERα was detected at 66 kDa using antibody MAB 461. C28/I2 cells were incubated without (lanes 2, 3) or with 10−11, 10−9, 10−7, 10−6 and 10−4 M 17β-estradiol (lanes 4–8). ERβ was detected at 57 kDa with antibody AB 1410. C28/I2 cells were incubated without (lanes 2, 3, 5, 6) or with 5 μg/ml insulin (lanes 4, 9). AR was detected at 72 kDa with antibody AR N-20. T/C-28a2 cells were incubated without (lanes 2, 3) or with 10−8 M dihydrotestosterone (lane 4). C-28/I2 cells were incubated without (lanes 5, 6) or with 10−8 M dihydrotestosterone (lane 7). IR was detected at 95 kDa with antibody IR GR 36. C-28/I2 cells were incubated without (lane 2) or with 10−11, 10−9, 10−7, 10−6 and 10−4 M 17β-estradiol (lanes 3–7).
Presence of AR protein was studied using the monoclonal antibody N-20. This antibody detected a 72 kDa product in both C-28/I2 and T/C-28a2 chondrocytes and in human primary articular chondrocytes (Fig. 2A). However, the 72 kDa band did not correspond to the expected product of 110 kDa. Western blot analysis with monoclonal antibody AR 441 revealed an identical 72 kDa band in both cell lines and prostate tissue detected with antibody N-20 (not shown). Compared to controls, the intensity of the 72 kDa band was not influenced by incubations with dihydrotestosterone in both T/C-28a2 and C-28/I2 chondrocytes (Fig. 2B).
Western blots for IR revealed that both cell lines and human primary articular chondrocytes expressed IR of the expected molecular weight of 95 kDa (Fig. 2A). Compared with liver tissue, where the antibody GR 36 detected a strong 95 kDa band, the respective band in C-28/I2 chondrocytes incubated without hormones showed approximately the same intensity (Fig. 2B). Note that compared to control, the intensity of the 95 kDa band was diminished if C-28/I2 cells were incubated with physiological and supraphysiological doses of 17β-estradiol (Fig. 2B).
3.3. Immunocytochemistry of sex hormone and insulin receptors
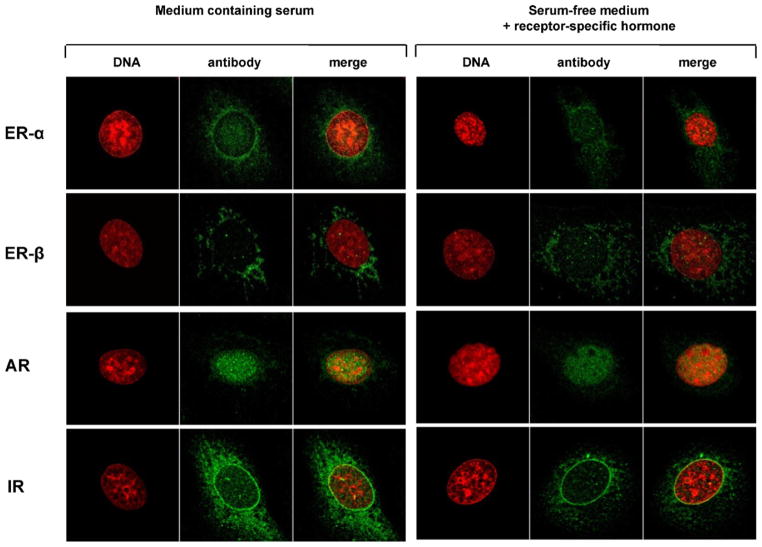
Nuclei of unstimulated C-28/I2 chondrocytes were immunostained by specific antibodies to ERα/β and AR (Fig. 3). Especially cytoplasm of unstimulated cells was stained by antibodies to IR (Fig. 3). Additionally, chondrocytes were incubated with 10−7 M 17β-estradiol, 10−7 M dihydrotestosterone or 10−7 M insulin. Staining of nuclei for ERα/β and AR or staining of cytoplasm for IR was diminished after incubation of chondrocytes with the receptor-specific hormone (Fig. 3). In T/C-28a2 chondrocytes similar results concerning immunostaining for sex hormone and insulin receptors were obtained (not shown).
Fig. 3.
Immunocytochemical staining of estrogen receptors alpha and beta (ERα, ERβ), androgen receptor (AR) and insulin receptor (IR) in chondrocytes of cell line C-28/I2 cultured on glass coverslips. The left tableau shows immunostaining of the respective receptors after cells were cultured in serum-containing medium (10% FCS) for 1 week. The right tableau presents receptor staining after cells were cultured in serum-free medium by adding the receptor-specific hormone for 2 days. Receptor-specific hormones and their concentrations: 10−7 M 17β-estradiol, 10−7 M dihydrotestosterone, 10−7 M insulin. The columns were inscribed with “DNA” (DNA staining), “antibody” (immunocytochemical staining with receptor-specific antibodies) and “merge” (fusion of DNA- and immunocytochemical staining). Note, that compared to culture in medium containing serum, immunostaining for ERα, ERβ, AR and IR is weaker after adding the receptor-specific hormone during culture in serum-free medium.
4. Discussion
Several lines of evidence suggest that hormones like insulin, and in particular sex hormones like 17β-estradiol and dihy-drotestosterone, are involved in the pathogenesis of OA. Previous immunohistochemical studies have shown that ERα is expressed in human articular chondrocytes (Claassen et al., 2001). Because it is difficult to obtain healthy human articular cartilage, particularly in larger amounts, for cell culture experiments, we analyzed the expression of sex hormone and insulin receptors in C-28/I2 and T/C-28a2 chondrocytes at mRNA and protein levels to find out whether both cell lines could serve as models for analysis of human articular cartilage metabolism. The results were compared with the expression of the respective hormone receptors in human primary articular chondrocytes. In addition, we stimulated C-28/I2 and T/C-28a2 chondrocytes with 17β-estradiol, dihydrotestosterone and insulin to see if sex hormone receptors react with the receptor specific hormone and if insulin receptor protein is influenced by 17β-estradiol incubation or vice versa.
The expression of the two controls, serum-free medium with or without solvent or vehicle, sometimes was different on mRNA and protein level. We assume that ethanol as solvent for 17β-estradiol can influence the gene expression of chondrocytes.
C-28/I2 and T/C-28a2 chondrocytes express ERα and ERβ at mRNA and protein levels as shown by RT-PCR and Western blot analysis. Furthermore human primary articular cartilage cells express both estrogen receptors. Specific antibodies to both estrogen receptors detected products of 66 or 46 kDa (Figtree et al., 2003) (ERα) and 57 kDa (ERβ), which have been described in breast cancer tissue as primarily estrogen-dependent (Skliris et al., 2002; Jarzabek et al., 2005).
In comparison to C-28/I2 cells incubated without 17β-estradiol, incubations with 10−6 and 10−4 M 17β-estradiol revealed a 66kDa band of diminished intensity. This result was confirmed by immunocytochemistry where staining of nuclei for ERα was diminished after incubation with 10−7 M 17β-estradiol. Down regulation of ERβ following estrogen treatment has also been shown in telomerase immortalized human endometrial cells (Hombach-Klonisch et al., 2005).
Both in C-28/I2 and T/C-28a2 chondrocytes and in human primary articular chondrocytes, AR mRNA was detected by specific primers. At the protein level, antibodies N-20 and AR 441 stained a 72 kDa band in both chondrocyte cell lines and in prostatic tissue. These results were confirmed by immunocytochemical staining for AR in chondrocytes of both cell lines using antibody AB-1. However, the molecular weight of AR was expected at 110 kDa as shown in female breast cancer tissue (Bry et al., 2001), in human endometria (Villavicencio et al., 2006) and in prostate cancer cells (He et al., 2006). By contrast, Wilson and McPhaul (1994) have shown that genital skin fibroblasts contain both the full-length 110 kDa protein (termed AR-B) and an 87 kDa N-terminally truncated AR isoform (termed AR-A). In this context, alternative RNA splicing can lead to multiple transcripts as it has been shown for the human endothelin-A receptor (Miyamoto et al., 1996). We therefore assume that the antibodies applied here detected a tissue-specific form of AR. Since previous studies have associated serum testosterone levels with tibial cartilage volume and tibial cartilage loss (Cicuttini et al., 2003; Hanna et al., 2005), it can be hypothesized that AR is involved in cartilage metabolism.
Incubations of C-28/I2 and T/C-28a2 chondrocytes with 10−8 M dihydrotestosterone did not show any effect in Western blot analysis but immunostaining of cells was diminished. We suppose that immunocytochemistry is more sensitive to detect different amounts of androgen receptor protein.
Insulin receptor was detected at the mRNA and protein levels in both cell lines and in human primary articular chondrocytes. Western blot analysis for IR revealed a product of the expected size of 95 kDa as described for other tissues, recently for example vascular smooth muscle cells (Johansson and Arnqvist, 2006). These results were also confirmed by immunocytochemistry.
Recently we observed that 17β-estradiol influenced the protein anabolic insulin effect negatively (Claassen et al., 2006b). Therefore C-28/I2 and T/C-28a2 chondrocytes were incubated with 17β-estradiol followed by examination of insulin receptor protein. Compared to control, incubations with 17β-estradiol diminished the 95 kDa band of IR in C-28/I2 chondrocytes. This finding suggests that ER signaling may have negative effects on cartilage anabolism during hormonal imbalance. The impact of both hormones on articular cartilage metabolism has been stressed by other investigators, who showed that estrogen replacement therapy increased the production of insulin-like growth factor protein 2 (Richmond et al., 2000) and that 17β-estradiol increased protein kinase C (Kinney et al., 2004). However, these results cannot explain our present findings. Further work is needed to adress the influence of ER on insulin-dependent signals, such as Akt, Grb2 (growth factor receptor bound protein 2) and MAP kinases ERK1/2.
Furthermore, sex hormone and insulin receptors of C-28/I2 and T/C-28a2 chondrocytes were functionally adressed after incubations with the receptor-specific hormones. Compared to unstimulated controls, immunostaining for ERα and β, AR and IR was decreased after cells were cultured with the receptor-specific hormones. With regard to ERα, such an effect has also been shown for the human endometrial carcinoma cell line ECC-1, where down regulation of ERα occurs after estrogen treatment (Dardes et al., 2002).
In conclusion, our results show that ERα/β, AR and IR are expressed at the mRNA and protein levels in human C-28/I2 and T/C-28a2 chondrocytes or in human primary articular cartilage chondrocytes. The findings suggest that chondrocytes may represent target cells for estrogens, androgens and insulin. In particular, an opportunity is presented to investigate a possible influence of estrogens on articular cartilage due to the clinical evidence of a higher prevalence of OA in post-menopausal women. Moreover, our findings provide a reproducible model for investigating sex hormone and insulin receptors, which are present in low concentrations in C-28/I2 and T/C-28a2 chondrocytes, in the tissue-specific context of cartilage metabolism. Furthermore, based on our present finding that 17β-estradiol diminishes the 95 kDa band of IR in C-28/I2 chondrocytes, and our recent finding that 17β-estradiol suppresses the protein anabolic effect of insulin in female bovine articular chondrocytes (Claassen et al., 2006b), the influence of interactions between ER and IR on cartilage metabolism can now be studied more definitively in a reproducible model.
Acknowledgments
We would like to thank Dr. J. Askaa (DAKO, Denmark) for providing us with antibody to ERβ, clone 8D5, and M. Beall for editing the English.
Footnotes
This study was supported by BMBF Wilhelm Roux program grant FKZ 13/17 to Horst Claassen as well as in part by DFG grant PA 738/6-1 and BMBF Wilhelm Roux program grant FKZ 24/25 to Friedrich Paulsen. Dr. Goldring’s research was supported by NIH grant R01-AG022021.
References
- Abu EO, Horner A, Kusec V, Triffitt JT, Compston JE. The localization of androgen receptors in human bone. J Clin Endocrinol Metab. 1997;82:3493–3497. doi: 10.1210/jcem.82.10.4319. [DOI] [PubMed] [Google Scholar]
- Athanasiou KA, Fleischli JG, Bosma J, Laughlin TJ, Zhu CF, Agrawal CM, Lavery LA. Effects of diabetes mellitus on the biomechanical properties of human ankle cartilage. Clin Orthop. 1999;368:182–189. [PubMed] [Google Scholar]
- Bonnelye E, Zirngibl RA, Jurdic P, Aubin JE. The orphan nuclear estrogen receptor-related receptor-alpha regulates cartilage formation in vitro: implication of Sox9. Endocrinology. 2007;148:1195–1205. doi: 10.1210/en.2006-0962. [DOI] [PubMed] [Google Scholar]
- Bry M, Wójcik M, Romanowicz-Makowska H, Krajewska WM. Androgen receptor status in female breast cancer: RT-PCR and Western blot studies. J Cancer Res Clin Oncol. 2001;128:85–90. doi: 10.1007/s004320100294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen FP, Hsu T, Hu Ch, Wrang WD, Wang KC, Teng LF. Expression of estrogen receptors alpha and beta in human osteoblasts: identification of exon-2-deletion variant of estrogen receptor beta in postmenopausal women. Chang Gung Med J. 2004;27:107–115. [PubMed] [Google Scholar]
- Claassen H, Hassenpflug J, Schünke M, Sierralta W, Thole H, Kurz B. Immunohistochemical detection of estrogen receptor alpha in articular chondrocytes from cows, pigs and humans: in situ and in vitro results. Ann Anat. 2001;183:223–227. doi: 10.1016/s0940-9602(01)80221-1. [DOI] [PubMed] [Google Scholar]
- Claassen H, Mönig H, Sel S, Werner JA, Paulsen F. Androgen receptors and gender-specific distribution of alkaline phosphatase in human thyroid cartilage. Histochem Cell Biol. 2006a;126:381–388. doi: 10.1007/s00418-006-0172-7. [DOI] [PubMed] [Google Scholar]
- Claassen H, Schlüter M, Schünke M, Kurz B. Influence of 17β-estradiol and insulin on type II collagen and protein synthesis of articular chondrocytes. Bone. 2006b;39:310–317. doi: 10.1016/j.bone.2006.02.067. [DOI] [PubMed] [Google Scholar]
- Cicuttini FM, Wluka A, Bailey M, O’Sullivan R, Poon C, Yeung S, Ebeling PR. Factors affecting knee cartilage volume in healthy men. Rheumatology. 2003;42:258–262. doi: 10.1093/rheumatology/keg073. [DOI] [PubMed] [Google Scholar]
- Dardes R, Schafer J, Pearce S, Osipo C, Chen B, Jordan V. Regulation of estrogen target genes and growth by selective estrogen receptor modulators in endometrial cancer cells. Gynaecol Oncol. 2002;85:498–506. doi: 10.1006/gyno.2002.6659. [DOI] [PubMed] [Google Scholar]
- Egerbacher M, Helmreich M, Rossmanith W, Haeusler G. Estrogen receptor-alpha and estrogen receptor-beta are present in the human growth plate in childhood and adolescence, in identical distribution. Horm Res. 2002;58:99–103. doi: 10.1159/000064661. [DOI] [PubMed] [Google Scholar]
- Figtree GA, McDonald D, Watkins H, Channon KM. Truncated estrogen receptor α 46-kDa isoform in human endothelial cells. Relationship to acute activation of nitric oxide synthase. Circulation. 2003;107:120–126. doi: 10.1161/01.cir.0000043805.11780.f5. [DOI] [PubMed] [Google Scholar]
- Gokhale JA, Frenkel SR, Dicesare PE. Estrogen and osteoarthritis. Am J Orthop. 2004;33:71–80. [PubMed] [Google Scholar]
- Goldring MB, Birkhead JR, Suen LF, Yamin R, Mizuno S, Glowacki J, Arbiser JL, Apperley JF. Interleukin-1β-modulated gene expression in immortalized human chondrocytes. J Clin Invest. 1994;94:2307–2316. doi: 10.1172/JCI117595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldring MB. Immortalization of human articular chondrocytes for generation of stable, differentiated cell lines. Methods Mol Med. 2004;100:23–35. doi: 10.1385/1-59259-810-2:023. [DOI] [PubMed] [Google Scholar]
- Hanna F, Ebeling PR, Wang Y, O’Sullivan R, Davis S, Wluka AE, Cicuttini FM. Factors influencing longitudinal change in knee cartilage volume measured from magnetic resonance imaging in healthy men. Ann Rheum Dis. 2005;64:1038–1042. doi: 10.1136/ard.2004.029355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He ML, Yuan HQ, Jiang AL, Gong AY, Chen WW, Zhang PJ, Young CYF, Zhang JY. Gum mastic inhibits the expression and function of the androgen receptor in prostate cancer cells. Cancer. 2006;106:2547–2555. doi: 10.1002/cncr.21935. [DOI] [PubMed] [Google Scholar]
- Holt GR, Aufdemorte TB, Sheridan PJ. Estrogen receptor in the larynx of the aged baboon (Papio cynocephalus) Ann Otol Rhinol Laryngol. 1986;95:608–617. doi: 10.1177/000348948609500614. [DOI] [PubMed] [Google Scholar]
- Hombach-Klonisch S, Kehlen A, Fowler PA, Huppertz B, Jugert JF, Bischoff G, Schlüter E, Buchmann J, Klonisch T. Regulation of functional steroid receptors and ligand-induced responses in telomerase-immortalized human endometrial epithelial cells. J Mol Endocrinol. 2005;34:517–534. doi: 10.1677/jme.1.01550. [DOI] [PubMed] [Google Scholar]
- Itagane Y, Inada H, Fujita K, Isshiki G. Interactions between steroid hormone and insulin-like growth factor-I in rabbit chondrocytes. Endocrinology. 1991;128:1419–1424. doi: 10.1210/endo-128-3-1419. [DOI] [PubMed] [Google Scholar]
- Jin JL, Tian CG. Testosterone and male osteoporosis. Zhonghua Nan Ke Xue. 2002;8:145–147. [PubMed] [Google Scholar]
- Jarzabek K, Koda M, Kozlowski L, Mittre H, Sulkowski S, Kottler ML, Wol-czynski S. Distinct mRNA, protein expression patterns and distribution of oestrogen receptors alpha and beta in human primary breast cancer: correlation with proliferation marker Ki-67 and clinicopathological factors. Eur J Cancer. 2005;41:2924–2934. doi: 10.1016/j.ejca.2005.09.010. [DOI] [PubMed] [Google Scholar]
- Johansson GS, Arnqvist HJ. Insulin and IGF-I action on insulin receptors. IGF-I receptors, and hybrid insulin/IGF-I receptors in vascular smooth muscle cells. Am J Physiol Endocrinol Metab. 2006;291:E1124–E1130. doi: 10.1152/ajpendo.00565.2005. [DOI] [PubMed] [Google Scholar]
- Kinney RC, Schwartz Z, Week K, Lotz MK, Boyan BD. Human articular chondrocytes exhibit sexual dimorphism in their responses to 17β-estradiol. Osteoarthr Cartil. 2004;13:330–337. doi: 10.1016/j.joca.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Liang M, Liao EY. Effects of progestin on proliferation and differentiation of osteoblasts. Zhonghua Fu Chan Ke Za Zhi. 2004;39:250–253. [PubMed] [Google Scholar]
- Latil A, Bièche I, Vidaud D, Lidereau R, Berthon P, Cussenot O, Vidaud M. Evaluation of androgen, estrogen (ERα and ERβ), and progesterone receptor expression in human prostate cancer by real-time quantitative reverse transcription-polymerase chain reaction assays. Cancer Res. 2001;61:1919–1926. [PubMed] [Google Scholar]
- Loeser RF, Sadiev S, Tan L, Goldring MB. Integrin expression by primary and immortalized human chondrocytes: evidence of a differential role for α1β1 and α2β1 integrins in mediating chondrocyte adhesion to types II and VI collagen. Osteoarthr Cartil. 2000;8:96–105. doi: 10.1053/joca.1999.0277. [DOI] [PubMed] [Google Scholar]
- Manolagas SC, Kousteni S, Jilka RL. Sex steroids and bone. Recent Prog Horm Res. 2002;57:385–409. doi: 10.1210/rp.57.1.385. [DOI] [PubMed] [Google Scholar]
- Miyamoto Y, Yoshimasa T, Arai H, Takaya K, Ogawa Y, Itoh H, Nakao K. Alternative RNA splicing of the human endothelin-A receptor generates multiple transcripts. Biochem J. 1996;313:795–801. doi: 10.1042/bj3130795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson E, Chrysis O, Pajulo D, Boman O, Holst A, Rubinstein M, Martin Ritzén J, Savendahl L. Localization of estrogen receptors-alpha and -beta and androgen receptor in the human growth plate at different pubertal stages. J Endocrinol. 2003;177:319–326. doi: 10.1677/joe.0.1770319. [DOI] [PubMed] [Google Scholar]
- Richmond RS, Carlson CS, Register TC, Shanker G, Loeser RF. Functional estrogen receptors in adult articular cartilage. Arthritis Rheum. 2000;43:2081–2090. doi: 10.1002/1529-0131(200009)43:9<2081::AID-ANR20>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Sassoon D, Segil N, Kelley D. Androgen-induced myogenesis and chondro-genesis in the larynx of Xenopus laevis. Dev Biol. 1986;113:135–140. doi: 10.1016/0012-1606(86)90115-6. [DOI] [PubMed] [Google Scholar]
- Silberberg M, Silberberg R. Effect of 17-ethyl-19-nortestosterone on articular aging and osteoarthrosis in orchiectomized mice. J Gerontol. 1961;16:20–31. [Google Scholar]
- Silberberg M, Silberberg R. Role of sex hormone in the pathogenesis of osteoarthrosis of mice. Lab Invest. 1963;12:285–295. [PubMed] [Google Scholar]
- Skliris GP, Parkes AT, Limer JL, Burdall SE, Carder PJ, Speirs V. Evaluation of seven oestrogen receptor β antibodies for immunohistochemistry, western blotting, and flow cytometry in human breast tissue. J Pathol. 2002;197:155–162. doi: 10.1002/path.1077. [DOI] [PubMed] [Google Scholar]
- Spector TD, Champion GD. Generalised osteoarthritis: a hormonally mediated disease. Ann Rheum Dis. 1989;48:523–527. doi: 10.1136/ard.48.6.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector TD, Perry LA, Jubb RW. Endogenous sex steroid levels in women with generalised osteoarthritis. Clin Rheum. 1991;10:316–319. doi: 10.1007/BF02208698. [DOI] [PubMed] [Google Scholar]
- Spector TD, Nandra D, Hart DJ, Doyle DV. Is hormone replacement therapy protective for hand and knee osteoarthritis in women? The Chingford study. Ann Rheum Dis. 1997;56:432–434. doi: 10.1136/ard.56.7.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villavicencio A, Bacallao K, Avellaira C, Gabler F, Fuentes A, Vega M. Androgen and estrogen receptors and co-regulators levels in endometria from patients with polycystic ovarian syndrome with and without endometrial hyperplasia. Gynecol Oncol. 2006;103:307–314. doi: 10.1016/j.ygyno.2006.03.029. [DOI] [PubMed] [Google Scholar]
- Wilson CM, McPhaul MJ. A and B forms of the androgen receptor are present in human genital fibroblasts. Proc Natl Acad Sci USA. 1994;91:1234–1238. doi: 10.1073/pnas.91.4.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wluka AE, Cicuttini FM, Spector TD. Menopause, oestrogens and arthritis. Maturitas. 2000;35:183–199. doi: 10.1016/s0378-5122(00)00118-3. [DOI] [PubMed] [Google Scholar]
- Zallone A. Direct and indirect estrogen actions on osteoblasts and osteoclasts. Ann NY Acad Sci. 2006;1068:173–179. doi: 10.1196/annals.1346.019. [DOI] [PubMed] [Google Scholar]
- Zhang H, Vollmer M, De Geyter M, Litzistorf Y, Ladewig A, Dürrenberger M, Guggenheim R, Miny P, Holzgreve W, De Geyter C. Characterization of an immortalized human granulosa cell line (COV434) Mol Hum Reprod. 2000a;6:146–153. doi: 10.1093/molehr/6.2.146. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Hannan MT, Chaisson CE, McAlindon TE, Evans SR, Aliabadi P, Levy D, Felson DT. Bone mineral density and risk of incident and progressive radiographic knee osteoarthritis in women: the Framingham Study. J Rheumatol. 2000b;27:1032–1037. [PubMed] [Google Scholar]