Abstract
Background Between 1997 and 2009, a number of key malaria control interventions were implemented in the Kilombero and Ulanga Districts in south central Tanzania to increase insecticide-treated nets (ITN) coverage and improve access to effective malaria treatment. In this study we estimated the contribution of these interventions to observed decreases in child mortality.
Methods The local Health and Demographic Surveillance Site (HDSS) provided monthly estimates of child mortality rates (age 1 to 5 years) expressed as cases per 1000 person-years (c/1000py) between 1997 and 2009. We conducted a time series analysis of child mortality rates and explored the contribution of rainfall and household food security. We used Poisson regression with linear and segmented effects to explore the impact of malaria control interventions on mortality.
Results Child mortality rates decreased by 42.5% from 14.6 c/1000py in 1997 to 8.4 c/1000py in 2009. Analyses revealed the complexity of child mortality patterns and a strong association with rainfall and food security. All malaria control interventions were associated with decreases in child mortality, accounting for the effect of rainfall and food security.
Conclusions Reaching the fourth Millenium Development Goal will require the contribution of many health interventions, as well as more general improvements in socio-environmental and nutritional conditions. Distinguishing between the effects of these multiple factors is difficult and represents a major challenge in assessing the effect of routine interventions. However, this study suggests that credible estimates can be obtained when high-quality data on the most important factors are available over a sufficiently long time period.
Keywords: Malaria, child mortality, malaria control interventions, rainfall, food security, mortality impact
Background
Child mortality is a leading indicator of child health and overall development. In sub-Saharan Africa, malaria, diarrhoea and pneumonia are responsible for more than half the deaths of children under five years of age, whereas more than a third of all child deaths are attributed to undernutrition. Malaria control strategies are therefore instrumental in reaching the fourth Millennium Development Goal of reducing the under-five mortality rate by two-thirds worldwide between 1990 and 2015, in combination with targeted efforts to fight pneumonia and diarrhoea while bolstering nutrition.1
The past 10 years have seen a steep rise in international funding for malaria, with disbursements reaching their highest ever levels in 2011 at US$ 2 billion.2 Since the Abuja Declaration in 2000, malaria control strategies have focused on achieving high coverage of insecticide treated nets (ITNs), access to prompt and effective treatment for malaria and the protection of pregnant mothers with intermittent presumptive treatment.3 In recent years, the number of tools available to fight malaria has increased, and now includes long lasting insecticidal nets (LLINs), rapid diagnostic tests (RDTs) and a renewed faith in the feasibility of implementing indoor residual spraying (IRS) in some settings.4
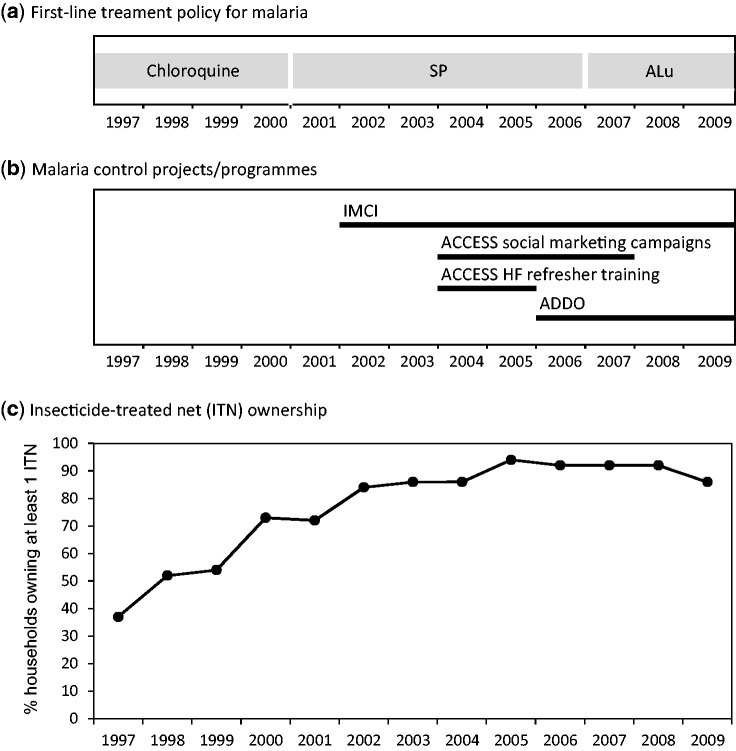
A number of key malaria control interventions have been implemented in the Kilombero and Ulanga districts in south central Tanzania to increase ITN coverage and improve access to effective malaria treatment. First, the KINET project5,6 was piloted to promote and subsidize ITNs between 1997 and 1999. The project was subsequently up-scaled nationwide within the frame of the Tanzania National Voucher Scheme (TNVS).7 There were two changes in the national policy for first-line treatment of malaria, from chloroquine to sulphadoxine-pyrimethamine (SP) in 2001 and from SP to the artemisinin-combination therapy (ACT) artemether-lumefantrine (ALu) in 2007. In 2002 the facility-based Integrated Management of Childhood Illness (IMCI) interventions for improved case management in health facilities and health system strengthening8,9 were introduced. Finally between 2004 and 2008 the ACCESS programme was implemented to improve access to prompt and effective malaria treatment by targeting both users and providers.10 Within the frame of the ACCESS programme, social marketing campaigns for improved treatment-seeking were conducted, nearly 200 Accredited Drug Dispensing Outlets (ADDOs) were opened11,12 and the staff from health facilities attended a refresher training on IMCI case management.10,13
A number of studies have been conducted on these malaria control interventions. Some studies used child mortality as an endpoint,6,8 some have focused on indicators of malaria transmission14–17 and others have assessed changes in treatment outcomes.18 However, no study to date has assessed the impact of all of these interventions on child mortality in the Kilombero and Ulanga Districts using a standardized methodology. In addition, other factors such as climatic conditions and agricultural production have not been considered as part of measuring impact on mortality. The availability of demographic time series and contextual data from the local Health and Demographic and Surveillance Site (HDSS)19 provides a unique opportunity to perform an interrupted time series analysis to assess the contribution of malaria interventions to decreases in child mortality.
Methods
Study setting
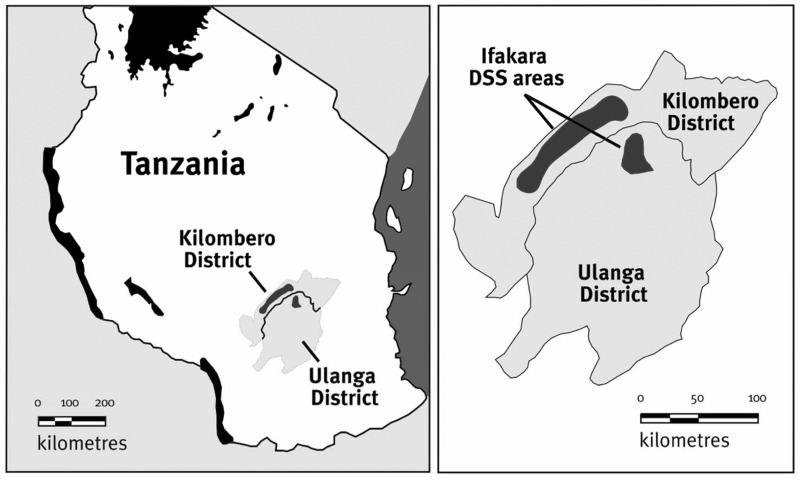
This study was conducted using data collected in the frame of the Ifakara HDSS in the Kilombero and Ulanga Districts in south central Tanzania (Figure 1). The HDSS was established in 1996 and covers 25 villages (13 in Kilombero and 12 in Ulanga).20 The population under surveillance in 1998 was just over 56 000 in 1997 and around 98 000 in 2009. The area is characterized by a rainy season from November to May. Historically, malaria transmission was intense and perennial21 but it has decreased considerably in recent years.22
Figure 1.
Location of the Ifakara Health and Demographic Surveillance System (HDSS) (source: INDEPTH Monograph19)
Data collection and processing
Mortality rates
The HDSS provided all data on all-cause mortality by month from January 1997 to December 2009. We calculated estimates of child mortality rates as the number of deaths occurring between 1 and 5 years of age per 1000 person-years of exposure (c/1000py).
Malaria control interventions
The HDSS routinely collected data on ITN ownership (percentage of households owning at least one mosquito net) for the 13 years’ observation period. From 2002, the majority of the nets were treated with insecticide. ITN household ownership increased steadily from 37% in 1997 to above 90% in 2005 and remained high thereafter. Dates for the two changes in first-line treatment policy for malaria were extracted from existing publications: August 2001 for the change from chloroquine to SP23 and January 2007 for the change from SP to ALu.24 The start date of the IMCI interventions was set in April 2002 (D. Maganga and M. Mawalla, Malaria and IMCI Focal Persons in 2002 in Kilombero and Ulanga Districts, respectively). The start date of the ACCESS interventions was set in November 2004, which coincides with the start of the ACCESS social marketing campaigns and health facility refresher trainings, whereas the ADDOs were piloted (and subsequently rolled out) in 2006.11,12 Figure 2 shows the coverage of these malaria control interventions over time.
Figure 2.
Major malaria control interventions in the Kilombero and Ulanga districts between 1997 and 2009
Rainfall and household food security data
The Kilombero Agricultural Training and Research Institute (KATRIN) provided monthly total rainfall data (mm) collected from gauges located just outside Ifakara town. We obtained yearly agricultural yield data (t/ha) of rice and maize from the Kilombero District Agricultural and Livestock Development Office (DALDO). Because of the proximity and environmental similarity of the districts, we extrapolated the values of the Kilombero District to the Ulanga District.
We constructed a proxy for monthly household food security (kg/household) on the basis of agricultural yield data and information describing the local farmers’ agricultural practices. Over 95% of the HDSS population reported farming as their main activity.20 Rice is the main staple food and the most important cash crop, but maize and other crops are also grown for household consumption.20 First we estimated average household agricultural production per year, as the sum of the products of a year’s crop yield times the average area dedicated to each crop per household, which is 1.5 hectare (ha) for rice and 0.24 ha for maize25 (data on other crops were not available for these calculations). We then calculated a proxy for household food security based on the fact that rice availability peaks in June and maize in March, when they are harvested.25,26 Although rice harvest is completed in June, we assumed that 1/10 of the rice harvest was available in May when harvesting starts. Given that, on average, farmers sell half of their rice crops,25,27 we applied a composite decay function for rice from June onwards. The half kept for personal consumption was assumed to decrease linearly every month (1/12 of half of the initial amount per month). The half sold was assumed to decrease at an exponential rate (1/2 of the amount in the previous month every month), to account for the fact that larger quantities are sold in the months immediately following harvest and most of the rice is sold by the beginning of the rainy season.26 We assumed that maize availability decreased linearly over time from March onwards (1/12 of the initial amount per month), given that it is primarily farmed for household consumption.26
Statistical analysis
First we graphed and described the time series of mortality, rainfall and agricultural yield to identify trends over time, interannual differences and correlations. Time series analyses were subsequently conducted on the monthly rates in two stages as described below. Intercooled Stata 12 (Stata Corp., College Station, TX, USA) was used for all data management and analyses.
Stage 1: Effect of rainfall and food security
In the first stage of analysis we built a multivariate time series model of child mortality rates accounting for the effects of rainfall and food security. We assumed that child deaths in month i arose from a Poisson distribution, and thus fitted the following multivariate model:
| (Model 1) |
where i = 1 to 156 and denotes the number of months from January 1997 to December 2009; µi is the expected value of the mortality rate at month i; Yi are the person-years exposed at month i; log(Yi) is an offset term that ensures that the log rate is being modelled on the log scale; α is the estimated baseline mortality rate at month i; the β parameters β1 to βn are the estimated regression coefficients for the independent covariates x1 to xn and ei denotes the error term, independently and normally distributed. As there was evidence of overdispersion in the data, we set the scale parameter to the Pearson χ2 statistic divided by the residual degrees of freedom.
Plots of rates over time revealed both a time trend and seasonal patterns. We accounted for the time trend by including a continuous variable denoting time at a certain month i, denoted as ti, as one of the covariates. To model seasonality, we included the following transformations of time: sin(2πt/T) and cos(2πt/T) with values T = 3, 6 and 12.28 To model the effect of rainfall and food security, we first plotted mortality rates against the following transformations of rainfall and food security: raw values, values lagged by 1 month, values lagged by 2 months, moving averages of the current and previous month and moving average of the current and 2 previous months. The effect of rainfall was estimated for every 500 mm in mean total monthly rainfall and the effect of food security for every 500 kg increase in household food security. Since plots showed approximately linear relationships with the log-transformed mortality rate (albeit with potential curvatures) we explored the effect of each variable in univariate regression models. Non-linear relationships were explored for each variable by introducing quadratic and cubic terms (variable exponentiated to the second and third power, respectively) in the regression. Interactions between rainfall and food security were tested, whereby the effect of rainfall was allowed to differ between months of higher food security (June–November) and months of lower food security (December–May).
Variables shown to have an effect on the mortality rates in the univariate analysis (Wald test probability values <0.05) were chosen as candidates for the multivariate model. If multiple transformations of the same variable were found to be associated with mortality rates, the variable with the strongest linear association (as estimated by the β parameter) in the univariate model was entered in the multivariate model, along with any significant quadratic or cubic effect. Collinearity was checked by calculating the correlation coefficient between the candidate variables to decide which variables to include in the multivariate model. In addition, the best fit pair of sine and cosine functions for seasonality were entered in the multivariate model (Wald test probability values of either function <0.05). The multivariate model was built by backward elimination of variables (Wald test probability values <0.2).
We performed various diagnostic tests to ensure that the model provided an adequate fit to the data. Serial autocorrelation of the residuals was checked by examining the autocorrelation function (ACF) plot and the partial autocorrelation (PACF) plot. In addition, a histogram of the residuals and scatterplots over time were examined. The goodness of fit of the models was tested using the Pearson’s χ2 test statistic.
Stage 2: Effect of malaria control interventions
In the second stage of analysis, we built upon the multivariate time series model above to estimate the effect of the various malaria control interventions. The effect of mosquito net ownership was estimated by including the annual variable denoting mosquito net ownership in the multivariate Model 1. The variable denoting time in months (t) was not included in the model. The effects of the other health interventions (change in treatment policy, IMCI intervention and ACCESS interventions) were estimated by means of a segmented regression analysis, a statistical method developed to estimate intervention effects in interrupted time series.29 In segmented regression analysis each segment of a series before and after the start of an intervention is allowed to exhibit both a level and a trend. The level is the value of the series at the beginning of a given time interval. The trend is the rate of change of a measure (i.e. the slope) during a segment. The model to estimate the effect of a single intervention is defined as the following extension to Model 1:
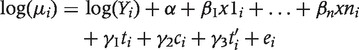 |
(Model 2) |
where ti is now explicitly stated in the model as denoting time in months from January 1997, c is an indicator variable in a given month i occurring before (c = 0) or after (c = 1) the malaria control intervention and t’i denotes time after the intervention, a continuous variable counting the number of months after the intervention in a given month i. In this model, γ1 estimates the change in the mean mortality rate per month before the implementation of a given intervention, γ2 estimates the level of change in the mean monthly rates after the intervention and γ3 estimates the change in trend in the mean mortality rate after the intervention. The effect of each intervention was estimated in separate models. In addition, a model taking all interventions into account simultaneously was also fitted. The goodness of fit of the models was re-tested using the Pearson’s χ2 test statistic.
Results
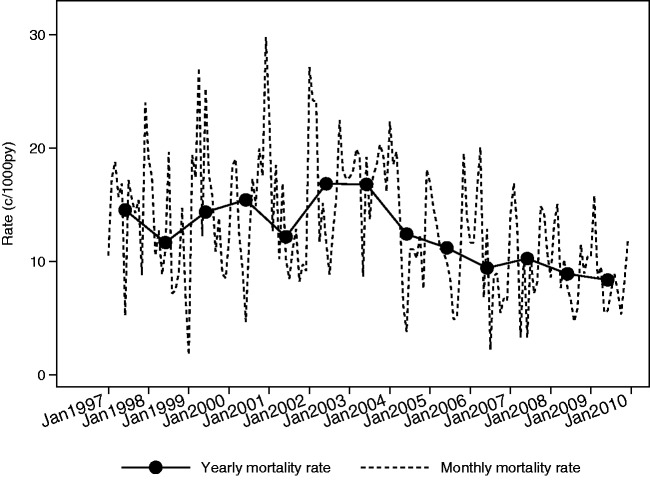
Between 1997 and 2009, child mortality rates decreased by 42.5% from 14.6 c/1000py to 8.4 c/1000py (Table 1 and Figure 3). Two peaks in mortality (in 2000 and 2003) coincided with below average rainfall and low agricultural yield (Figure 4). Average child mortality rates exhibited strong intra-annual variation between 1997 and 2009. Child mortality rates were lower between June and September, which corresponds to the months with lower rainfall and higher food security (Table 2).
Table 1.
Child mortality rates by year between 1997 and 2009
| Year | Cases (c) | Person-years (py) | Rate (c/1000py) |
|---|---|---|---|
| 1997 | 103 | 7075.8 | 14.6 |
| 1998 | 80 | 6848.0 | 11.7 |
| 1999 | 100 | 6958.2 | 14.4 |
| 2000 | 122 | 7901.5 | 15.4 |
| 2001 | 106 | 8699.3 | 12.2 |
| 2002 | 162 | 9607.2 | 16.9 |
| 2003 | 163 | 9695.4 | 16.8 |
| 2004 | 123 | 9889.5 | 12.4 |
| 2005 | 113 | 10 076.1 | 11.2 |
| 2006 | 102 | 10 806.0 | 9.4 |
| 2007 | 120 | 11 681.8 | 10.3 |
| 2008 | 115 | 12 889.2 | 8.9 |
| 2009 | 112 | 13 355.7 | 8.4 |
Figure 3.
Child mortality rates in the Kilombero and Ulanga HDSS areas between 1997 and 2009
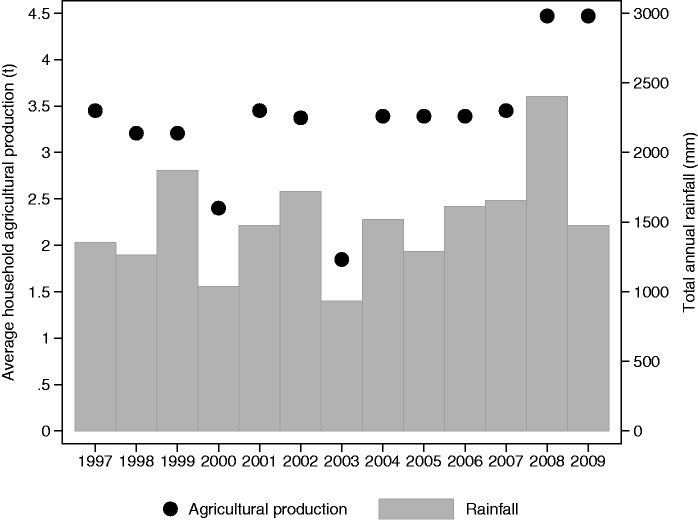
Figure 4.
Annual rainfall and agricultural production in the Kilombero district between 1997 and 2009
Table 2.
Child mortality, rainfall and estimated food security by month between 1997 and 2009
| Month | Child mortality rate (c/1000py) | Total rainfall (mm) median (IQR) | Household food security (kg) median (IQR) |
|---|---|---|---|
| Jan | 11.0 (38/3.463) | 215.1 (110.0) | 525 (40) |
| Feb | 12.0 (40/ 3.089) | 203.0 (136.1) | 360 (30) |
| Mar | 13.9 (47/3.379) | 307.2 (228.5) | 730 (75) |
| Apr | 12.3 (40/3.255) | 380.4 (289.0) | 570 (65) |
| May | 10.7 (36/3.375) | 83.9 (88.5) | 980 (70) |
| Jun | 8.9 (29/3.245) | 14.1 (22.3) | 1810 (130) |
| Jul | 9.5 (31/3.263) | 1.1 (6.0) | 1860 (130) |
| Aug | 9.4 (31/3.282) | 1.1 (24.8) | 1520 (110) |
| Sep | 7.9 (25/3.148) | 0.2 (5.5) | 1260 (90) |
| Oct | 12.4 (40/3.216) | 1.2 (14) | 1060 (80) |
| Nov | 7.7 (24/3.098) | 27.6 (80) | 870 (60) |
| Dec | 11.1 (36/3.229) | 159.1 (206.8) | 690 (40) |
Over the 13 years’ study period, the mean annual rice yield was 1.86 t/ha (SD = 0.400) and the mean annual maize yield was 2.32 t/ha (SD = 0.446), which corresponds to an estimated annual household agricultural production of 2.79 t of rice (SD = 0.600) and 0.56 t of maize (SD = 0.107). Agricultural production varied across the years but there was no secular trend (Figure 3). According to our proxy measure, household food security was at its maximum in June after the harvest and decreased constantly thereafter, reaching its minimum in January and February (Table 2). The rainy season was shown to last from November to May with peaks in March and April, but there was strong year on year variability in monthly rainfall (Table 2). Annual agricultural production and rainfall were highly positively correlated (r = 0.718, P < 0.0001), with years of high production coinciding with years of high rainfall (Figure 4). However, the correlation between monthly values was lower, and negative, given that crops are usually harvested in the dry season (r = −0.528, P < 0.0001).
Effect of rainfall and food security
Univariate analyses showed evidence of a decrease in mortality rates over time, and a seasonal pattern over 12 months (Table 3). Rainfall in the same month and the moving average of rainfall in the current and previous month were shown to be strong risk factors and there was a slight but not significant evidence of a quadratic relationship (data not shown). There was no significant association with rainfall lagged by 1 or 2 months, but the corresponding moving averages did emerge as risk factors. Food security at all lags and all its moving average transformations had a protective effect. The variables considered as candidates for the multivariate model were: time in months since January 1997 (t), sin(2πt/12), cos(2πt/12), rainfall and food security. Rainfall in the same month was chosen over the moving average of the current and previous month, with which it is highly correlated, as it showed a stronger effect in the univariate analysis. Food security in the same month was chosen over the other food security variables to minimize collinearity as it was less correlated with rainfall (r = −0.528, P < 0.0001). There was no evidence of an interaction between rainfall and food security.
Table 3.
Estimated effect of rainfall and food security on child mortality rates (Model 1)
| Univariate model |
Multivariate model | ||||
|---|---|---|---|---|---|
| (n = 156) |
|||||
| n | IRR (95% CI) | P-value | IRR (95% CI) | P-value | |
| Time in months since January 1997 (t) | 156 | 0.996 (0.994 to 0.997) | <0.001 | 0.996 (0.995 to 0.998) | <0.001 |
| Sin(2πt/12) | 156 | 1.021 (0.927 to 1.125) | 0.670 | 0.916 (0.835 to 1.001) | 0.066 |
| Cos(2πt/12) | 156 | 1.269 (1.158 to 1.390) | <0.001 | ||
| Rainfall | 156 | 1.470 (1.122 to 1.774) | <0.001 | 1.364 (1.087 to 1.712) | 0.007 |
| Rainfall Lag 1 | 155 | 1.093 (0.887 to 1.348) | 0.404 | ||
| Rainfall Lag 2 | 154 | 0.921 (0.740 to 1.146) | 0.460 | ||
| Rainfall MA(2) | 156 | 1.363 (1.089 to 1.708) | 0.007 | ||
| Rainfall MA(3) | 156 | 1.244 (0.962 to 1.612) | 0.098 | ||
| Food security | 156 | 0.826 (0.779 to 0.875) | <0.001 | 0.885 (0.830 to 0.944) | <0.001 |
| Food security Lag 1 | 155 | 0.853 (0.804 to 0.904) | <0.001 | ||
| Food security Lag 2 | 154 | 0.891 (0.839 to 0.945) | <0.001 | ||
| Food security MA(2) | 156 | 0.812 (0.763 to 0.865) | <0.001 | ||
| Food security MA(3) | 156 | 0.805 (0.753 to 0.861) | <0.001 | ||
IRR, incidence rate ratio.
The effect of rain was estimated for every 500-mm increase in mean total monthly rainfall, and the effect of food security for every 500-kg increase in household food security.
Lag(x) refers to the value of the variable lagged by x months; MA(2) refers to the moving average of the variable in the given month and the month before; MA(3) refers to the moving average of the variable in the given month and the previous 2 months.
The final multivariate model estimated a 0.4% decrease in child mortality rates per month, equivalent to a 5% decrease per year (Table 3). Although the cosine function best captured seasonality in the univariate model, the sine function remained the only predictor of seasonality in the multivariate model, albeit only borderline significant. Rainfall in the same month emerged as a statistically significant independent risk factor for child mortality, increasing rates by 36.4% for every 500-mm increase in rain. Food security, on the other hand, had a significant independent protective effect, with an estimated 11.5% decrease in child mortality rates for every 500 kg increase in household food security.
The residuals were approximately normally distributed and there was no evidence of autocorrelation. The Pearson’s goodness of fit test was statistically significant (χ2 = 189, P = 0.019) suggesting that that there is still unexplained variance in mortality not accounted for by the factors in the model.
Effect of malaria control interventions
In the segmented regression analyses, all malaria control interventions were associated with decreases in child mortality (Table 4). There was an estimated 5% decrease in mortality per year for every 10% increase in mosquito net ownership. In the three regression models fitted for the introduction of SP (August 2001), the introduction of IMCI (April 2002) and the start of the ACCESS programme (November 2004), there was no evidence of a trend prior to the implementation of each intervention, but there was evidence of a significant change in trend after each intervention was implemented. The introduction of SP as a first-line treatment for malaria was associated with 0.8% significant change in trend per month, the introduction of IMCI was associated with a 0.9% significant change in trend and the start of the ACCESS interventions was associated with a 0.6% significant change in trend per month. In addition, the start of the ACCESS interventions was also associated with a 23.4% decrease in level of mortality rates. In the model fitted to estimate the effect of ALu introduction (January 2007), there was evidence of a pre-intervention decreasing trend. The introduction of ALu was associated with both a change in level and a change in trend, but these were not statistically significant. In the model estimating the effect of all interventions simultaneously, none of the interventions were significant (results not shown).
Table 4.
Estimated effect of individual malaria control interventions on child mortality rates (Model 2) [n = 156]
| IRR (95% CI) | P-value | |
|---|---|---|
| ITN ownership | ||
| Effect of every 10% increase per year | 0.950 (0.917 to 0.984) | 0.004 |
| Introduction of SP (August 2001) | ||
| Trend before the intervention | 1.001 (0.995 to 1.007) | 0.766 |
| Change in level | 1.169 (0.942 to 1.449) | 0.156 |
| Change in trend | 0.992 (0.986 to 0.998) | 0.009 |
| Introduction of IMCI (April 2002) | ||
| Trend before the intervention | 1.001 (0.996 to 1.005) | 0.764 |
| Change in level | 1.179 (0.960 to 1.449) | 0.116 |
| Change in trend | 0.991 (0.986 to 0.996) | 0.001 |
| Start of ACCESS programme (November 2004) | ||
| Trend before the intervention | 1.000 (0.998 to 1.003) | 0.502 |
| Change in level | 0.766 (0.619 to 0.948) | 0.014 |
| Change in trend | 0.994 (0.988 to 0.999) | 0.031 |
| Introduction of ALu (January 2007) | ||
| Trend before the intervention | 0.997 (0.996 to 0.999) | 0.010 |
| Change in level | 0.876 (0.672 to 1.141) | 0.326 |
| Change in trend | 0.998 (0.986 to 1.010) | 0.692 |
IRR, incidence rate ratio.
The effects of the interventions are estimated in separate models. The effect of each intervention is adjusted for Sin(2πt/12), rainfall and food security.
The Pearson’s goodness of fit test for the model estimating the effect of mosquito nets was highly statistically significant (χ2 = 213, P < 0.001), but it was not significant for the models estimating the effect of the other interventions (SP: χ2 = 163, P = 0.199; IMCI: χ2 = 161, P = 0.232; ACCESS: χ2 = 163, P = 0.204; ALu: χ2 = 176, P = 0.066). There was only a negligible difference in the estimated effect of rainfall and food security when fitting Model 1 compared with Model 2 (data not shown).
Discussion
Between 1997 and 2009, child mortality decreased by 42.5% in the Kilombero and Ulanga HDSS. Our time series modelling suggests that rainfall is a strong risk factor of child mortality whereas household food security has a strong protective effect. The increase in mosquito net coverage was significantly associated with decreases in mortality. The segmented regression analyses suggest that the other malaria control interventions implemented in the study area were also associated with statistically significant decreases in child mortality. The switch to SP, the introduction of IMCI and the start of the ACCESS interventions had effects on the trend above and beyond decreasing pre-intervention secular trends. In addition, the ACCESS interventions were associated with a decrease in the level of the mortality rates. Although we did observe a change in both trend and level after the introduction of ALu, neither were statistically significant (at a 5% level).
Although the endpoint of these analyses was all-cause child mortality, a concurrent decrease in malaria morbidity and transmission indicators in the same area provide an independent argument that the decrease in child mortality is at least in part driven by a decrease in the malaria burden. The incidence of fever in all ages in the area decreased from 47 cases per 1000 weeks (c/1000pw) in 2005 to 34 c/1000pw in 2008 and the incidence of convulsions in young children decreased from 4263 to 2320 cases for every 100 000 children per year.30 An older study reported an entomological inoculation rate (EIR) of 349 infective bites per person per year (ib/p/y) between 2001 and 2003,21 but by 2008 it had declined to 81 ib/p/y.22 Similarly, data from two other projects documented a decrease in parasitaemia in children under the age of 5 years from 25% in 200431,32 to 10% in 2008.33 However, it is important to acknowledge that this decrease had started already in the mid 90ss when an EIR of up to 1400 ib/p/y was recorded34 and parasitaemia in infants and children under the age of 2 years was over 60%.35,36
Any attribution of the observed mortality decreases to specific interventions should be interpreted with caution, given the observational nature of our study, the multiple potential drivers of child mortality and the lack of yearly data documenting the coverage of malaria control interventions. Observational study designs are often the only feasible way to assess the impact of health interventions at population level, but they do not provide a strong argument for causality. The main concern is that there may be other secular factors explaining the observed decrease in mortality which were neither identified nor measured and hence that could not be factored into the analyses. For example, between 1999 and 2004 there were important improvements in Tanzania's health system, including: doubled public expenditure on health; decentralization and sector-wide basket funding; and increased vitamin A supplementation, immunization and exclusive breastfeeding.37 Unfortunately annual serial data on these changes were not available from HDSS household surveys nor from other surveys such as the Tanzanian Household Budget Survey,38 the Demographic and Health Survey39 and the Malaria Indicator Surveys.40 Similarly, verbal autopsy data collected by the HDSS on malaria-related mortality were not available for all the years between 1997 and 2009.
Furthermore, although the application of segmented regression analysis of interrupted times series is a validated approach to evaluate the effect of health interventions,29,41 it is not without limitations. In this study we were unable to estimate the effect of all interventions simultaneously, which would ensure that effect of each intervention would be corrected for the effect of the previous interventions. Indeed, the model fitted with all the variables necessary for that (all ci, and t’i variables) gave inconclusive results since none of the interventions had a significant effect any more. A possible reason may be the high collinearity between the various time variables and the resulting model instability. Despite this limitation, it is important to bear in mind that the effect of each intervention, as reported here, assesses the change in trend adjusted for prior trend, and thus to a certain extent does take prior interventions (or better, their effect on the time trend) into account. However, a more general limitation about time series analyses for biological data is that methodologies to deal with the presence of potential unmeasured time-varying confounders, to model harvesting and delayed non-linear effects, are still in development and typically require a larger time series of observations than was available for this study. The fact that the Pearson’s goodness of fit test of some of our models was significant confirms that the models we fitted did not capture all the variability in the data.
The results of our analyses should therefore be interpreted as part as the existing body of evidence on malaria control.42 The 5% decrease in mortality observed for every 10% increase in mosquito net ownership is in line with Cochrane review on ITNs which documented a nearly 20% reduction in child mortality at high ITN use.43 The impact of the change of treatment policy from chloroquine to SP is credible given the much higher treatment efficacy of SP upon its introduction14 and the high resistance to chloroquine before it was replaced.16 Likewise the efficacy of the IMCI interventions has been extensively documented.8,9 As far as ACCESS is concerned, two studies suggest that the combination of social marketing campaigns and the roll-out of ADDOs resulted in improved availability and accessibility of malaria treatment24 as well as very high treatment outcomes.18 Although the lack of impact of the introduction of ALu is surprising given the much higher treatment efficacies of ALu upon its introduction15 and the high resistance to and SP before it was replaced,17 it may be explained by the low access to ALu in the years following the change in treatment policy.18 An alternative explanation is that analyses based on only 2 years post-intervention may not have sufficient power to show an effect.
Our time series analyses confirmed that child mortality patterns are complex and multifactorial. Rainfall (in the same month) and household food security (in the same month) were both found to be independent predictors of child mortality. Various studies have linked intra-annual differences in malaria to rainfall patterns.22,44–47 A 1 month lag is considered the absolute minimum for a biologically plausible link between creating suitable breeding conditions for mosquito development and the onset of disease symptoms in humans.45,47 However, we could not observe an independent effect of rainfall lagged by 1 or 2 months (or moving averages including these values) on child mortality despite the fact that, according to verbal autopsies conducted in the HDSS, confirmed malaria is the leading cause of under-five mortality.48,49 This unexpected finding may be the result of some un-measured confounder but it is also possible that rainfall alone does not adequately capture variations in malaria transmission in our study setting.
The fact that child deaths are more likely to occur in months of high rainfall and low food security rather than in months of high malaria risk, despite malaria being a leading cause of mortality, can be argued in different ways. On one hand, low food security and high rainfall are risk factors for what are considered to be the two most important causes of mortality in the study area after malaria, namely acute respiratory infections and anaemia.50–53 On the other hand, it cannot be excluded that malaria deaths also occur in months of high rainfall and low food security. Children who are malnourished are more susceptible to malaria morbidity and mortality54,55 and, in a cash crop society, household food security is strongly related to the ability to mobilize resources to afford treatment.56 In addition, excess rainfall is known to create considerable flooding which limits access to care and increases the risk of diarrhoea, which in turn makes children weaker and more prone to succumb to deadly diseases such as malaria.57,58
Conclusions
Between 1997 and 2009, childhood mortality decreased substantially in the Kilombero and Ulanga Districts. Our analyses support the existing body of evidence suggesting that malaria control interventions contribute to substantial decreases in child mortality and are essential to reach the fourth Millenium Development Goal. Given the observational nature of our study, it is difficult to quantify the individual contribution of any given malaria control intervention, as well as the contribution of the other major determinants of child mortality (mainly environmental and nutritional, but also more generally socio-economic). This represents a major challenge when attempting to assess the effect of routine interventions as well as the cost effectiveness of public health programmes and other development interventions. However, this study suggests that credible estimates can be obtained when high-quality data on the most important factors are available over a sufficiently long time period.
Funding
All authors participated in the implementation or monitoring and evaluation activities of the ACCESS Programme, which was funded by the Novartis Foundation for Sustainable Development (NFSD). The Foundation works independently from the company’s business and supports not-for-profit health programmes in developing countries. A.S. is employed by NFSD and contributed to the study design, decision to publish and preparation of the manuscript.
Acknowledgements
This article is dedicated to the memory of our colleague Mathew Alexander, whose hard work and good spirits, as well as a strong commitment to the Ifakara HDSS, made it possible for us to have these data and to conduct all other ACCESS-related surveys. We thank the communities of the Kilombero and Ulanga districts for their time and patient collaboration, and for providing informed consent allowing us to use their data for research. We are also very grateful to the staff of the Kilombero DALDO and KATRIN for providing agricultural production and rainfall data, respectively. Special thanks to Tom Smith, Blaise Genton, Ente Rood, Neva Coello, Federica Giardina, Manuel Hetzel, Iddy Mayumana and Ashley Warren and the three anonymous IJE reviewers for the various inputs provided during data collection, analysis or manuscript preparation/revision. S.A. analysed the data, drafted and finalized the manuscript. R.N. headed the Ifakara HDSS and oversaw the collection and entry of all HDSS-related data between 1996 and 2008, contributed to the study design and reviewed the manuscript. C.L. conceptualized the study design and contributed to the data analysis as well as to the writing of the manuscript. A.S. and H.M. contributed to the design of the study and the interpretation of findings. The final manuscript was approved by all authors.
Conflict of interest: None declared.
KEY MESSAGES.
Between 1997 and 2009, a number of key malaria control interventions were implemented in the Kilombero and Ulanga Districts in south central Tanzania.
Child mortality decreased by 42.5% from 14.6 c/1000py in 1997 to 8.4 c/1000py in 2009.
All malaria control interventions were associated with decreases in child mortality, accounting for the effect of rainfall and food security.
Reaching the fourth Millenium Development Goal will require the contribution of many health interventions, as well as more general improvements in socio-environmental and nutritional conditions.
References
- 1.United Nations. The Millenium Development Goals Report 2011. New York: UNO; 2011. [Google Scholar]
- 2.World Health Organization. World Malaria Report 2011. Geneva: WHO; 2011. [Google Scholar]
- 3.Roll Back Malaria. The African Summit on Roll Back Malaria. Geneva: WHO, 2000. [Google Scholar]
- 4.Roll Back Malaria. The Global Malaria Action Plan. Geneva: WHO; 2008. [Google Scholar]
- 5.Armstrong Schellenberg JRM, Abdulla S, Minja H, et al. KINET: a social marketing programme of treated nets and net treatment for malaria control in Tanzania, with evaluation of child health and long-term survival. Trans R Soci TropMed Hyg. 1999;93:225–31. doi: 10.1016/s0035-9203(99)90001-9. [DOI] [PubMed] [Google Scholar]
- 6.Armstrong Schellenberg JRM, Abdulla S, Nathan R, et al. 2001) Effect of large-scale social marketing of insecticide-treated nets on child survival in rural Tanzania. Lancet. 2001;357:1241–47. doi: 10.1016/S0140-6736(00)04404-4. [DOI] [PubMed] [Google Scholar]
- 7.Magesa SM, Lengeler C, deSavigny D, et al. Creating an “enabling environment” for taking insecticide treated nets to national scale: the Tanzanian experience. Malar J. 2005;4:34. doi: 10.1186/1475-2875-4-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Armstrong Schellenberg JRM, Adam T, Mshinda H, et al. Effectiveness and cost of facility-based Integrated Management of Childhood Illness (IMCI) in Tanzania. Lancet. 2004;364:1583–94. doi: 10.1016/S0140-6736(04)17311-X. [DOI] [PubMed] [Google Scholar]
- 9.Tanzania IMCI Multi-Country Evaluation Health Facility Survey Study Group. The effect of Integrated Management of Childhood Illness on observed quality of care of under-fives in rural Tanzania. Health Pol Plan. 2004;19:1–10. doi: 10.1093/heapol/czh001. [DOI] [PubMed] [Google Scholar]
- 10.Hetzel MW, Iteba N, Makemba A, et al. Understanding and improving access to prompt and effective malaria treatment and care in rural Tanzania: the ACCESS Programme. Malar J. 2007;6:83. doi: 10.1186/1475-2875-6-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rutta Edmund, Senauer Katie, Johnson Keith, et al. Creating a New Class of Pharmaceutical Services Provider for Underserved Areas: The Tanzania Accredited Drug Dispensing Outlet Experience. Progress in Community Health Partnerships. Res Edu Action. 2009;3:145–53. doi: 10.1353/cpr.0.0063. [DOI] [PubMed] [Google Scholar]
- 12.Rutta E, Kibassa B, McKinnon B, et al. Increasing Access to Subsidized Artemisinin-based Combination Therapy through Accredited Drug Dispensing Outlets in Tanzania. Health Res Policy Syst. 2011;9:22. doi: 10.1186/1478-4505-9-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alba S. An evaluation of integrated interventions to improve access to malaria treatment in Tanzania (ACCESS programme) PhD Thesis. Swiss Tropical Institute, University of Basel, Switzerland, 2010. [Google Scholar]
- 14.Mugittu K, Ndejembi M, Malisa A, et al. Therapeutic efficacy of sulfadoxine-pyrimethamine and prevalence of resistance markers in Tanzania prior to revision of malaria treatment policy: Plasmodium falciparum dihydrofolate reductase and dihydropteroate synthase mutations in monitoring in vivo resistance. Am J Trop Med Hyg. 2004;71:696–702. [PubMed] [Google Scholar]
- 15.Kabanywanyi AM, Mwita A, Sumari D, et al. Efficacy and safety of artemisinin-based antimalarial in the treatment of uncomplicated malaria in children in southern Tanzania. Malar J. 2007;6:146. doi: 10.1186/1475-2875-6-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hatz C, Abdulla S, Mull R, et al. Efficacy and safety of CGP 56697 (artemether and benflumetol) compared with chloroquine to treat acute falciparum malaria in Tanzanian children aged 1-5 years. Trop Med Int Health. 1998;3:498–504. doi: 10.1046/j.1365-3156.1998.00250.x. [DOI] [PubMed] [Google Scholar]
- 17.Mugittu K, Abdulla S, Falk N, et al. Efficacy of sulfadoxine-pyrimethamine in Tanzania after two years as first-line for uncomplicated malaria: assessment protocol and implication for treatment policy strategies. Malar J. 2005;4:55. doi: 10.1186/1475-2875-4-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alba S, Dillip A, Hetzel M, et al. Improvements in access to malaria treatment in Tanzania following community, retail sector and health facility interventions – a user perspective. Malar. 2010;9:163. doi: 10.1186/1475-2875-9-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Network I. Population and Health in Developing Countries. Vol 1. Population, Health, and Survival at INDEPTH Sites. Ottawa: International Development Research Centre; 2002. [Google Scholar]
- 20.Armstrong Schellenberg J, Mukasa O, et al. In: Population and Health in Developing Countries. Ifakara DSS, Tanzania. Vol 1: Population, Health, and Survival in INDEPTH Sites. Ottawa: International Development Research Centre, 2002. [Google Scholar]
- 21.Killeen GF, Tami A, Kihonda J, et al. Cost-sharing strategies combining targeted public subsidies with private-sector delivery achieve high bednet coverage and reduced malaria transmission in Kilombero Valley, southern Tanzania. BMC Infect Dis. 2007;7:121. doi: 10.1186/1471-2334-7-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Russell T, Lwetoijera D, Maliti D, et al. Impact of promoting longer-lasting insecticide treatment of bed nets upon malaria transmission in a rural Tanzanian setting with pre-existing high coverage of untreated nets. Malar J. 2010;9:187. doi: 10.1186/1475-2875-9-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mulligan J-A, Mandike R, Palmer N, et al. The costs of changing national policy: lessons from malaria treatment policy guidelines in Tanzania. Trop Med Int Health. 2006;11:452–61. doi: 10.1111/j.1365-3156.2006.01590.x. [DOI] [PubMed] [Google Scholar]
- 24.Alba S, Hetzel M, Goodman C, et al. Improvements in access to malaria treatment in Tanzania after switch to artemisinin combination therapy and the introduction of accredited drug dispensing outlets - a provider perspective. Malar J. 2010;9:164. doi: 10.1186/1475-2875-9-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mbapila JC. Crop Protection Programme – Development and Promotion of Wild Rice Management Strategy for the Lowlands of Southern Tanzania. Ifakara, Tanzania: Ministry of Agriculture and Food Security, Kilombero Agricultural Training and Research Institute (KATRIN); 2005. [Google Scholar]
- 26.Kato F. Development of a major rice cultivation area in the Kilombero Valley, Tanzania. Afr Stud Monog Suppl. 2007;36:3–18. [Google Scholar]
- 27.Ashimogo GC, Isinika AC, Mlangwa JED. Africa in Transition – Micro Study Tanzania Research Report. Lund, Sweden: Lund University; 2003. [Google Scholar]
- 28.Stolwijk AM, Straatman H, Zielhuis GA. Studying seasonality by using sine and cosine functions in regression analysis. J Epidemiol Community Health. 1999;53:235–38. doi: 10.1136/jech.53.4.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wagner AK, Soumerai SB, Zhang F, Ross-Degnan D. Segmented regression analysis of interrupted time series studies in medication use research. J Clin Pharm Ther. 2002;27:299–309. doi: 10.1046/j.1365-2710.2002.00430.x. [DOI] [PubMed] [Google Scholar]
- 30.Alba S, Hetzel MW, Nathan R, Alexander M, Lengeler C. Assessing the impact of malaria interventions on morbidity through a community-based surveillance system. Int J Epidemiol. 2011;40:405–16. doi: 10.1093/ije/dyq240. [DOI] [PubMed] [Google Scholar]
- 31.Khatib R. Malaria control dynamics in rural Tanzania: evaluation of implementation of artemisinin based antimalarial combination therapy. PhD Thesis. Swiss Tropical Institute, University of Basel, Switzerland, 2009. [Google Scholar]
- 32.Khatib RA, Skarbinski J, Njau JD, et al. Routine delivery of artemisinin-based combination treatment at fixed health facilities reduces malaria prevalence in Tanzania: an observational study. Malar J. 2012;11:140. doi: 10.1186/1475-2875-11-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mulokozi A. Pharmaco-epidemiology of artemisinin-based combination therapy in the context of impact evaluation of artemether-lumefantrine on malaria morbidity and mortality during programmatic implementation in rural Tanzania. PhD Thesis. Swiss Tropical Institute, University of Basel, Switzerland, 2010. [Google Scholar]
- 34.Smith T, Charlwood JD, Kihonda J, et al. Absence of seasonal variation in malaria parasitaemia in an area of intense seasonal transmission. Acta Trop. 1993;54:55–72. doi: 10.1016/0001-706x(93)90068-m. [DOI] [PubMed] [Google Scholar]
- 35.Kitua AY, Smith T, Alonso PL, et al. Plasmodium falciparum malaria in the first year of life in an area of intense and perennial transmission. Trop Med Int Health. 1996;1:475–84. doi: 10.1046/j.1365-3156.1996.d01-89.x. [DOI] [PubMed] [Google Scholar]
- 36.Abdulla S, Schellenberg JA, Nathan R, et al. Impact on malaria morbidity of a programme supplying insecticide treated nets in children aged under 2 years in Tanzania: community cross sectional study. BMJ. 2001;322:270–73. doi: 10.1136/bmj.322.7281.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Masanja H, De Savigny D, Smithson P, et al. Child survival gains in Tanzania: analysis of data from demographic and health surveys. Lancet. 2008;371:1276–83. doi: 10.1016/S0140-6736(08)60562-0. [DOI] [PubMed] [Google Scholar]
- 38.National Bureau of Statistics. 2000/01 Tanzanian Household Budget Survey. Dar es Salaam, Tanzania: NBS; 2002. [Google Scholar]
- 39.National Bureau of Statistics, Dar es Salaam, Tanzania and ORC Macro, Calverton, MDd. Tanzania Demographic and Health Survey 2004-2005. Dar es Salaam, Tanzania: NBS; 2005. [Google Scholar]
- 40.Tanzania Commission for AIDS, National Bureau of Statistics, Tanzania and ORC Macro. Tanzania HIV/AIDS Indicator Survey 2003-04. Calverton, MD: ORC Macro; 2005. 2005. [Google Scholar]
- 41.Ramsay CR, Matowe L, Grilli R, Grimshaw JM, Thomas RE. Interrupted time series designs in health technology assessment: lessons from two systematic reviews of behavior change strategies. Int J Technol Assess Health Care. 2003;19:613–23. doi: 10.1017/s0266462303000576. [DOI] [PubMed] [Google Scholar]
- 42.Habicht JP, Victora CG, Vaughan JP. Evaluation designs for adequacy, plausibility and probability of public health programme performance and impact. Int Epidemiol. 1999;28:10–18. doi: 10.1093/ije/28.1.10. [DOI] [PubMed] [Google Scholar]
- 43.Lengeler C. Insecticide-treated bednets and curtains for preventing malaria. Cochrane Database of Systematic Reviews. 2000:CD000363. doi: 10.1002/14651858.CD000363. [DOI] [PubMed] [Google Scholar]
- 44.Craig MH, Kleinschmidt I, Nawn JB, Le Sueur D, Sharp BL. Exploring 30 years of malaria case data in KwaZulu-Natal, South Africa: part I. The impact of climatic factors. Trop Med Int Health. 2004;9:1247–57. doi: 10.1111/j.1365-3156.2004.01340.x. [DOI] [PubMed] [Google Scholar]
- 45.Briet O, Vounatsou P, Gunawardena D, Galappaththy G, Amerasinghe P. Temporal correlation between malaria and rainfall in Sri Lanka. Malar J. 2008;7:77. doi: 10.1186/1475-2875-7-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Worrall E, Connor SJ, Thomson MC. A model to simulate the impact of timing, coverage and transmission intensity on the effectiveness of indoor residual spraying (IRS) for malaria control. Trop Med Int Health. 2007;12:75–88. doi: 10.1111/j.1365-3156.2006.01772.x. [DOI] [PubMed] [Google Scholar]
- 47.Teklehaimanot H, Lipsitch M, Teklehaimanot A, Schwartz J. Weather-based prediction of Plasmodium falciparum malaria in epidemic-prone regions of Ethiopia I. Patterns of lagged weather effects reflect biological mechanisms. Malar J. 2004;3:41. doi: 10.1186/1475-2875-3-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ministry of Health and Social Welfare. District Health Services Profile 2005 for Kilombero and Ulanga Districts, Morogoro Region, Tanzania. Ifakara, Tanzania: Ministry of Health and Social Welfare; 2005. [Google Scholar]
- 49.Ministry of Health and Social Welfare. District Health Services Profile 2010 for Ulanga and Kilombero Districts, Morogoro Region, Tanzania. Ifakara, Tanzania: Ministry of Health and Social Welfare; 2010. [Google Scholar]
- 50.Armstrong JR, Campbell H. Indoor air pollution exposure and lower respiratory infections in young Gambian children. Int J Epidemiol. 1991;20:424–29. doi: 10.1093/ije/20.2.424. [DOI] [PubMed] [Google Scholar]
- 51.Armstrong Schellenberg JR, Nathan R, Abdulla S, Mukasa O, Marchant TJ, et al. Risk factors for child mortality in rural Tanzania. Tropical Medicine & International Health. 2002;7:506–11. doi: 10.1046/j.1365-3156.2002.00888.x. [DOI] [PubMed] [Google Scholar]
- 52.Azizi BH, Zulkifli HI, Kasim MS. Protective and risk factors for acute respiratory infections in hospitalized urban Malaysian children: a case control study. Southeast Asian J Trop Med Public Health. 1995;26:280–85. [PubMed] [Google Scholar]
- 53.Morris CR, Singer ST, Walters MC. Clinical hemoglobinopathies: iron, lungs and new blood. Curr Opin Hematol. 2006;13:407–18. doi: 10.1097/01.moh.0000245685.24462.4e. [DOI] [PubMed] [Google Scholar]
- 54.Caulfield LE, De Onis M, Blössner M, Black RE. Undernutrition as an underlying cause of child deaths associated with diarrhea, pneumonia, malaria, and measles. Am J Clin Nutr. 2004;80:193–98. doi: 10.1093/ajcn/80.1.193. [DOI] [PubMed] [Google Scholar]
- 55.Shankar AH. Nutritional modulation of malaria morbidity and mortality. J Infect Dis. 2000;182(Suppl 1):S37–53. doi: 10.1086/315906. [DOI] [PubMed] [Google Scholar]
- 56.Obrist B, Mayumana I, Kessy F. Livelihood, malaria and resilience. Prog Dev Stud. 2010;10:325–43. [Google Scholar]
- 57.Wang L, Kanji S, Bandyopadhyay S. The Health Impact of Extreme Weather Events in Sub-Saharan Africa. New York: World Bank; 2009. [Google Scholar]
- 58.Checkley W, Epstein LD, Gilman RH, Cabrera L, Black RE. Effects of Acute Diarrhea on Linear Growth in Peruvian Children. Am J Epidemiol. 2003;157:166–75. doi: 10.1093/aje/kwf179. [DOI] [PubMed] [Google Scholar]