Abstract
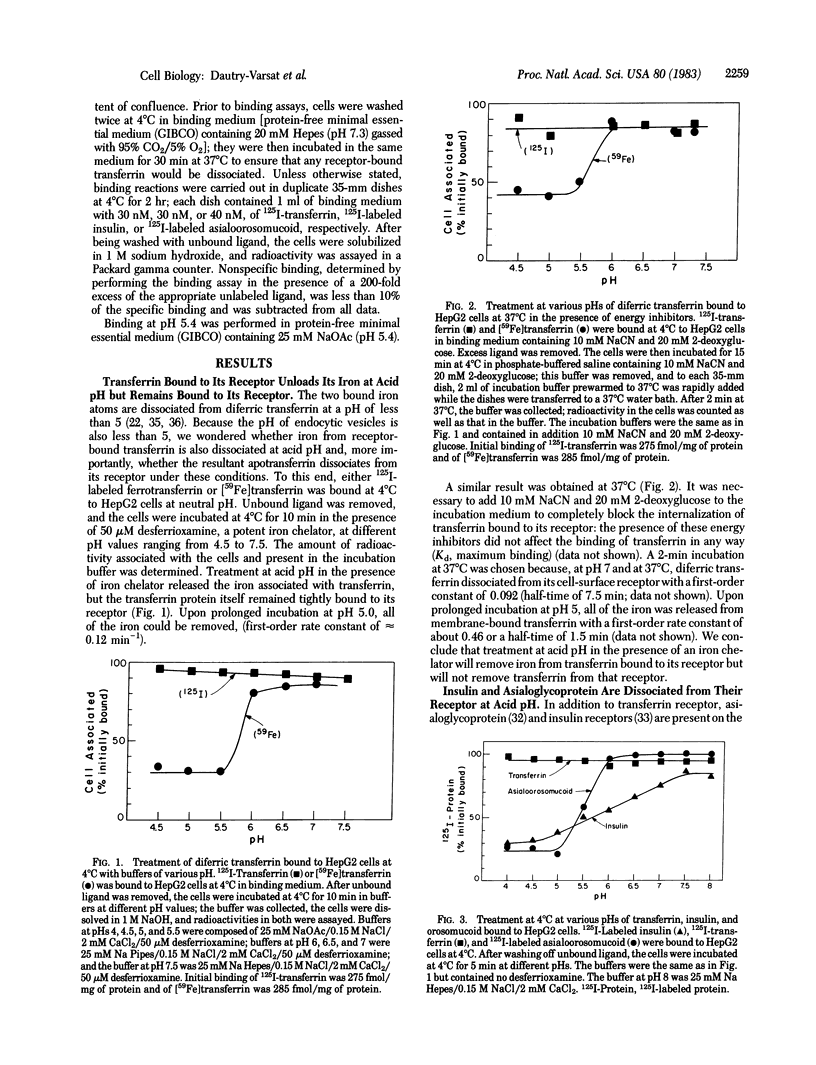
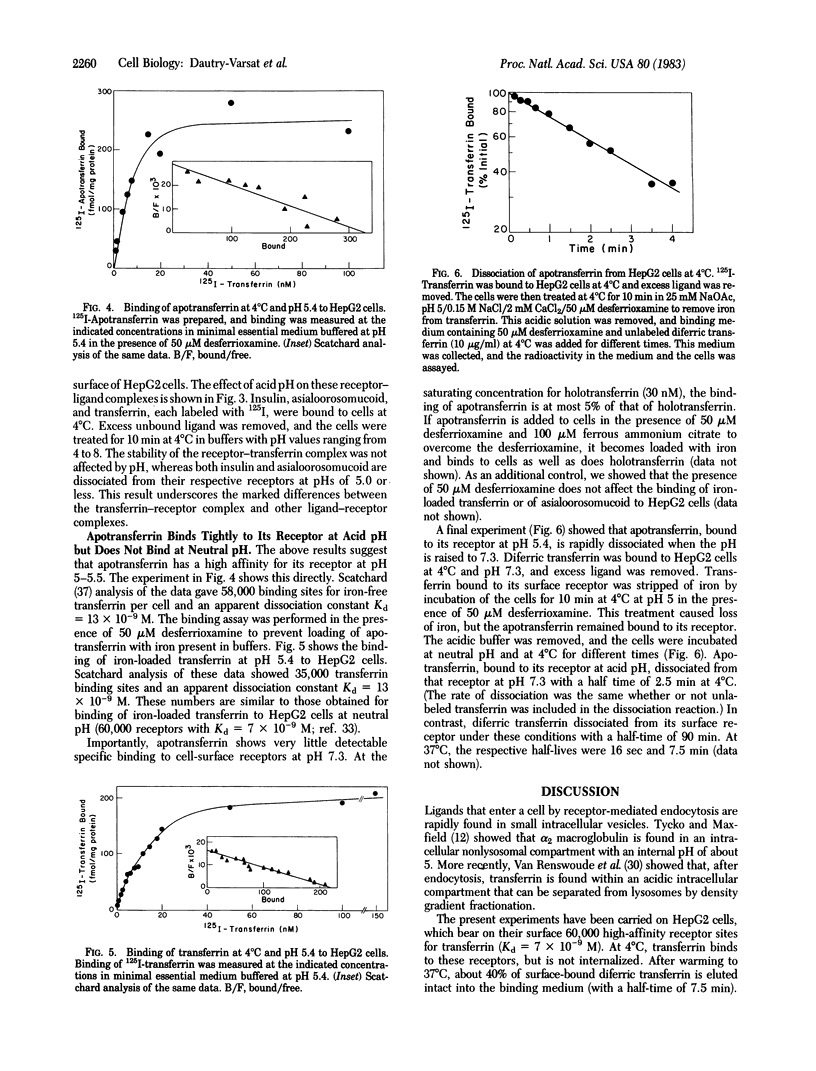
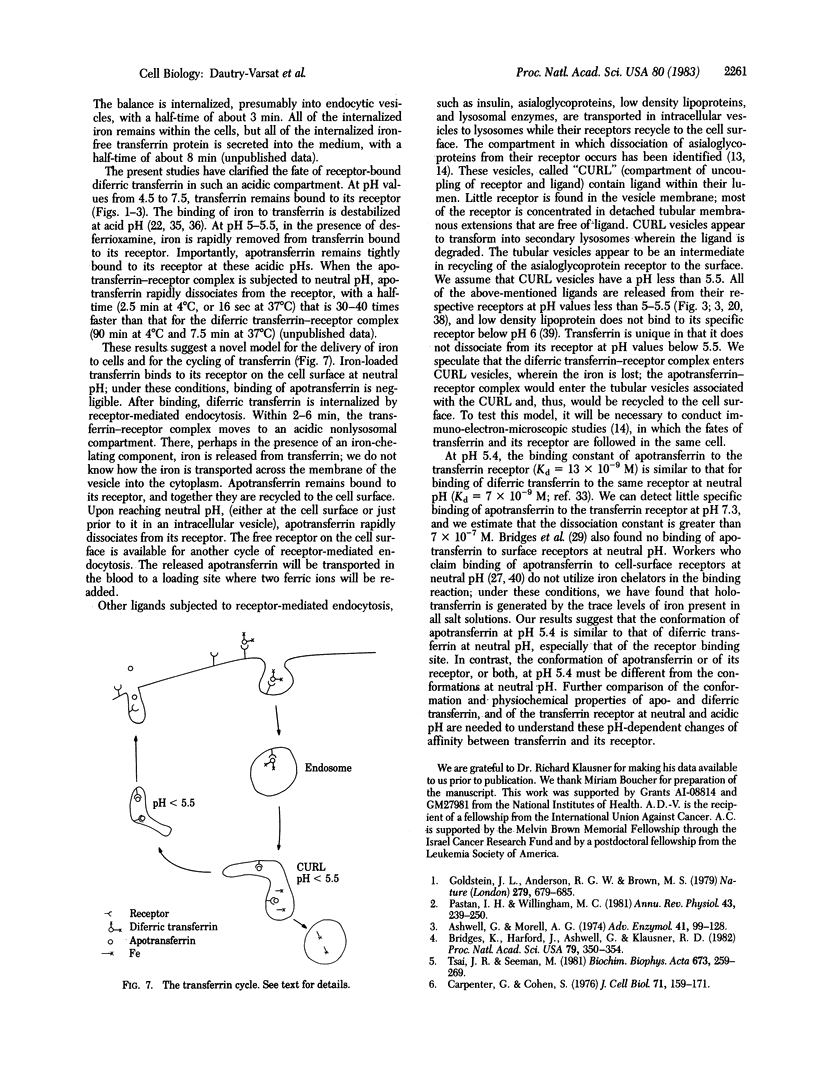
At pH 5.4 apotransferrin (iron-free transferrin) binds to cell-surface transferrin receptors to the same extent and with the same affinity as does diferric transferrin at pH 7.0. Apotransferrin is quickly dissociated from its receptor when the pH is raised to 7.0. These and other results strongly support a simple model that explains the cycling of transferrin during a single cycle of receptor-mediated endocytosis. Diferric transferrin binds to cell-surface receptors, and the transferrin-receptor complex is endocytosed. The pH of the endocytic vesicle is lowered to 5.5 or below; this causes dissociation of iron from the transferrin-receptor complex, but apotransferrin remains bound to its receptor. The iron remains within the cell, and the apotransferrin-receptor complex is recycled to the cell surface. Upon encountering the neutral pH of the medium, apotransferrin is dissociated from the cell.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ascoli M., Puett D. Degradation of receptor-bound human choriogonadotropin by murine Leydig tumor cells. J Biol Chem. 1978 Jul 25;253(14):4892–4899. [PubMed] [Google Scholar]
- Ashwell G., Morell A. G. The role of surface carbohydrates in the hepatic recognition and transport of circulating glycoproteins. Adv Enzymol Relat Areas Mol Biol. 1974;41(0):99–128. doi: 10.1002/9780470122860.ch3. [DOI] [PubMed] [Google Scholar]
- Basu S. K., Goldstein J. L., Brown M. S. Characterization of the low density lipoprotein receptor in membranes prepared from human fibroblasts. J Biol Chem. 1978 Jun 10;253(11):3852–3856. [PubMed] [Google Scholar]
- Bridges K., Harford J., Ashwell G., Klausner R. D. Fate of receptor and ligand during endocytosis of asialoglycoproteins by isolated hepatocytes. Proc Natl Acad Sci U S A. 1982 Jan;79(2):350–354. doi: 10.1073/pnas.79.2.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. S., Anderson R. G., Basu S. K., Goldstein J. L. Recycling of cell-surface receptors: observations from the LDL receptor system. Cold Spring Harb Symp Quant Biol. 1982;46(Pt 2):713–721. doi: 10.1101/sqb.1982.046.01.068. [DOI] [PubMed] [Google Scholar]
- Carpenter G., Cohen S. 125I-labeled human epidermal growth factor. Binding, internalization, and degradation in human fibroblasts. J Cell Biol. 1976 Oct;71(1):159–171. doi: 10.1083/jcb.71.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciechanover A., Schwartz A. L., Lodish H. F. The asialoglycoprotein receptor internalizes and recycles independently of the transferrin and insulin receptors. Cell. 1983 Jan;32(1):267–275. doi: 10.1016/0092-8674(83)90517-2. [DOI] [PubMed] [Google Scholar]
- Fehlmann M., Carpentier J. L., Van Obberghen E., Freychet P., Thamm P., Saunders D., Brandenburg D., Orci L. Internalized insulin receptors are recycled to the cell surface in rat hepatocytes. Proc Natl Acad Sci U S A. 1982 Oct;79(19):5921–5925. doi: 10.1073/pnas.79.19.5921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geuze H. J., Slot J. W., Strous G. J., Lodish H. F., Schwartz A. L. Intracellular site of asialoglycoprotein receptor-ligand uncoupling: double-label immunoelectron microscopy during receptor-mediated endocytosis. Cell. 1983 Jan;32(1):277–287. doi: 10.1016/0092-8674(83)90518-4. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Anderson R. G., Brown M. S. Coated pits, coated vesicles, and receptor-mediated endocytosis. Nature. 1979 Jun 21;279(5715):679–685. doi: 10.1038/279679a0. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Brown M. S. The low-density lipoprotein pathway and its relation to atherosclerosis. Annu Rev Biochem. 1977;46:897–930. doi: 10.1146/annurev.bi.46.070177.004341. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Noriega A., Grubb J. H., Talkad V., Sly W. S. Chloroquine inhibits lysosomal enzyme pinocytosis and enhances lysosomal enzyme secretion by impairing receptor recycling. J Cell Biol. 1980 Jun;85(3):839–852. doi: 10.1083/jcb.85.3.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JANDL J. H., KATZ J. H. The plasma-to-cell cycle of transferrin. J Clin Invest. 1963 Mar;42:314–326. doi: 10.1172/JCI104718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin M., Mintz B. Receptor-mediated endocytosis of transferrin in developmentally totipotent mouse teratocarcinoma stem cells. J Biol Chem. 1981 Apr 10;256(7):3245–3252. [PubMed] [Google Scholar]
- Knowles B. B., Howe C. C., Aden D. P. Human hepatocellular carcinoma cell lines secrete the major plasma proteins and hepatitis B surface antigen. Science. 1980 Jul 25;209(4455):497–499. doi: 10.1126/science.6248960. [DOI] [PubMed] [Google Scholar]
- Krupp M., Lane M. D. On the mechanism of ligand-induced down-regulation of insulin receptor level in the liver cell. J Biol Chem. 1981 Feb 25;256(4):1689–1694. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lestas A. N. The effect of pH upon human transferrin: selective labelling of the two iron-binding sites. Br J Haematol. 1976 Mar;32(3):341–350. doi: 10.1111/j.1365-2141.1976.tb00937.x. [DOI] [PubMed] [Google Scholar]
- Morgan E. H., Huebers H., Finch C. A. Differences between the binding sites for iron binding and release in human and rat transferrin. Blood. 1978 Dec;52(6):1219–1228. [PubMed] [Google Scholar]
- Octave J. N., Schneider Y. J., Crichton R. R., Trouet A. Transferrin uptake by cultured rat embryo fibroblasts. The influence of temperature and incubation time, subcellular distribution and short-term kinetic studies. Eur J Biochem. 1981 Apr;115(3):611–618. [PubMed] [Google Scholar]
- Octave J. N., Schneider Y. J., Hoffmann P., Trouet A., Crichton R. R. Transferrin uptake by cultured rat embryo fibroblasts. The influence of lysosomotropic agents, iron chelators and colchicine on the uptake of iron and transferrin. Eur J Biochem. 1982 Apr 1;123(2):235–240. doi: 10.1111/j.1432-1033.1982.tb19758.x. [DOI] [PubMed] [Google Scholar]
- Pastan I. H., Willingham M. C. Journey to the center of the cell: role of the receptosome. Science. 1981 Oct 30;214(4520):504–509. doi: 10.1126/science.6170111. [DOI] [PubMed] [Google Scholar]
- Pastan I. H., Willingham M. C. Receptor-mediated endocytosis of hormones in cultured cells. Annu Rev Physiol. 1981;43:239–250. doi: 10.1146/annurev.ph.43.030181.001323. [DOI] [PubMed] [Google Scholar]
- Posner B. I., Josefsberg Z., Bergeron J. J. Intracellular polypeptide hormone receptors. Characterization of insulin binding sites in Golgi fractions from the liver of female rats. J Biol Chem. 1978 Jun 10;253(11):4067–4073. [PubMed] [Google Scholar]
- Princiotto J. V., Zapolski E. J. Difference between the two iron-binding sites of transferrin. Nature. 1975 May 1;255(5503):87–88. doi: 10.1038/255087a0. [DOI] [PubMed] [Google Scholar]
- Schwartz A. L., Fridovich S. E., Knowles B. B., Lodish H. F. Characterization of the asialoglycoprotein receptor in a continuous hepatoma line. J Biol Chem. 1981 Sep 10;256(17):8878–8881. [PubMed] [Google Scholar]
- Stahl P., Schlesinger P. H., Sigardson E., Rodman J. S., Lee Y. C. Receptor-mediated pinocytosis of mannose glycoconjugates by macrophages: characterization and evidence for receptor recycling. Cell. 1980 Jan;19(1):207–215. doi: 10.1016/0092-8674(80)90402-x. [DOI] [PubMed] [Google Scholar]
- Sullivan A. L., Grasso J. A., Weintraub L. R. Micropinocytosis of transferrin by developing red cells: an electron-microscopic study utilizing ferritin-conjugated transferrin and ferritin-conjugated antibodies to transferrin. Blood. 1976 Jan;47(1):133–143. [PubMed] [Google Scholar]
- Tsai J. S., Seeman M. In vitro characterization of the mechanism of insulin degradation and the effect of chloroquine. Biochim Biophys Acta. 1981 Mar 18;673(3):259–269. [PubMed] [Google Scholar]
- Tycko B., Maxfield F. R. Rapid acidification of endocytic vesicles containing alpha 2-macroglobulin. Cell. 1982 Mar;28(3):643–651. doi: 10.1016/0092-8674(82)90219-7. [DOI] [PubMed] [Google Scholar]
- Van Leuven F., Cassiman J. J., Van Den Berghe H. Primary amines inhibit recycling of alpha 2M receptors in fibroblasts. Cell. 1980 May;20(1):37–43. doi: 10.1016/0092-8674(80)90232-9. [DOI] [PubMed] [Google Scholar]
- Wall D. A., Wilson G., Hubbard A. L. The galactose-specific recognition system of mammalian liver: the route of ligand internalization in rat hepatocytes. Cell. 1980 Aug;21(1):79–93. doi: 10.1016/0092-8674(80)90116-6. [DOI] [PubMed] [Google Scholar]
- Ward J. H., Kushner J. P., Kaplan J. Regulation of HeLa cell transferrin receptors. J Biol Chem. 1982 Sep 10;257(17):10317–10323. [PubMed] [Google Scholar]
- Willingham M. C., Pastan I. The receptosome: an intermediate organelle of receptor mediated endocytosis in cultured fibroblasts. Cell. 1980 Aug;21(1):67–77. doi: 10.1016/0092-8674(80)90115-4. [DOI] [PubMed] [Google Scholar]
- van Renswoude J., Bridges K. R., Harford J. B., Klausner R. D. Receptor-mediated endocytosis of transferrin and the uptake of fe in K562 cells: identification of a nonlysosomal acidic compartment. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6186–6190. doi: 10.1073/pnas.79.20.6186. [DOI] [PMC free article] [PubMed] [Google Scholar]



