Abstract
Background: Artificial fluoridation of drinking water to improve dental health has long been a topic of controversy. Opponents of this public health measure have cited the possibility of bone cancer induction. The study objective was to examine whether increased risk of primary bone cancer was associated with living in areas with higher concentrations of fluoride in drinking water.
Methods: Case data on osteosarcoma and Ewing sarcoma, diagnosed at ages 0–49 years in Great Britain (GB) (defined here as England, Scotland and Wales) during the period 1980–2005, were obtained from population-based cancer registries. Data on fluoride levels in drinking water in England and Wales were accessed through regional water companies and the Drinking Water Inspectorate. Scottish Water provided data for Scotland. Negative binomial regression was used to examine the relationship between incidence rates and level of fluoride in drinking water at small area level.
Results: The study analysed 2566 osteosarcoma and 1650 Ewing sarcoma cases. There was no evidence of an association between osteosarcoma risk and fluoride in drinking water [relative risk (RR) per one part per million increase in the level of fluoride = 1·001; 90% confidence interval (CI) 0·871, 1·151] and similarly there was no association for Ewing sarcoma (RR = 0·929; 90% CI 0·773, 1·115).
Conclusions: The findings from this study provide no evidence that higher levels of fluoride (whether natural or artificial) in drinking water in GB lead to greater risk of either osteosarcoma or Ewing sarcoma.
Keywords: Osteosarcoma, Ewing sarcoma, bone cancer, children, young people, artificial fluoridation, fluoride, drinking water, Great Britain, small area analysis
Key Messages.
There was no evidence of an association between fluoride in drinking water and osteosarcoma or Ewing sarcoma before or after adjustment for small area level deprivation.
33% of artificially fluoridated water supply zones in Great Britain were found to be supplying water that was below 0·7 parts per million of fluoride, the lower limit of the optimal level for dental health benefit.
There was no evidence that those who lived in an artificially fluoridated area of Great Britain were at increased risk of osteosarcoma or Ewing sarcoma.
There was no evidence that those living in an area of Great Britain with naturally occurring fluoride within the optimal level for dental health benefit were at increased risk of osteosarcoma or Ewing sarcoma.
Introduction
Primary bone cancer is the third most common cancer in 10–24-year-olds, mainly comprising osteosarcoma and Ewing sarcoma (ES).1 The aetiology is unclear, but both genetic and environmental factors are likely to be involved.1,2
Fluoride occurs naturally in drinking water at varying concentrations. Water is a primary dietary fluoride source. Populations supplied with high levels of naturally occurring fluoride in drinking water have low levels of dental caries.3 The optimum range for dental health benefit is 0·7–1·2 parts per million (ppm). Concentrations below 0·3 ppm may provide no benefit.4
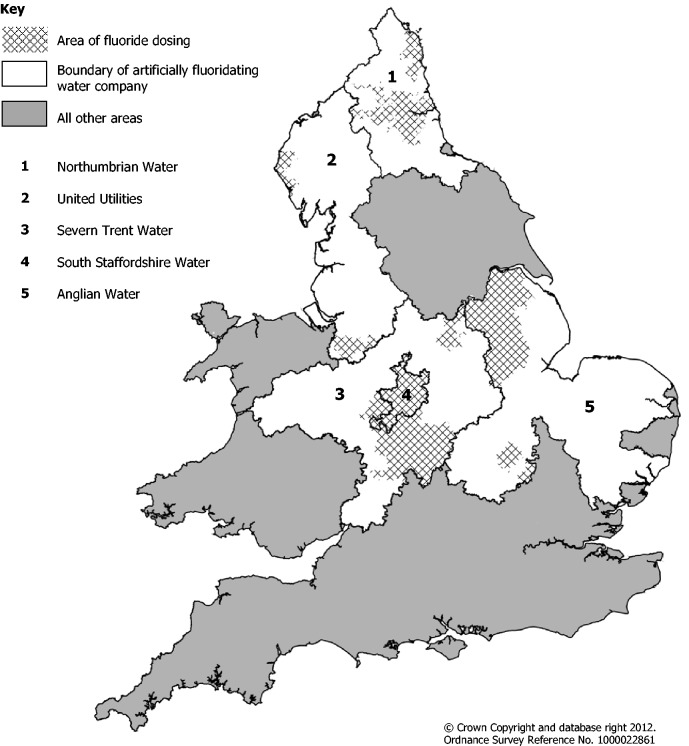
The recognition of an association between prevalence of dental caries and areas of greater socio-economic deprivation led to artificial fluoridation of water supplies in some countries, including in parts of Great Britain (GB), defined here as England, Scotland and Wales.5 Pilot schemes established in the 1950s included: Kilmarnock (1956–62); part of Anglesey (1955–92); Andover (1955–58) and Watford (1956–89).6–8 Artificial fluoridation programmes were introduced during the 1960s. Currently five water companies artificially fluoridate some drinking water supplies under the instruction of health authorities (Figure 1).9
Figure 1.
Map of England and Wales illustrating the boundaries of the water companies with artificial fluoridation programmes in place.
This practice has been controversial and there is speculation that adding fluoride to community water supplies could result in adverse health outcomes including increased risk of cancer, particularly osteosarcoma.10 Two systematic reviews concluded there was no clear association between water fluoridation and osteosarcoma incidence or mortality, but both advised caution in view of heterogeneity and other methodological concerns.11,12
The present study used the same case dataset as a previous demographic analysis that tested whether spatial variation among osteosarcoma and ES was associated with population density and area-based deprivation. Area-based deprivation is an ecological measure used for examining socio-economic data according to a small geographical unit. The previous ecological study found higher incidence of osteosarcoma for females in less deprived areas and of ES in areas of low population density and high car ownership. The putative association between osteosarcoma and ES risk and fluoride in drinking water was not analysed.13
The present study objective was to examine whether osteosarcoma risk was associated with fluoride levels in drinking water. For comparison, ES was also studied. This study is the first to analyse fluoride monitoring data as part of the exposure measurement and the putative association with osteosarcoma risk in small geographical areas across the whole of GB. No distinction was made between artificial or naturally occurring fluoride in drinking water, and novel geographical information system (GIS) methodologies were utilised to assign fluoride levels at small area level. The following hypotheses were tested: (i) geographical heterogeneity of osteosarcoma incidence is modulated by differences in fluoride levels in drinking water; and (ii) geographical heterogeneity of ES incidence is not modulated by differences in fluoride levels.
Methods
Study subjects
Data for patients diagnosed with osteosarcoma or ES in GB during 1980–2005 were analysed. Cases were limited to ages 0–49 years, as there are few ES cases above these ages and osteosarcoma over the age of 50 years is usually associated with Paget’s disease or is secondary to radiotherapy.2,14 To ensure sufficient case numbers in each category at small area level, cases were sub-divided into age groups 0–14, 15–29 and 30–49 years at diagnosis.
Regulatory and ethical approvals were gained (UK National Research Ethics Service reference number 09/H0904/5). Case data were accessed from the 10 regional cancer registries in GB. Case data from the National Registry of Childhood Tumours were extracted and used to cross-check accuracy of the regional registry cases aged 0–14 years.
Diagnostic groups
Cases were grouped using the International Classification of Diseases for Oncology, third edition (ICD-O-3).15 Osteosarcoma and ES were specified a priori and the associated topography and morphology codes are given elsewhere.13
Population data
Denominator data were derived from national decennial census data.16–21 Analyses over a prolonged time span are impeded by boundary changes, especially at small area level.22 Therefore, population counts from previous censuses and geo-referenced bone cancer registration data were adjusted to be compatible with 2001 census boundaries.23 The small area units (SAU) used in the analyses were census wards in England and Wales (0–49-years-old population ranges from 297 to 29 300, median = 3090), and postcode sectors in Scotland (0–49-years-old population ranges from 23 to 15 916, median = 3201).
Similarly, to adjust for deprivation, a time-series of indicators was obtained from each census during the study period and geographically converted to be compatible with 2001 SAUs. The Townsend index (comprising four components: unemployment, non-car ownership, non-home ownership and household overcrowding) is commonly used in health studies.24 To track changes in deprivation for every SAU at the different time points, each variable was expressed as a z-score relative to the GB average level over the study period, then summed and equally weighted to a single deprivation score.25
Fluoride monitoring data
At the time of the study, 25 companies in GB supplied water to 2265 statutorily demarcated ‘water supply zones’ (WSZs) with a population supply threshold of 100 000 (Water Regulation Zones in Scotland). Less than 1% of households have private water supplies.26 Fluoride level in drinking water is continuously monitored and required to be less than 1·5 ppm on a 3–month average basis.27,28 These routine fluoride monitoring data were utilised in this study and obtained through Scottish Water and a regulatory body, the Drinking Water Inspectorate (DWI) for companies in England and Wales.
Digital boundary data
Digital boundaries for census areas facilitated geo-referenced data linkage for statistical analysis and were accessed from UK Borders.29,30 Digital boundaries for WSZs were accessed through the DWI and Scottish Water.
Data linkage: assignment of fluoride level to census small area units
Postcode distributions were used to link WSZs to each SAU which was subsequently assigned a fluoride level. The population centroid of each SAU was calculated in ESRI ArcMap 9.331 to assign a weighted average for SAUs with water supply from more than one WSZ. The Nondetects and Data Analysis (NADA) add-on package for estimating censored environmental data was used to compute values for left-censored or missing data in R 2·8·1.32,33 Bone cancer cases were linked and aggregated for each 2001 census SAU.
Statistical analysis
Negative binomial regression was implemented using STATA version 12.34 The logarithm of the incidence rate was modelled in two parts: (i) variation explained by the data was expressed as a linear function of gender, age-group and deprivation; (ii) unexplained variation was modelled by a negative binomial distribution. The number of cases observed in each SAU was the dependent variable and the logarithm of the ‘at risk’ population was used as the offset. Independent variables were the census-derived SAU attributes that were allocated to the 2001 census geography.22
The previously determined best-fitting models that explained bone cancer variation (the final models in the hierarchical series for osteosarcoma and ES)13 were used as the base models. In the present study, these models were extended to include fluoride level in drinking water by SAU. For osteosarcoma, the base model included gender, age-group, the interaction gender*age-group, the Townsend score and the interaction Townsend*female. For ES, the base model included age-group, gender, the interaction age-group*gender, Scotland, East Midlands, population density and non-car ownership. Fluoride, which had not been analysed in the previous analysis, was then added to these base models. The effect of fluoride level in drinking water was tested in multivariable models whilst adjusting for gender, age-group, population density and interactions. The effect of fluoride was assessed, after adjustment for these covariates, using likelihood ratio tests and the Akaike Information Criterion (AIC).35 Relative risks (RRs) and associated 90% confidence intervals (CIs) were calculated.36
The mean fluoride level in drinking water was determined using monitoring data sampled between 2004 and 2006 and modelled as a continuous variable, under the assumption that any association was linear. The effect of fluoride level in drinking water was modelled with and without adjustment for deprivation. To test the linearity assumption, fluoride was also modelled as an ordinal variable (dividing the distribution into fifths). To test for a possible threshold effect, the most fluoridated fifth of the population was compared with less fluoridated fifths. Also, areas with a level of at least 0·7 ppm, the lower limit of the range for optimum dental health benefit4 were compared with less fluoridated areas.
Sensitivity analyses tested for effects of age-group. The data were restricted to cases born within the following non-overlapping cohorts: (i) before 1970; (ii) 1970–79; and (iii) 1980 onwards. To test for constancy of fluoride exposures in regions where levels may have fluctuated, artefactually inflated levels (1 ppm) were analysed. Finally, an analysis was carried out on the osteosarcoma data using age-groups 0–9, 10-14, … 45-49 years to assess whether the three age-groups 0–14, 15–29 and 30–49 years were too broad to detect increases limited to specific ages. This additional analysis examined age-group interactions with fluoride to test if the age-group 0–9 years was more affected by fluoride than other age-groups. For all analyses, P-values were two-sided.
Results
The study analysed 2566 osteosarcoma cases (1493 males, 1073 females) and 1650 ES cases (988 males, 662 females) aged 0–49 years. The numbers of cases by age-group and gender are given in Table 1, with full descriptive data given elsewhere.13 For osteosarcoma the overall age-standardized rate (ASR) for all persons was 2·64 per million persons per year (90% CI 2·55, 2·72), and 1·76 per million persons per year (90% CI 1·68, 1·83) for ES. Most analyses included an adjustment for deprivation.
Table 1.
Number of cases of osteosarcoma and Ewing sarcoma by age-group and gender
| Age-group (years) |
Number of osteosarcoma cases |
Number of Ewing sarcoma cases |
||||
|---|---|---|---|---|---|---|
| Males | Females | Total | Males | Females | Total | |
| 0-14 | 406 | 411 | 817 | 356 | 303 | 659 |
| 15-29 | 821 | 494 | 1315 | 516 | 284 | 800 |
| 30-49 | 266 | 168 | 434 | 116 | 75 | 191 |
| 0-49 | 1493 | 1073 | 2566 | 988 | 662 | 1650 |
After adjustment for gender, age-group, the interaction gender*age-group, the Townsend score and the interaction Townsend*female, no association was found between osteosarcoma and fluoride levels in drinking water (P = 0·987). The RR for osteosarcoma for 1-ppm increase in fluoride level was 1·001 (90% CI 0·871 to 1·151). After adjustment for age-group, gender, the interaction age-group*gender, Scotland, East Midlands, population density and non-car ownership, no association was found between ES incidence and fluoride levels in drinking water (P = 0·503). The RR for ES for 1-ppm increase in fluoride levels was 0·929 (90% CI 0·773 to 1·115).
There was no effect without adjustment for deprivation. Similarly, using ordinal measures there was no evidence of any threshold effect. When testing cohorts born before 1970, 1970–79 and 1980 onwards, there was no differential effect of cohort on the association between bone cancer and fluoride. An effect was not found when testing with artefactually raised levels of fluoride (Tables 2–4), nor when using narrower age-bands (0–9, 10–14 … 45–49 years). There was no evidence of an interaction between age-group and fluoride.
Table 2.
Description of models used in the sensitivity analyses that tested the assumption that: (a) a continuous scale is an appropriate level of measurement for quantity of fluoride in drinking water; (b) there was no change in the effect of fluoride by cohort; and (c) fluoride levels in drinking water are constant over the study period
| Model | Test | Factora,b |
|---|---|---|
| 1 | (a) | Fluoride level in drinking water as a continuous variable with adjustment for deprivation |
| 2 | Fluoride level in drinking water as a continuous variable without adjustment for deprivation | |
| 3 | Fluoride level in drinking water as a discrete variable (quintiles) with adjustment for deprivation and comparison of quintile 1 with quintile 2, 3, 4 and 5 | |
| 4 | Fluoride level in drinking water as a binary variable based on the highest (5th) quintile with adjustment for deprivation | |
| 5 | Fluoride level in drinking water higher than 0·7 ppm are compared to levels that are less than 0·7 ppm (where fluoride level as binary based on 0·7 ppm threshold) with adjustment for deprivation | |
| 6 | Fluoride level in drinking water as a discrete variable (quintiles) without adjustment for deprivation and comparison of quintile 1 with quintile 2, 3, 4 and 5 | |
| 7 | Fluoride level in drinking water as a binary variable based on the highest (5th) quintile without adjustment for deprivation | |
| 8 | Fluoride levels in drinking water higher than 0·7 ppm are compared with levels that are less than 0·7 ppm (where fluoride level as binary is based on 0·7 ppm threshold) without adjustment for deprivation | |
| 9 | (b) | Fluoride level in drinking water (where cohort is restricted to include cases born before 1970) |
| 10 | Fluoride level in drinking water (where cohort is restricted to include cases born 1970 to 1979) | |
| 11 | Fluoride level in drinking water (where cohort is restricted to include cases born 1980 or later) | |
| 12 | (c) | Fluoride level in drinking water (where 10% of AF SAUs assigned a fluoride level of 1 ppm) |
| 13 | Fluoride level in drinking water (where 20% of AF SAUs assigned a fluoride level of 1 ppm) | |
| 14 | Fluoride level in drinking water (where 30% of AF SAUs assigned a fluoride level of 1 ppm) | |
| 15 | Fluoride level in drinking water (where 40% of AF SAUs assigned a fluoride level of 1 ppm) | |
| 16 | Fluoride level in drinking water (where 100% of AF SAUs assigned a fluoride level of 1 ppm) | |
| 17 | Fluoride level in drinking water (where pilot areas Watford, Kilmarnock & all Anglesey assigned a fluoride level of 1 ppm) | |
| 18 | Fluoride level in drinking water (where pilot areas Watford, Kilmarnock & wards in north Anglesey assigned a fluoride level of 1 ppm) | |
| 19 | Fluoride level in drinking water (where pilot areas Watford, Kilmarnock & wards in south Anglesey assigned a fluoride level of 1 ppm) | |
| 20 | Fluoride level in drinking water (where pilot areas Watford, Kilmarnock & wards in east Anglesey assigned a fluoride level of 1 ppm) | |
| 21 | Fluoride level in drinking water (where pilot areas Watford, Kilmarnock & wards in west Anglesey assigned a fluoride level of 1 ppm) | |
| 22 | Fluoride level in drinking water (excluding all AF SAUs including those in Three Valleys) | |
| 23 | Artificial fluoridation as a binary variable with adjustment for deprivation | |
Table 3.
Examination of the effect of fluoride level in drinking water on osteosarcoma incidence: comparison of fluoride models and test results
|
Model |
dfb | deviance | AICc |
Difference in: |
Coefficient (with 90% confidence interval) | Relative risk (with 90% confidence interval) | Likelihood ratio: | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Numbera | Comparison | df | deviance | P-value | |||||||
| 1 | d | 175665 | 17940·9 | 24754·8 | 1 | 0·000 | 0·001 (−0·138,0·141) | 1.001 (0.871,1.151) | 0·987 | ||
| 2 | e | 175667 | 17976·3 | 24786·2 | 1 | 0·006 | −0·006 (−0·145,0·133) | 0·994 (0·865,1·142) | 0·940 | ||
| 3f | d | 175662 | 17936·5 | 24756·4 | 4 | 4·368 | (a) | −0·122 (−0·227,−0·016) | (a) | 0·885 (0·797,0·984) | 0·359 |
| (b) | −0·097 (−0·202,0·009) | (b) | 0·908 (0·817,1·009) | ||||||||
| (c) | −0·056 (−0·160,0·048) | (c) | 0·946 (0·853,1·049) | ||||||||
| (d) | −0·042 (−0·146,0·061) | (d) | 0·958 (0·864,1·063) | ||||||||
| 4 | d | 175665 | 17940·6 | 24754·5 | 1 | 0·273 | 0·026 (−0·056,0·108) | 1·026 (0·946,1·114) | 0·602 | ||
| 5 | d | 175665 | 17940·8 | 24754·7 | 1 | 0·114 | −0·024 (−0·140,0·093) | 0·976 (0·869,1·097) | 0·736 | ||
| 6f | e | 175664 | 17973·3 | 24789·1 | 4 | 3·051 | (a) | −0·102 (−0·207,0·004) | (a) | 0·903 (0·813,1·004) | 0·549 |
| (b) | −0·038 (−0·142,0·067) | (b) | 0·963 (0·867,1·069) | ||||||||
| (c) | −0·058 (−0·161,0·046) | (c) | 0·944 (0·851,1·047) | ||||||||
| (d) | −0·016 (−0·119,0·088) | (d) | 0·984 (0·888,1·092) | ||||||||
| 7 | e | 175667 | 17975·9 | 24785·8 | 1 | 0·440 | 0·033 (−0·049,0·115) | 1·034 (0·952,1·122) | 0·507 | ||
| 8 | e | 175667 | 17975·9 | 24785·8 | 1 | 0·367 | −0·043 (−0·159,0·074) | 0·958 (0·853,1·076) | 0·545 | ||
| 9 | g | 175667 | 8669·8 | 11462·7 | 1 | 3·425 | −0·193 (−0·421,0·036) | 0·825 (0·656,1·036) | 0·064 | ||
| 10 | h | 175665 | 6587·3 | 8606·0 | 1 | 0·965 | 0·148 (−0·097,0·393) | 1·160 (0·908,1·482) | 0·326 | ||
| 11 | i | 117108 | 6439·1 | 8603·9 | 1 | 1·656 | 0·197 (−0·051,0·445) | 1·218 (0·951,1·560) | 0·198 | ||
| 12 | d | 175665 | 17940·9 | 24754·7 | 1 | 0·024 | −0·013 (−0·150,0·124) | 0·987 (0·860,1·132) | 0·876 | ||
| 13 | d | 175665 | 17940·8 | 24754·7 | 1 | 0·066 | −0·021 (−0·158,0·115) | 0·979 (0·854,1·122) | 0·798 | ||
| 14 | d | 175665 | 17940·9 | 24754·8 | 1 | 0·010 | −0·008 (−0·141,0·125) | 0·992 (0·868,1·133) | 0·921 | ||
| 15 | d | 175665 | 17940·9 | 24754·7 | 1 | 0·021 | 0·011 (−0·118,0·141) | 1·011 (0·889,1·151) | 0·885 | ||
| 16 | d | 175665 | 17940·8 | 24754·7 | 1 | 0·030 | 0·013 (−0·106,0·131) | 1·013 (0·899,1·140) | 0·862 | ||
| 17 | d | 175665 | 17940·9 | 24754·8 | 1 | 0·006 | 0·006 (−0·131,0·143) | 1·006 (0·877,1·154) | 0·941 | ||
| 18 | d | 175665 | 17940·9 | 24754·7 | 1 | 0·017 | 0·011 (−0·126,0·148) | 1·011 (0·881,1·160) | 0·896 | ||
| 19 | d | 175665 | 17940·9 | 24754·8 | 1 | 0·005 | −0·006 (−0·143,0·132) | 0·994 (0·866,1·141) | 0·947 | ||
| 20 | d | 175665 | 17940·9 | 24754·8 | 1 | 0·002 | 0·004 (−0·134,0·141) | 1·004 (0·875,1·152) | 0·963 | ||
| 21 | d | 175665 | 17940·9 | 24754·8 | 1 | 0·000 | 0·001 (−0·136,0·139) | 1·001 (0·873,1·149) | 0·986 | ||
| 22 | j | 148137 | 14904·9 | 20527·2 | 1 | 1·090 | 0·156 (−0·086,0·397) | 1·168 (0·917,1·488) | 0·297 | ||
| 23 | g | 175667 | 8669·8 | 11400·8 | 1 | 3·425 | −0·189 (−0·361,−0·017) | 0·828 (0·697,0·983) | 0·064 | ||
| h | 175665 | 6588·1 | 8606·8 | 1 | 0·160 | 0·045 (−0·139,0·229) | 1·046 (0·870,1·257) | 0·689 | |||
| i | 117108 | 6437·5 | 8603·9 | 1 | 3·216 | 0·197 (0·020,0·373) | 1·218 (1·021,1·452) | 0·073 | |||
aPlease see Table 2a–c for model description.
bDegree of freedom.
cAkaike Information Criterion
dCompared with the non-fluoride model that contained age-group, gender, gender*age-group, Townsend & Townsend*female—cohort includes all cases in GB for the whole of the study period.
eCompared with the non-fluoride model that contained age-group, gender, gender*age-group—cohort includes all cases in GB for the whole of the study period.
fCoefficients and relative risks are reported for quintiles (a) 2, (b) 3, (c) 4 and (d) 5.
gCompared with the non-fluoride model that contained age-group, gender, unemployment—cohort is restricted to include cases born before 1970.
hCompared with the non-fluoride model that contained age-group, gender, gender*age-group, non-home ownership, non-home ownership *age-group—cohort is restricted to include cases born between 1970 and 1979.
iCompared with the non-fluoride model that contained age-group, gender, gender*age-group, unemployment, unemployment*age-group—cohort is restricted to include cases born 1980 or later.
Table 4.
Examination of the effect of fluoride level in drinking water on Ewing sarcoma incidence: comparison of fluoride models and test results
|
Model |
dfb | deviance | AICh |
Difference in: |
Coefficient (with 90% confidence interval) | Relative risk (with 90% confidence interval) | Likelihood ratio: | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Numbera | Comparison | df | deviance | P-value | |||||||
| 1 | d | 175663 | 12874·8 | 17301·6 | 1 | 0·448 | −0·074 (−0·257,0·109) | 0·929 (0·773,1·115) | 0·503 | ||
| 2 | e | 175664 | 12883·0 | 17307·8 | 1 | 0·617 | −0·086 (−0·269,0·096) | 0·917 (0·764,1·101) | 0·432 | ||
| 3f | d | 175660 | 12869·6 | 17302·4 | 4 | 5·630 | (a) | 0·071 (−0·068,0·211) | (a) | 1·074 (0·934,1·235) | 0·229 |
| (b) | −0·120 (−0·268,0·027) | (b) | 0·887 (0·765,1·028) | ||||||||
| (c) | −0·019 (−0·174,0·136) | (c) | 0·981 (0·840,1·146) | ||||||||
| (d) | −0·002 (−0·150,0·146) | (d) | 0·998 (0·861,1·157) | ||||||||
| 4 | d | 175663 | 12875·2 | 17302·0 | 1 | 0·039 | 0·013 (−0·094,0·120) | 1·013 (0·910,1·128) | 0·843 | ||
| 5 | d | 175663 | 12874·9 | 17301·7 | 1 | 0·351 | −0·053 (−0·202,0·095) | 0·948 (0·817,1·100) | 0·554 | ||
| 6f | e | 175661 | 12880·2 | 17311·0 | 4 | 3·416 | (a) | 0·069 (−0·071,0·209) | (a) | 1·071 (0·931,1·233) | 0·491 |
| (b) | −0·076 (−0·222,0·070) | (b) | 0·927 (0·801,1·073) | ||||||||
| (c) | 0·028 (−0·126,0·182) | (c) | 1·028 (0·881,1·199) | ||||||||
| (d) | 0·011 (−0·137,0·159) | (d) | 1·011 (0·872,1·173) | ||||||||
| 7 | e | 175664 | 12883·6 | 17308·4 | 1 | 0·002 | 0·003 (−0·104,0·110) | 1·003 (0·901,1·116) | 0·967 | ||
| 8 | e | 175664 | 12882·8 | 17307·6 | 1 | 0·761 | −0·078 (−0·225,0·070) | 0·925 (0·798,1·073) | 0·383 | ||
| 9 | g | 175666 | 5138·1 | 6543·7 | 1 | 0·679 | −0·152 (−0·460,0·156) | 0·859 (0·631,1·168) | 0·410 | ||
| 10 | h | 175665 | 5288·3 | 6797·4 | 1 | 0·164 | −0·072 (−0·366,0·222) | 0·930 (0·693,1·249) | 0·685 | ||
| 11 | i | 117108 | 5127·0 | 6707·6 | 1 | <0·001 | 0·022 (−0·293,0·337) | 1·023 (0·746,1·401) | 0·996 | ||
| 12 | d | 175663 | 12874·7 | 17301·5 | 1 | 0·564 | −0·082 (−0·262,0·098) | 0·921 (0·769,1·103) | 0·453 | ||
| 13 | d | 175663 | 12874·6 | 17301·4 | 1 | 0·586 | −0·083 (−0·261,0·096) | 0·921 (0·770,1·101) | 0·444 | ||
| 14 | d | 175663 | 12874·7 | 17301·2 | 1 | 0·807 | −0·095 (−0·271,0·080) | 0·909 (0·763,1·084) | 0·369 | ||
| 15 | d | 175663 | 12874·7 | 17301·4 | 1 | 0·640 | −0·083 (−0·253,0·088) | 0·921 (0·776,1·092) | 0·424 | ||
| 16 | d | 175663 | 12874·5 | 17301·2 | 1 | 0·780 | −0·083 (−0·240,0·073) | 0·920 (0·787,1·076) | 0·377 | ||
| 17 | d | 175663 | 12874·8 | 17301·6 | 1 | 0·396 | −0·068 (−0·248,0·111) | 0·934 (0·780,1·118) | 0·529 | ||
| 18 | d | 175663 | 12874·7 | 17301·5 | 1 | 0·513 | −0·078 (−0·259,0·103) | 0·925 (0·772,1·108) | 0·474 | ||
| 19 | d | 175663 | 12874·8 | 17301·6 | 1 | 0·420 | −0·071 (−0·251,0·110) | 0·932 (0·778,1·116) | 0·517 | ||
| 20 | d | 175663 | 12874·7 | 17301·5 | 1 | 0·520 | −0·079 (−0·260,0·102) | 0·924 (0·771,1·108) | 0·471 | ||
| 21 | d | 175663 | 12874·8 | 17301·6 | 1 | 0·413 | −0·070 (−0·251,0·110) | 0·932 (0·778,1·117) | 0·520 | ||
| 22 | j | 148135 | 10798·1 | 14474·9 | 1 | 0·037 | 0·039 (−0·294,0·372) | 1·040 (0·745,1·451) | 0·847 | ||
| 23 | g | 175666 | 5137·1 | 6542·7 | 1 | 1·627 | −0·178 (−0·412,0·057) | 0·837 (0·662,1·058) | 0·202 | ||
| h | 175665 | 5288·5 | 6795·6 | 1 | 0·010 | −0·013 (−0·228,0·202) | 0·987 (0·796,1·223) | 0·919 | |||
| i | 117108 | 5127·0 | 6707·6 | 1 | <0·001 | −0·001 (−0·229,0·227) | 0·999 (0·796,1·255) | 0·996 | |||
aPlease see Table 2a–c for model description.
bDegree of freedom.
cAkaike Information Criterion.
dCompared with the model that contains age-group, gender, age-group *gender, Scotland, East Midlands, population density and non-car ownership—cohort includes all cases for whole of study period.
eCompared with the model that contains age-group, gender, age-group *gender, Scotland, East Midlands, population density—cohort includes all cases for whole of study period.
fCoefficients and relative risks are reported for quintiles (a) 2, (b) 3, (c) 4 and (d) 5.
gCompared with the non-fluoride model that contained age-group, gender, population density, unemployment—cohort is restricted to include cases born before 1970.
hCompared with the non-fluoride model that contained age-group, gender, age-group *gender, population density, unemployment—cohort is restricted to include cases born between 1970 and 1979.
iCompared with the non-fluoride model that contained age-group, gender, age-group *gender, Scotland, unemployment—cohort is restricted to include cases born 1980 or later.
Table 5 presents the mean fluoride level assigned to each SAU in GB. The means range from 0·00 to 1·26 ppm; 48% of the SAUs had a fluoride level less than 0·10 ppm and 80% had a level below 0·26 ppm. Approximately 70% of the SAUs belonging to the upper fifth had fluoride levels considered to provide the optimal dental decay prevention benefit (0·70–1·20 ppm). This equated to approximately 14% of the total number of SAUs in GB having a fluoride level within optimal limits. Five water companies in England currently operate artificial fluoridation programmes but not all their WSZs are artificially fluoridated (AF) (Figure 1). The AF WSZs ranged from 0·04 to 1·08 ppm; 67% of the AF WSZs had a level within optimal limits.
Table 5.
The upper and lower limits of each fluoride category (the average level of fluoride assigned to each census small area unit (SAU) in parts per million (ppm) and then the SAU population distribution ranked and divided into fifths)
| Fluoride category (SAU population distribution divided into fifths) | Average level of fluoride assigned to each census SAU (ppm) (upper and lower limits of fluoride category) |
|---|---|
| 1 (lower fifth) | 0·000000 – 0·048969 |
| 2 | 0·048970 – 0·078770 |
| 3 | 0·078771 – 0·138820 |
| 4 | 0·138821 – 0·254040 |
| 5 (upper fifth) | 0·254041 – 1·268000 |
Discussion
This ecological analysis used high-quality population-based osteosarcoma and ES case data from 0–49-year-olds diagnosed 1980–2005 in GB. The demographic profile of the study population has previously been published.13 There was no evidence of an association between fluoride in drinking water and osteosarcoma or ES. Thus, there was no support for prior hypothesis (i) that geographical heterogeneity of osteosarcoma is modulated by differences in fluoride levels. There was support for prior hypothesis (ii) that geographical heterogeneity of ES is not modulated by differences in fluoride levels.
This is the first study that has assessed fluoride levels in drinking water across the whole of GB. Novel methodologies were developed within a GIS framework to enable fluoride levels to be assigned to each SAU. Such an approach reduced the potential of misclassification of exposure data when compared with previous studies that took simpler approaches.37–39
The monitoring data suggested that levels in some AF areas were much lower than 1 ppm. Indeed, 33% of AF WSZs were below 0·7 ppm, the lower limit of the optimum range for dental health benefit.4 It is noteworthy that this corresponded to only 14% of all SAUs in GB having a fluoride level that may confer a dental health benefit, and 61% of AF SAUs had such a level. This suggests that 35% of populations residing in AF areas were being supplied with AF water dosed below the optimal level.
The relationship between fluoride and osteosarcoma risk has been examined in a small number of animal40,41 and human studies37–39,41–48 with conflicting results. Disagreement could be linked to fundamental differences in study design; some studies were laboratory-based,40,41 some ecological,37,38,43–45 others were case-control.46–48 Moreover, investigations to date had several methodological limitations. These included limited statistical power,37,46–48 the potential of selection bias in choice of cases47,48 and controls48 or method of exposure categorization leading to misclassification.
In a recent case-control study, Bassin and colleagues analysed 103 cases (60 males, 43 females) aged under 20 years and found increased osteosarcoma risk with fluoride in drinking water for males only, with a peak in the age-group 6–8 years.47 However, the number of cases within this age-group would have been extremely small.49,50 A further limitation acknowledged by the authors was potential selection bias because of differences in case and control referral patterns to participating hospitals.47
Another case-control study using the same dataset found no association, but again methods used introduced limitations. There were 109 cases aged under 30 years, but only 21 controls in this age range (median age for male controls = 41·3 years; male cases = 17·0).48 It is also possible that the use of other newly diagnosed malignant bone tumours as the controls masked any differences if risk also increased in those tumours.21 Furthermore, although the overall results contradict those from Bassin’s study,51 the use of total accumulated fluoride dose rather than a specific time in the life course prevents any direct comparisons being made.
A study did find a link between mean fluoride levels in blood serum and increased osteosarcoma risk, but finding this association does not infer causality.52 Mean fluoride levels in cases with other bone-forming tumours were significantly higher when compared with the control group consisting of patients with musculo-skeletal pain only. Although the mean fluoride level was only approximately 50% of the osteosarcoma group, it highlights caution when selecting controls.
Two ecological studies from the USA and from Ireland concluded water fluoridation status had no influence on osteosarcoma incidence rates.38,39 However, both studies had shortcomings due to the methods of exposure data categorization. The US study categorized states according to high and low community water fluoridation.37 Other studies merely compared bone cancer incidence in areas with and without artificial fluoridation programmes.38,39 The bioavailability and chemistry have been assessed, with no difference being found between naturally occurring and artificial fluoride.53 Similarly, another experimental double-blind cross-over trial reported no difference in bioavailability although this finding needed to be treated cautiously due to small case numbers.54
The present study had some limitations. It is a small area study and assumed that the characteristics of each individual within a designated area were represented by the aggregate statistics for that area. However, although these studies are susceptible to the ecological fallacy,55 ecological analyses are suited to initial investigation of causal hypotheses.56 Artificial fluoridation programmes are necessarily ecological but are considered an effective method of providing populations with the dental health benefit of fluoride.22,23
Lack of availability and inconsistency of individual sampling data across the whole of GB during the study period (only 2004–06 data were used) meant an assumption was made of no change in fluoride levels within the study time-frame. However, this assumption was substantiated through sensitivity analyses (Tables 2-4). The fluctuations in fluoride levels over time were assessed. Fluoride levels in England fluctuated plus or minus 10%. Since source data for Scotland were limited to 2004–06 and more variation was found in Wales, the main analysis was repeated using data for England only. It found the best-fitting models had the same predictors as the whole of GB.
It is well established that fluoride concentrations in bone increase with age.57 To determine whether age or time of putative exposure had influenced the findings, the analyses were repeated using non-overlapping birth cohorts (before 1970, 1970–79 and 1980 onwards). For all three cohorts, there was no evidence of any association with risk (Tables 2–4). This confirmed that making an assumption of stable fluoride concentrations over time was reasonable.
Finding no association might be because of attenuation due to exposure measurement error, arising through the imprecision in allocating fluoride levels to specific areas during specific periods. For example, the WSZ boundaries have changed over time but it was not possible to represent these changes, as digital boundary data have only been archived since 2004. Lack of data availability also made it impossible to take any local changes within artificial fluoridation supply areas into account.
SAU of residence at time of diagnosis may not represent the true lifetime or shorter period of fluoride exposure for each case. Other sources of fluoride are not taken into consideration. In the 1950s when the first pilot studies were carried out in GB, there was much less availability from other sources. Fluoride started to be added to toothpaste in the 1970s and by 1978 approximately 96% of all toothpastes were fluoridated. Nevertheless, it is believed that drinking water is still the primary source of fluoride in GB, particularly in areas with fluoride levels of 1 ppm and over.4
In conclusion, this small area analysis used high-quality population-based osteosarcoma and ES case data. Novel GIS methodologies were developed to enable fluoride level in drinking water to be assigned to each SAU in GB. No association was found between fluoride level and osteosarcoma or ES before and after adjustment for deprivation. The findings from this study provide no evidence that higher levels of fluoride (whether natural or artificial) in drinking water in GB lead to greater risk of osteosarcoma or ES. Ecological design was appropriate for this initial investigation but also introduced limitations. Further research, such as large case-control studies that incorporate the GIS methodologies developed during this study, is recommended.
Funding
This work was supported by the Bone Cancer Research Trust (BCRT) [grant reference number: BCRT/08/08], North of England Children's Cancer Research (NECCR) Fund, the National Institute for Health Research (NIHR) and Children with Cancer UK.
Acknowledgements
We are most grateful to Public Health England Knowledge and Intelligence Team (West Midlands, formerly known as West Midlands Cancer Intelligence Unit), in particular to Dr Gill Lawrence and Sally Vernon who co-ordinated the data request from cancer registries in England and Wales. Likewise, we would like to thank the Scottish Registry for facilitating the acquisition of case data for Scotland. We are indebted to David Martin, Professor of Geography at the University of Southampton, for his guidance with geo-conversion methodologies and also to Richard Hardy (funded by the NECCR at the Institute of Health and Society, Newcastle University) for providing information technology support. Finally, we would also like thank all water companies in GB who provided data and the DWI, particularly Andy Taylor, Water Quality Data Manager and Mark Kozlowski, for construction of the map. The work is based on census data which are copyright of the Crown. The work is also based on data provided with the support of the ESRC and Joint Information Systems Committee (JISC) and uses census boundary material, which is copyright of the Crown and the ED-LINE Consortium.
Conflict of interest: None declared.
References
- 1.Eyre R, Feltbower RG, Mubwandarikwa E, Eden TO, McNally RJ. Epidemiology of bone tumours in children and young adults. Pediatr Blood Cancer 2009;53:941–52 [DOI] [PubMed] [Google Scholar]
- 2.Savage SA, Mirabello L. Using epidemiology and genomics to understand osteosarcoma etiology. Sarcoma 2011;2011:548151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ainsworth N. Mottled teeth. Br Dent J 1933;55:233–50 [Google Scholar]
- 4.Harrison PT. Fluoride in water: A UK perspective. J Fluor Chem 2005;126:1448–56 [Google Scholar]
- 5.Pizzo G, Piscopo MR, Pizzo I, Giuliana G. Community water fluoridation and caries prevention: a critical review. Clin Oral Invest 2007;11:189–93 [DOI] [PubMed] [Google Scholar]
- 6.Ministry of Health, Scottish Office, Ministry of Housing and Local Government The Conduct of the Fluoridation Studies in the United Kingdom and the Results Achieved After Five Years . London: HMSO; 1962 [Google Scholar]
- 7.Department of Health and Social Security, Scottish Office, Ministry of Housing and Local Government The Fluoridation Studies in the United Kingdom and the Results Achieved After Eleven Years . London: HMSO; 1969 [Google Scholar]
- 8.Murray JJ, Nunn JH, Steele JG. (eds). Prevention of Oral Disease. New York: Oxford University Press; 2003 [Google Scholar]
- 9.Mullen J, European Association for Paediatric Dentistry History of water fluoridation. Br Dent J 1995;199(Suppl 7):1–4 [DOI] [PubMed] [Google Scholar]
- 10.Committee to Coordinate Environmental Health and Related Programs, Ad Hoc Subcommittee on Fluoride Review of Fluoride: Benefits and Risks. Washington, DC: Public Health Service, Department of Health and Human Services; 1991 [Google Scholar]
- 11.McDonagh MS, Whiting PF, Wilson PM, et al. Systematic review of water fluoridation. BMJ 2000;321:855–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yeung CA. A systematic review of the efficacy and safety of fluoridation. Evid Based Dent 2008;9:39. [DOI] [PubMed] [Google Scholar]
- 13.McNally RJ, Blakey K, Parslow RC, et al. Small area analyses of bone cancer diagnosed in 0-49 year olds from Great Britain provide clues to aetiology. BMC Cancer 2012;12:270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ottaviani G, Jaffe N. The epidemiology of osteosarcoma. Canc Treat Res 2009;152:3–13 [DOI] [PubMed] [Google Scholar]
- 15.Fritz A, Percy C, Jack A, et al. International Classification of Diseases for Oncology (ICD-O) . 3rd edn Geneva: World Health Organization; 2000 [Google Scholar]
- 16.Office of Population Censuses and Surveys 1981 Census: Small Area Statistics (England and Wales). Manchester, UK: Census Dissemination Unit, Mimas, University of Manchester; 1981 [Google Scholar]
- 17.Registrar General for Scotland 1981 Census: Small Area Statistics (Scotland) . Manchester, UK: Census Dissemination Unit, Mimas, University of Manchester, 1981 [Google Scholar]
- 18.Office for Population Censuses and Surveys 1991 Census: Small Area Statistics (England and Wales). Manchester, UK: Census Dissemination Unit, Mimas, University of Manchester; 1991 [Google Scholar]
- 19.General Register Office Scotland 1991 Census: Small Area Statistics (Scotland) . Manchester, UK: Census Dissemination Unit, Mimas, University of Manchester; 1991 [Google Scholar]
- 20.Office for National Statistics 2001 Census: Standard Area Statistics (England and Wales). Manchester, UK: Census Dissemination Unit, Mimas, University of Manchester; 2001 [Google Scholar]
- 21.General Register Office for Scotland . 2001 Census: Standard Area Statistics (Scotland) . Manchester, UK: Census Dissemination Unit, Mimas, University of Manchester; 2001 [Google Scholar]
- 22.Simpson L. Geography conversion tables: a framework for conversion of data between geographical units. Int J Popul Geog 2002;8 69–82 [Google Scholar]
- 23.Norman P, Rees P, Boyle P. Achieving data compatibility over space and time: creating consistent geographical zones. Int JPopul Geog 2003;9:365–86 [Google Scholar]
- 24.Townsend P, Phillimore P, Beattie A. Health and Deprivation: Inequality and the North. London: Croom Helm; 1988 [Google Scholar]
- 25.Norman P. Identifying change over time in small area socio-economic deprivation. Appl Spatial Anal Policy 2010;3:107–38 [Google Scholar]
- 26.Toledano MB, Nieuwenhuijsen MJ, Best N, et al. Relation of trihalomethane concentrations in public water supplies to stillbirth and birth weight in three water regions in England. Environ Health Perspect 2005;113:225–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.The Stationery Office Scottish Statutory Instruments, Water Supply Water Quality (Scotland) Regulations No. 95 . 2010. http://www.legislation.gov.uk/ssi/2010/95/pdfs/ssi_20100095_en.pdf (5 November 2013, date last accessed) [Google Scholar]
- 28.The Stationery Office Statutory Instruments Water, England and Wales, Water Supply Water Quality Regulations No. 991 . 2010. http://dwi.defra.gov.uk/stakeholders/legislation/wsr2010.pdf (5 November 2013, date last accessed) [Google Scholar]
- 29.Office for National Statistics 2001 Census: Digitised Boundary Data (England and Wales). Edinburgh: Census Geography Data Unit, EDINA, University of Edinburgh; 2001 [Google Scholar]
- 30.General Register Office for Scotland 2001 Census: Digitised Boundary Data (Scotland) . Edinburgh: Census Geography Data Unit, EDINA, University of Edinburgh; 2001 [Google Scholar]
- 31.Environmental Systems Research Institute ArcGIS Desktop: Release 10. Redlands, CA: Environmental Systems Research Institute; 2011 [Google Scholar]
- 32.Lee L. NADA: Nondetects and Data Analysis for Environmental Data . R package version 1.5-3, 2010. http://cran.r-project.org/src/contrib/Archive/NADA/ (5 November 2013, date last accessed) [Google Scholar]
- 33.Core Team R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing; 2013 [Google Scholar]
- 34.StataCorp Stata Statistical Software: Release 12. College Station, TX: StataCorp LP; 2011 [Google Scholar]
- 35.Akaike H. A new look at the statistical model identification. IEEE Trans Automatic Control 1974;19:716–23 [Google Scholar]
- 36.Sterne JA, Davey Smith G. Sifting the evidence—what's wrong with significance tests? BMJ 2001;322:226–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cohn PD. A Brief Report on the Association of Drinking Water Fluoridation and the Incidence of Osteosarcoma Among Young Males. Trenton, NJ: New Jersey Department of Health and Environmental Health Services; 1992 [Google Scholar]
- 38.Comber H, Deady S, Montgomery E, Gavin A. Drinking water fluoridation and osteosarcoma incidence on the island of Ireland. Cancer Causes Control 2011;22:919–24 [DOI] [PubMed] [Google Scholar]
- 39.Levy M, Leclerc BS. Fluoride in drinking water and osteosarcoma incidence rates in the continental United States among children and adolescents. Cancer Epidemiol 2012;36:e83–e88 [DOI] [PubMed] [Google Scholar]
- 40.National Toxicology Program (NTP) Toxicology and Carcinogenesis Studies of Sodium Fluoride in F344/N Rats and B6C3f1 Mice . Technical report Series No. 393. Research Triangle Park, NC: National Institute of Environmental Health Sciences, 1990 [Google Scholar]
- 41.Maurer JK, Cheng MC, Boysen BG, Anderson RL. Two-year carcinogenicity study of sodium fluoride in rats. J Natl Cancer Inst 1990;82:1118–26 [DOI] [PubMed] [Google Scholar]
- 42.Hoover RN, Devesa SS, Cantor KP, Lubin JH, Fraumeni JF. Time trends for bone and joint cancers and osteosarcomas in the Surveillance, Epidemiology and End Results (SEER) Program. In: National Cancer Institute. Benefits and Risks Report of the Ad Hoc Committee on Fluoride of the Committee to Coordinate Environmental Health and Related Programs. Washington, DC: US Public Health Service; 1991 [Google Scholar]
- 43.Mahoney MC, Nasca PC, Bumett WS, Melius JM. Bone cancer incidence rates in New York State: time trends and fluoridated drinking water. Am J Public Health 1991;81:475–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Freni SC, Gaylor DW. International trends in the incidence of bone cancer are not related to drinking water fluoridation. Cancer 1992;70:611–18 [DOI] [PubMed] [Google Scholar]
- 45.Moss ME, Kanarek MS, Anderson HA, Hanrahan LP, Remington PL. Osteosarcoma, seasonality and environmental factors in Wisconsin, 1979-1989. Arch Environ Health 1995;50:235–41 [DOI] [PubMed] [Google Scholar]
- 46.Gelberg KH, Fitzgerald EF, Hwang SA, Dubrow R. Fluoride exposure and childhood osteosarcoma: a case-control study. Am J Public Health 1995;85:1678–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bassin EB, Wypij D, Davis RB, Mittleman MA. Age-specific fluoride exposure in drinking water and osteosarcoma (United States). Cancer Causes Control 2006;17:421–28 [DOI] [PubMed] [Google Scholar]
- 48.Kim FM, Hayes C, Williams PL, et al. National Osteosarcoma Etiology Group. An assessment of bone fluoride and osteosarcoma. J Dent Res 2011;90:1171–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ries LA, Smith MA, Gurney JG, et al. (eds). Cancer Incidence and Survival Among Children and Adolescents: United States SEER Program 1975-1995, NIH Pub. No. 99-4649. Bethesda, MD: National Cancer Institute, SEER Program, 1999 [Google Scholar]
- 50.Mirabello L, Troisi RJ, Savage SA. Osteosarcoma incidence and survival rates from 1973 to 2004: data from the Surveillance, Epidemiology, and End Results Program Cancer 2009;115:1531–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Douglass CW, Joshipura K. Caution needed in fluoride and osteosarcoma study. Cancer Causes Control 2006;17:481–82 [DOI] [PubMed] [Google Scholar]
- 52.Sandhu R, Lal H, Kundu ZS, Kharb S. Serum fluoride and sialic acid levels in osteosarcoma. Biol Trace Elem Res 2011;144:1–5 [DOI] [PubMed] [Google Scholar]
- 53.Jackson PJ, Harvey PW, Young WF. Chemistry and Bioavailability Aspects of Fluoride in Drinking-Water. Report No. CO5037. Marlow, Bucks, UK: Water Research Centre–National Sanitation Foundation (WRc-NSF) 2002 [Google Scholar]
- 54.Maguire A, Zohouri FV, Mathers JC, Steen IN, Hindmarch PN, Moynihan PJ. Bioavailability of fluoride in drinking water: a human experimental study. J Dent Res 2005;84:989–93 [DOI] [PubMed] [Google Scholar]
- 55.Greenland S, Robins J. Invited commentary: ecological studies – biases, misconceptions, and counterexamples. Am J Epidemiol 1994;139:747–60 [DOI] [PubMed] [Google Scholar]
- 56.Wakefield J. Ecologic studies revisited. Annu Rev Public Health 2008;29:75–90 [DOI] [PubMed] [Google Scholar]
- 57.Hac E, Czarnowski W, Gos T, Krechniak J. Lead and fluoride content in human bone and hair in the Gdansk region. Sci Total Environ 1997;206:249–54 [PubMed] [Google Scholar]