Abstract
Despite the common use of exercise as a weight loss strategy, little is known about its neuronal effects, and how these may be related to cognitive changes that impact food intake. The current study assessed the effects of a 6-month exercise intervention on intrinsic activity in the default mode network (DMN), a functionally connected network of brain regions including posterior cingulate cortex, cuneus/precuneus, medial prefrontal cortex, medial temporal lobe, and inferior parietal cortices, and salience network, which includes the anterior cingulate cortex and insula. Resting-state functional magnetic resonance imaging (fMRI) data were acquired in 12 overweight/obese individuals. The intervention was associated with a reduction in DMN activity in the precuneus (p=0.003, FWE-corrected), which was associated with greater fat mass loss (p=0.013) as well as reduced perceived hunger (Three Factor Eating Questionnaire, p=0.024) and hunger ratings in response to a meal (p=0.013). No changes were observed in the salience network in response to the exercise intervention. The association between DMN change and both fat mass loss and reduction of hunger ratings suggests that DMN function may be involved in the regulation of food intake behaviors. Given previous reports of DMN overactivity in overweight/obese individuals, the present findings may indicate an exercise-related “normalization” of network function.
Keywords: exercise, obesity, default network, salience network, fMRI
Introduction
Weight loss in obese individuals is associated with a reduction in comorbid conditions, such as cardiovascular disease and hypertension [1]. Weight loss can be difficult, however, and preventing subsequent weight regain even more challenging [2]. Understanding the neuronal mechanisms involved in food intake behaviors and weight loss could help to identify successful weight loss maintenance strategies.
Despite the common use of exercise as a weight loss strategy, little is known about the mechanisms of its effects. In addition to physiological effects such as reduced adiposity, reduced leptin, and improved insulin sensitivity [3-5] exercise exerts effects on the central nervous system (CNS). A potential mechanism through which exercise may induce cognitive and metabolic alterations is by increasing the expression of brain-derived neurotrophic factor (BDNF), critical for neurogenesis and synaptic plasticity [6,7]. Exercise may also increase serotonin levels, angiogenesis, and the production of other neurotrophic factors, such as insulin-like growth factor 1 (IGF-1) and fibroblast growth factor 2 (FGF-2) [6-8].
Neuroimaging studies have begun to identify the effects of exercise on neuronal function in humans. Davis et al. found 3 months of exercise to increase prefrontal cortex activity and decrease posterior parietal cortex activity during an executive function task [9]. Evero et al. found acute exercise to reduce the neuronal response to visual food cues in lean adults in brain regions important in food reward, including the insula [5]. We have recently reported that a 6-month exercise intervention was associated with significantly reduced neuronal response to visual food cues in overweight/obese adults in the insula and parietal cortex [4]. In addition to examining neuronal responses during specific cognitive tasks, our understanding of the neurobiology of food intake behaviors and weight-loss maintenance can be improved by studying the brain’s intrinsic network activity.
The human brain is organized into functionally connected networks, in which intrinsic activity can be measured either in the resting state or across tasks [10]. Studies are increasingly demonstrating the importance of these large-scale brain networks as fundamental organizational features of the human brain [11]. Understanding the function of these networks in overweight/obese individuals and changes in network activity associated with weight loss may provide insight into mechanisms of food intake behavior. Previous studies have found overweight/obese individuals to show altered activity in two commonly studied networks, the default mode network (DMN) and salience network (SN).
The DMN is a functionally connected network of brain regions including posterior cingulate cortex, cuneus/precuneus, medial prefrontal cortex, medial temporal lobe, and inferior parietal cortices [12]. Activity in this network is thought to reflect a baseline state of brain function, in which subjects are focused on their internal mental state, such as in self-relevant mentalizing and interoception[12]. We and others have previously found default network activity to be increased in obese and reduced-obese compared to lean individuals [13,14]. The impact of exercise on DMN function is, however, unknown.
The salience network is also of particular interest in obesity, given the relevance of this network to feeding behavior and reward [15,16]. The salience network includes the anterior cingulate cortex and insula, and is involved in assessing relevance of internal and external stimuli [10,15]. Increased salience network activation has been reported in obese individuals [16,17]. We previously found chronic exercise to be associated with significantly reduced activation in the insula in response to visual food cues [4]. Evero et al. found similar results following acute exercise [5]. The insula is a primary hub (part of a network with a high number of connections to other parts of the network) of the salience network. It is unknown, however, if exercise affects intrinsic, resting-state insula function, or if this effect is specific to task-related activation.
Given our previous finding of greater DMN activity in overweight/obese individuals [13], we hypothesized that a 6-month exercise intervention would be associated with a reduction in DMN activity. Since previous studies have found increased SN activity in overweight/obese individuals [16,17] and exercise has been found to reduce activity in the insula [4,5], a main hub of the SN, we also hypothesized that the intervention would be associated with a reduction in SN activity. Furthermore, we hypothesized that these reductions would be related to the amount of body fat lost during the intervention, with those showing the greatest physiological alterations also showing the greatest changes in intrinsic network activity.
Methods
Participants
Twelve overweight/obese adults (5 women, 7 men; mean body mass index (BMI) 33.3±4.3 mg/kg2; mean age 38.2±9.5 years) participated in the study. Participants were free of metabolic and psychiatric disease and eating disorders and not actively dieting. Participants provided informed consent and all procedures were approved by the Colorado Multiple Institutional Review Board.
Experimental design
The following measures were completed at baseline and after a 6-month exercise intervention: 3-day diet diary; body composition by dual-energy X-ray absorptiometry (DPX whole-body scanner, Lunar Radiation Corp.); resting metabolic rate (RMR) by standard hood indirect calorimetry (TrueOne 2400 metabolic cart, Parvometrics); food intake and eating-related behaviors; and neuronal responses as measured by functional magnetic resonance imaging (fMRI).
Exercise intervention
Participants were recruited from a larger study evaluating the effects of a 6-month exercise intervention on components of total daily energy expenditure. Individualized exercise prescriptions targeted 2500 kcal per week. Participants performed a supervised treadmill-walking program that gradually increased in intensity (60% to 75%) and duration (~15-20 min/day to 40-60 min/day) to achieve a target workload (500 kcal/day at 75% of V02max) by week 18. The exercise prescription was calculated from a maximal aerobic capacity test at baseline and updated according to submaximal tests every 6 weeks. Participants were required to attend more than 75% of the scheduled exercise sessions.
Behavioral, hormonal, and body measures
Measures were completed before and after the intervention, following an overnight fast and no exercise for 24 h, on a separate day from fMRI measures. Participants completed the Three Factor Eating Inventory (TFEQ [18]), Power of Food Scale (PFS [19]), Craving and Mood Questionnaire (CMQ [20]), and Food Craving Inventory (FCQ-S [21]). Fasting blood sampling was performed and analyzed for leptin concentration as determined by radioimmunoassay (Linco Research, Inc.). Participants also completed hunger, satiety, and prospective food consumption ratings by visual analog scale (VAS) before and every 30 min for 180 min following a test meal breakfast. The test meal was served at 7:30 AM and provided 30% of daily energy intake (55% carbohydrate, 35% fat, 15% protein), estimated using baseline RMR and lean body mass plus an activity factor of 1.4. The entire meal was required to be consumed and was prepared by the University of Colorado Clinical Translational Research Center kitchen.
Functional magnetic resonance imaging
Within a week of behavioral, hormonal, and body measures, participants completed imaging the morning after an overnight fast (approximately 8:00 AM; asked to not consume any food after 10:00 PM the night before). Measures were assessed in the fasted state to be consistent with prior studies examining resting-state networks in overweight/obese individuals [13,14]. Prior to scanning, fasting VAS appetite measures were performed. fMRI was performed using a GE 3.0T MR scanner. A high-resolution, T1-weighted 3D anatomical scan was acquired for each participant, after which functional images were acquired with an echo-planar gradient-echo T2* blood oxygenation level dependent (BOLD) imaging contrast technique, with TR = 2000 ms, TE = 30 ms, 642 matrix, 240 mm2 FOV, 27 axial slices angled parallel to the planum sphenoidale, 2.6 mm thick, 1.4 mm gap. An inversion-recovery echo-planar image (IR-EPI; TI = 505 ms) volume was acquired to improve coregistration between the echo-planar images and gray matter templates used in preprocessing. Participants completed fMRI during 10 minutes of rest with eyes open. Of the 12 participants completing resting-state scans, data from one participant in the first session were excluded due to technical difficulties. Final analyses included scans for 11 participants at baseline and 12 participants post-intervention.
Data analyses
fMRI data were preprocessed and analyzed using SPM8 (Wellcome Dept. of Imaging Neuroscience, London, UK). Functional data were realigned to the first echo-planar image, normalized to the Montreal Neurological Institute (MNI) template, using the gray-matter-segmented IR-EPI as an intermediate to improve registration, and smoothed with an 8 mm full width at half maximum (FWHM) Gaussian kernel. Group independent component analysis (ICA) was conducted using the GIFT toolbox (http:icatb.sourceforge.net). Data for each session (pre- and post-exercise) were processed separately. The dimensionality of the data from each subject was reduced using principle component analysis and concatenated into an aggregate dataset. Twenty independent components were estimated using the infomax algorithm [22], then individual subject ICA datasets were back-reconstructed. DMN and SN were identified using spatial sorting in GIFT to assess the spatial correlation to network masks, defined anatomically from the Wake Forest University Pickatlas (http://www.fmri/wfubmc.edu). The SN mask consisted of the anterior cingulate cortex and bilateral insula [14,15]. The DMN mask consisted of the lateral inferior parietal cortex, precuneus, posterior cingulate cortex, medial temporal cortex, frontal pole, and occipitotemporal junction [13,14]. For each network, the component with the highest correlation to the mask was selected for further analyses. Components for all participants, as z-score maps, were evaluated across the entire brain on a voxel-wise basis with directional contrasts (SPM t-contrasts). The term “activity” as used here reflects the amplitude of the network signals identified by ICA and spatial template matching. To restrict results to the network of interest, results were masked with the map of within-network brain regions demonstrating significant activity for all subjects (p<0.001, uncorrected). Results were considered significant at a whole-brain level if they exceeded a voxel-wise threshold of p<0.01 and cluster-level familywise error (FWE) correction for multiple comparisons of p<0.05.
Analyses for behavioral, hormonal, and body measures were performed with SPSS 21.0 (IBM Corp.). Total area under the curve for appetite VAS ratings using all post-meal time points was used. Effects of exercise as compared to baseline were compared with a paired t-test (alpha of 0.05). For correlations between fMRI and behavioral data, regression analyses were performed in SPSS using z-scores reflecting network activity extracted from SPM at the local maxima for each network of interest.
Results
Body composition and behavioral measures
Fat mass and percent body fat were significantly reduced following exercise (fat mass: 36.4±2.8 to 33.7±3.2kg, p=0.04; percent body fat: 36.5±1.9 to 34.4±2.0%, p=0.01), with a trend towards a reduction in body weight (101.5±4.9 to 98.7±5.8kg, p=0.09) [9]. There were also significant reductions in leptin concentration (32.1±5.7 to 20.3±4.6 ng/ml, p=0.03). No changes were observed in behavioral measures (see Table 1), but mean self-reported energy intake (3-day diet diary) was significantly reduced after exercise (2192±208 to 1980±159 kcal/day, p=0.049). This was still the case when expressed per fat free mass (36.7±2.7 to 31.4±2.0 kcal/day, p=0.046).
Table 1.
Eating behaviors and appetite
| Baseline | Post-Exercise | |||
|---|---|---|---|---|
| Measure | Mean | SEM | Mean | SEM |
| TFEQ: Restraint | 8.4 | 1.1 | 7.8 | 1.4 |
| TFEQ: Disinhibition | 7.9 | 1.2 | 7.5 | 1.0 |
| TFEQ: Hunger | 6.5 | 1.0 | 5.1 | 0.82 |
| CMQ | 37.9 | 7.4 | 35.2 | 5.6 |
| FCQ-S | 41.2 | 4.4 | 34.9 | 4.2 |
| PFS | 52.7 | 6.1 | 48.2 | 4.5 |
| Hunger AUC | 166.2 | 35.4 | 187.0 | 35.7 |
| Satiety AUC | 412.4 | 48.3 | 390.0 | 44.4 |
| PFC AUC | 222.7 | 37.2 | 226.8 | 32.8 |
TFEQ: Three Factor Eating Questionnaire; CMQ: Craving and Mood Questionnaire; FCQ-S: Food Craving Inventory; PFS: Power of Food Scale; AUC: area under the curve in response to a meal; PFC: prospective food consumption
fMRI
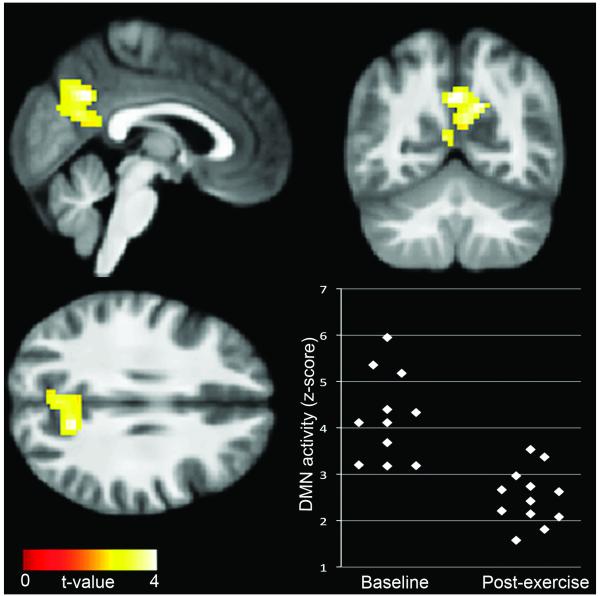
DMN activity across sessions was observed in the precuneus, lateral inferior parietal cortex, posterior cingulate cortex, and medial prefrontal cortex (see Figure, Supplemental Digital Content 1, which shows DMN and SN components), consistent with prior reports of the network [13,14,17]. SN activity was observed in the insula, anterior cingulate cortex, and middle frontal gyri, also consistent with prior studies [15-17]. As shown in Figure 1, the primary finding of the study was significantly decreased DMN activity in the precuneus following exercise, compared to baseline (t=4.50, p=0.003, FWE-corrected; x=-3, y=-61, z=34). No differences were observed in SN.
Figure 1.
Reduced default network activity in the precuneus post-exercise, compared to baseline. Data are thresholded at a voxel-wise threshold of p<0.01, and a cluster-extent threshold of p<0.01, FWE-corrected, and overlaid onto a group-average anatomical image for visualization.
Greater reduction in DMN activity was associated with greater reduction in fat mass (r=0.72, p=0.012). A trend towards DMN change being associated with weight change (r=0.58, p=0.064) also was observed. Greater reduction in DMN activity was also associated with a greater reduction in hunger, as measured by the TFEQ (r=0.77, p=0.024) and area under the curve measures of hunger response to a meal (r=0.82, p=0.013).
Discussion
The current study investigated the effects of a 6-month exercise intervention on intrinsic resting-state activity in functionally connected brain networks in overweight/obese individuals. The intervention was associated with a reduction in resting-state DMN activity, specifically in the precuneus, which was positively associated with fat mass loss and reduction in hunger ratings. No changes were observed, however, in resting-state SN activity. As previously reported [4], the exercise intervention was associated with significant reductions in fat mass and percent body fat, with a trend towards a reduction in body weight. Leptin concentrations were also significantly reduced following the intervention.
We previously found increased intrinsic DMN activity in overweight/obese individuals [13]. As such, the reduction in DMN activity in the precuneus observed in the current study may represent an exercise-related improvement or “normalization” of network function. The precuneus is a core hub of the DMN, thought to play a key role in internally-directed, self-referential processes [23]. Given previous speculation that increased DMN activity in overweight/obese individuals may indicate increased focus on internal states such as appetite or food-related cognitive factors [13], an exercise-related decrease in precuneus activity may reflect a reduction in this maladaptive behavior. Greater fat mass loss was associated with greater reductions in DMN activity, suggesting that those most responsive to exercise in terms of fat loss were also most responsive neuronally. Alternatively, it could be that the exercise intervention impacted DMN activity, which then influenced fat loss.
While mechanisms underlying neuronal effects of exercise are unknown, animal studies suggest that exercise promotes neurogenesis, neuroprotection, and synaptic plasticity, potentially through increased expression of BDNF [6-8]. Exercise-induced BDNF expression has been shown in cerebral cortex, cerebellum, and spinal cord (Vaynman, 2005}, but these effects are most robust in the hippocampus, which is part of the DMN [6,8]. As such, exercise-induced effects observed in the precuneus may result from the high degree of functional connectivity between the precuneus and hippocampus, even though effects were not observed in the hippocampus itself, possibly due to the decreased fMRI sensitivity in the region.
Interestingly, BDNF plays a critical role in homeostatic signaling and metabolic function, which could underlie the association between DMN alterations and fat mass loss. Low BDNF levels have been associated with obesity [6]. Additionally, exercise has been found to increase BDNF in both animal and human studies [6,7]. Both exercise and BDNF administration have been found to improve insulin sensitivity in animal models of obesity [3,6]. As such, exercise may affect metabolic factors and neuronal circuitry through induction of such neurotrophic factors as BDNF. In addition to BDNF, exercise may also exert positive effects on neuronal networks by reducing expression of inflammatory cytokines, increasing production of other neurotrophic factors (e.g., IGF-1 and FGF-2), or increasing neurotransmitter levels, such as serotonin or dopamine[6-8]. Although little is known regarding the neurochemical mechanisms of the DMN, studies have suggested the network to be modulated, at least in part, by both serotonin and dopamine [25]. This suggests a possible role for exercise-induced neurotransmitter effects on the DMN.
Laboratory measures of eating-related behaviors were not significantly altered following exercise, which could suggest the neuronal measures were a more sensitive predictor of appetitive changes, particularly given that DMN change was related to fat mass change. Changes in hunger ratings were also associated with DMN change. As noted, the precuneus plays an important role in internally-directed cognitive processes [23]. Thus, alterations in an area important in introspection may lead to changes in interpretations of hunger feelings, both in general, and in response to a meal. Measures of hunger were not significantly changed by the intervention, but this could again indicate the greater sensitivity of neuronal measures. That exercise effects were observed to a greater degree on neuronal responses than on behavioral or physiological responses may indicate sufficient intervention length/intensity for neuronal change, but not for less sensitive measures.
The current study did not find exercise to influence intrinsic SN activity. Our recent study of the effects of this intervention on neuronal responses during a visual food cues task did, however, find exercise to reduce activity in the insula, a main salience network hub [4]. Evero et al. also found acute exercise to reduce insula activation during a similar task [5]. This suggests that exercise alters task-specific activation of the insula, but not resting-state activity. The insula contains primary taste cortex and is involved in feeding behavior regulation, including food reward processing, neuronal responses to food stimuli, hunger, tastes, smells, and assignment of valence to external stimuli [2,4,5,26]. As such, it is not surprising that this area may show greater exercise-related changes in response to viewing food cues than during rest. While the DMN operates on internal stimuli, present in the resting state, the insula is more reactive to external sensory stimuli (e.g., taste), not as present in the resting state. It could be that exercise impacts SN activity when the network is more engaged, but that this is not evident during rest.
Sample size is a potential limitation of this study. However, the observed changes in intrinsic network activity following the intervention suggest that the sample size was sufficient to detect effects. Another potential limitation is a session effect; that is, resting-state activity could have changed over time, regardless of intervention. However, previous studies have found activity in intrinsic networks, including DMN and SN, to be reliable and consistent across fMRI recording sessions timed 45 minutes apart to 16 months apart [27-29]. That we did not see changes in SN between sessions also suggests that DMN decreases were not simply due to session effects. However, to investigate this possibility, we assessed DMN activity in a control group (N=12; mean BMI: 28.47±6.72; mean age: 41.58±13.20) not participating in the exercise study completing resting state fMRI on two separate occasions between 6 months and 1 year apart. No significant between-session differences were found in any areas of the DMN, further supporting that alterations were not due to session effects.
Conclusion
In conclusion, the current study found a 6-month exercise intervention to be associated with reduced DMN activity in the precuneus, during rest. This suggests that exercise can alter intrinsic, resting-state activity of functionally connected brain networks, independent of externally directed cognitive tasks. This is the first study to investigate the potential of exercise to alter obesity-related DMN impairments and supports a role for exercise in improved function of brain networks underlying human behavior. While no significant changes in short-term, laboratory measures of food intake behavior were observed, change in DMN activity was associated with greater reductions in fat mass and hunger ratings. The association between fat mass and DMN activity suggests activity of the network may be a marker for longer-term changes in food intake behavior. As such, further study of the involvement of intrinsic brain networks in regulation of food intake behaviors and weight maintenance and loss is warranted.
Supplementary Material
Acknowledgments
We thank Debra Singel and Yiping Du for their assistance with fMRI and Andrea Salzberg for her work on study design and data collection.
Funding: Funding for this study was provided by NIH/NCRR Colorado CTSI grant UL1 RR025780, NIH/NIDDK Clinical Nutrition Research Unit grant DK48520, and NIH/NIDDK grants R01DK07708, R01DK089095, and R01DK072174.
Footnotes
Conflicts of interest: None declared
References
- [1].Sjostrom L, Lindroos AK, Peltonen M, Torgerson J, Bouchard C, Carlsson B, et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004;351:2683–2693. doi: 10.1056/NEJMoa035622. [DOI] [PubMed] [Google Scholar]
- [2].Cornier MA. Is your brain to blame for weight regain? Physiol Behav. 2011;104:608–612. doi: 10.1016/j.physbeh.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Bradley RL, Jeon JY, Liu FF, Maratos-Flier E. Voluntary exercise improves insulin sensitivity and adipose tissue inflammation in diet-induced obese mice. Am J Physiol Endocrinol Metab. 2008;295:E586–594. doi: 10.1152/ajpendo.00309.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Cornier MA, Melanson EL, Salzberg AK, Bechtell JL, Tregellas JR. The effects of exercise on the neuronal response to food cues. Physiol Behav. 2012;105:1028–1034. doi: 10.1016/j.physbeh.2011.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Evero N, Hackett LC, Clark RD, Phelan S, Hagobian TA. Aerobic exercise reduces neuronal responses in food reward brain regions. J Appl Physiol. 2012;112:1612–1619. doi: 10.1152/japplphysiol.01365.2011. [DOI] [PubMed] [Google Scholar]
- [6].Vaynman S, Gomez-Pinilla F. License to run: exercise impacts functional plasticity in the intact and injured central nervous system by using neurotrophins. Neurorehabil Neural Repair. 2005;19:283–295. doi: 10.1177/1545968305280753. [DOI] [PubMed] [Google Scholar]
- [7].Hillman CH, Erickson KI, Kramer AF. Be smart, exercise your heart: exercise effects on brain and cognition. Nat Rev Neurosci. 2008;9:58–65. doi: 10.1038/nrn2298. [DOI] [PubMed] [Google Scholar]
- [8].van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci U S A. 1999;96:13427–13431. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Davis CL, Tomporowski PD, McDowell JE, Austin BP, Miller PH, Yanasak NE, et al. Exercise improves executive function and achievement and alters brain activation in overweight children: a randomized, controlled trial. Health Psychol. 2011;30:91–98. doi: 10.1037/a0021766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Bressler SL, Menon V. Large-scale brain networks in cognition: emerging methods and principles. Trends Cogn Sci. 2010;14:277–290. doi: 10.1016/j.tics.2010.04.004. [DOI] [PubMed] [Google Scholar]
- [11].Sporns O. The human connectome: a complex network. Ann N Y Acad Sci. 2011;1224:109–125. doi: 10.1111/j.1749-6632.2010.05888.x. [DOI] [PubMed] [Google Scholar]
- [12].Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- [13].Tregellas JR, Wylie KP, Rojas DC, Tanabe J, Martin J, Kronberg E, et al. Altered default network activity in obesity. Obesity (Silver Spring) 2011;19:2316–2321. doi: 10.1038/oby.2011.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kullmann S, Heni M, Veit R, Ketterer C, Schick F, Haring HU, et al. The obese brain: association of body mass index and insulin sensitivity with resting state network functional connectivity. Hum Brain Mapp. 2012;33:1052–1061. doi: 10.1002/hbm.21268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Garcia-Garcia I, Jurado MA, Garolera M, Segura B, Sala-Llonch R, Marques-Iturria I, et al. Alterations of the salience network in obesity: A resting-state fMRI study. Hum Brain Mapp. 2012 doi: 10.1002/hbm.22104. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Kullmann S, Pape AA, Heni M, Ketterer C, Schick F, Haring HU, et al. Functional network connectivity underlying food processing: disturbed salience and visual processing in overweight and obese adults. Cereb Cortex. 2013;23:1247–1256. doi: 10.1093/cercor/bhs124. [DOI] [PubMed] [Google Scholar]
- [18].Stunkard AJ, Messick S. The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. J Psychosom Res. 1985;29:71–83. doi: 10.1016/0022-3999(85)90010-8. [DOI] [PubMed] [Google Scholar]
- [19].Lowe MR, Butryn ML, Didie ER, Annunziato RA, Thomas JG, Crerand CE, et al. The Power of Food Scale. A new measure of the psychological influence of the food environment. Appetite. 2009;53:114–118. doi: 10.1016/j.appet.2009.05.016. [DOI] [PubMed] [Google Scholar]
- [20].Sobik L, Hutchison K, Craighead L. Cue-elicited craving for food: a fresh approach to the study of binge eating. Appetite. 2005;44:253–261. doi: 10.1016/j.appet.2004.12.001. [DOI] [PubMed] [Google Scholar]
- [21].White MA, Whisenhunt BL, Williamson DA, Greenway FL, Netemeyer RG. Development and validation of the food-craving inventory. Obes Res. 2002;10:107–114. doi: 10.1038/oby.2002.17. [DOI] [PubMed] [Google Scholar]
- [22].Bell AJ, Sejnowski TJ. An information-maximization approach to blind separation and blind deconvolution. Neural Comput. 1995;7:1129–1159. doi: 10.1162/neco.1995.7.6.1129. [DOI] [PubMed] [Google Scholar]
- [23].Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129:564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- [24].Pieramico V, Esposito R, Sensi F, Cilli F, Mantini D, Mattei PA, et al. Combination training in aging individuals modifies functional connectivity and cognition, and is potentially affected by dopamine-related genes. PLoS One. 2012;7:e43901. doi: 10.1371/journal.pone.0043901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Hahn A, Wadsak W, Windischberger C, Baldinger P, Hoflich AS, Losak J, et al. Differential modulation of the default mode network via serotonin-1A receptors. Proc Natl Acad Sci U S A. 2012;109:2619–2624. doi: 10.1073/pnas.1117104109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Hinton EC, Parkinson JA, Holland AJ, Arana FS, Roberts AC, Owen AM. Neural contributions to the motivational control of appetite in humans. Eur J Neurosci. 2004;20:1411–1418. doi: 10.1111/j.1460-9568.2004.03589.x. [DOI] [PubMed] [Google Scholar]
- [27].Damoiseaux JS, Rombouts SA, Barkhof F, Scheltens P, Stam CJ, Smith SM, et al. Consistent resting-state networks across healthy subjects. Proc Natl Acad Sci U S A. 2006;103:13848–13853. doi: 10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Chen S, Ross TJ, Zhan W, Myers CS, Chuang KS, Heishman SJ, et al. Group independent component analysis reveals consistent resting-state networks across multiple sessions. Brain Res. 2008;1239:141–151. doi: 10.1016/j.brainres.2008.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Shehzad Z, Kelly AM, Reiss PT, Gee DG, Gotimer K, Uddin LQ, et al. The resting brain: unconstrained yet reliable. Cereb Cortex. 2009;19:2209–2229. doi: 10.1093/cercor/bhn256. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.