Abstract
Glycoproteins expressed by Cryptosporidium parvum are immunogenic in infected individuals but the nature of the epitopes recognised in C. parvum glycoproteins is poorly understood. Since a known immunodominant antigen of Cryptosporidium, the 17 kDa glycoprotein, has previously been shown to bind to lectins that recognise the Tn antigen (GalNAcα1-Ser/Thr-R), a large number of glycopeptides with different Tn valency and presentation were prepared. In addition, glycopeptides were synthesised based on a 40 kDa cryptosporidial antigen, a polymorphic surface glycoprotein with varying numbers of serine residues, to determine the reactivity with sera from C. parvum-infected humans. These glycopeptides and non-glycosylated peptides were used to generate a glycopeptide microarray to allow screening of sera from C. parvum-infected individuals for the presence of IgM and IgG antibodies. IgG but not IgM in sera from C. parvum-infected individuals bound to multivalent Tn antigen epitopes presented on glycopep-tides, suggesting that glycoproteins from C. parvum that contain the Tn antigen induce immune responses upon infection. In addition, molecular differences in glycosylated peptides (e.g. substituting Ser for Thr) as well as the site of glycosylation had a pronounced effect on reactivity. Lastly, pooled sera from individuals infected with either Toxoplasma or Plasmodium were also tested against the modified Cryptosporidium peptides and some sera showed specific binding to glycopeptide epitopes. These studies reveal that specific anti-glycopeptide antibodies that recognise the Tn antigen may be useful diagnostically and in defining the roles of parasite glycoconjugates in infections.
Keywords: Cryptosporidium parvum, Tn antigen, Glycopeptide microarrays, Toxoplasma, Plasmodium
1. Introduction
Cryptosporidium parvum is a protozoan parasite that infects the epithelial cells of the small intestine causing diarrheal illness in humans. It has a worldwide distribution and is considered an emerging zoonosis with reservoirs in cattle, domestic animals and in faecally-contaminated environments (Davies and Chalmers, 2009). Transmission via the faecal–oral route results from the ingestion of Cryptosporidium oocysts through the consumption of contaminated food or water (drinking and recreational water) or through direct person-to-person or animal-to-person contact. In immunocompetent individuals, cryptosporidiosis is usually an acute self-limiting gastroenteritis, resolving within 2–3 weeks. Symptoms include diarrhoea (3–6 stools per day), weight loss, fever and fatigue. In immunocompromised persons, however, infection is associated with more persistent symptoms and serious illness, especially in those with HIV, primary immunodeficiency or those undergoing solid-organ transplantation (Leitch and He, 2012). Drug treatment is very limited; nitazoxanide has demonstrated some efficacy in immunocompetent individuals and is approved for children under the age of 12 years. However, it has not proven effective for treatment of immunocompromised persons (Huang et al., 2004; Fox and Saravolatz, 2005). Additionally, no vaccines are currently available for use against C. parvum.
While sequencing of the Cryptosporidium genome has facilitated the characterisation of the protein components of these antigens, little is known about the glycan moieties in parasite glycoconjugates. Several C. parvum membrane proteins/antigens that are associated with invasion and/or protection include various mucin-like proteins (Boulter-Bitzer et al., 2007). Two Cryptosporidium immunodominant antigens, Cp17 and Cp40, are major surface glycoproteins (Cevallos et al., 2000; Priest et al., 2000; Strong et al., 2000) that carry multiple GalNAcα1-Ser/Thr-R epitopes, here termed O-linked GalNAc glycans. They also represent potential vaccine targets (Benitez et al., 2009; Manque et al., 2011). These antigens are encoded by a single gene, Cp40/17, and subsequently cleaved into the Cp40 and Cp17 proteins. Within the Cp40 antigen there is a hypervariable region with predicted O-glycosylation sites following the conserved polyserine domain (Leav et al., 2002). The C. parvum 17 kDa antigen glycosylphosphatidylinositol (GPI) anchor is composed of a very basic GPI anchor core comprised of Mana1–2Manα1–6Manα1–4-glucosamine (Priest et al., 2006), but there are also minor GPI phospholipids that are recognised by serum antibodies in infected humans (Priest et al., 2003).
In the present study, antibody responses were evaluated against GalNAc-containing glycopeptides using defined synthetic glycan microarrays as a mechanism for characterising and identifying glycans that may be important antigenic components. Additionally, we synthesised glycopeptides of the Cp15/17 and Cp40/45 antigens, including novel synthetic poly-Tn sequences, based on the observations that these two immunodominant antigens contain potential Tn (GalNAcα-Ser/Thr-R) antigen sites (Gut and Nelson, 1999) and that sera from infected individuals reacted to glycosylated antigens (Moss et al., 1994). Our results show that such defined glycopeptide microarrays can help to characterise the effect of glycosylation on immunoreactivity and could be useful in future developments of diagnostic assays and understanding of host–parasite interactions.
2. Materials and methods
2.1. Synthesis of peptides
Cp17 and Cp40 Tn glycopeptides were synthesised by standard Fmoc-protected solid phase peptide synthesis (Liu et al., 2005; Bolscher et al., 2010; Borgert et al., 2012). The glycosylated Ser or Thr residues (Sussex Research Laboratories, Ontario, Canada) were incorporated into the growing peptide chain in the same manner as the non-glycosylated sequences, creating defined site-specific glycosylation, meaning that the glycosylated residues could be added at specific places in the sequence rather than indiscriminately enzymatically glycosylating a whole peptide. This allowed us to determine which glycosylation site(s) is important for binding. A unique feature of the Cp40 peptides is the repeating poly-Ser-GalNAc residues, where multiple glycosylated serine residues are found in sequence in the native protein and which were therefore synthesised (Strong et al., 2000). These long chains of Ser-GalNAc were difficult to synthesise and required longer and repeated reaction times compared with standard Fmoc synthesis, and have not been previously reported to be synthesised in the literature. However, peptides were made with up to 23 Ser-GalNAc residues in succession to compare the differential binding to these varying glycosylated repeats. Cp17 #1–7 contained different combinations of glycosylated Ser/Thr residues, and Cp17 #9 and 10 had the Ser and Thr residues switched from the native sequence to determine whether the GalNAc linkage to the Ser or Thr is critical for binding. Cp40 #1, 3 and 5 contained 7, 16 and 23 repeating units of Ser-GalNAc, respectively. As controls, the non-glycosylated sequences were also produced and printed (Cp17 #8 and 10, Cp40 #2, 4 and 6).
2.2. Glycan and glycopeptide arrays
To identify glycans and glycopeptides that are recognised by antibodies in serum of Cryptosporidium-infected people compared with uninfected people, multiple sets of glycan and glycopeptide microarrays were used. The mammalian glycan microarray avail able from the Consortium for Functional Glycomics (CFG) (http://www.functionalglycomics.org/static/index.shtml) was comprised of over 500 glycans. A mannose-6-phosphate array containing high mannose-type N-glycans of defined structure containing zero, one or two mannose-6-phosphate-GlcNAc phosphodiester or mannose-6-phosphate phosphomonoester residues was utilised (Bohnsack et al., 2009; Song et al., 2009a). A Tn antigen (GalNAc-Ser/Thr) glycopeptide array was constructed with a variety of glycopeptides and control peptides, containing sequences of various origins that are O-glycosylated at Ser/Thr sites (Borgert et al., 2012). The Tn glycopeptide array was expanded to contain the new Cp17- and Cp40-specific sequences (see Table 1) as well as recombinant Cp17 and Cp23 proteins (printed at 200 lg/ml) (Priest et al., 1999, 2000).
Table 1.
Synthesis of Cryptosporidium parvum Cp17 and Cp40 peptides. Cryptosporidial peptides Cp17 (Cp17 #1–10) and Cp40 (Cp40 #1–6) were synthesised with different Tn valency (Cp17) and length of repeating serine residues (Cp40) to determine how these groups affect sera antibody binding.
| Peptide number | Sequence, (*) denotes Tn antigen on S or T |
|---|---|
| Cp17_#1 | H-ETS*EAAAT*VDLFAFT*LDGGK-NH2 |
| Cp17_#2 | H-ETSEAAAT*VDLFAFTLDGGK-NH2 |
| Cp17_#3 | H-ETS*EAAATVDLFAFT*LDGGK-NH2 |
| Cp17_#4 | H-ETSEAAATVDLFAFT*LDGGK-NH2 |
| Cp17_#5 | H-ETS*EAAAT*VDLFAFTLDGGK-NH2 |
| Cp17_#6 | H-ETSEAAArVDLFAFTLDGGK-NH2 |
| Cp17_#7 | H-ETS*EAAATVDLFAFTLDGGK-NH2 |
| Cp17_#8 | H-ETSEAAATVDLFAFTLDGGK-NH2 |
| Cp17_#9 | H-ETT*EAAAS*VDLFAFS*LDGGK-NH2 |
| Cp17_#10 | H-ETTEAAASVDLFAFSLDGGK-NH2 |
| Cp40_#1 | H-DVPVEGSS* (7)TSTVAPANK-NH2 |
| Cp40_#2 | H-DVPVEGSS(7)TSTVAPANK-NH2 |
| Cp40_#3 | H-DVPVEGSS* (16)TSTVAPANK-NH2 |
| Cp40_#4 | H-DVPVEGSS(16)TSTVAPANK-NH2 |
| Cp40_#5 | H-DVPVEGSS* (23)TSTVAPANK-NH2 |
| Cp40_#6 | H-DVPVEGSS(23)TSTVAPANK-NH2 |
Glycans containing a reducing alkyl amine or homogeneous peptides and glycopeptides containing N-terminal amino groups or lysine residues were directly printed by covalent attachment to microarray slides containing N-hydroxysuccinimide derivatised surfaces (Song et al., 2009a,b). For peptide/glycopeptide micro-array studies, all materials were printed at 100 μM concentration (except as noted above), as previously described (Song et al., 2008, 2009b) using precision printing by a piezoelectric printing approach in which 1/3 nl of samples were deposited in ~100 micron-sized spots. The slides were washed, dried, and stored indefinitely in anhydrous conditions.
2.3. Human sera
For Cryptosporidium sera, individual, anonymised sera were used for this study. Positive samples (P; n = 10) were collected during two cryptosporidiosis outbreaks from donors who met the outbreak case definition but who were not stool-confirmed. Five additional positive sera (G) were included from patients who reacted strongly to the C. parvum glycosylinositol phospholipids (GIPLs) (Priest et al., 2003). These samples were collected during three different cryptosporidiosis outbreaks and included two donors who were stool-confirmed. All positive samples (collectively called P samples) were assayed using the ‘gold standard’ western blot assay (Moss et al., 1998) and were determined to be strongly reactive. Negative samples (N; n = 5) were from western blot negative donors. Additionally, pools of high titer serum samples (10 serum samples per pool) from individuals acutely infected with Plasmodium falciparum or Toxoplasma gondii were tested. All serum samples were diluted 1:100 before analysis and IgM and IgG binding assays were quantitated separately, based on previous CFG array data using serum and preliminary data to optimise the signal. Written informed consent was obtained from the C. parvum outbreak study participants and the study was approved by the Centers for Disease Control and Prevention Institutional Review Board, USA.
2.4. Analysis of sera
For analysis on the CFG glycan microarray slides, the human serum samples were diluted 1:100 in TSM binding buffer (20 mM Tris-HCl pH 7.4, 150 mM sodium chloride, 2 mM calcium chloride, 2 mM magnesium chloride, 1% BSA, 0.05% Tween 20) and 70 μl was added to the array slide for 1 h at room temperature in a humidified chamber. Slides were washed four times with TSM wash buffer 1 (20 mM Tris–HCl pH 7.4, 150 mM sodium chloride, 2 mM calcium chloride, 2 mM magnesium chloride, 0.05% Tween 20) and four times with TSM wash buffer 2 (20 mM Tris–HCl pH 7.4, 150 mM sodium chloride, 2 mM calcium chloride, 2 mM magnesium chloride). Samples were detected with fluorescently labelled anti-human IgG antibody (Invitrogen, USA; 5 μg/ml) for 1 h at room temperature in a humidified chamber and then washed as above, followed by a water wash. Dried slides were read on a Perk-inElmer ProScanArray XL4000 microarray-scanner. For the other types of arrays, printed microarray slides were covered with Flex-Well-16 (Grace Bio-Labs, USA) chambers. Wells were rehydrated with TSM wash buffer 1. The wells were incubated with the human serum diluted 1:100 in TSM binding buffer and added to the wells for 1 h at room temperature on an orbital rotator. After washing five times with TSM wash buffer 1 and five times with TSM wash buffer 2, the slides were incubated with fluorescently labelled anti-human IgG (Alexa-633 or Alexa-488), IgM (Alexa-594 or Alexa-647), and IgA (FITC) antibodies (Invitrogen, 5 μg/ml) for 1 h at room temperature on an orbital rotator and then washed as above. Dried slides were read on a PerkinElmer ProScanArray XL4000 microarray-scanner at appropriate wavelengths for each label. Images were analysed with quantitation software (ScanArrayExpress, Perkin Elmer and Imagene, BioDiscovery) (Smith et al., 2010; Heimburg-Molinaro et al., 2011).
2.5. Statistical analysis
Data from sera reactivity to microarray slides were analysed by performing log transformations of the data and comparing positive and negative data sets using the Mann–Whitney non-parametric tests (GraphPad Prism or Stat 9 software). P < 0.05 was considered significant.
3. Results
3.1. Reactivity of serum antibodies screened against glycan microarrays
Sera from 15 cryptosporidiosis patients (five G and 10 P samples) and five normal controls were screened against several glycan libraries. The arrays included the CFG version 4.2 array, consisting of 511 glycans (http://www.functionalglycomics.org/static/index.shtml) and a mannose/mannose-6-phosphate (Man/M6P) glycan microarray (Song et al., 2012). On the Man/M6P glycan microarray array, binding was observed but no overall pattern differences emerged between positive and normal control sera (data not shown). For the CFG array, only a subset of samples was tested. GIPL-reactive serum samples (n = 2) bound weakly to a few blood group-related glycans such as lacto-N-tetraose (LNT), blood group A- and H-related structures, glycans with terminal α-Gal structures and charged lactosamine structures (3-sulfated Galβ1–3GlcNAc, KDNα2–3Galβ1–3GlcNAc). Overall the binding was only marginally greater than the negative controls and as with the M/M6P arrays, no distinct patterns in reactivity were observed. Anti-glycan antibodies have been found previously in pooled IgG from normal control sera and perhaps represent the common anti-blood group type of immunoglobulins to be expected (von Gunten et al., 2009). These results indicate that sera from control and Cryptosporidium-infected individuals do not strongly target the wide range of isolated or peptide-bound glycans represented in these micro-arrays in the absence of specific cryptosporidial peptide backbones.
3.2. Distinct reactivity of serum antibodies observed against Tn glycopeptides
The most distinct binding was generated by screening the glycopeptide microarray that contained glycopeptides presenting the Tn antigen. Of the 15 positive serum samples (including all five G sera), reactivity was seen to three distinct compounds: an α-dystroglycan-derived peptide modified with four adjacent GalNAc-threonine residues Ac-PPT*T*T*T*KKP-HN (#13) (*=GalNAc residue on the amino acid), and two MUC-based peptides, glycopeptides #6 and #7 (displaying two and three adjacent GalNAc-threonines, respectively). Normal control sera showed little reactivity (Table 2), especially to #13. IgG antibodies in several normal control samples bound to two constructs that have three repeating Tn antigens, HG(d-Ala)K(cyclohexyl-Ala)VAAWTLKAA(d-Ala)T*T*T*G-NH2 (#19) and Ac-T*T*T*-NH(CH2)3NH2 (#20). Additionally, a low level of IgA binding was observed in some of the positive samples, directed mainly at peptide #13, Ac-PPT*T*T*T*KKP-HN (four adjacent Tn antigens, data not shown). No significant binding of IgM antibodies was detected in any of the samples (data not shown). Most IgG anti-Tn reactivity was directed to glycopeptides with multiple Gal-NAc residues (some adjacent on Thr/Ser residues) and some closely spaced GalNAc residues. These results suggested that sera from Cryptosporidium-infected individuals contained antibodies that interact with particular multivalent Tn antigen epitopes presented on peptides and that Cryptosporidium antigens may express glycoepitopes or glycopeptides that contain the Tn antigen. Through lectin binding studies, C. parvum sporozoites have been shown to contain Tn antigen-positive glycopeptides and these may play a role in invasion (Gut and Nelson, 1999). We therefore opted to synthesise peptides with different Tn groups on the 17 kDa antigen (a surface glycoprotein with possible Tn antigen sites) and the 40-kDa antigen (a polymorphic surface glycoprotein with different serine residues) to see how these changes in glycosylation and serine residues affect antibody binding.
Table 2.
Glycan array analysis of anti-glycan antibodies in human sera from Cryptosporidium sp.-infected individuals. Reactivity of human sera from individuals seropositive for cryptosporidiosis (n = 15), as well as from healthy blood donors (n = 5) were diluted 1:100 and analysed for the presence of anti-glycan IgG antibodies using a Tn glycopeptide array. Three distinct compounds, a peptide with four adjacent GalNAc-threonine residues Ac-PPT*T*T*T*KKP-HN (#13), and two MUC-based peptides, glycopeptides #6 and #7 (displaying two and three adjacent GalNAc-threonines, respectively) were found to have distinct reactivity.
| Mean RFU ±
S.E.M. |
P value | ||
|---|---|---|---|
| G, P samples | N samples | ||
| Peptide #6 | 179 ± 107 | 25.6 ± 3.6 | P < .093 |
| Peptide #7 | 432 ± 235 | 18.60 ± 1.86 | P < .006 |
| Peptide #13 | 1953 ± 1231 | 432 ± 235 | P < .003 |
RFU, relative fluorescent units.
3.3. Synthesis of Tn peptides and reactivity of Cryptosporidium sera
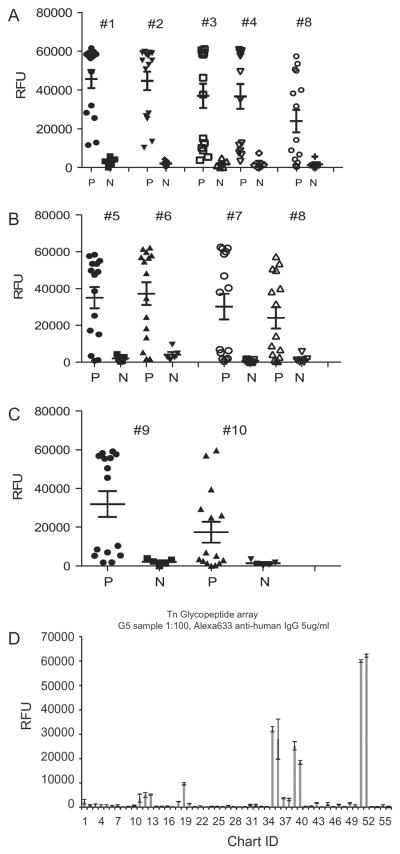
As shown in Table 1, glycopeptides of the C. parvum glycoprotein antigens Cp17 and Cp40 were successfully synthesised with different Tn valency (Cp17) and varying lengths of repeating serine residues (Cp40) to determine how these groups may affect serum binding. For the Cp17 glycopeptides, differences in glycosylation and Tn expression significantly affected the binding of serum antibodies to the immunodominant 17 kDa antigen in some individual samples. In general, mean increases in reactivity in sera from infected humans were observed for glycosylated peptides (Fig. 1) with approximately a two-fold increase overall in the average response to glycosylated peptides #1–4 versus non-glycosylated (#8) in the P samples. Increases were significant or approached significance (see Fig. 1 for P values). However, in some individuals, glycosylation of the second and third threonines (#1 and #2) resulted in up to a five- or 10-fold increase in serum reactivity. For example in one individual serum sample reactivity was 49,452 and 50,767 relative fluorescent units (RFU) for glycosylated peptides #1 and #2 binding respectively, versus 6,755 RFU for the non-glycosylated peptide (#8). Likewise, the antibody binding for another individual sample was 28,314 RFU and 26,567 RFU for glycosylated peptides 1 and 2, respectively, versus 2,606 RFU for nonglycosylated peptide (#8).
Fig. 1.
Reactivity to Cryptosporidium parvum 17 kDa synthetic peptides with different Tn glycosylation. (A) Differences in serum responses to the glycosylated Cp17 peptides #1–4 compared with non-glycosylated peptide #8 (P < 0.04, 0.04, 0.04 and 0.05, respectively, compared with #8). (B) Responses to the glycosylated Cp17 peptides #5–7 compared with non-glycosylated peptide #8 (P < 0.07, 0.4 and 0.075, respectively). (C) Responses to the glycosylated Cp17 peptide #9 compared with non-glycosylated peptide #10 (P < 0.06). Sera from cryptosporidiosis patients who were antibody positive (P) (n = 15), as well as from antibody-negative healthy blood donors (N) (n = 5) were diluted 1:100 and analysed for the presence of anti-IgG antibodies using the printed array of synthesised Cp17 peptides (Table 1). (D) Representative example of Tn glycopeptide array data for one patient serum sample. RFU, relative fluorescent units. See Supplementary Table S1 for complete data set. Chart ID numbers 12, 13, 19 and 35–40 where binding is observed all contain varying numbers and presentations of the Tn antigen.
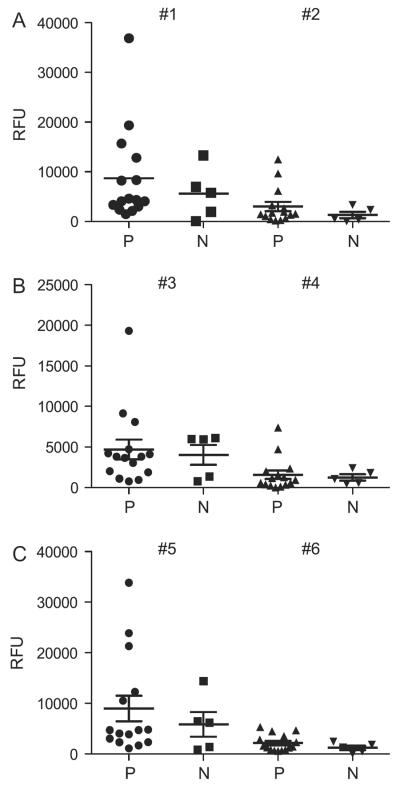
We compared the response of each serum sample to the specific Cp17 and Cp40 glycopeptides (patient versus control and glycosylated versus non-glycosylated) and analysed the statistical distribution between the groups. Significant reactivity to the Cp17 peptides and glycopeptides (#1–10) was observed in the patient sera compared with the normal control (P < 0.05) and the response was higher to the glycosylated compared with the non-glycosylated peptides. Although differences in responses to the Cp40 peptide between positive and negative sera were not as distinct, significant increased reactivity of positive sera was observed for the glycosylated peptides compared with the non-glycosylated peptides (Cp40 #1 versus Cp40 #2 peptides, P < 0.0032; Cp40 #3 versus Cp40 #4 peptides, P < 0.0094; Cp40 #5 versus Cp40 #6 peptides, P < 0.0026) (Fig. 2). The patient reaction to the non-glycosylated Cp17 peptides also indicates that these C. parvum proteins are immunogenic even without the glycans present. The relative lack of reactivity to the non-glycosylated Cp40 peptide by both positive and negative sera suggests that this portion of the Cp40 protein in a non-glycosylated form may be less immunogenic, while the glycosylated form was significantly more reactive with both sera.
Fig. 2.
Reactivity to Cryptosporidium parvum 40 kDa synthetic peptides with different Tn valency. (A) Differences in serum responses to the glycosylated Cp40 peptide #1 compared with the non-glycosylated Cp40 peptide #2 (P < 0.0032). (B) Glycosylated Cp40 peptide #3 compared with the non-glycosylated Cp40 peptide #4 (P < 0.0094). (C) Glycosylated Cp40 peptide #5 compared with the non-glycosylated Cp40 peptide #6 (P < 0.0026). Sera from cryptosporidiosis patients who were antibody positive (P) (n = 15), as well as from antibody-negative healthy blood donors (N) (n = 5) were diluted 1:100 and analysed for the presence of anti-IgG antibodies using the printed array of synthesised Cp40 peptides (Table 1). RFU, relative fluorescent units.
3.4. Reactivity of serum antibodies from Toxoplasma- and Plasmodium-infected individuals screened against the Cryptosporidium glycan microarrays
Pooled serum samples from individuals with known infections of T. gondii and P. falciparum were also evaluated for reactivity against the expanded Tn glycopeptide array (Table 3). Strong binding of the Toxoplasma and Plasmodium pools to the Cp17 peptide set was observed. In particular, increased binding was observed to the peptides with glycosylated threonines, particularly the second glycosylated threonine (Cp17 #1–4) compared with the nonglycosylated peptide (Cp17 #8, #10). Sera showed lower binding to glycopeptides lacking glycosylation on the second glycosylated threonine (Cp17 #5–7). Lower reactivity was also observed when the glycosylated threonines were substituted with serine (Cp17 #9), similar to reactivity in sera from Cryptosporidium-infected individuals against non-glycosylated sequences. For the Cp40 peptides, binding was modest and less distinct. The T. gondii-infected serum antibodies bound noticeably more to the sequences with 16 and 23 repeating GalNAc-Ser (9,540 RFUs and 13,020 RFUs, respectively) compared with non-glycosylated peptides (2,333 and 1,136 RFUs, respectively), whereas the P. falciparum sera showed no marked increased reactivity to the repeating GalNAc-Ser peptides (data not shown).
Table 3.
Reactivity (relative fluorescent units; RFU) of pooled human sera from individuals seropositive for cryptosporidiosis, toxoplasmosis and malaria. Pooled serum samples from individuals with known infections of Cryptosporidium sp., Toxoplasma gondii and Plasmodium falciparum were evaluated for reactivity against the Tn glycopeptide array. SD, standard deviation for toxoplasma and plasmodium data.
| Peptide | Sequence | Cryptosporidium sera (RFU) | Negative sera (RFU) | Toxoplasma sera (RFU) | SD | Plasmodium sera (RFU) | SD |
|---|---|---|---|---|---|---|---|
| Cp17_#1 | H-ETS*EAAAT*VDLFAFT*LDGGK-NH2 | 57,119 | 3,230 | 27,938 | 3,312 | 51,097 | 4,736 |
| Cp17_#2 | H-ETSEAAAT*VDLFAFT*LDGGK-NH2 | 55,206 | 2,369 | 21,454 | 4,694 | 38,133 | 11,834 |
| Cp17_#3 | H-ETS*EAAATVDLFAFT*LDGGK-NH2 | 46,362 | 974 | 35,579 | 2,017 | 57,692 | 877 |
| Cp17_#4 | H-ETSEAAATVDLFAFTLDGGK-NH2 | 49,458 | 1,329 | 24,072 | 2,674 | 56,813 | 550 |
| Cp17_#5 | H-ETS*EAAAT*VDLFAFTLDGGK-NH2 | 47,425 | 1,741 | 4,078 | 975 | 7,031 | 6,967 |
| Cp17_#6 | H-ETSEAAAT*VDLFAFTLDGGK-NH2 | 48,340 | 2,628 | 9,086 | 1,719 | 16,292 | 5,424 |
| Cp17_#7 | H-ETS*EAAATVDLFAFTLDGGK-NH2 | 37,957 | 822 | 3,524 | 3,001 | 2,150 | 1,126 |
| Cp17_#8 | H-ETSEAAATVDLFAFTLDGGK-NH2 | 14,250 | 1,112 | 3,691 | 1,422 | 2,402 | 1,974 |
| Cp17_#9 | H-ETT*EAAAS*VDLFAFS*LDGGK-NH2 | 45,472 | 2,237 | 3,675 | 843 | 7,391 | 2,626 |
| Cp17_#10 | H-ETTEAAASVDLFAFSLDGGK-NH2 | 5,239 | 1,115 | 2,324 | 938 | 2,416 | 2,319 |
| Cp17 | Recombinant Cp17 protein control | 60,353 | 13,384 | 24,683 | 3,066 | 57,535 | 3,698 |
| Cp23 | Recombinant Cp23 protein control | 58,873 | 3,877 | 39,228 | 2,031 | 58,923 | 2,974 |
| PBS | 425 | 254 | 350 | 344 | 380 | 743 | |
| Tn3-linker | Ac-T*T*T*-CONH2(CH2)3NH2 | 9,618 | 477 | 2,204 | 1,500 | 11,346 | 13,493 |
| Tn-linker | Ac-T*-CONH2(CH2)3NH2 | 1,532 | 73 | 638 | 300 | 1,178 | 1,483 |
| S-GalNAc | C41H72N8O22 | 123 | 82 | 710 | 145 | 845 | 1,965 |
| T-GalNAc | C17H32N4O8 | 165 | 188 | 964 | 1,037 | 355 | 1,063 |
4. Discussion
Little is known overall about the structures of the glycomes of Cryptosporidium. GalNAc and mannose are important components in several parasitic surface antigens (Casaravilla et al., 2003) including the GPI anchor of C. parvum 17 kDa antigen (Priest et al., 2006). In this study we began by using multiple sets of existing glycan and glycopeptide microarrays to analyse antibody recognition, including the CFG glycan array. However binding was relatively modest and an overall pattern could not be established among the different sera.
The most distinct binding occurred with glycopeptides expressing the Tn antigen which is among the common cores of all O-glycans in animal cells. It is usually terminally extended to contain galactose and other sugars on normal cells but on neoplastic cells the unextended Tn antigen is often observed (Ju et al., 2011). It is interesting that animal hosts do not commonly express the Tn antigen. However, Tn antigen has been identified in other parasites including Schistosoma mansoni (Nyame et al., 1987), Echinococcus granulosus (Alvarez et al., 2001), the cestode responsible for cystic echinococcosis, and Fasciola hepatica (Freire et al., 2003). In C. parvum infection, it was shown that T or Tn antigen reactive lectins irreversibly inhibit the invasion of epithelial cells by C. parvum, suggesting that Tn antigen-containing sporozoite surface glycoproteins play an important role in infectivity and could be neutralising targets for antibodies (Gut and Nelson, 1999). While most of the reactivity seen in our glycan/glycopeptide arrays was due to IgG, a low level IgA response was observed in some of the positive samples, directed mainly at peptide #13 with four adjacent GalNAc-Thr residues. IgA reactive to Cryptosporidium antigens has been found in the sera and faeces of individuals infected with Cryptosporidium (Priest et al., 1999; Dann et al., 2000). One study showed that IgA reactivity was mostly to the 17 kDa glycoprotein (Moss et al., 1998). No significant levels of IgM antibodies to Cryptosporidium glycopeptides were detected in this study.
The 17 kDa antigen protein sequence contains numerous serine and threonine residues that might serve as potential modification sites for addition of O-glycans (Strong et al., 2000). Apparent molecular weight differences between the native antigen and the deduced protein sequence are attributable to these Tn glycosylation sites, probably at positions Ser-223, Thr-228 and Thr-235 (Priest et al., 2003). Within the Cp40 antigen there is a hypervariable region following the conserved polyserine domain, with predicted O-glycosylation sites (Leav et al., 2002). Because it is likely that Tn antigen occurs on these glycoproteins, we synthesised Cp17 and Cp40 peptides with differences in Tn glycosylation to determine how antibody binding was affected by these changes. In addition, novel Cp40 peptides containing different lengths of repeating GalNAc-serine residues were synthesised and analysed.
It was found that for the Cp17 antigen, differences in glycosylation and Tn expression could significantly affect the binding of serum antibodies to the immunodominant 17-kDa antigen-derived sequences. In some individuals, glycosylation resulted in up to 10-fold increases in reactivity and in general, multiple glycosylation sites increased the binding reactivity (Fig. 1). Binding events between carbohydrates and proteins are typically weak but may be enhanced if two or more carbohydrates are involved in the binding of antibody to the glycoprotein, resulting in the formation of a high avidity multivalent complex (Oyelaran et al., 2009).
For the different Cp40 peptides synthesised in this study, binding of sera was enhanced with the addition of glycosylated serine residues (7, 16 and 23). It has been previously suggested that differences in Tn glycosylation of Cp40 could provide greater diversity to adhere to different host tissues or as a way to modulate (e.g. antigenic variation) antibody response (Strong et al., 2000). Little is known about the antibody response to the Cp40 antigen in humans or whether differences in polymorphisms affect binding and immunoreactivity. One study evaluating antibody responses in children from a birth cohort study in southern India found that despite minor differences in reactivity to Cp40 subtypes, there was significant cross-reactivity to the two major subtypes of Cp40 antigen (Ajjampur et al., 2011).
In the present study it was found that sera from T. gondii and P. falciparum-infected individuals recognised Cryptosporidium peptides with different degrees of reactivity depending on the sites and valency of the Tn groups that were attached. This is perhaps not unexpected since cross-reactivity of antibody has been shown to occur with the apicomplexan proteins (Lorenzo et al., 1998; Sasai et al., 1998; Matsubayashi et al., 2005). It is also possible that some reactivity is due to previous infection with Cryposporidium since prevalence is high in the adult population (Isaac-Renton et al., 1999). As with C. parvum, an enhanced response was observed with addition of Tn antigen, particularly with an addition of GalNAc to the third threonine (second glycosylated threonine) of the Cp17 antigen sequence. Although we show that sera from individuals infected with T. gondii can bind Tn antigens on different peptides, this does not demonstrate the presence of Tn in this parasite. However, the existence of a T. gondii ppGalNAc-T isoform, ppGalNAc-T3, one of a family of enzymes that initiates mucin-type O-linked protein glycosylation by catalysing the transfer of GalNAc to specific threonine and serine residues on proteins, is expressed in both tachyzoites and bradyzoites of T. gondii (Stwora-Wojczyk et al., 2004a). Additionally, a family of four ppGalNAc-Ts has been identified in the C. parvum genome (Stwora-Wojczyk et al., 2004b), suggesting that the biochemical machinery necessary for this type of glycosylation is present.
In summary, it was found that antibodies in sera from C. parvum-infected individuals bound to multivalent Tn antigen epitopes presented on glycopeptides and that the molecular differences in glycosylated peptides as well as the site of glycosylation had a pronounced effect on reactivity. In addition, pooled sera from individuals infected with either Toxoplasma or Plasmodium were found to recognise the modified Cryptosporidium peptides as well as specifically binding to glycopeptide epitopes. This reactivity suggests that there is a conservation of immunogenicity in these glycosylated peptides among these apicomplexan parasites.
Supplementary Material
Acknowledgements
This work was supported in part by Emory's and Children's Pediatrics Research Center, USA, Children's Center for Immunology and Vaccines, USA, the Department of Veteran's Affairs, USA, the Georgia Research Alliance, USA, the National Institutes of Health, USA, Grant RO1CA088986 (G.-J.B.), and the National Institutes of Health Grant GM098791 (R.D.C). We thank Hong Ju and Yi Lasanajak for technical assistance. Use of trade names is for identification only and does not imply endorsement by the Public Health Service, USA or by the U.S. Department of Health and Human Services. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention, USA.
Footnotes
Appendix A. Supplementary data Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.ijpara.2013.05.012.
References
- Ajjampur SS, Sarkar R, Allison G, Banda K, Kane A, Muliyil J, Naumova E, Ward H, Kang G. Serum IgG response to Cryptosporidium immunodominant antigen gp15 and polymorphic antigen gp40 in children with cryptosporidosis in South India. Clin. Vaccine Immunol. 2011;18:633–639. doi: 10.1128/CVI.00464-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez E, Medeiros DA, Miguez M, Casaravilla C, Malgor R, Carmona C, Nieto A, Osinaga E. O-glycosylation in Echinococcus granulosus: identification and characterization of the carcinoma associated Tn antigen. Exp. Parasitol. 2001;98:100–109. doi: 10.1006/expr.2001.4620. [DOI] [PubMed] [Google Scholar]
- Benitez AJ, McNair N, Mead JR. Oral immunization with attenuated Salmonella enterica Serovar typhimurium encoding Cryptosporidium parvum Cp23 and Cp40 antigens induces a specific immune response in mice. Clin. Vaccine Immunol. 2009;16:1272–1278. doi: 10.1128/CVI.00089-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohnsack RN, Song X, Olson LJ, Kudo M, Gotschall RR, Canfield WM, Cummings RD, Smith DF, Dahms NM. Cation-independent mannose 6-phosphate receptor: a composite of distinct phosphomannosyl binding sites. J. Biol. Chem. 2009;284:35215–35226. doi: 10.1074/jbc.M109.056184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolscher JGM, Brevoord J, Nazmi K, Ju T, Veerman ECI, van Wijk JAE, Cummings RD, van Die I. Solid-phase synthesis of a pentavalent GalNAc-containing glycopeptide (Tn antigen) representing the nephropathy-associated IgA hinge region. Carbohydr. Res. 2010;345:1998–2003. doi: 10.1016/j.carres.2010.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgert A, Heimburg-Molinaro J, Song X, Lasanajak Y, Ju T, Liu M, Thompson P, Ragupathi G, Barany G, Smith DF, Cummings RD, Live D. Deciphering structural elements of mucin glycoprotein recognition. ACS Chem. Biol. 2012;7:1019–1031. doi: 10.1021/cb300076s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulter-Bitzer JI, Lee H, Trevors JT. Molecular targets for detection and immunotherapy in Cryptosporidium parvum. Biotechnol. Adv. 2007;1:13–44. doi: 10.1016/j.biotechadv.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Casaravilla C, Freire T, Malgor R, Medeiros A, Osinaga E, Carmona C. Mucin-type O-glycosylation in helminth parasites from major taxonomic groups: evidence for widespread distribution of the Tn antigen (GalNAc-Ser/Thr) and identification of UDP-GalNAc:polypeptide N-acetylgalactosaminyltransferase activity. J. Parasitol. 2003;89:709–715. doi: 10.1645/GE-2970. [DOI] [PubMed] [Google Scholar]
- Cevallos AM, Zhang X, Waldor MK, Jaison S, Zhou X, Tzipori S, Neutra MR, Ward HD. Molecular cloning and expression of a gene encoding Cryptosporidium parvum glycoproteins gp40 and gp15. Infect. Immun. 2000;68:4108–4116. doi: 10.1128/iai.68.7.4108-4116.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dann SM, Okhuysen PC, Salameh BM, DuPont HL, Chappell CL. Fecal antibodies to Cryptosporidium parvum in healthy volunteers. Infect. Immun. 2000;68:5068–5074. doi: 10.1128/iai.68.9.5068-5074.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies AP, Chalmers RM. Cryptosporidiosis. BMJ. 2009;339:b4168. doi: 10.1136/bmj.b4168. [DOI] [PubMed] [Google Scholar]
- Fox LM, Saravolatz LD. Nitazoxanide: a new thiazolide antiparasitic agent. Clin. Infect. Dis. 2005;40:1173–1180. doi: 10.1086/428839. [DOI] [PubMed] [Google Scholar]
- Freire T, Casaravilla C, Carmona C, Osinaga E. Mucin type O-glycosylation in Fasciola hepatica: characterization of carcinoma associated Tn and sialyl-Tn antigens and evaluation of UDP-GalNAc:polypeptide N-acetylgalactosaminyltransferase activity. Int. J. Parasitol. 2003;33:47–56. doi: 10.1016/s0020-7519(02)00231-x. [DOI] [PubMed] [Google Scholar]
- Gut J, Nelson RG. Cryptosporidium parvum: synchronized excystation in vitro and evaluation of sporozoite infectivity with a new lectin-based assay. J. Eukaryot. Microbiol. 1999;46:56S–57S. [PubMed] [Google Scholar]
- Heimburg-Molinaro J, Song X, Smith DF, Cummings RD. Preparation and analysis of glycan microarrays. Curr. Protoc. Protein Sci. 2011;64:12.10.1–12.10.29. doi: 10.1002/0471140864.ps1210s64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang DB, Chappell C, Okhuysen PC. Cryptosporidiosis in children. Semin. Pediatr. Infect. Dis. 2004;4:253–259. doi: 10.1053/j.spid.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Isaac-Renton J, Blatherwick J, Bowie WR, Fyfe M, Khan M, Li A, McLean M, Medd L, Moorehead W, Ong CS, Robertson W. Epidemic and andemic seroprevalence of antibodies to Cryptosporidium and Giardia in residents of three communities with different drinking water supplies. Am. J. Trop. Med. Hyg. 1999;60:578–583. doi: 10.4269/ajtmh.1999.60.578. [DOI] [PubMed] [Google Scholar]
- Ju T, Otto VI, Cummings RD. The Tn antigen-structural simplicity and biological complexity. Angew. Chem. Int. Ed. Engl. 2011;50:1770–1791. doi: 10.1002/anie.201002313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leav BA, Mackay MR, Anyanwu A, O' Connor RM, Cevallos AM, Kindra G, Rollins NC, Bennish ML, Nelson RG, Ward HD. Analysis of sequence diversity at the highly polymorphic Cpgp40/15 locus among Cryptosporidium isolates from human immunodeficiency virus-infected children in South Africa. Infect. Immun. 2002;70:3881–3890. doi: 10.1128/IAI.70.7.3881-3890.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitch GJ, He Q. Cryptosporidosis – an overview. J. Biomed. Res. 2012;1:1–16. doi: 10.1016/S1674-8301(11)60001-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Barany G, Live D. Parallel solid-phase synthesis of mucin-like glycopeptides. Carbohydr. Res. 2005;340:2111–2122. doi: 10.1016/j.carres.2005.05.023. [DOI] [PubMed] [Google Scholar]
- Lorenzo MJ, Casal JA, Freire F, Castro JA, Vergara CA, Ares-Mazás ME. Determination of immunocrossreactivity between Cryptosporidium and Eimeria spp. Vet. Parasitol. 1998;76:1–8. doi: 10.1016/s0304-4017(97)00225-2. [DOI] [PubMed] [Google Scholar]
- Manque PA, Tenjo F, Woehlbier U, Lara AM, Serrano MG, Xu P, Alves JM, Smeltz RB, Conrad MG, Buck GA. Identification and immunological characterization of three potential vaccinogens against Cryptosporidium species. Clin. Vaccine Immunol. 2011;18:1796–1802. doi: 10.1128/CVI.05197-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubayashi M, Kimata I, Iseki M, Lillehoj HS, Matsuda H, Nakanishi T, Tani H, Sasai K, Bab E. Cross-reactivities with Cryptosporidium spp. by chicken monoclonal antibodies that recognize avian Eimeria spp. Vet. Parasitol. 2005;128:47–57. doi: 10.1016/j.vetpar.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Moss DM, Bennett SN, Arrowood MJ, Hurd MR, Lammie PJ, Wahlquist SP, Addiss DG. Kinetic and isotypic analysis of specific immunoglobulins from crew members with cryptosporidiosis on a US Coast Guard cutter. J. Eukaryot. Microbiol. 1994;41:52S–55S. [PubMed] [Google Scholar]
- Moss DM, Chappell CL, Okhuysen PC, DuPont HL, Arrowood MJ, Hightower AW, Lammie PJ. The antibody response to 27-, 17-, and 15-kDa Cryptosporidium antigens following experimental infection in humans. J. Infect. Dis. 1998;178:827–833. doi: 10.1086/515377. [DOI] [PubMed] [Google Scholar]
- Nyame K, Cummings RD, Damian RT. Schistosoma mansoni synthesizes glycoproteins containing terminal O-linked N-acetylglucosamine residues. J. Biol. Chem. 1987;262:7990–7995. [PubMed] [Google Scholar]
- Oyelaran O, Li Q, Farnsworth D, Gildersleeve JC. Microarray with varying carbohydrate density reveal distinct subpopulations of serum antibodies. J. Proteome Res. 2009;8:3529–3538. doi: 10.1021/pr9002245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priest JW, Kwon JP, Moss DM, Roberts JM, Arrowood MJ, Dworkin MS, Juranek DD, Lammie PJ. Detection by enzyme immunoassay of serum immunoglobulin G antibodies that recognize specific Cryptosporidium parvum antigens. J. Clin. Microbiol. 1999;37:1385–1392. doi: 10.1128/jcm.37.5.1385-1392.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priest JW, Kwon JP, Arrowood MJ, Lammie PJ. Cloning of the immunodominant 17-kDa antigen from Cryptosporidium parvum. Mol. Biochem. Parasitol. 2000;106:261–271. doi: 10.1016/s0166-6851(99)00223-6. [DOI] [PubMed] [Google Scholar]
- Priest JW, Mehlert A, Arrowood MJ, Riggs MW, Ferguson MA. Characterization of a low molecular weight glycolipid antigen from Cryptosporidium parvum. J. Biol. Chem. 2003;278:52212–52222. doi: 10.1074/jbc.M306835200. [DOI] [PubMed] [Google Scholar]
- Priest JW, Mehlert A, Moss DM, Arrowood MJ, Ferguson MA. Characterization of the glycosylphosphatidylinositol anchor of the immunodominant Cryptosporidium parvum 17-kDa antigen. Mol. Biochem. Parasitol. 2006;149:108–112. doi: 10.1016/j.molbiopara.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Sasai K, Lillehoj HS, Hemphill A, Matsuda H, Hanioka Y, Fukata T, Baba E, Arakawa A. A chicken anti-conoid monoclonal antibody identifies a common epitope which is present on motile stages of Eimeria, Neospora, and Toxoplasma. J. Parasitol. 1998;84:654–656. [PubMed] [Google Scholar]
- Smith DF, Song X, Cummings RD. Use of glycan microarrays to explore specificity of glycan-binding proteins. Methods Enzymol. 2010;480:417–444. doi: 10.1016/S0076-6879(10)80033-3. [DOI] [PubMed] [Google Scholar]
- Song X, Xia B, Lasanajak Y, Smith DF, Cummings RD. Quantifiable fluorescent glycan microarrays. Glycoconj. J. 2008;25:15–25. doi: 10.1007/s10719-007-9066-8. [DOI] [PubMed] [Google Scholar]
- Song X, Lasanajak Y, Olson LJ, Boonen M, Dahms NM, Kornfeld S, Cummings RD, Smith DF. Glycan microarray analysis of P-type lectins reveals distinct phosphomannose glycan recognition. J. Biol. Chem. 2009a;284:35201–35214. doi: 10.1074/jbc.M109.056119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X, Xia B, Stowell SR, Lasanajak Y, Smith DF, Cummings RD. Novel fluorescent glycan microarray strategy reveals ligands for galectins. Chem. Biol. 2009b;16:36–47. doi: 10.1016/j.chembiol.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X, Heimburg-Molinaro J, Dahms NM, Smith DF, Cummings RD. Preparation of a mannose-6-phosphate glycan microarray through fluorescent derivation, phosphorylation, and immobilization of natural high-mannose N-glycans and application in ligand identification of P-type lectins. Methods Mol. Biol. 2012;808:137–148. doi: 10.1007/978-1-61779-373-8_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strong WB, Gut J, Nelson RG. Cloning and sequence analysis of a highly polymorphic Cryptosporidium parvum gene encoding a 60-kilodalton glycoprotein and characterization of its 15- and 45-kilodalton zoite surface antigen products. Infect. Immun. 2000;68:4117–4134. doi: 10.1128/iai.68.7.4117-4134.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stwora-Wojczyk MM, Dzierszinski F, Roos DS, Spitalnik SL, Wojczyk BS. Functional characterization of a novel Toxoplasma gondii glycosyltransferase: UDP-N-acetyl-D-galactosamine:polypeptide N-acetylgalactosaminyltransferase-T3. Arch. Biochem. Biophys. 2004a;15(426):231–240. doi: 10.1016/j.abb.2004.02.013. [DOI] [PubMed] [Google Scholar]
- Stwora-Wojczyk MM, Kissinger JC, Spitalnik SL, Wojczyk BS. O-glycosylation in Toxoplasma gondii: Identification and analysis of a family of UDP-GalNAc:polypeptide N-acetylgalactosaminyltransferases. Int. J. Parasitol. 2004b;34:309–322. doi: 10.1016/j.ijpara.2003.11.016. [DOI] [PubMed] [Google Scholar]
- von Gunten S, Smith DF, Cummings RD, Riedel S, Miescher S, Schaub A, Hamilton RG, Bochner BS. Intravenous immunoglobulin contains a broad repertoire of anti-carbohydrate antibodies that is not restricted to the IgG2 subclass. J. Allergy Clin. Immunol. 2009;123:1268–1276. doi: 10.1016/j.jaci.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.