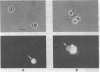
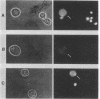
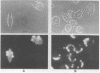
Abstract
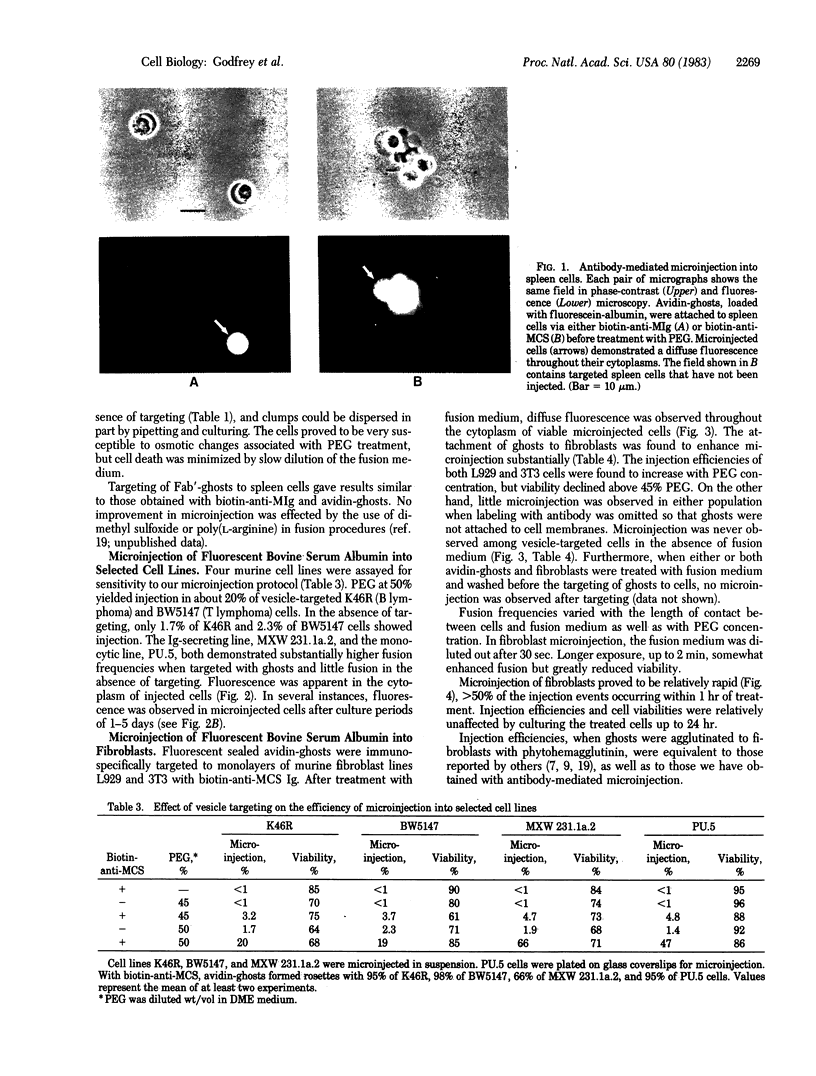
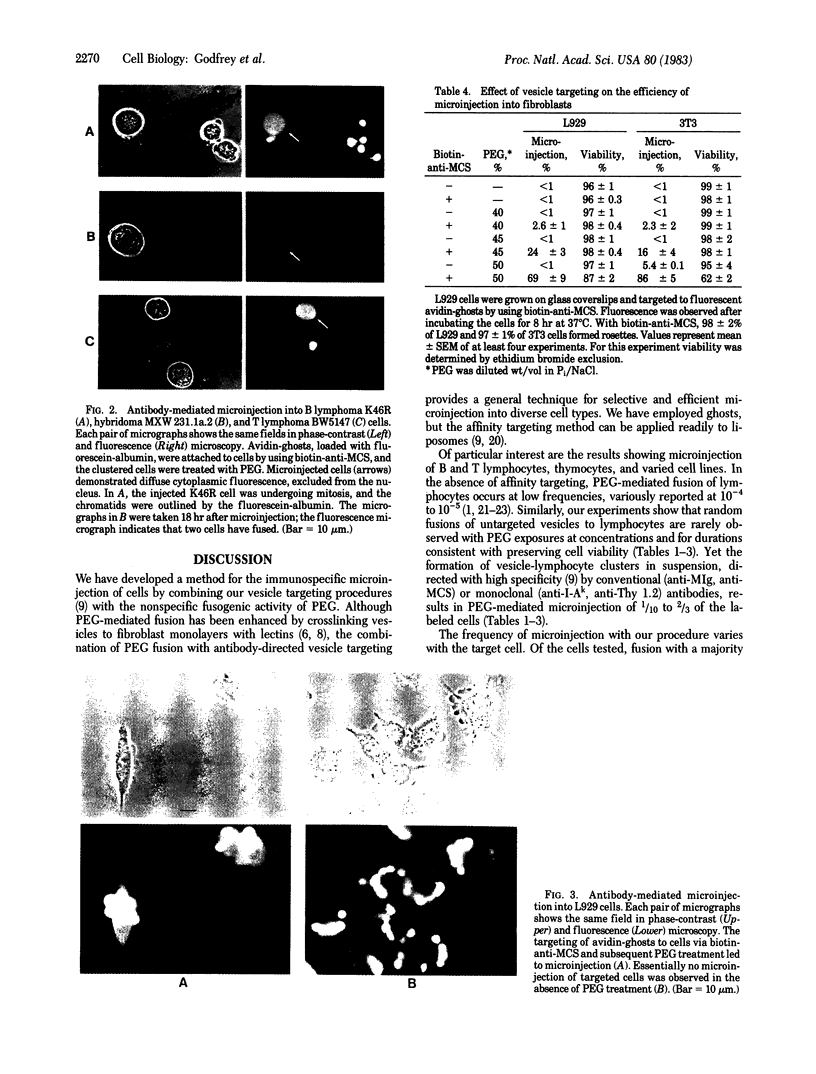
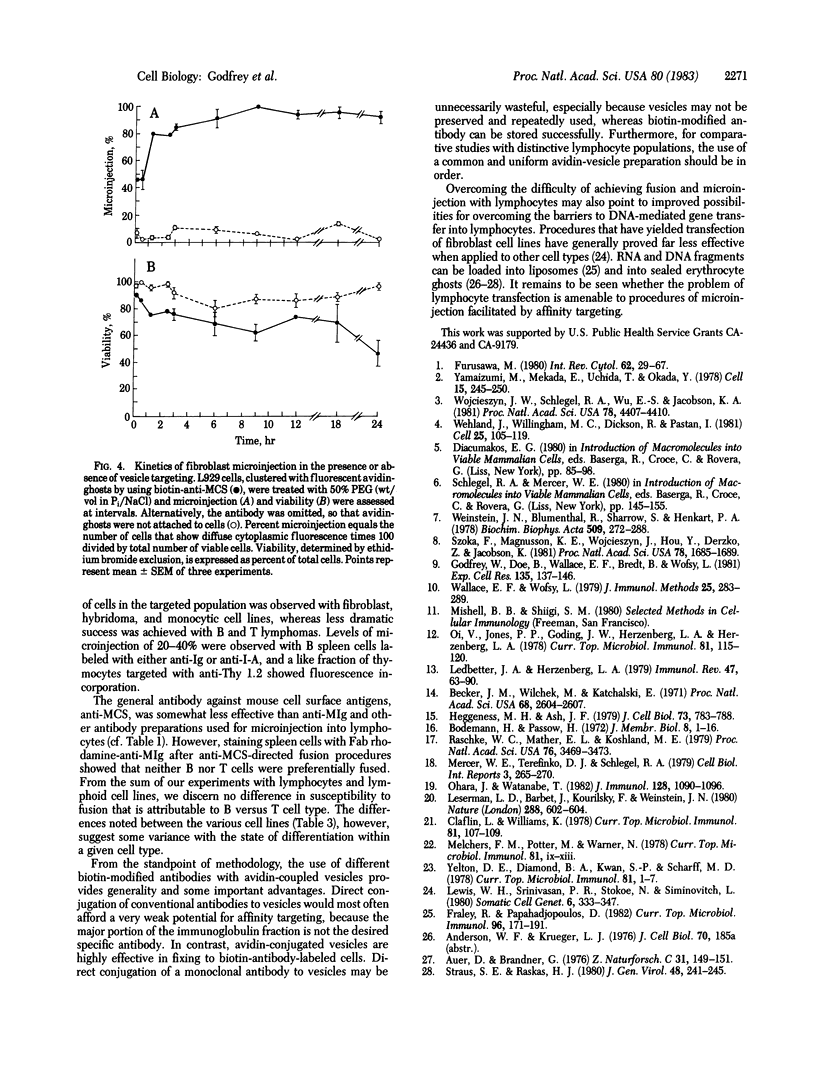
Antibody-directed targeting of vesicles to cells dramatically enhances polyethylene glycol-mediated fusion and microinjection. Sealed erythrocyte ghosts, containing fluorescent bovine serum albumin, were targeted to murine spleen and thymus cells, and to lymphocyte, monocyte, and fibroblast cell lines. In all cases, targeted cell populations showed substantial levels of microinjection, whereas populations treated with the fusogen in the absence of targeting were not significantly microinjected. To achieve attachment of vesicles to selected cells, the cells were first labeled with biotin-modified antibody then treated with sealed ghosts prepared from avidin-coupled erythrocytes. This procedure should prove useful when the injection of specific cell populations is desired, or with cell types such as lymphocytes that are difficult to fuse, or when the use of limited reagents necessitates high injection efficiencies.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auer D., Brandner G. Loading of human red blood cells with DNA and RNA. Z Naturforsch C. 1976 Mar-Apr;31(3-4):149–151. doi: 10.1515/znc-1976-3-410. [DOI] [PubMed] [Google Scholar]
- Becker J. M., Wilchek M., Katchalski E. Irreversible inhibition of biotin transport in yeast by biotinyl-p-nitrophenyl ester. Proc Natl Acad Sci U S A. 1971 Oct;68(10):2604–2607. doi: 10.1073/pnas.68.10.2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodemann H., Passow H. Factors controlling the resealing of the membrane of human erythrocyte ghosts after hypotonic hemolysis. J Membr Biol. 1972;8(1):1–26. doi: 10.1007/BF01868092. [DOI] [PubMed] [Google Scholar]
- Claflin L., Williams K. Mouse myeloma--spleen cell hybrids: Enhanced hybridization frequencies and rapid screening procedures. Curr Top Microbiol Immunol. 1978;81:107–109. doi: 10.1007/978-3-642-67448-8_16. [DOI] [PubMed] [Google Scholar]
- Fraley R., Papahadjopoulos D. Liposomes: the development of a new carrier system for introducing nucleic acid into plant and animal cells. Curr Top Microbiol Immunol. 1982;96:171–191. doi: 10.1007/978-3-642-68315-2_11. [DOI] [PubMed] [Google Scholar]
- Furusawa M. Cellular microinjection by cell fusion: technique and applications in biology and medicine. Int Rev Cytol. 1980;62:29–67. doi: 10.1016/s0074-7696(08)61898-7. [DOI] [PubMed] [Google Scholar]
- Godfrey W., Doe B., Wallace E. F., Bredt B., Wofsy L. Affinity targeting of membrane vesicles to cell surfaces. Exp Cell Res. 1981 Sep;135(1):137–145. doi: 10.1016/0014-4827(81)90306-2. [DOI] [PubMed] [Google Scholar]
- Heggeness M. H., Ash J. F. Use of the avidin-biotin complex for the localization of actin and myosin with fluorescence microscopy. J Cell Biol. 1977 Jun;73(3):783–788. doi: 10.1083/jcb.73.3.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledbetter J. A., Herzenberg L. A. Xenogeneic monoclonal antibodies to mouse lymphoid differentiation antigens. Immunol Rev. 1979;47:63–90. doi: 10.1111/j.1600-065x.1979.tb00289.x. [DOI] [PubMed] [Google Scholar]
- Leserman L. D., Barbet J., Kourilsky F., Weinstein J. N. Targeting to cells of fluorescent liposomes covalently coupled with monoclonal antibody or protein A. Nature. 1980 Dec 11;288(5791):602–604. doi: 10.1038/288602a0. [DOI] [PubMed] [Google Scholar]
- Lewis W. H., Srinivasan P. R., Stokoe N., Siminovitch L. Parameters governing the transfer of the genes for thymidine kinase and dihydrofolate reductase into mouse cells using metaphase chromosomes or DNA. Somatic Cell Genet. 1980 May;6(3):333–347. doi: 10.1007/BF01542787. [DOI] [PubMed] [Google Scholar]
- Melchers F., Potter M., Warner N. L. Lymphocyte hybridomas. Second workshop on "functional properties of tumors of T and B lymphocytes." Preface. Curr Top Microbiol Immunol. 1978;81:IX–XXIII. [PubMed] [Google Scholar]
- Mercer W. E., Terefinko D. J., Schlegel R. A. Red cell-mediated microinjection of macromolecules into monolayer cultures of mammalian cells. Cell Biol Int Rep. 1979 May;3(3):265–270. doi: 10.1016/0309-1651(79)90039-0. [DOI] [PubMed] [Google Scholar]
- Ohara J., Watanabe T. Microinjection of macromolecules into normal murine lymphocytes by cell fusion technique. I. Quantitative microinjection of antibodies into normal splenic lymphocytes. J Immunol. 1982 Mar;128(3):1090–1096. [PubMed] [Google Scholar]
- Oi V. T., Jones P. P., Goding J. W., Herzenberg L. A., Herzenberg L. A. Properties of monoclonal antibodies to mouse Ig allotypes, H-2, and Ia antigens. Curr Top Microbiol Immunol. 1978;81:115–120. doi: 10.1007/978-3-642-67448-8_18. [DOI] [PubMed] [Google Scholar]
- Raschke W. C., Mather E. L., Koshland M. E. Assembly and secretion of pentameric IgM in a fusion between a nonsecreting B cell lymphoma and an IgG-secreting plasmacytoma. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3469–3473. doi: 10.1073/pnas.76.7.3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straus S. E., Raskas H. J. Transfection of KB cells by polyethylene glycol-induced fusion with erythrocyte ghosts containing adenovirus type 2 DNA. J Gen Virol. 1980 May;48(1):241–245. doi: 10.1099/0022-1317-48-1-241. [DOI] [PubMed] [Google Scholar]
- Szoka F., Magnusson K. E., Wojcieszyn J., Hou Y., Derzko Z., Jacobson K. Use of lectins and polyethylene glycol for fusion of glycolipid-containing liposomes with eukaryotic cells. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1685–1689. doi: 10.1073/pnas.78.3.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace E. F., Wofsy L. Hapten-sandwich labeling. IV. Improved procedures and non-cross-reacting hapten reagents for double-labeling cell surface antigens. J Immunol Methods. 1979;25(3):283–289. doi: 10.1016/0022-1759(79)90116-9. [DOI] [PubMed] [Google Scholar]
- Wehland J., Willingham M. C., Dickson R., Pastan I. Microinjection of anticlathrin antibodies into fibroblasts does not interfere with the receptor-mediated endocytosis of alpha2-macroglobulin. Cell. 1981 Jul;25(1):105–119. doi: 10.1016/0092-8674(81)90235-x. [DOI] [PubMed] [Google Scholar]
- Weinstein J. N., Blumenthal R., Sharrow S. O., Henkart P. A. Antibody-mediated targeting of liposomes. Binding to lymphocytes does not ensure incorporation of vesicle contents into the cells. Biochim Biophys Acta. 1978 May 18;509(2):272–288. doi: 10.1016/0005-2736(78)90047-0. [DOI] [PubMed] [Google Scholar]
- Wojcieszyn J. W., Schlegel R. A., Wu E. S., Jacobson K. A. Diffusion of injected macromolecules within the cytoplasm of living cells. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4407–4410. doi: 10.1073/pnas.78.7.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaizumi M., Mekada E., Uchida T., Okada Y. One molecule of diphtheria toxin fragment A introduced into a cell can kill the cell. Cell. 1978 Sep;15(1):245–250. doi: 10.1016/0092-8674(78)90099-5. [DOI] [PubMed] [Google Scholar]