Abstract
BACKGROUND
Signal transducer and activator of transcription 3 (Stat3) is an oncogenic transcriptional factor that plays a critical role in carcinogenesis and cancer progression and is a potential therapeutic target. Sanguinarine, a benzophenanthridine alkaloid derived primarily from the bloodroot plant, was identified previously as a novel inhibitor of survivin that selectively kills prostate cancer cells over “normal” prostate epithelial cells.
METHODS
DU145, C4-2B, and LNCaP cells were treated with sanguinarine. The phosphorylation status of Stat3 and related proteins were measured with Western blots. Activation of transcription by Stat3 was measured with luciferase reporter assay. The effect of sanguinarine on anchorage-independent growth was examined with soft agar assay, and on cell migration and invasion of DU145 cells were measured with scratch assay and invasion assay, respectively.
RESULTS
In this study, we identified sanguinarine as a potent inhibitor of Stat3 activation which was able to suppress prostate cancer growth, migration, and invasion. Sanguinarine inhibits constitutive as well as IL6-induced phosphorylation of Stat3 at both Tyr705 and Ser727 in prostate cancer cells. The inhibition of Stat3 phosphorylation by sanguinarine correlates with reduction of Janus-activated Kinase 2 (Jak2) and Src phosphorylation. Sanguinarine downregulates the expression of Stat3-mediated genes such as c-myc and survivin and inhibits the Stat3 responsive element luciferase reporter activity. Sanguinarine inhibits the anchorage-independent growth of DU145 and LN-S17 cells expressing constitutively activated Stat3. Migration and invasion abilities of DU145 cells were also inhibited by sanguinarine in a manner similar to the dominant negative form of Stat3.
CONCLUSIONS
These data demonstrate that sanguinarine is a potent Stat3 inhibitor and it could be developed as a therapeutic agent for prostate cancer with constitutive activation of Stat3.
Keywords: prostate cancer, Stat3, sanguinarine, invasion
INTRODUCTION
Prostate cancer is the most frequently diagnosed cancer, and the second leading cause of cancer-related death in American men [1]. Current treatments have proved inadequate in controlling prostate cancer, and search for novel therapeutic agents for the management of this disease has become a priority for researchers. Studies from various groups have shown that Stat3 activation plays a pivotal role in the survival, proliferation, and metastatic progression of prostate cancer [2–10]. Stat3, a member of the signal transducers and activators of transcription (STAT) family, is a key signal transduction protein that mediates signaling by many cytokines, hormones, growth factors, and oncoproteins [11]. Upon stimulation by cytokines such as Interleukin-6 (IL-6), Stat3 can be activated by tyrosine and serine phosphorylation and acetylation [11–13]. Stat3 activation in cancer cells has been demonstrated to influence important processes such as survival, proliferation, apoptosis, angiogenesis, and invasion [14–16]. Stat3 activity is increased in androgen independent prostate cancer cells compared to androgen sensitive cells [3,4]. IL-6 induced androgen receptor (AR)-mediated gene activation requires the activation of Stat3 in LNCaP cells [5,6]. Constitutively active Stat3 (Stat3C) enhances tumorigenesis and enhances cell motility of prostate epithelial cells [10]. Blockade of Stat3 expression in human prostate cancer cells suppresses proliferation in vitro and tumorigenicity in vivo [7].
The critical role of Stat3 activation in oncogenesis makes it an attractive target for prostate cancer therapy. Inhibitors are being developed to target various steps in the Stat3 activation pathway. These inhibitors include upstream tyrosine kinase inhibitors such as tyrphostin AG490 [17] and cucurbitacin I [18], anti-sense oligonucleotides [4], and decoy oligonucleotides [19], dominant negative Stat3 protein [7,20], and various small molecule inhibitors [21,22].
We have previously identified that sanguinarine, a benzophenanthridine alkaloid derived primarily from the bloodroot plant, suppresses prostate cancer cell growth and inhibits survivin expression by screening compounds from the Preswich 1120 compound library [23]. Sanguinarine (13-methyl[1,3] benzodioxolo[5,6-c]-1,3-dioxolo[4,5-i] phenanthridinium) has been shown to possess anti-microbial, anti-inflammatory, and anti-cancer activities [24–27]. Studies from others have shown that sanguinarine has anti-proliferative effect on human prostate cancer cells [28].
In this study, we demonstrate that sanguinarine is a potent inhibitor of Stat3 activation and is able to suppress prostate cancer growth, migration, and invasion.
MATERIAL ANDMETHODS
Chemicals, Reagents, and Antibodies
Sanguinarine chloride (#S5890) was purchased from Sigma–Aldrich. Antibodies against p-Stat3 (tyr705, #SC7993-R), Stat3 (#SC482), survivin (#SC81417), and c-myc (#SC764) were purchased from Santa Cruz Biotechnology. Antibodies against p-Jak2 (#CS3776), p-Src (#CS2101S), p-Stat3 (ser727, #CS9136), and GAPDH (#CS2118) were purchased from Cell Signaling.
Cell Culture and Transfection
DU145, C4-2B, and LNCaP prostate cancer cells were cultured in RPMI1640 containing 10% fetal bovine serum (FBS) and penicillin/streptomycin. LN-S17 cells were generated from LNCaP cells transfected with IL-6 cDNA and described previously and cultured in RPMI1640 media supplemented with 300 μg/ml G418 [29]. For transfection studies, DU145 cells were transiently transfected with Stat3F (dominant negative mutant) or the control vector using Lipofectamine 2000 (Invitrogen).
Luciferase Assay
C4-2B cells (1 × 105 cells per well of 12-well plate) were transfected with 0.8 μg of pLucTKS3 reporter plasmid containing specific responsive elements for Stat3 [30,31] or the control plasmid. The luciferase activity was determined 24–48 hr after transfection using a dual-luciferase reporter assay system (Promega).
Western Blot
Whole-cell protein extracts were resolved on 8% or 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). Proteins were then transferred to nitrocellulose membranes. After blocking for 1 hr at room temperature in 5% milk in phosphate-buffered saline (PBS)/0.1% Tween-20, membranes were incubated overnight at 4°C with the indicated primary antibodies diluted in PBS with 1% BSA. Following secondary antibody incubation, immuno-reactive proteins were visualized with an enhanced chemiluminescence detection system (Amersham Pharmacia Biotech, Buckinghamshire, England).
Wound Healing Assay
DU145 and LN-S17 cells were cultured under standard condition as described above and plated onto 60 mm2 dishes. Scratch wounds were introduced into the confluent monolayers the day after plating or the day after transfection. The wounds were created with a 200 μl pipette tip. Cells were kept in regular culture media with various concentrations of sanguinarine. Wound closure was monitored over time and photographed using Olympus 1 × 81 microscope at 40× magnification.
Invasion Assay
DU145 cells (1 × 104) were suspended in growth medium containing 0, 0.5, or 1 μM sanguinarine into the cell culture inserts for 24-well plates containing 8 μm pores. The inserts were coated with Matrigel (2–3 mg/ml protein) and allowed to solidify for 30 min before cell plating. The lower chambers were filled with 300 μl growth medium containing DMSO or sanguinarine. After 48 h, cells were fixed with 5% glutaraldehyde in PBS, washed with PBS, and stained with 0.5% toluidine blue in 2% Na2CO3 solution. Invasive cells that penetrated the membrane were counted under the microscope.
Soft Agar Assay
DU145 cells (1 × 104) or LN-S17 cells (2 × 104) were plated in 0.3% agar in cell culture media with or without sanguinarine in 60 mm2 dishes. Colony formation was examined after 2 weeks. The colonies were stained with 0.005% crystal violet for 1 hr. Scanned images of the colonies were analyzed with the Image software.
Statistical Analysis
All data are presented as means ± standard deviation of the mean (SD). Statistical analyses were performed with Microsoft Excel analysis tools, differences between individual groups were analyzed by paired t-test. P < 0.05 was considered statistically significant.
RESULTS
Sanguinarine Inhibits Constitutive and IL-6-Induced Stat3 Phosphorylation
We previously demonstrated that sanguinarine inhibited expression of survivin, a target gene of Stat3. To determine whether sanguinarine inhibits Stat3 activation, human DU145 prostate cancer cells that express constitutively activated Stat3 were treated with different doses of sanguinarine for 4 hr and then stimulated with or without 10 ng/ml IL-6 for 30 min. As shown in Figure 1A, treatment of DU145 cells with sanguinarine resulted in a significant decrease in the levels of both constitutive and IL-6-induced tyrosine 705 phosphorylation of Stat3. Sanguinarine also inhibited Serine 727 phosphorylation of Stat3 in DU145 cells, although less effective compared to inhibition of the tyrosine 705 phosphorylation of Stat3. Phosphorylation of Stat3 at Ser727 has been shown to play an important role in optimal activation of Stat3 and cell invasion [32,33]. Sanguinarine decreased the level of IL-6-induced p-Stat3 to the resting state within 1 hr. The p-Stat3 was completely abolished after 6 hr of sanguinarine treatment (Fig. 1B). In LNCaP cells where Stat3 is not constitutively active, exogenous addition of IL-6 induced phosphorylation of Stat3 at Tyr705, which was reduced by sanguinarine (Fig. 1C). These data demonstrate that sanguinarine inhibits both constitutive and IL-6-induced Stat3 activation.
Fig. 1.
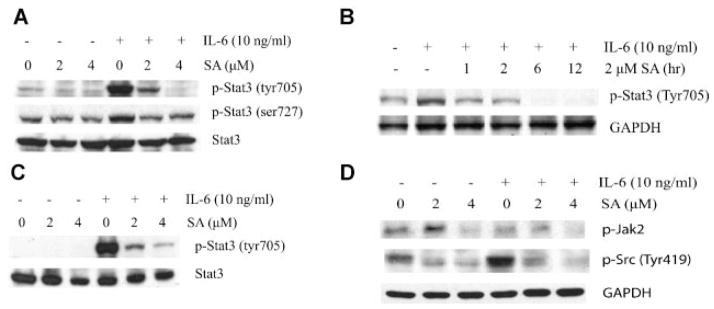
Sanguinarine inhibits constitutive and IL-6-induced Stat3 phosphorylation. A: Immunoblot for phospho-Stat3 at Tyr705 and Ser727. DU145 cells were treated with 2 and 4 μM sanguinarine for 4 hr and stimulated with 10 ng/ml IL-6 for 30 min. B: Time-dependent inhibition of Stat3 phosphorylation by sanguinarine in DU145 cells. DU145 cells were treated with 2 μM sanguinarine for various time periods (from1 hr up to12 hr). C: Immunoblot for phosho-Stat3 in LNCaP cells. D: Sanguinarine inhibits both Jak2 and Src phosphorylation. Immunoblot for p-Jak2, p-Src, and GAPDH. DU145 cells were treated with 2 and 4 μM sanguinarine for 4 hr and stimulated with10 ng/ml IL-6 for 30 min. Whole cell lysates were used for Western blot analysis (SA, sanguinarine; IL-6, interleukin-6).
Stat3 is activated by both Jak2 and Src family kinases. It has been shown that neither inhibition of Jak2 nor Src alone resulted in complete inactivation of p-Stat3 [34,35], suggesting that the complete inhibition of p-Stat3 has to be achieved by suppression of both Jak2 and Src kinases. As shown in Figure 1D, sanguinarine decreased both Jak2 and Src phosphorylation. These data suggest that inhibition of Stat3 activation by sanguinarine is through suppression of both Jak2 and Src phosphorylation.
Sanguinarine Inhibits Stat3-Mediated Gene Expression
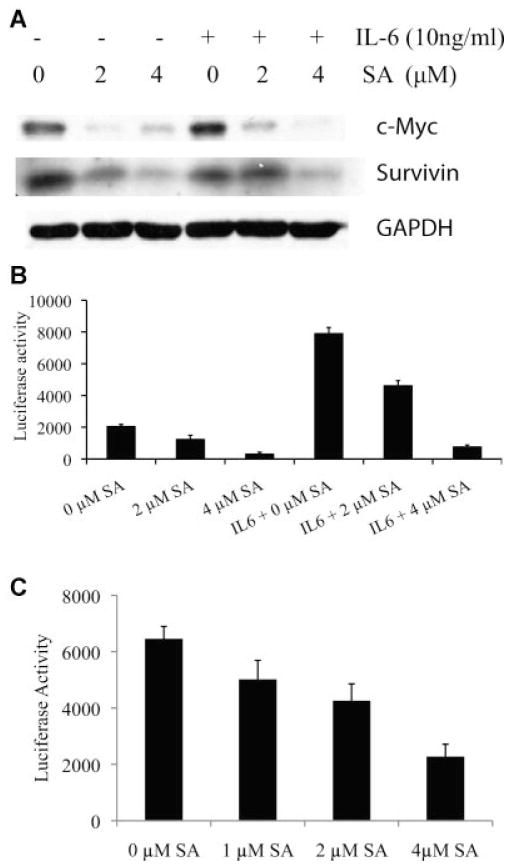
Stat3 is an oncogenic transcription factor that induces expression of target genes by binding to Stat3-responsive elements in their promoters. As a transcription factor, Stat3 activation contributes to cancer progression through upregulation of its target genes such as c-myc and survivin [36]. Sanguinarine decreased the levels of both endogenous c-myc and survivin proteins and those induced by IL-6 (Fig. 2A). To examine the effect of sanguinarine on the activity of Stat3-responsive genes, we transfected castration resistant C4-2B prostate cancer cells with the pLucTKS3 luciferase reporter containing the Stat3 responsive elements [30,31], or the control plasmid, and treated the cells with sanguinarine with or without IL-6. As shown in Figure 2B, sanguinarine inhibited Stat3-responsive luciferase reporter activity. IL-6 strongly induced the Stat3-responsive luciferase reporter activity, which was reduced by sanguinarine treatment (Fig. 2B). We have previously generated a subline of LNCaP (LN-S17) that express high levels of IL-6 and exhibit constitutive activation of Stat3 [6]. Sanguinarine decreased the Stat3-responsive luciferase activity in LN-S17 cells in a dose-dependent manner (Fig. 2C).
Fig. 2.
Effect of sanguinarine on the expression of Stat3 target genes. A: Immunoblot for c-myc and survivin and GAPDH. DU145 cells were treated with 2 and 4 μM sanguinarine for 4 hr and stimulated with 10 ng/ml IL-6 for 30 min. Whole cell lysates were used for Western blot analysis. B: C4-2B cells were transfected with the Stat3-responsive luciferase reporter plasmid pLucTKS3 and treated with 0, 2, or 4 μM of sanguinarine for 6 hr in the presence or absence of 10 ng/ml IL-6. C: LN-S17 cells were transfected with the Stat3-responsive luciferase reporter plasmid pLucTKS3 and treated with 0, 2, or 4 μM of sanguinarine for 6 hr. Luciferase activity was measured and shown in arbitrary units.
Sanguinarine Inhibits Prostate Cancer Cells Migration and Invasion
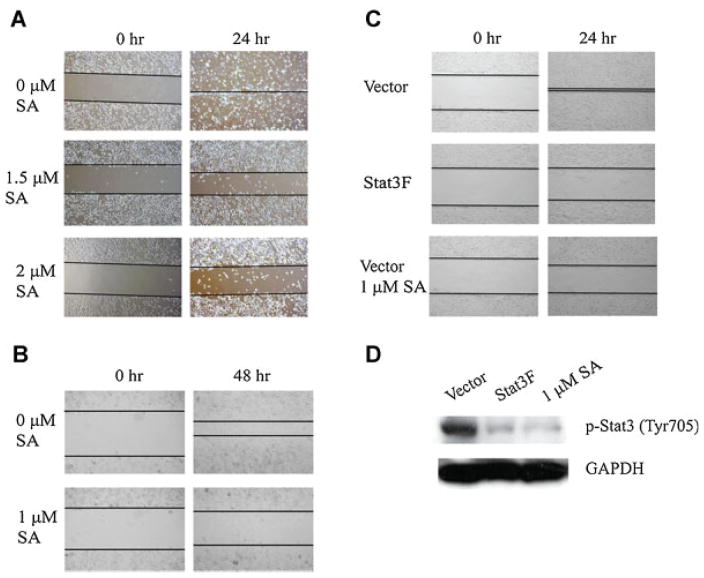
Given the fact that Stat3 is constitutively active in the majority of human clinical prostate cancer metastases and the role of Stat3 in cancer migration and invasion [2], and that sanguinarine can effectively inhibit Stat3 activation, we examined the effect of sanguinarine on prostate cancer cell migration and invasion. As shown in Figure 3A, sanguinarine inhibited wound healing in DU145 cells that express constitutively activated Stat3 at both 1.5 and 2 μM in 24 hr. Sanguinarine treatment also blocked wound healing in LN-S17 cells that express constitutively activated Stat3 (Fig. 3B). To further determine the role of Stat3 activation in DU145 cell migration, we transfected DU145 cells with Stat3F, a dominant negative mutant of Stat3 with a point mutation at Y705F. This dominant negative form of Stat3 has been shown to be able to inhibit the phosphorylation of Tyr705 of endogenous Stat3 and inhibit expression of Stat3 target genes [7,20,37]. We transfected DU145 cells with Stat3F or control vector and then treated the cells with or without sanguinarine for 24 hr. As shown in Figure 3C, Stat3F completely blocked the migration of DU145 cells, suggesting that Stat3 activation is required for DU145 cell migration. Figure 3D showed that Stat3F inhibited phosphorylation of Stat3.
Fig. 3.
Sanguinarine inhibits prostate cancer cells migration in vitro. A: Sanguinarine inhibited DU145 cells migration in the wound healing assay. DU145 cells were treated with DMSO, 1.5 or 2 μM sanguinarine after the wounds were introduced. Photographs of the cells were taken at time zero or 24 hr .B: Sanguinarine inhibited LN-S17 cells migration in 24 hr. C: Both Stat3F and sanguinarine inhibited DU145 cell migration. DU145 cells were transfected with Stat3F or the empty vector and then treated with 1 μM sanguinarine for 24 hr. D: Immunoblot for p-Stat3 from the cells transfected with Stat3F or vector and treated with 1 μM sanguinarine. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
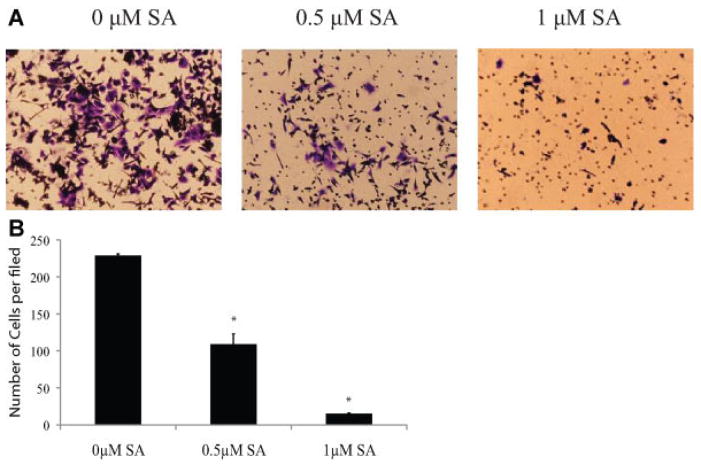
In a Boyden chamber based invasion assay, we examined the effect of sanguinarine on DU145 cells invasion. As shown in Figure 4A,B, sanguinarine significantly reduced the number of invasive cells in a dose-dependent manner. The suppression of invasion was not due to apoptosis or growth arrest as 0.5 μM sanguinarine exhibited minimal toxicity in DU145 cells [23]. Collectively, these data suggest that Stat3 activation is required for DU145 cell migration and invasion and that inhibition of Stat3 activation by sanguinarine suppresses cell migration and invasion.
Fig. 4.
Sanguinarine inhibits DU145 cells invasion in vitro. A: Representative photographs of invasive DU145 cells. The cells were allowed to migrate through matrigel coated membrane with 8 μm pores for 48 hr in the presence of 0, 0.5, or 1 μM sanguinarine. B: Quantification of invasive DU145 cells.* Indicates statistical significance. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Sanguinarine Inhibits DU145 and LN-S17 Colony Formation in Soft Agar
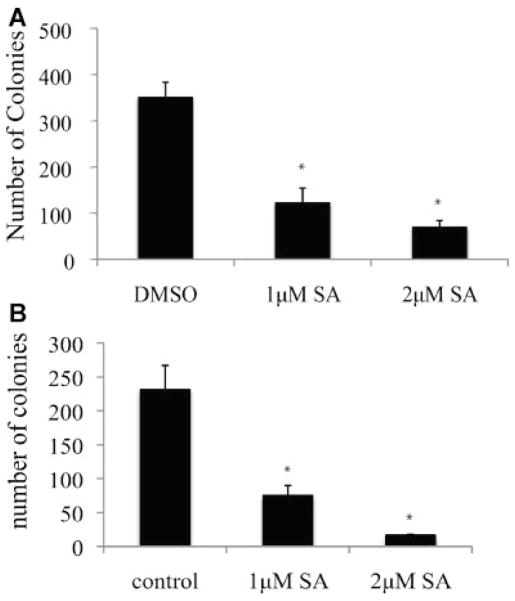
Stat3 has been shown to mediate anchorage-independent growth in human cancer cells [38,39]. To test the effect of sanguinarine on anchorage-independent growth of prostate cancer cells that express constitutively active Stat3, we performed soft agar colony formation assay in DU145 and LN-S17 cells that express constitutively active Stat3. As shown in Figure 5, sanguinarine significantly inhibited colony formation in both DU145 (Fig. 5A) and LN-S17 cells (Fig. 5B). These data suggest that sanguinarine inhibits the tumorigenicity of prostate cancer cells expressing constitutively activated Stat3.
Fig. 5.
Sanguinarine inhibits the anchorage-independent growth of prostate cancer cells. A: 1 × 104 DU145 cells were plated in 0.3% agar in cell culture media containing 0, 1, or 2 μM sanguinarine. B: 2 × 104 LN-S17 cells were plated in 0.3% agar in cell culture media containing 0, 1, or 2 μM sanguinarine. The colonies were stained with crystal violet after 4 weeks. The scanned images of the colonies were quantified with the image software. *Indicates statistical significance.
DISCUSSION
Accumulating studies provide strong evidence that Stat3 activation plays an important role in the development and progression of prostate cancer. We previously demonstrated that increased Stat3 activation frequently occurs in prostate cancer and that constitutively activated Stat3 promotes prostate cancer cell tumor growth [3,40]. Blockade of Stat3 activation by a dominant negative Stat3 mutant resulted in suppression of prostate cancer growth both in vitro and in vivo [7]. Numerous studies also demonstrate that Stat3 activates AR-mediated gene expression and prevents apoptosis [4,40,41]. Collectively, these findings indicate that targeting Stat3 signaling may represent a novel approach to treat prostate cancer.
In this study, we have identified that sanguinarine inhibits constitutively activated Stat3 and suppresses cell growth and invasion. Sanguinarine has the ability to inhibit Stat3 phosphorylation at both tyrosine 705 and serine 727 sites, suggesting that sanguinarine has the potential to completely block Stat3 activation since maximal activation of Stat3 requires phosphorylation at both tyrosine 705 and serine 727. To understand how sanguinarine inhibits Stat3 activation, we examined the effect of sanguinarine on Jak2, a kinase that activates Stat3, and found that sanguinarine effectively inhibits Jak2 phosphorylation, suggesting that inhibition of Stat3 activation by sanguinarine is through suppression of Jak2 expression. In addition to inhibition of Jak2, sanguinarine can reduce Src phosphorylation, another kinase that activates Stat3.
The oncogenic effect of Stat3 is mostly through dys-regulation of its target genes such as c-myc and survivin. We have previously demonstrated that sanguinarine inhibits survivin expression in prostate cancer cells [23]. In this study, we showed that sanguinarine can also inhibit c-myc expression. Furthermore, analyzing the effect of sanguinarine on Stat3 responsive elements, we found that sanguinarine can inhibit transactivation of Stat3 responsive elements, suggesting that sanguinarine is capable of inhibition of Stat3 target gene expression.
Stat3 plays an important role in cancer cell motility, migration, and invasion in many types of cancer such as breast, bladder, ovarian, and brain [42–45]. In prostate cancer, Stat3 activation has been found to be associated with lymph node and bone metastases of clinical human prostate cancer, and Stat3 promotes human prostate cancer cell migration and metastases [2,46,47]. Introduction of an activating mutant form of Stat3 (Stat3c) into immortalized prostate epithelial cells resulted in tumorigenesis and enhanced migration through integrin beta 6 [10]. Inhibition of Stat3 activation by Stat3 specific shRNA or AG490 suppressed migration of prostate cancer cells that express constitutively activated Stat3 [2]. We showed that sanguinarine inhibited cell motility in DU145 cells expressing constitutively activated Stat3 and LN-s17 cells expressing activated Stat3 induced by IL-6. Furthermore, invasion ability of DU145 cells was also inhibited by sanguinarine. The ability of inhibition of migration and invasion by sanguinarine is comparable to that of the Stat3F, the dominant negative Stat3. These results further confirm that inhibition of Stat3 activation by sanguinarine results in suppression of cell migration and invasion. In addition to the newly identified target of Stat3, sanguinarine has been shown to possess anti-microbial, anti-oxidant, anti-inflammatory, and anti-tumor properties and is widely used in toothpaste and mouthwash to prevent/treat gingivitis and other inflammatory conditions of the mouth [24,48,49].
In conclusion, we have identified that sanguinarine inhibits constitutively activated Stat3 and suppresses cell proliferation, migration, and invasion. Since sanguinarine has been approved and used clinically, our study holds therapeutic promise for repositioning of sanguinarine for treatment of advanced prostate cancer by targeting constitutively activated Stat3. We are currently testing the efficacy of sanguinarine in mouse models of prostate cancer with constitutively activated Stat3.
Acknowledgments
This work was supported in part by grants from NIH CA109441, CA118887, CA140468, and VA merits I01 BX000526.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60(5):277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Abdulghani J, Gu L, Dagvadorj A, Lutz J, Leiby B, Bonuccelli G, Lisanti MP, Zellweger T, Alanen K, Mirtti T, Visakorpi T, Bubendorf L, Nevalainen MT. Stat3 promotes metastatic progression of prostate cancer. Am J Pathol. 2008;172(6):1717–1728. doi: 10.2353/ajpath.2008.071054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dhir R, Ni Z, Lou W, DeMiguel F, Grandis JR, Gao AC. Stat3 activation in prostatic carcinomas. Prostate. 2002;51(4):241–246. doi: 10.1002/pros.10079. [DOI] [PubMed] [Google Scholar]
- 4.Mora LB, Buettner R, Seigne J, Diaz J, Ahmad N, Garcia R, Bowman T, Falcone R, Fairclough R, Cantor A, Muro-Cacho C, Livingston S, Karras J, Pow-Sang J, Jove R. Constitutive activation of Stat3 in human prostate tumors and cell lines: Direct inhibition of Stat3 signaling induces apoptosis of prostate cancer cells. Cancer Res. 2002;62(22):6659–6666. [PubMed] [Google Scholar]
- 5.Chen T, Wang LH, Farrar WL. Interleukin 6 activates androgen receptor-mediated gene expression through a signal transducer and activator of transcription 3-dependent pathway in LNCaP prostate cancer cells. Cancer Res. 2000;60(8):2132–2135. [PubMed] [Google Scholar]
- 6.Lou W, Ni Z, Dyer K, Tweardy DJ, Gao AC. Interleukin-6 induces prostate cancer cell growth accompanied by activation of stat3 signaling pathway. Prostate. 2000;42(3):239–242. doi: 10.1002/(sici)1097-0045(20000215)42:3<239::aid-pros10>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 7.Ni Z, Lou W, Leman ES, Gao AC. Inhibition of constitutively activated Stat3 signaling pathway suppresses growth of prostate cancer cells. Cancer Res. 2000;60(5):1225–1228. [PubMed] [Google Scholar]
- 8.De Miguel F, Lee SO, Onate SA, Gao AC. Stat3 enhances trans-activation of steroid hormone receptors. Nucl Recept. 2003;1(1):3. doi: 10.1186/1478-1336-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sekine Y, Suzuki K, Remaley AT. HDL and sphingosine-1-phosphate activate stat3 in prostate cancer DU145 cells via ERK1/2 and S1P receptors, and promote cell migration and invasion. Prostate. 2011;71(7):690–699. doi: 10.1002/pros.21285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Azare J, Leslie K, Al-Ahmadie H, Gerald W, Weinreb PH, Violette SM, Bromberg J. Constitutively activated Stat3 induces tumorigenesis and enhances cell motility of prostate epithelial cells through integrin beta 6. Mol Cell Biol. 2007;27(12):4444–4453. doi: 10.1128/MCB.02404-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Darnell JE., Jr STATs and gene regulation. Science. 1997;277(5332):1630–1635. doi: 10.1126/science.277.5332.1630. [DOI] [PubMed] [Google Scholar]
- 12.Yuan ZL, Guan YJ, Chatterjee D, Chin YE. Stat3 dimerization regulated by reversible acetylation of a single lysine residue. Science. 2005;307(5707):269–273. doi: 10.1126/science.1105166. [DOI] [PubMed] [Google Scholar]
- 13.Wang R, Cherukuri P, Luo J. Activation of Stat3 sequence-specific DNA binding and transcription by p300/CREB-binding protein-mediated acetylation. J Biol Chem. 2005;280(12):11528–11534. doi: 10.1074/jbc.M413930200. [DOI] [PubMed] [Google Scholar]
- 14.Bowman T, Garcia R, Turkson J, Jove R. STATs in oncogenesis. Oncogene. 2000;19(21):2474–2488. doi: 10.1038/sj.onc.1203527. [DOI] [PubMed] [Google Scholar]
- 15.Kujawski M, Kortylewski M, Lee H, Herrmann A, Kay H, Yu H. Stat3 mediates myeloid cell-dependent tumor angiogenesis in mice. J Clin Invest. 2008;118(10):3367–3377. doi: 10.1172/JCI35213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Devarajan E, Huang S. STAT3 as a central regulator of tumor metastases. Curr Mol Med. 2009;9(5):626–633. doi: 10.2174/156652409788488720. [DOI] [PubMed] [Google Scholar]
- 17.Meydan N, Grunberger T, Dadi H, Shahar M, Arpaia E, Lapidot Z, Leeder JS, Freedman M, Cohen A, Gazit A, Levitzki A, Roifman CM. Inhibition of acute lymphoblastic leukaemia by a Jak-2 inhibitor. Nature. 1996;379(6566):645–648. doi: 10.1038/379645a0. [DOI] [PubMed] [Google Scholar]
- 18.Blaskovich MA, Sun J, Cantor A, Turkson J, Jove R, Sebti SM. Discovery of JSI-124 (cucurbitacin I), a selective Janus kinase/signal transducer and activator of transcription 3 signaling pathway inhibitor with potent antitumor activity against human and murine cancer cells in mice. Cancer Res. 2003;63(6):1270–1279. [PubMed] [Google Scholar]
- 19.Leong PL, Andrews GA, Johnson DE, Dyer KF, Xi S, Mai JC, Robbins PD, Gadiparthi S, Burke NA, Watkins SF, Grandis JR. Targeted inhibition of Stat3 with a decoy oligonucleotide abrogates head and neck cancer cell growth. Proc Natl Acad Sci USA. 2003;100(7):4138–4143. doi: 10.1073/pnas.0534764100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakajima K, Yamanaka Y, Nakae K, Kojima H, Ichiba M, Kiuchi N, Kitaoka T, Fukada T, Hibi M, Hirano T. A central role for Stat3 in IL-6-induced regulation of growth and differentiation in M1 leukemia cells. EMBO J. 1996;15(14):3651–3658. [PMC free article] [PubMed] [Google Scholar]
- 21.Siddiquee KA, Gunning PT, Glenn M, Katt WP, Zhang S, Schrock C, Sebti SM, Jove R, Hamilton AD, Turkson J. An oxazole-based small-molecule Stat3 inhibitor modulates Stat3 stability and processing and induces antitumor cell effects. ACS Chem Biol. 2007;2(12):787–798. doi: 10.1021/cb7001973. [DOI] [PubMed] [Google Scholar]
- 22.Leeman RJ, Lui VW, Grandis JR. STAT3 as a therapeutic target in head and neck cancer. Expert Opin Biol Ther. 2006;6(3):231–241. doi: 10.1517/14712598.6.3.231. [DOI] [PubMed] [Google Scholar]
- 23.Sun M, Lou W, Chun JY, Cho DS, Nadiminty N, Evans CP, Chen J, Yue J, Zhou Q, Gao AC. Sanguinarine suppresses prostate tumor growth and inhibits survivin expression. Genes Cancer. 2010;1(3):283–292. doi: 10.1177/1947601910368849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mitscher LA, Park YH, Clark D, Clark GW, III, Hammesfahr PD, Wu WN, Beal JL. Antimicrobial agents from higher plants. An investigation of Hunnemannia fumariaefolia pseudoalcoholates of sanguinarine and chelerythrine. Lloydia. 1978;41(2):145–150. [PubMed] [Google Scholar]
- 25.Chaturvedi MM, Kumar A, Darnay BG, Chainy GB, Agarwal S, Aggarwal BB. Sanguinarine (pseudochelerythrine) is a potent inhibitor of NF-kappaB activation, IkappaBalpha phosphorylation, and degradation. J Biol Chem. 1997;272(48):30129–30134. doi: 10.1074/jbc.272.48.30129. [DOI] [PubMed] [Google Scholar]
- 26.Han MH, Yoo YH, Choi YH. Sanguinarine-induced apoptosis in human leukemia U937 cells via Bcl-2 downregulation and caspase-3 activation. Chemotherapy. 2008;54(3):157–165. doi: 10.1159/000140359. [DOI] [PubMed] [Google Scholar]
- 27.Choi WY, Kim GY, Lee WH, Choi YH. Sanguinarine, a benzophenanthridine alkaloid, induces apoptosis in MDA-MB-231 human breast carcinoma cells through a reactive oxygen species-mediated mitochondrial pathway. Chemotherapy. 2008;54(4):279–287. doi: 10.1159/000149719. [DOI] [PubMed] [Google Scholar]
- 28.Adhami VM, Aziz MH, Reagan-Shaw SR, Nihal M, Mukhtar H, Ahmad N. Sanguinarine causes cell cycle blockade and apoptosis of human prostate carcinoma cells via modulation of cyclin kinase inhibitor-cyclin-cyclin-dependent kinase machinery. Mol Cancer Ther. 2004;3(8):933–940. [PubMed] [Google Scholar]
- 29.Lee SO, Lou W, Hou M, de Miguel F, Gerber L, Gao AC. Interleukin-6 promotes androgen-independent growth in LNCaP human prostate cancer cells. Clin Cancer Res. 2003;9(1):370–376. [PubMed] [Google Scholar]
- 30.Turkson J, Bowman T, Garcia R, Caldenhoven E, De Groot RP, Jove R. Stat3 activation by Src induces specific gene regulation and is required for cell transformation. Mol Cell Biol. 1998;18(5):2545–2552. doi: 10.1128/mcb.18.5.2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee SO, Lou W, Qureshi KM, Mehraein-Ghomi F, Trump DL, Gao AC. RNA interference targeting Stat3 inhibits growth and induces apoptosis of human prostate cancer cells. Prostate. 2004;60(4):303–309. doi: 10.1002/pros.20072. [DOI] [PubMed] [Google Scholar]
- 32.Li L, Shaw PE. A STAT3 dimer formed by inter-chain disulphide bridging during oxidative stress. Biochem Biophys Res Commun. 2004;322(3):1005–1011. doi: 10.1016/j.bbrc.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 33.Aziz MH, Hafeez BB, Sand JM, Pierce DB, Aziz SW, Dreckschmidt NE, Verma AK. Protein kinase Cvarepsilon mediates Stat3 Ser727 phosphorylation, Stat3-regulated gene expression, and cell invasion in various human cancer cell lines through integration with MAPK cascade (RAF-1, MEK1/2, and ERK1/2) Oncogene. 2010;29(21):3100–3109. doi: 10.1038/onc.2010.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hedvat M, Huszar D, Herrmann A, Gozgit JM, Schroeder A, Sheehy A, Buettner R, Proia D, Kowolik CM, Xin H, Armstrong B, Bebernitz G, Weng S, Wang L, Ye M, McEachern K, Chen H, Morosini D, Bell K, Alimzhanov M, Ioannidis S, McCoon P, Cao ZA, Yu H, Jove R, Zinda M. The JAK2 inhibitor AZD1480 potently blocks Stat3 signaling and oncogenesis in solid tumors. Cancer Cell. 2009;16(6):487–497. doi: 10.1016/j.ccr.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Purnell PR, Mack PC, Tepper CG, Evans CP, Green TP, Gumerlock PH, Lara PN, Gandara DR, Kung HJ, Gautschi O. The Src inhibitor AZD0530 blocks invasion and may act as a radiosensitizer in lung cancer cells. J Thorac Oncol. 2009;4(4):448–454. doi: 10.1097/JTO.0b013e31819c78fb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Turkson J. STAT proteins as novel targets for cancer drug discovery. Expert Opin Ther Targets. 2004;8(5):409–422. doi: 10.1517/14728222.8.5.409. [DOI] [PubMed] [Google Scholar]
- 37.Lee SO, Lou W, Johnson CS, Trump DL, Gao AC. Interleukin-6 protects LNCaP cells from apoptosis induced by androgen deprivation through the Stat3 pathway. Prostate. 2004;60(3):178–186. doi: 10.1002/pros.20045. [DOI] [PubMed] [Google Scholar]
- 38.Scholz A, Heinze S, Detjen KM, Peters M, Welzel M, Hauff P, Schirner M, Wiedenmann B, Rosewicz S. Activated signal transducer and activator of transcription 3 (STAT3) supports the malignant phenotype of human pancreatic cancer. Gastroenterology. 2003;125(3):891–905. doi: 10.1016/s0016-5085(03)01064-3. [DOI] [PubMed] [Google Scholar]
- 39.Cheng GZ, Zhang WZ, Sun M, Wang Q, Coppola D, Mansour M, Xu LM, Costanzo C, Cheng JQ, Wang LH. Twist is transcriptionally induced by activation of STAT3 and mediates STAT3 oncogenic function. J Biol Chem. 2008;283(21):14665–14673. doi: 10.1074/jbc.M707429200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.DeMiguel F, Lee SO, Lou W, Xiao X, Pflug BR, Nelson JB, Gao AC. Stat3 enhances the growth of LNCaP human prostate cancer cells in intact and castrated male nude mice. Prostate. 2002;52(2):123–129. doi: 10.1002/pros.10110. [DOI] [PubMed] [Google Scholar]
- 41.Chen T, Wang LH, Farrar WL. Interleukin 6 activates androgen receptor-mediated gene expression through a signal transducer and activator of transcription 3-dependent pathway in LNCaP prostate cancer cells [In Process Citation] Cancer Res. 2000;60(8):2132–2135. [PubMed] [Google Scholar]
- 42.Itoh M, Murata T, Suzuki T, Shindoh M, Nakajima K, Imai K, Yoshida K. Requirement of STAT3 activation for maximal collagenase-1 (MMP-1) induction by epidermal growth factor and malignant characteristics in T24 bladder cancer cells. Oncogene. 2006;25(8):1195–1204. doi: 10.1038/sj.onc.1209149. [DOI] [PubMed] [Google Scholar]
- 43.Silver DL, Naora H, Liu J, Cheng W, Montell DJ. Activated signal transducer and activator of transcription (STAT) 3: Localization in focal adhesions and function in ovarian cancer cell motility. Cancer Res. 2004;64(10):3550–3558. doi: 10.1158/0008-5472.CAN-03-3959. [DOI] [PubMed] [Google Scholar]
- 44.Zhang F, Li C, Halfter H, Liu J. Delineating an oncostatin M-activated STAT3 signaling pathway that coordinates the expression of genes involved in cell cycle regulation and extra-cellular matrix deposition of MCF-7 cells. Oncogene. 2003;22(6):894–905. doi: 10.1038/sj.onc.1206158. [DOI] [PubMed] [Google Scholar]
- 45.Xie TX, Wei D, Liu M, Gao AC, Ali-Osman F, Sawaya R, Huang S. Stat3 activation regulates the expression of matrix metallo-proteinase-2 and tumor invasion and metastasis. Oncogene. 2004;23(20):3550–3560. doi: 10.1038/sj.onc.1207383. [DOI] [PubMed] [Google Scholar]
- 46.Gu L, Dagvadorj A, Lutz J, Leiby B, Bonuccelli G, Lisanti MP, Addya S, Fortina P, Dasgupta A, Hyslop T, Bubendorf L, Nevalainen MT. Transcription factor Stat3 stimulates meta-static behavior of human prostate cancer cells in vivo, whereas Stat5b has a preferential role in the promotion of prostate cancer cell viability and tumor growth. Am J Pathol. 2010;176(4):1959–1972. doi: 10.2353/ajpath.2010.090653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou W, Grandis JR, Wells A. STAT3 is required but not sufficient for EGF receptor-mediated migration and invasion of human prostate carcinoma cell lines. Br J Cancer. 2006;95(2):164–171. doi: 10.1038/sj.bjc.6603234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Larsson DE, Lovborg H, Rickardson L, Larsson R, Oberg K, Granberg D. Identification and evaluation of potential anti-cancer drugs on human neuroendocrine tumor cell lines. Anticancer Res. 2006;26(6B):4125–4129. [PubMed] [Google Scholar]
- 49.Eun JP, Koh GY. Suppression of angiogenesis by the plant alkaloid, sanguinarine. Biochem Biophys Res Commun. 2004;317(2):618–624. doi: 10.1016/j.bbrc.2004.03.077. [DOI] [PubMed] [Google Scholar]