Abstract
Background
Data describing urinary health in elderly, community-dwelling prostate cancer (PCa) survivors are limited.
Objective
To elucidate the prevalence of lower urinary tract symptoms, urinary bother, and incontinence in elderly PCa survivors compared with peers without PCa.
Design, setting, and participants
A cross-sectional analysis of 5990 participants in the Osteoporotic Fractures in Men Research Group, a cohort study of community-dwelling men ≥65 yr.
Outcome measurements and statistical analysis
We characterized urinary health using self-reported urinary incontinence and the American Urological Association Symptom Index (AUA-SI). We compared urinary health measures according to type of PCa treatment in men with PCa and men without PCa using multivariate log-binomial regression to generate prevalence ratios (PRs).
Results and limitations
At baseline, 706 men (12%) reported a history of PCa, with a median time since diagnosis of 6.3 yr. Of these men, 426 (60%) reported urinary incontinence. In adjusted analyses, observation (PR: 1.92; 95% confidence interval [CI], 1.15–3.21; p = 0.01), surgery (PR: 4.68; 95% CI, 4.11–5.32; p < 0.0001), radiation therapy (PR: 1.64; 95% CI, 1.20– 2.23; p = 0.002), and androgen-deprivation therapy (ADT) (PR: 2.01; 95% CI, 1.35–2.99; p = 0.0006) were each associated with daily incontinence. Daily incontinence risk increased with time since diagnosis independently of age. Observation (PR: 1.33; 95% CI, 1.00–1.78; p = 0.05), surgery (PR: 1.25; 95% CI, 1.10–1.42; p = 0.0008), and ADT (PR: 1.50; 95% CI, 1.26–1.79; p < 0.0001) were associated with increased AUA-SI bother scores. Cancer stage and use of adjuvant or salvage therapies were not available for analysis.
Conclusions
Compared with their peers without PCa, elderly PCa survivors had a two-fold to five-fold greater prevalence of urinary incontinence, which rose with increasing survivorship duration. Observation, surgery, and ADT were each associated with increased urinary bother. These data suggest a substantially greater burden of urinary health problems among elderly PCa survivors than previously recognized.
Keywords: Aging male, Elderly, Epidemiology, Incontinence, Lower urinary tract symptoms, Prostate cancer, Prostate cancer treatment, Urinary bother
1. Introduction
Prostate cancer (PCa) is the second most frequently diagnosed cancer and the sixth leading cause of cancer death in males worldwide [1,2]. Because of prostate-specific antigen (PSA) screening, most men are diagnosed with early-stage disease, the majority of whom experience lengthy progression-free and overall survival regardless of treatment modality [3–7]. Men who undergo aggressive treatment with surgery or radiation therapy are at increased risk of chronic, treatment-related adverse effects [3,6]. There are >2.7 million PCa survivors in the United States, and this number is expected to increase to >3.9 million by 2022 [8]. The combination of frequent and early detection, lengthy PCa survivorship marked by treatment-related adverse effects, and an expanding population of aging survivors highlights the importance of elucidating the chronic health burdens of PCa in elderly PCa survivors.
A common and costly adverse effect of treatment of localized PCa is diminished urinary health: urinary incontinence (most often from surgery) and lower urinary tract symptoms (LUTS) including urinary frequency, urinary urgency, and nocturia (most often from radiation therapy) [9–13]. Prior investigations of urinary health in PCa survivors have focused primarily on pretreatment and posttreatment comparisons in patients with localized disease who undergo surgery, radiation therapy, or cryosurgery [5–8]. In these studies, the prevalence of clinically significant urinary bother 2 yr after treatment is as high as 8% after radical prostatectomy and 11% after external-beam radiation therapy [9,14]; urinary incontinence occurs in as many as 69% and 25% of men after radical prostatectomy and radiation therapy, respectively [12].
However, the true prevalence of chronic urinary health problems among elderly PCa survivors remains unclear for at least three reasons. First, urinary health data comparing elderly PCa survivors with similarly aged peers without PCa are limited [11,13,15]. Second, there are no comprehensive studies of urinary health in PCa survivors who are on observation or are receiving androgen-deprivation therapy (ADT). Finally, because LUTS occur among 15%–80% of males >40 yr without PCa, it is unclear whether urinary health problems in elderly PCa survivors exceed the problems that might be expected with normal aging [16–18].
Further analyses of urinary incontinence, LUTS, and urinary bother in older PCa survivors may elucidate the long-term impacts of PCa diagnosis and treatment on urinary health in the elderly and inform strategies to enhance the quality of survivorship in this population. Therefore, we performed a cross-sectional study to examine the effects of PCa diagnosis and treatment on urinary incontinence, LUTS, and urinary bother in a large cohort of community-dwelling elderly men.
2. Patients and methods
2.1. Study population
The Osteoporotic Fractures in Men (MrOS) study is a prospective cohort study of 5994 community-dwelling men ≥65 yr that includes data on fractures, falls, and prostatic disease [19,20]. Participants enrolled in the study between March 2000 and April 2002 at an academic medical center at one of six US sites: Birmingham, Alabama; Minneapolis, Minnesota; Palo Alto, California; Pittsburgh, Pennsylvania; Portland, Oregon; and San Diego, California.
2.2. Measurements
At baseline, all participants completed a questionnaire that included demographics; medical history; and lifestyle, including alcohol use, tobacco use, and self-rated health. Medical history included queries for benign prostatic hyperplasia (BPH) diagnosed by a physician or other health care provider and BPH treatment. We characterized LUTS using the validated American Urological Association Symptom Index (AUA-SI) [21]. We defined LUTS as mild (AUA-SI <8), moderate (AUA-SI 8–19), or severe (AUA-SI ≥20) and defined clinically significant urinary bother as a bother score ≥3 points [21].
Self-report of urinary incontinence over the prior month incorporated multiple measures, including use of absorbent pads or diapers, ability to control urine, frequency of urine leakage, and the extent to which urine leakage caused problems. The medical history also assessed for a history of PCa diagnosed by a physician or other health care provider, including age at time of diagnosis and history of treatment with surgery, radiation therapy, ADT, observation, or other treatment. Body mass index was calculated as kilograms divided by square meters. Height was measured by wall-mounted stadiometers; weight was measured by a balance beam or digital scale.
2.3. Statistical analysis
We examined baseline characteristics of patients with self-reported PCa and patients without self-reported PCa. Descriptive statistics included the Pearson χ2 test for contingency tables and the t test for continuous variables. We used log-binomial regression models to compare the prevalence of LUTS and incontinence among men with PCa and men without PCa. Although we examined, and report the results from, several different measures of urinary incontinence, we used “at least one episode of daily urinary incontinence” as the primary definition. Prevalence ratios (PRs) and their 95% confidence intervals (CIs) were estimated as the measure of association [22]. During descriptive analyses, we evaluated all the previously noted demographic, body size, lifestyle, and medical conditions as potential confounding factors. A variable was determined to be a confounder if the variable changed the PR estimates by ≥10%. Based on this selection procedure, the final multivariable PRs were adjusted for age, previous BPH diagnosis, self-reported health status, and smoking status. To evaluate the effect on urinary incontinence of time from treatment in men with PCa, we performed a time- and age-stratified analysis. Statistical analysis was performed using SAS software (SAS Institute Inc., Cary, NC, USA).
3. Results
3.1. Study population
Our analysis included 5990 participants who completed the baseline AUA-SI questionnaire (Table 1). At baseline, 706 participants (12%) reported a history of PCa. Compared with men without PCa, men with PCa were more likely to be ≥80 yr; less likely to be current smokers; and more likely to report fair or poor self-rated health, a prior diagnosis of BPH, or a history of cancers other than PCa. Among men with PCa, the majority reported a history of surgery (Table 1).
Table 1.
Distribution of baseline characteristics among men who did not and did report a history of prostate cancer in the Osteoporotic Fractures in Men study
| PCa |
|||
|---|---|---|---|
| Yes | No | ||
| No. (%) | 706 (12) | 5284 (88) | |
|
|
|||
| Baseline characteristic | p value | ||
| Age, no. (%) | |||
| 65–69 yr | 131 (18.6) | 1638 (31.0) | <0.0001 |
| 70–79 yr | 377 (53.4) | 2777 (52.6) | |
| ≥80 yr | 198 (28.0) | 869 (16.5) | |
| Caucasian race, no. (%) | 625 (88.5) | 4733 (89.6) | 0.40 |
| BMI, kg/m2, mean (SD) | 27.4 (3.8) | 27.4 (3.8) | 0.76 |
| Alcohol consumption, drinks per week, median (IQR) | 1.0 (0.0–7.0) | 1.0 (0.0–7.0) | 0.69 |
| No | 280 (39.7) | 1967 (37.2) | 0.03 |
| Past | 413 (58.5) | 3124 (59.1) | |
| Current | 13 (1.8) | 192 (3.6) | |
| Self-reported health fair or poor, no. (%) | 125 (17.7) | 732 (13.9) | 0.006 |
| Previous BPH diagnosis, no. (%) | 389 (55.1) | 2539 (48.1) | 0.0004 |
| Medical history, no. (%) | |||
| Any heart disease | 159 (22.5) | 1269 (24.0) | 0.38 |
| Self-reported diabetes | 67 (9.5) | 586 (11.1) | 0.20 |
| Self-reported hypertension | 304 (43.1) | 2276 (43.1) | 0.99 |
| Self-reported cancer, not PCa | 216 (30.6) | 1036 (19.6) | <0.0001 |
| Years since PCa diagnosis, mean (SD) | 6.3 (4.8) | – | – |
| PCa major treatment, no. (%) | |||
| Surgery | 362 (51.3) | – | – |
| Radiation therapy | 204 (29.0) | – | – |
| Hormones | 68 (9.6) | – | – |
| Observation | 42 (6.0) | – | – |
| Other treatment | 30 (4.3) | – | – |
PCa = prostate cancer; BMI = body mass index; SD = standard deviation; IQR = interquartile range; BPH = benign prostatic hyperplasia.
3.2. Lower urinary tract symptoms and urinary incontinence
In unadjusted analyses, there were no significant differences in median AUA-SI scores or the proportions of men reporting moderate or severe LUTS. However, men with PCa, in comparison with men without PCa, were significantly more likely to report substantial urinary bother and urinary incontinence as represented by lack of urinary control, frequency of daily leaking, pad/diaper use, and extent of leaking problems (Table 2).
Table 2.
Distribution of American Urological Association Symptom Index scores and measure of urinary incontinence among men who did not and did report a history of prostate cancer in the Osteoporotic Fractures in Men study
| PCa history |
|||
|---|---|---|---|
| Yes | No | ||
| No. (%) | 706 (12) | 5284 (88) | |
|
|
|||
| p value | |||
| AUA-SI symptom score, median (IQR) | 7 (3–12) | 7 (3–12) | 0.73 |
| AUA-SI symptom category, no. (%) | 0.48 | ||
| None/mild: 0–7 points | 389 (55.1) | 2847 (53.9) | |
| Moderate: 8–19 points | 265 (37.5) | 2091 (39.6) | |
| Severe: ≥20 points | 52 (7.4) | 346 (6.6) | |
| Urinary bother, no. (%) | <0.0001 | ||
| Delighted/pleased/mostly satisfied | 398 (56.4) | 3566 (67.5) | |
| Mixed | 163 (23.1) | 1044 (19.8) | |
| Dissatisfied/unhappy/terrible | 145 (20.5) | 674 (12.8) | |
| Urinary control in past month, no. (%) | <0.0001 | ||
| Total control | 280 (39.7) | 3473 (65.7) | |
| Occasional leaking | 343 (48.6) | 1683 (31.9) | |
| Frequent leaking/no control | 83 (11.8) | 128 (2.4) | |
| Frequency of leaking urine past month, no. (%) | <0.0001 | ||
| Not all | 237 (33.6) | 3113 (58.9) | |
| Less than once per week | 157 (22.2) | 1086 (20.6) | |
| Once per week | 100 (14.2) | 596 (11.3) | |
| At least once per day | 212 (30.0) | 489 (9.3) | |
| Pad/diaper use in past month, no. (%) | <0.0001 | ||
| None | 583 (82.6) | 5200 (98.4) | |
| At least one pad per day | 123 (17.4) | 84 (1.6) | |
| Extent of problem with leaking urine, no. (%) | <0.0001 | ||
| No problem | 331 (46.9) | 3715 (70.3) | |
| Very small or small problem | 300 (42.5) | 1440 (27.3) | |
| Moderate to big problem | 75 (10.6) | 129 (2.4) | |
PCa = prostate cancer; AUA-SI = American Urological Association Symptom Index; IQR = interquartile range.
In unadjusted analyses stratified by type of treatment, median AUA-SI scores varied considerably and were highest in the observation and ADT groups, in which more patients reported severe (AUA-SI ≥20) LUTS (Table 3). Prevalence estimates of mixed feelings or of being unsatisfied with LUTS were highest among PCa survivors, except those reporting “other” treatment. Similarly, measures of urinary incontinence were more prevalent among men with all types of PCa treatment except men reporting other treatment. Notably, men treated with surgery reported the highest prevalence of occasional or frequent leaking and use of at least one pad per day, and men treated with surgery or ADT most often reported leaking at least once per day.
Table 3.
Distribution of lower urinary tract symptoms and urinary incontinence according to prostate cancer treatment history in the Osteoporotic Fractures in Men study
| PCa treatment |
|||||||
|---|---|---|---|---|---|---|---|
| Prostate health symptoms | No PCa | Observation | Surgery | Radiation therapy |
ADT | Other | p value |
| Patients, no. | 5284 | 42 | 362 | 204 | 68 | 30 | |
| AUA-SI symptom score, median (IQR) |
7.0 (3.0–12.0) |
9.5 (3.0– 16.0) |
5.5 (3.0– 10.0) |
8.0 (4.5–13.0) |
11.0 (6.5–16.5) |
4.5 (2.0–12.0) |
<0.0001 |
| AUA-SI symptom category, no. (%) | |||||||
| None/mild: 0–7 points | 2847 (53.9) | 18 (42.9) | 233 (64.4) |
97 (47.6) | 20 (29.4) | 21 (70.0) | <0.0001* |
| Moderate: 8–19 points | 2091 (39.6) | 18 (42.9) | 115 (31.8) |
88 (43.1) | 36 (52.9) | 8 (26.7) | |
| Severe: ≥20 points | 346 (6.6) | 6 (14.3) | 14 (3.9) | 19 (9.3) | 12 (17.7) | 1 (3.3) | |
| Urinary bother, no. (%) | |||||||
| Satisfied: 0–2 points | 3566 (67.5) | 20 (47.6) | 213 (58.8) |
121 (59.3) | 23 (33.8) | 21 (70.0) | <0.0001* |
| Mixed: 3 points | 1044 (19.8) | 14 (33.3) | 79 (21.8) | 43 (21.1) | 21 (30.9) | 6 (20.0) | |
| Dissatisfied: 4–6 points | 674 (12.8) | 8 (19.1) | 70 (19.3) | 40 (19.6) | 24 (35.3) | 3 (10.0) | |
| Urinary control, no. (%) | |||||||
| Total control | 3473 (65.7) | 19 (45.2) | 96 (26.5) | 110 (53.9) | 30 (44.1) | 25 (83.3) | <0.0001* |
| Occasional leaking | 1683 (31.9) | 22 (52.4) | 204 (56.4) |
83 (40.7) | 29 (42.7) | 5 (16.7) | |
| Frequent leaking/no control |
128 (2.4) | 1 (2.4) | 62 (17.1) | 11 (5.4) | 9 (13.2) | 0 (0.0) | |
| Frequency of leaking | |||||||
| Not all | 3113 (58.9) | 19 (45.2) | 76 (21.0) | 94 (46.1) | 26 (38.2) | 22 (73.3) | <0.0001* |
| Less than once per week | 1086 (20.6) | 9 (21.4) | 79 (21.8) | 51 (25.0) | 13 (19.1) | 5 (16.7) | |
| Once per week | 596 (11.3) | 4 (9.5) | 56 (15.5) | 27 (13.2) | 12 (17.7) | 1 (3.3) | |
| At least once per day | 489 (9.3) | 10 (23.8) | 151 (41.7) |
32 (15.7) | 17 (25.0) | 2 (6.7) | |
| Pad/diaper use in past month, no. (%) | |||||||
| None | 5200 (98.4) | 39 (92.9) | 264 (72.9) |
190 (93.1) | 60 (88.2) | 30 (100.0) | <0.0001* |
| At least one pad per day | 84 (1.6) | 3 (7.1) | 98 (27.1) | 14 (6.9) | 8 (11.8) | 0 (0.0) | |
| Extent of problem with leaking urine, no. (%) | |||||||
| No problem | 3715 (70.3) | 19 (45.2) | 130 (35.9) |
124 (60.8) | 34 (50.0) | 24 (80.0) | <0.0001* |
| Very small or small problem |
1440 (27.3) | 21 (50.0) | 181 (50.0) |
68 (33.3) | 25 (36.8) | 5 (16.7) | |
| Moderate to big problem | 129 (2.4) | 2 (4.8) | 51 (14.1) | 12 (5.9) | 9 (13.2) | 1 (3.3) | |
PCa = prostate cancer; ADT = androgen-deprivation therapy; AUA-SI = American Urological Association Symptom Index; IQR = interquartile range.
3.3. Multivariable regression
Multivariable regression analyses demonstrated that the prevalence of moderate or severe LUTS was significantly higher among men with ADT compared with men with no PCa history (Table 4). Urinary incontinence remained most prevalent in surgical patients, although observation, radiation therapy, and ADT were also significantly associated with multiple metrics of urinary incontinence, including lack of urinary control, frequency of urine leakage, and problems with urine and leakage (Table 4). In addition, observation (PR: 2.11; 95% CI, 1.22–3.65; p = 0.007), surgery (PR: 4.41; 95% CI, 3.79–5.13; p < 0.0001), radiation therapy (PR: 1.49; 95% CI, 1.06– 2.08; p = 0.02), and ADT (PR: 2.02; 95% CI, 1.31–3.13; p = 0.002) were each associated with an increased risk of at least one episode of daily urinary incontinence (Table 4).
Table 4.
Prevalence ratios and 95% confidence intervals of urinary symptom severity and measures of urinary incontinence among 5990 men with or without prostate cancer*
| PCa treatment, n = 706 |
||||||
|---|---|---|---|---|---|---|
| No PCa, n = 5284 |
Observation, n = 42 |
Surgery, n = 362 |
Radiation therapy, n = 204 |
ADT, n = 68 | Other, n = 30 |
|
| AUA-SI ≥8 | ||||||
| PR | Ref | 1.07 | 0.76 | 1.06 | 1.23 | 0.58 |
| 95% CI | 0.84–1.37 | 0.66–0.87 | 0.92–1.22 | 1.05–1.45 | 0.34–0.98 | |
| p value | 0.59 | 0.001 | 0.41 | 0.001 | 0.04 | |
| AUA-SI ≥20 | ||||||
| PR | Ref | 1.66 | 0.58 | 1.23 | 1.72 | 0.39 |
| 95% CI | 0.79–3.51 | 0.34–0.98 | 0.79–1.92 | 1.01–2.91 | 0.06–2.42 | |
| p value | 0.18 | 0.04 | 0.36 | 0.04 | 0.31 | |
| AUA-SI urinary bother score ≥3 |
||||||
| PR | Ref | 1.33 | 1.25 | 1.12 | 1.50 | 0.78 |
| 95% CI | 1.00–1.78 | 1.10–1.42 | 0.94–1.33 | 1.26–1.79 | 0.48–1.25 | |
| p value | 0.05 | 0.0008 | 0.19 | <0.0001 | 0.30 | |
| Urinary control: any leaking/lack of control |
||||||
| PR | Ref | 1.37 | 2.10 | 1.20 | 1.26 | 0.42 |
| 95% CI | 1.04–1.79 | 1.95–2.26 | 1.03–1.40 | 1.01–1.57 | 0.19–0.93 | |
| p value | 0.02 | <0.0001 | 0.02 | 0.04 | 0.03 | |
| Leaking frequency at least once per day vs less than once per day |
||||||
| PR | Ref | 2.11 | 4.41 | 1.49 | 2.02 | 0.61 |
| 95% CI | 1.22–3.65 | 3.79–5.13 | 1.06–2.08 | 1.31–3.13 | 0.16–2.30 | |
| p value | 0.007 | <0.0001 | 0.02 | 0.002 | 0.50 | |
| Extent of problem with leaking: any size problem vs no problem |
||||||
| PR | Ref | 1.57 | 2.11 | 1.19 | 1.30 | 0.58 |
| 95% CI | 1.20–2.06 | 1.93–2.31 | 0.99–1.42 | 1.02–1.66 | 0.29–1.20 | |
| p value | 0.001 | < 0.0001 | 0.06 | 0.04 | 0.14 | |
PCa = prostate cancer; ADT = androgen-deprivation therapy; AUA-SI = American Urological Association Symptom Index; PR = prevalence ratio; CI = confidence interval; Ref = reference.
Model adjusted for age, previous benign prostatic hyperplasia diagnosis, self-reported health, and smoking status.
Absorbent pad use occurred too infrequently to examine according to individual treatment categories except for surgery. In multivariable analyses comparing pad use prevalence among PCa survivors and men without PCa, cancer survivors were almost 10 times more likely to report absorbent pad or diaper use in the prior month (PR: 9.60; 95% CI, 7.18–12.82; p < 0.0001); men specifically reporting surgery were 17 times more likely to report absorbent pad use than men without PCa (PR: 17.01; 95% CI, 12.93–22.38; p < 0.001).
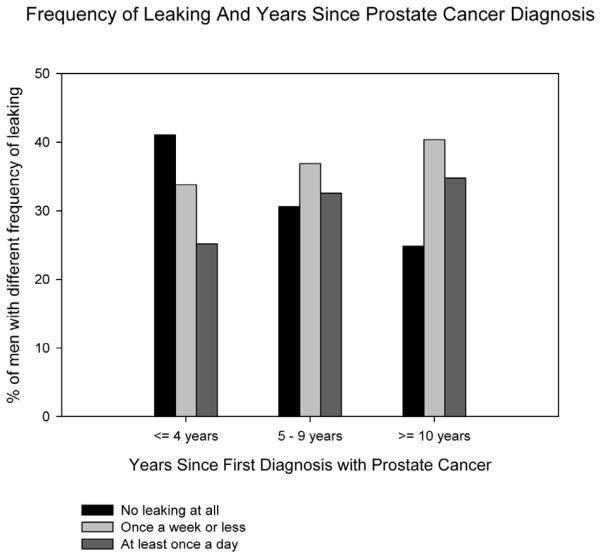
The risk of urinary incontinence increased with longer duration since first diagnosis with PCa—from 25% to 35% prevalence, respectively—in men ≤4 yr and >10 yr from diagnosis (Fig. 1) and was independent of age (Table 5).
Fig. 1.
Association of urinary incontinence with time since prostate cancer diagnosis among elderly prostate cancer survivors.
Table 5.
Frequency of urinary leakage stratified by time since first prostate cancer diagnosis and age at diagnosis among elderly prostate cancer survivors
| Frequency of leaking, no. (%) | ||||
|---|---|---|---|---|
| Time since first diagnosis, yr | No leaking at all | Once per week or less |
At least once per day |
p value |
| ≤4 | 119 (41.0) | 98 (33.8) | 73 (25.2) | 0.72 |
| <70 yr at first diagnosis with PCa | 77 (42.5) | 61 (33.7) | 43 (23.8) | |
| ≥70 yr at first diagnosis with PCa | 42 (38.5) | 37 (33.9) | 30 (27.5) | |
| 5–9 | 78 (30.6) | 94 (36.9) | 83 (32.6) | 0.89 |
| <70 yr at first diagnosis with PCa | 35 (29.9) | 45 (38.5) | 37 (31.6) | |
| ≥70 yr at first diagnosis with PCa | 43 (31.2) | 49 (35.5) | 46 (33.3) | |
| ≥10 | 40 (24.8) | 65 (40.4) | 56 (34.8) | 0.67 |
| <70 yr at first diagnosis with PCa | 16 (29.1) | 21 (38.2) | 18 (32.7) | |
| ≥70 yr at first diagnosis with PCa | 24 (22.6) | 44 (41.5) | 38 (35.9) | |
PCa = prostate cancer.
4. Discussion
This study is the largest to date that evaluates multiple aspects of urinary health among community-dwelling PCa survivors. In this cohort of elderly men, the prevalence of urinary incontinence was significantly higher among PCa survivors compared with similarly aged peers without PCa, regardless of treatment type and previous BPH diagnosis. The prevalence of daily urine leakage was two-fold greater in PCa survivors treated with observation or ADT and more than four-fold greater in men treated with surgery. Incontinence prevalence increased with time since treatment, independent of age at time of treatment. In addition, PCa survivors treated with observation, surgery, or ADT were significantly more likely than men with no PCa history to report urinary bother associated with LUTS.
These data inform PCa survivorship paradigms by elucidating the burden of LUTS, urinary bother, and incontinence in elderly PCa patients. Diminished urinary health in older PCa survivors represents an important public health issue. First, our data emphasize that economic and quality-of-life burdens in these patients will persist years after initial diagnosis and treatment; as the prevalence of PCa survivors grows (with almost 4 million PCa survivors expected by the end of the decade) [8] and the survivorship population ages, this situation will potentially strain finite health care resources. Second, LUTS, urinary bother, and urinary incontinence affect both quality of life and the global health of older men. LUTS are independently associated with increased risks of mortality, falls, depression, and impaired activities of daily living [8,16,23–27].
MrOS provides a unique and contemporary repository of urinary health data in elderly men. Prior data comparing patients with a variety of PCa treatments and controls without PCa are limited, particularly in an older age group with a high prevalence of LUTS unrelated to PCa or its treatment [16,27]. A case–control study of 915 men demonstrated that men with PCa had significantly diminished urinary health compared with controls without cancer, with radical prostatectomy patients reporting the largest deficits in adjusted mean composite urinary function scores, likely because of the incontinence domains [13]. However, these men were younger and demonstrated more robust urinary health at baseline than MrOS participants.
An important finding of this study is that elderly men on observation have significantly increased urinary incontinence and urinary bother compared with men without PCa, independent of previous BPH diagnosis. These data suggest that a diagnosis of PCa is a risk factor for diminished urinary health and produces negative consequences even in patients not actively pursuing treatment. PSA screening and early detection of PCa might substantially diminish urinary health in elderly men. Our observation is supported by a recent analysis of cancer survivors from the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial, in which screened survivors had significantly greater urinary dysfunction compared with screened noncancer controls as measured by the Expanded Prostate Cancer Index Composite Short Form [15]. Two potential explanations for this finding are diagnosis-related anxiety contributing to urinary dysfunction [28] and chronic LUTS caused by serial prostate biopsies performed for diagnosis and staging.
Another notable observation in this cohort is that urinary incontinence was more prevalent in patients who were farther out from diagnosis and treatment. These data contradict previous longitudinal studies that reported decreasing or stable urinary incontinence up to 3 yr following treatment [9,11,12] A more recent study observed steadily increasing urinary incontinence at 15 yr of follow-up among patients undergoing surgery or radiation therapy [29]. It is not necessarily counterintuitive to posit that urinary incontinence would increase over time among elderly men. It is possible, for example, that the pelvic floor musculature in these older men—already stressed by surgery or radiation therapy—would further weaken with continued aging. It may also be that incontinence progresses with longer duration after treatment and possibly less aggressive follow-up over time; there are data to support this finding after radiation treatment [14] but not after observation, surgery, or ADT. With PCa being frequently diagnosed and aggressively treated in younger men, our longer-term data suggest that the prevalence of urinary incontinence will continue to rise as these survivors age.
Urinary incontinence likely accounted for the comparatively worse urinary bother scores in the surgery group, as reflected in the additional survey items for incontinence. This supposition is supported by studies that link urinary incontinence after prostatectomy to decreased quality of life [12,13]. In a cohort of 435 PCa patients who underwent surgery, external-beam radiation therapy, or brachytherapy with 3 yr of follow-up, Pardo and colleagues reported increased urinary incontinence but decreased LUTS; in addition, problems with incontinence were nearly twice as common in the surgical group compared with other treatments [12]. Using an instrument that included six items measuring quality of life related to LUTS, Litwin and colleagues noted that men who underwent treatment of clinically localized PCa exhibited a decrease in urinary quality of life compared with men without PCa [30].
Our study has several strengths, including a large analytic cohort, multiple measures of urinary health, and data from multiple different types of PCa treatment, including observation and ADT. Previous BPH diagnosis was accounted for in the multivariable analyses, so benign LUTS is an unlikely source of confounding among men with PCa in the observation group.
Potential limitations merit discussion. First, it is not known how many patients had locally advanced disease or how many in the surgery or radiation therapy groups may have received adjuvant, neoadjuvant, or salvage therapy. Second, the relatively healthy composition of MrOS may have limited the external validity of the results. Still, a healthy cohort may strengthen the applicability of the findings to the general population, since the majority of community-dwelling older men are relatively healthy compared with less independent, age-matched peers. Third, we could not distinguish among different types of urinary incontinence, such as stress or urge. Fourth, our study included only men who were PCa survivors and were healthy enough to participate in the MrOS cohort. Thus the prevalence of severe LUTS or incontinence that we observed in these men may be lower than the prevalence in all PCa survivors living in the community, in which case the differences in prevalence of urinary incontinence we observed between PCa survivors and men without PCa may be underestimates. Fifth, we could not perform an exact time-to-event analysis (ie, hazard regression analysis) because we could not precisely identify the exact onset of incontinence. Finally, we did not have details regarding the observation protocols or other treatments.
5. Conclusions
In this cohort of healthy, community-dwelling men, including men on observation, a diagnosis of PCa was associated with a substantially increased prevalence of urinary bother and incontinence. Incontinence prevalence increased with survivorship duration. These data suggest a greater burden of urinary health problems in elderly PCa survivors then previously recognized and emphasize the need for continued evaluation of urinary health in elderly PCa survivors.
Take-home message.
Urinary incontinence and bother are highly prevalent in elderly prostate cancer survivors, including men on watchful waiting. These data suggest that prostate cancer diagnosis is associated with diminished quality of life in the elderly, even in the absence of treatment.
Acknowledgments
Funding/Support and role of the sponsor: The Osteoporotic Fractures in Men (MrOS) study is supported by National Institutes of Health funding. The following institutes provide support: the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), National Institute on Aging (NIA), National Center for Research Resources (NCRR), and NIH Roadmap for Medical Research (grant numbers U01 AR45580, U01 AR45614, U01 AR45632, U01 AR45647, U01 AR45654, U01 AR45583, U01 AG18197, U01-AG027810, and UL1 RR024140). The sponsors were involved in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and approval of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions: J. Kellogg Parsons had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Parsons, Marshall, Kopp.
Acquisition of data: Marshall.
Analysis and interpretation of data: Parsons, Kopp, Marshall.
Drafting of the manuscript: Kopp, Parsons.
Critical revision of the manuscript for important intellectual content: Barrett-Connor, Bauer, Marshall, Wang.
Statistical analysis: Marshall, Wang.
Obtaining funding: Parsons, Marshall.
Administrative, technical, or material support: None.
Supervision: None.
Other (specify): None.
Financial disclosures: J. Kellogg Parsons certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/ affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
References
- [1].Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- [2].Center MM, Jemal A, Lortet-Tieulent J, et al. International variation in prostate cancer incidence and mortality rates. Eur Urol. 2012;61:1079–92. doi: 10.1016/j.eururo.2012.02.054. [DOI] [PubMed] [Google Scholar]
- [3].Thompson I, Thrasher JB, Aus G, et al. Guideline for the management of clinically localized prostate cancer: 2007 update. J Urol. 2007;177:2106–31. doi: 10.1016/j.juro.2007.03.003. [DOI] [PubMed] [Google Scholar]
- [4].Miller DC, Gruber SB, Hollenbeck BK, Montie JE, Wei JT. Incidence of initial local therapy among men with lower-risk prostate cancer in the United States. J Natl Cancer Inst. 2006;98:1134–41. doi: 10.1093/jnci/djj308. [DOI] [PubMed] [Google Scholar]
- [5].Cooperberg MR, Broering JM, Carroll PR. Time trends and local variation in primary treatment of localized prostate cancer. J Clin Oncol. 2010;28:1117–23. doi: 10.1200/JCO.2009.26.0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Wilt TJ, MacDonald R, Rutks I, Shamliyan TA, Taylor BC, Kane RL. Systematic review: comparative effectiveness and harms of treatments for clinically localized prostate cancer. Ann Intern Med. 2008;148:435–48. doi: 10.7326/0003-4819-148-6-200803180-00209. [DOI] [PubMed] [Google Scholar]
- [7].Alibhai SM, Klotz LH. A systematic review of randomized trials in localized prostate cancer. Can J Urol. 2004;11:2110–7. [PubMed] [Google Scholar]
- [8].Siegel R, Desantis C, Virgo K, et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin. 2012;62:220–41. doi: 10.3322/caac.21149. [DOI] [PubMed] [Google Scholar]
- [9].Sanda MG, Dunn RL, Michalski J, et al. Quality of life and satisfaction with outcome among prostate-cancer survivors. N Engl J Med. 2008;358:1250–61. doi: 10.1056/NEJMoa074311. [DOI] [PubMed] [Google Scholar]
- [10].Malcolm JB, Fabrizio MD, Barone BB, et al. Quality of life after open or robotic prostatectomy, cryoablation or brachytherapy for localized prostate cancer. J Urol. 2010;183:1822–8. doi: 10.1016/j.juro.2009.12.102. [DOI] [PubMed] [Google Scholar]
- [11].Litwin MS, Gore JL, Kwan L, et al. Quality of life after surgery, external beam irradiation, or brachytherapy for early-stage prostate cancer. Cancer. 2007;109:2239–47. doi: 10.1002/cncr.22676. [DOI] [PubMed] [Google Scholar]
- [12].Pardo Y, Guedea F, Aguilo F, et al. Quality-of-life impact of primary treatments for localized prostate cancer in patients without hormonal treatment. J Clin Oncol. 2010;28:4687–96. doi: 10.1200/JCO.2009.25.3245. [DOI] [PubMed] [Google Scholar]
- [13].Hoffman RM, Gilliland FD, Penson DF, Stone SN, Hunt WC, Potosky AL. Cross-sectional and longitudinal comparisons of health-related quality of life between patients with prostate carcinoma and matched controls. Cancer. 2004;101:2011–9. doi: 10.1002/cncr.20608. [DOI] [PubMed] [Google Scholar]
- [14].Miller DC, Sanda MG, Dunn RL, et al. Long-term outcomes among localized prostate cancer survivors: health-related quality-of-life changes after radical prostatectomy, external radiation, and brachytherapy. J Clin Oncol. 2005;23:2772–80. doi: 10.1200/JCO.2005.07.116. [DOI] [PubMed] [Google Scholar]
- [15].Taylor KL, Luta G, Miller AB, et al. Long-term disease-specific functioning among prostate cancer survivors and noncancer controls in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial. J Clin Oncol. 2012;30:2768–75. doi: 10.1200/JCO.2011.41.2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Wei JT, Calhoun E, Jacobsen SJ. Urologic Diseases in America project: benign prostatic hyperplasia. J Urol. 2005;173:1256–61. doi: 10.1097/01.ju.0000155709.37840.fe. [DOI] [PubMed] [Google Scholar]
- [17].Kupelian V, Wei JT, O’Leary MP, et al. Prevalence of lower urinary tract symptoms and effect on quality of life in a racially and ethnically diverse random sample: the Boston Area Community Health (BACH) Survey. Arch Intern Med. 2006;166:2381–7. doi: 10.1001/archinte.166.21.2381. [DOI] [PubMed] [Google Scholar]
- [18].Parsons JK, Bergstrom J, Silberstein J, Barrett-Connor E. Prevalence and characteristics of lower urinary tract symptoms in men aged > or = 80 years. Urology. 2008;72:318–21. doi: 10.1016/j.urology.2008.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Orwoll E, Blank JB, Barrett-Connor E, et al. Design and baseline characteristics of the Osteoporotic Fractures in Men (MrOS) study—a large observational study of the determinants of fracture in older men. Contemp Clin Trials. 2005;26:569–85. doi: 10.1016/j.cct.2005.05.006. [DOI] [PubMed] [Google Scholar]
- [20].Blank JB, Cawthon PM, Carrion-Petersen ML, et al. Overview of recruitment for the Osteoporotic Fractures in Men study (MrOS) Contemp Clin Trials. 2005;26:557–68. doi: 10.1016/j.cct.2005.05.005. [DOI] [PubMed] [Google Scholar]
- [21].Barry MJ, Fowler FJ, Jr, O’Leary MP, et al. The American Urological Association Symptom Index for benign prostatic hyperplasia. The Measurement Committee of the American Urological Association. J Urol. 1992;148:1549–57. doi: 10.1016/s0022-5347(17)36966-5. discussion 1564. [DOI] [PubMed] [Google Scholar]
- [22].Spiegelman D, Hertzmark E. Easy SAS calculations for risk or prevalence ratios and differences. Am J Epidemiol. 2005;162:199–200. doi: 10.1093/aje/kwi188. [DOI] [PubMed] [Google Scholar]
- [23].Engstrom G, Henningsohn L, Steineck G, Leppert J. Self-assessed health, sadness and happiness in relation to the total burden of symptoms from the lower urinary tract. BJU Int. 2005;95:810–5. doi: 10.1111/j.1464-410X.2005.05406.x. [DOI] [PubMed] [Google Scholar]
- [24].Taylor BC, Wilt TJ, Fink HA, et al. Prevalence, severity, and health correlates of lower urinary tract symptoms among older men: the MrOS study. Urology. 2006;68:804–9. doi: 10.1016/j.urology.2006.04.019. [DOI] [PubMed] [Google Scholar]
- [25].Parsons JK, Mougey J, Lambert L, et al. Lower urinary tract symptoms increase the risk of falls in older men. BJU Int. 2009;104:63–8. doi: 10.1111/j.1464-410X.2008.08317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kupelian V, Fitzgerald MP, Kaplan SA, Norgaard JP, Chiu GR, Rosen RC. Association of nocturia and mortality: results from the Third National Health and Nutrition Examination Survey. J Urol. 2011;185:571–7. doi: 10.1016/j.juro.2010.09.108. [DOI] [PubMed] [Google Scholar]
- [27].Parsons JK, Wilt TJ, Wang PY, Barrett-Connor E, Bauer DC, Marshall LM. Progression of lower urinary tract symptoms in older men: a community based study. J Urol. 2010;183:1915–20. doi: 10.1016/j.juro.2010.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Coyne KS, Kvasz M, Ireland AM, Milsom I, Kopp ZS, Chapple CR. Urinary incontinence and its relationship to mental health and health-related quality of life in men and women in Sweden, the United Kingdom, and the United States. Eur Urol. 2012;61:88–95. doi: 10.1016/j.eururo.2011.07.049. [DOI] [PubMed] [Google Scholar]
- [29].Resnick MJ, Koyama T, Fan KH, et al. Long-term functional outcomes after treatment for localized prostate cancer. N Engl J Med. 2013;368:436–45. doi: 10.1056/NEJMoa1209978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Litwin MS, Hays RD, Fink A, et al. Quality-of-life outcomes in men treated for localized prostate cancer. JAMA. 1995;273:129–35. doi: 10.1001/jama.273.2.129. [DOI] [PubMed] [Google Scholar]