Abstract
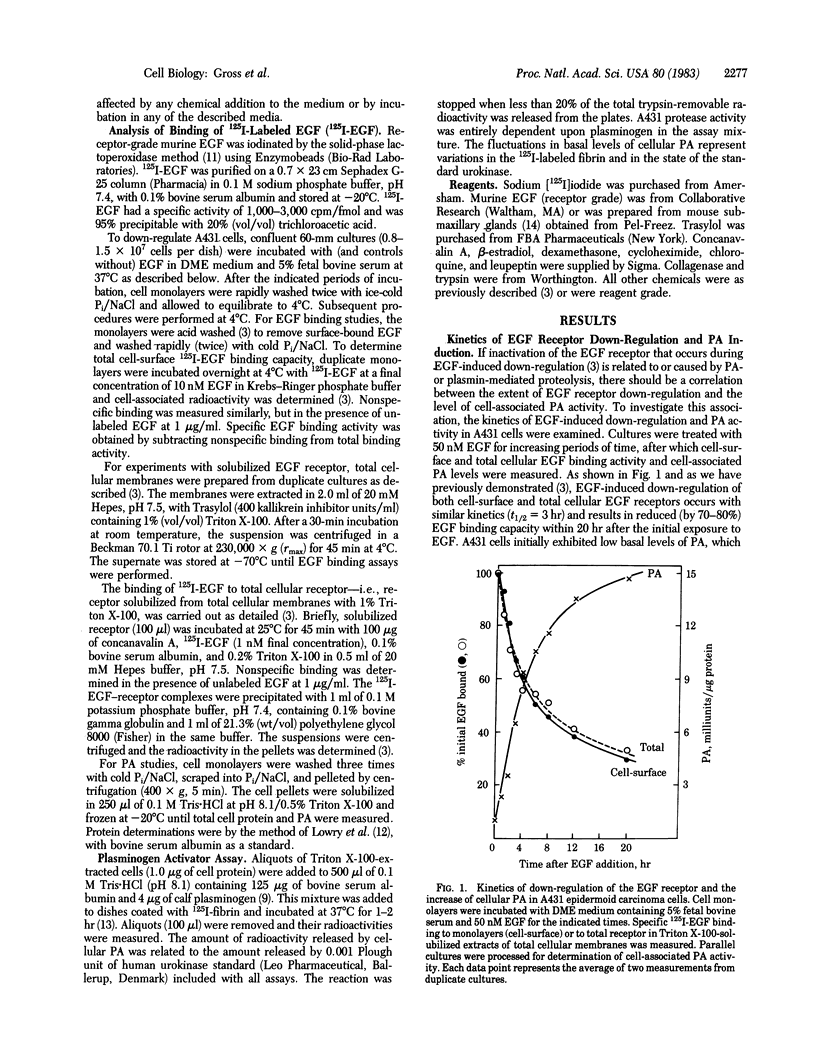
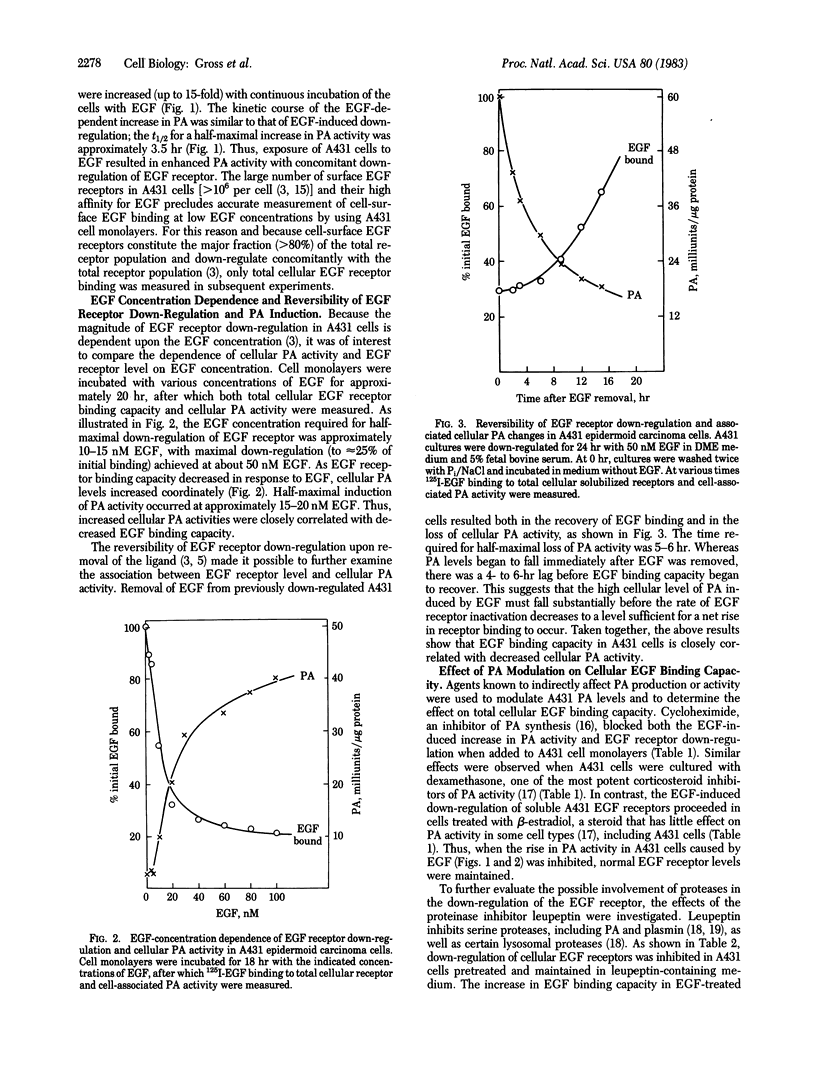
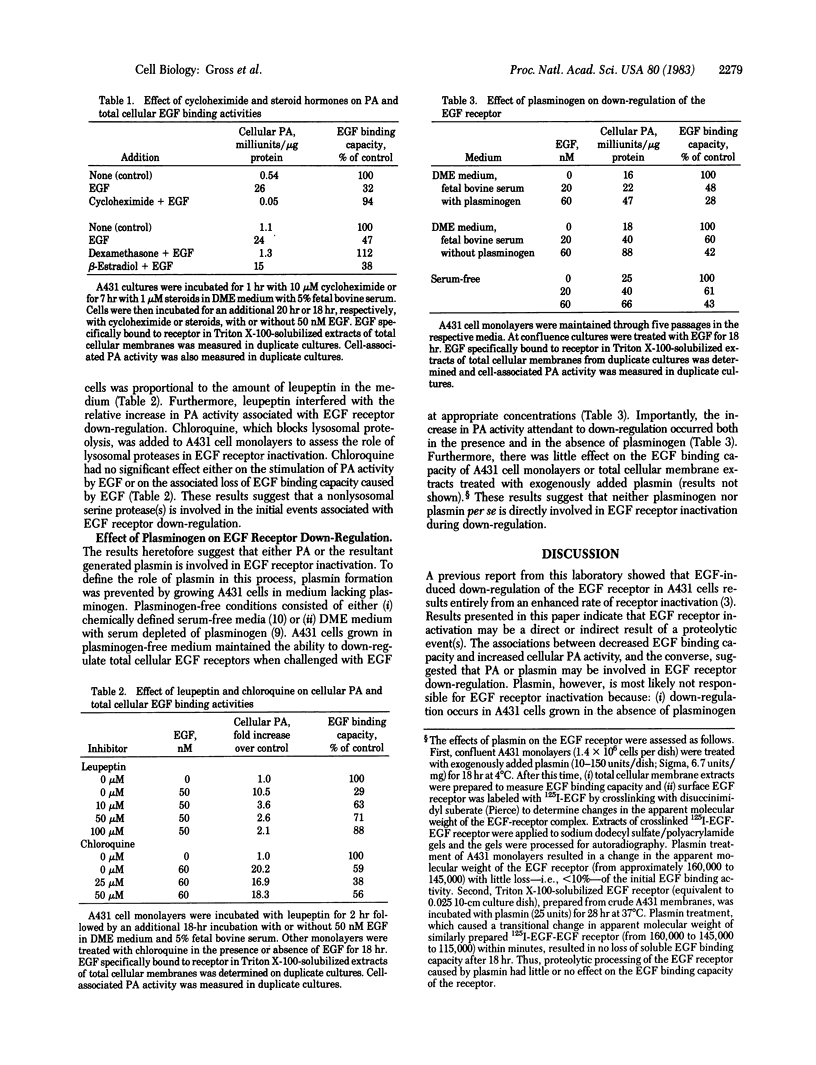
Human A431 epidermoid carcinoma cells in culture exhibit epidermal growth factor (EGF)-induced "down-regulation" of cell-surface and total cellular (Triton X-100 extractable) EGF receptors caused entirely by an enhanced rate (4-fold) of receptor inactivation [Krupp, M. N., Connolly, D. T. & Lane, M. D. (1982) J. Biol. Chem. 257, 11489-11496]. The following observations show that this enhanced rate of EGF receptor inactivation is closely correlated with an increased cellular activity of plasminogen activator (PA), a serine protease. First, EGF-induced down-regulation of cell-surface and total cellular EGF receptors and the concomitant increase in cellular PA activity occur with identical kinetics, the t 1/2 for both processes being 3-3.5 hr. Second, the EGF dose-response curves for down-regulation of total cellular EGF receptor and increased PA activity are similar. The EGF concentrations for half-maximal responses of both processes are 10-15 nM and 20 nM, respectively. Third, the removal of EGF from previously down-regulated cells results in the recovery of total cellular EGF binding activity with a concurrent loss of cellular PA activity. Fourth, blocking PA synthesis or activity with cycloheximide or dexamethasone prevents down-regulation of the EGF receptor. Fifth, the addition of leupeptin, an inhibitor of PA and plasmin action, blocks EGF-induced receptor down-regulation as well as the increase of PA activity. That EGF receptor down-regulation is independent of plasminogen per se in the culture medium suggests that PA-mediated events may initiate the rapid inactivation of the EGF receptor that occurs during down-regulation.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aharonov A., Pruss R. M., Herschman H. R. Epidermal growth factor. Relationship between receptor regulation and mitogenesis in 3T3 cells. J Biol Chem. 1978 Jun 10;253(11):3970–3977. [PubMed] [Google Scholar]
- Barnes D. W. Epidermal growth factor inhibits growth of A431 human epidermoid carcinoma in serum-free cell culture. J Cell Biol. 1982 Apr;93(1):1–4. doi: 10.1083/jcb.93.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter G., Cohen S. 125I-labeled human epidermal growth factor. Binding, internalization, and degradation in human fibroblasts. J Cell Biol. 1976 Oct;71(1):159–171. doi: 10.1083/jcb.71.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter G., Cohen S. Human epidermal growth factor and the proliferation of human fibroblasts. J Cell Physiol. 1976 Jun;88(2):227–237. doi: 10.1002/jcp.1040880212. [DOI] [PubMed] [Google Scholar]
- Cassel D., Glaser L. Proteolytic cleavage of epidermal growth factor receptor. A Ca2+-dependent, sulfhydryl-sensitive proteolytic system in A431 cells. J Biol Chem. 1982 Aug 25;257(16):9845–9848. [PubMed] [Google Scholar]
- Chapman H. A., Jr, Vavrin Z., Hibbs J. B., Jr Macrophage fibrinolytic activity: identification of two pathways of plasmin formation by intact cells and of a plasminogen activator inhibitor. Cell. 1982 Mar;28(3):653–662. doi: 10.1016/0092-8674(82)90220-3. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P., Hollenberg M. D. Membrane receptors and hormone action. Adv Protein Chem. 1976;30:251–451. doi: 10.1016/s0065-3233(08)60481-7. [DOI] [PubMed] [Google Scholar]
- Deutsch D. G., Mertz E. T. Plasminogen: purification from human plasma by affinity chromatography. Science. 1970 Dec 4;170(3962):1095–1096. doi: 10.1126/science.170.3962.1095. [DOI] [PubMed] [Google Scholar]
- Fehlmann M., Carpentier J. L., Van Obberghen E., Freychet P., Thamm P., Saunders D., Brandenburg D., Orci L. Internalized insulin receptors are recycled to the cell surface in rat hepatocytes. Proc Natl Acad Sci U S A. 1982 Oct;79(19):5921–5925. doi: 10.1073/pnas.79.19.5921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haigler H., Ash J. F., Singer S. J., Cohen S. Visualization by fluorescence of the binding and internalization of epidermal growth factor in human carcinoma cells A-431. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3317–3321. doi: 10.1073/pnas.75.7.3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatzfeld J., Miskin R., Reich E. Acetylcholine receptor: effects of proteolysis on receptor metabolism. J Cell Biol. 1982 Jan;92(1):176–182. doi: 10.1083/jcb.92.1.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knauer D. J., Cunningham D. D. Epidermal growth factor carrier protein binds to cells via a complex with released carried protein nexin. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2310–2314. doi: 10.1073/pnas.79.7.2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupp M. N., Connolly D. T., Lane M. D. Synthesis, turnover, and down-regulation of epidermal growth factor receptors in human A431 epidermoid carcinoma cells and skin fibroblasts. J Biol Chem. 1982 Oct 10;257(19):11489–11496. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Low D. A., Baker J. B., Koonce W. C., Cunningham D. D. Released protease-nexin regulates cellular binding, internalization, and degradation of serine proteases. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2340–2344. doi: 10.1073/pnas.78.4.2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchalonis J. J. An enzymic method for the trace iodination of immunoglobulins and other proteins. Biochem J. 1969 Jun;113(2):299–305. doi: 10.1042/bj1130299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miskin R., Easton T. G., Maelicke A., Reich E. Metabolism of acetylcholine receptor in chick embryo muscle cells: effects of RSV and PMA. Cell. 1978 Dec;15(4):1287–1300. doi: 10.1016/0092-8674(78)90054-5. [DOI] [PubMed] [Google Scholar]
- Quigley J. P. Association of a protease (plasminogen activator) with a specific membrane fraction isolated from transformed cells. J Cell Biol. 1976 Nov;71(2):472–486. doi: 10.1083/jcb.71.2.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley J. P. Phorbol ester-induced morphological changes in transformed chick fibroblasts: evidence for direct catalytic involvement of plasminogen activator. Cell. 1979 May;17(1):131–141. doi: 10.1016/0092-8674(79)90301-5. [DOI] [PubMed] [Google Scholar]
- Rifkin D. B., Crowe R. M. Studies on the control of plasminogen activator production by cultured human embryonic lung cells: requirements for inhibition by corticosteroids. J Cell Physiol. 1980 Dec;105(3):417–422. doi: 10.1002/jcp.1041050305. [DOI] [PubMed] [Google Scholar]
- Savage C. R., Jr, Cohen S. Epidermal growth factor and a new derivative. Rapid isolation procedures and biological and chemical characterization. J Biol Chem. 1972 Dec 10;247(23):7609–7611. [PubMed] [Google Scholar]
- Solomon J. A., Chou I. N., Schroder E. W., Black P. H. Evidence for membrane association of plasminogen activator activity in mouse macrophages. Biochem Biophys Res Commun. 1980 May 30;94(2):480–486. doi: 10.1016/0006-291x(80)91256-5. [DOI] [PubMed] [Google Scholar]


