Abstract
Background
Omega-3 fatty acids are known to prevent cardiac death. However, previous observational studies have suggested that omega-3 fatty acids are associated with cancer risk in adults. We conducted a meta-analysis based on randomized controlled trials to evaluate the effect of omega-3 fatty acids on the risk of cancer incidence, nonvascular death, and total mortality.
Methods
In February 2013, we performed electronic searches in PubMed, EmBase, and the Cochrane Library to identify randomized controlled trials on cancer incidence, nonvascular death, and total mortality. Relative risk (RR) was used to measure the effect of omega-3 fatty acid supplementation on the risk of cancer incidence, nonvascular death, and total mortality using a random-effect model. The analysis was further stratified by factors that could affect the treatment effects.
Results
Of the 8,746 identified articles, we included 19 trials reporting data on 68,954 individuals. These studies reported 1,039 events of cancer, 2,439 events of nonvascular death, and 7,025 events of total mortality. Omega-3 fatty acid supplementation had no effect on cancer incidence (RR, 1.10; 95% CI: 0.97–1.24; P = 0.12), nonvascular death (RR, 1.00; 95% CI: 0.93–1.08; P = 1.00), or total mortality (RR, 0.95; 95% CI: 0.88–1.03; P = 0.24) when compared to a placebo. Subgroup analysis indicated that omega-3 fatty acid supplementation was associated with a reduction in total mortality risk if the proportion of men in the study population was more than 80%, or participants received alpha-linolenic acid.
Conclusions
Omega-3 fatty acid supplementation does not have an effect on cancer incidence, nonvascular death, or total mortality.
Keywords: Omega-3 fatty acid, Cancer, Mortality, Meta-analysis
Background
Omega-3 fatty acid supplementation has been suggested to reduce the risk of cancer incidence [1-3], including that of colorectal [1], lung [2], and prostate [3] cancers. However, observational studies often overestimate the size of the effect and do not prove causality. Omega-3 fatty acid-derived eicosanoids influence many physiological processes such as calcium transport across cell membranes, angiogenesis, apoptosis, cell proliferation, and immune cell function [4-6], all of which might play a role in cancer risk.
Omega-3 fatty acid supplementation has been studied in numerous, large-scale, randomized, controlled trials for primary and secondary prevention of cardiovascular outcomes [7-17]. We could gain insight into the risk of cancer between omega-3 fatty acid supplementation and a placebo, with a study involving a long-term follow-up period and proper collection of cancer data. Therefore, we conducted a systematic review and meta-analysis of pooled data from randomized controlled trials to evaluate the possible effect of omega-3 fatty acid supplementation on cancer incidence, nonvascular death, and total mortality.
Methods
Data sources, search strategy, and selection criteria
This review was conducted and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) Statement [18], issued in 2009 (Additional file 1: Table S1). Randomized controlled trials of omega-3 fatty acid supplementation, written in the English language, were eligible for inclusion in our meta-analysis, regardless of the publication status (published, in press, or in progress), and the effects of omega-3 fatty acid supplementation on cancer incidence, nonvascular death, and total mortality were examined. Relevant trials were identified using the following procedure:
(1) Electronic searches: we searched the PubMed, EmBase, and Cochrane Central Register of Controlled Trials electronic databases for articles published through February 2013 and used “linolenic acid” OR “timnodonic acid” OR “ALA” OR “EPA” OR “docosahexaenoic acid” OR “DHA” OR “omega-3 fatty acid” OR “fish oil” AND “randomized controlled trials” AND “clinical trials” AND “human” as the search terms. All reference lists from reports on non-randomized controlled trials were searched manually for additional eligible studies.
(2) Other sources: we searched ongoing randomized controlled trials in the metaRegister of Controlled Trials, which lists trials that are registered as completed but not yet published. Furthermore, we reviewed bibliographies of publications for potentially relevant trials. Medical subject headings, methods, patient population, interventions, and outcomes variables of these studies were used to identify relevant trials.
The literature search, data extraction, and quality assessment were undertaken by two authors (YFZ and AJH), independently with a standardized approach. Any inconsistencies between these 2 authors (YFZ and AJH) were settled by the primary author (YHZ) until a consensus was reached. We restricted our research to randomized controlled trials, which were less likely to be subjected to confounding variables or bias views than observational studies. The study was eligible for inclusion if the following criteria were met: (1) the study was a randomized controlled trial; (2) the trial evaluated the effects of omega-3 fatty acid supplementation compared to a placebo; (3) the duration of the study’s follow-up period was at least 6 months; and (4) the trial reported at least 1 outcome as either cancer incidence, nonvascular death, or total mortality.
Data collection and quality assessment
All data from eligible trials were independently abstracted, in duplicate, by 2 independent investigators (YFZ and HFG) with a standard protocol and reviewed by a third investigator (YHZ). Any discrepancies were resolved by a group discussion. The data collected included baseline characteristics (first author or study group’s name, publication year, study design, type of blinding, number of patients, mean age, percentage of males, patient diseases, intervention regimes, and the duration of the follow-up period). The outcomes investigated included cancer incidence, nonvascular death, and total mortality. Study quality was assessed using the Jadad score [19], which is based on the 5 following subscales: randomization (1 or 0), concealment of the treatment allocation (1 or 0), blinding (1 or 0), completeness of follow-up (1 or 0), and the use of intention-to-treat analysis (1 or 0). A “score system” (ranging from 1 to 5) has been developed for quality assessment. In our study, we considered a study given a score of 4 or above to be a high-quality study.
Statistical analysis
We allocated the results of each randomized controlled trial as dichotomous frequency data. Individual study relative risks (RRs) and 95% confidence intervals (CIs) were calculated from event numbers extracted from each trial before data pooling. The overall RR and 95% CIs of cancer incidence, nonvascular death, and total mortality were also calculated. Both fixed-effect and random-effects models were used to assess the pooled RR for omega-3 fatty acid supplementation compared to a placebo. Although both models yielded similar findings, results from the random-effects model presented here assume that the true underlying effect varies among included trials [20,21]. Heterogeneity of the treatment effects between studies was investigated visually by a scatter plot analysis as well as statistically using the heterogeneity I2 statistic [22,23]. We explored potential heterogeneity in estimates of the treatment effects with univariate meta-regression (for sample size, mean age, percent men, and duration of the follow-up period) [24]. Subsequently, subgroup analyses were conducted on the basis of publication year, number of patients, percent men, mean age, intervention, primary or secondary prevention, duration of the follow-up period, and the Jadad score [19]. Interaction tests [25] were performed to compare differences between estimates of the 2 subsets, which were based on Student t distribution rather than on normal distribution because the number of inclusive studies was small. We also performed a sensitivity analysis by removing each individual trial from the meta-analysis. Visual inspections of funnel plots for cancer incidence, nonvascular death, and total mortality were conducted. The Egger [26] and Begg tests [27] were also used to statistically assess publication bias for cancer incidence, nonvascular death, and total mortality. All reported P values are two-sided, and P values of <0.05 were considered statistically significant for all included studies. Statistical analyses were performed using STATA software (version 10.0).
Results
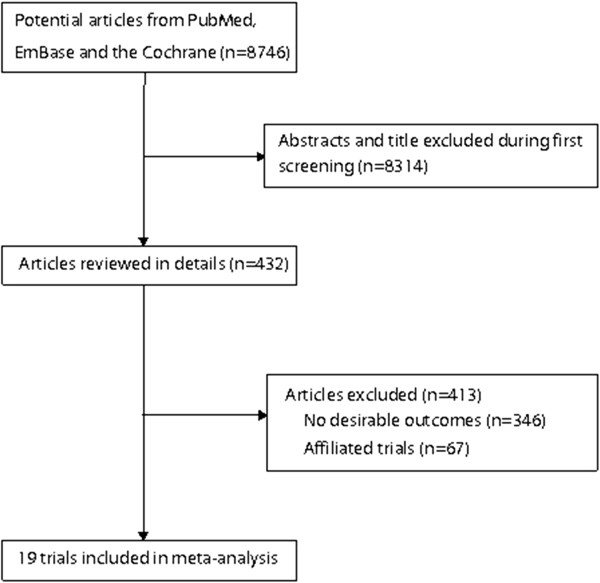
We identified 8,746 articles from our initial electronic search, of which 8,314 were excluded during an initial review (title and abstract). We retrieved the full text for the remaining 432 articles, and 19 randomized controlled trials [7-17,28-35] met the inclusion criteria (Figure 1 and Additional file 2: Figure S1). Table 1 summarizes the characteristics of these trials and the important baseline information of the included 68,954 individuals. The trials included in this study compared omega-3 fatty acid supplementation to a placebo for cancer incidence, nonvascular death, and total mortality. The follow-up period for participants ranged from 10 to 74.4 months, and the number of individuals included in each trial ranged from 36 to 18645. The breakdown for the number of trials available for each outcome were 11, 16, and 17 for cancer incidence [7-17], nonvascular death [7,8,11-16,28-35], and total mortality [7,8,11-17,28-35], respectively. We restricted the inclusion criteria to randomized controlled trials with a 6-month minimum follow-up to ensure a reliable conclusion. Although the included trials scarcely reported on the key indicators of trial quality, the quality of the trials was also assessed by the Jadad score [19]. Overall, 4 trials had a Jadad score of 5, 7 trials had a score of 4, 4 trials had a score of 3, 2 trials had a score of 2, and the remaining 2 trials had a score of 1.
Figure 1.
Flow diagram of the literature search and trials selection process.
Table 1.
Design and baseline characteristic of trials included in the systematic review and meta-analysis
| Source | No. of patients | Mean age, y | Sex (male, %) | Disease status | Intervention | Control | Follow-up (months) | Reporting outcomes | Jaded score |
|---|---|---|---|---|---|---|---|---|---|
| Borchgrevink CF (1966) [7] |
100/100 |
57.4 |
100 |
Coronary heart disease |
10 ml linseed oil |
10 ml corn oil |
10 |
Cancer incidence; nonvascular death; total death |
2 |
| Burr ML (1989) [8] |
1015/1018 |
56.5 |
100 |
Recovered from myocardial infarction |
Fish dietary |
Non-fish dietary |
24 |
Cancer incidence; Nonvascular death; total death |
1 |
| de Lorgeril M (1994) [28] |
302/303 |
53.5 |
90.8 |
Myocardial infarction within 6 months |
Alpha-linolenic acid rich diet |
Post-infarct prudent diet |
27 |
Nonvascular death; total death |
1 |
| Rossing P (1996) [9] |
18/18 |
33.0 |
65.5 |
Diabetic nephropathy |
N–3 fatty acids |
Olive oil |
12 |
Cancer incidence; |
4 |
| Leng GC (1998) [29] |
60/60 |
65.7 |
68.3 |
Stable intermittent claudication |
Gamma-linolenic and eicosapentaenoic acids |
Placebo |
24 |
Nonvascular death; total death |
3 |
| von Schacky (1999) [10] |
111/112 |
58.4 |
80.3 |
Coronary artery disease |
Fish oil concentrate (55% eicosapentaenoic and docosahexaenoic acids) |
Placebo |
24 |
Cancer incidence; |
5 |
| GISSI-Prevenzione Investigators (1999) [11] |
5666/5658 |
60.0 |
85.3 |
Recovered from myocardial infarction |
N-3 polyunsaturated fatty acids witho or without vitamin E |
Vitamin E or no treatment |
42 |
Cancer incidence; Nonvascular death; total death |
3 |
| Nilsen DWT (2001) [12] |
150/150 |
64.0 |
79.4 |
Acute myocardial infarction |
4 g highly concentrated n-3 fatty acids |
Corn oil |
24 |
Cancer incidence; nonvascular death; total death |
2 |
| Burr ML (2003) [13] |
1571/1543 |
61.1 |
100 |
Angina |
Two portions of oily fish each week, or to take three fish oil capsule daily with or without more fruit, vegetables and oats |
no specific dietary advice with or without more fruit, vegetables and oats |
60 |
Cancer incidence; nonvascular death; total death |
3 |
| Raitt MH (2005) [14] |
100/100 |
62.5 |
86 |
Implantable cardioverter defibrillator and a recent episode of sustained VT or VF |
Fish oil, 1.8 g/d, 72% omega-3 PUFAs |
Placebo |
24 |
Cancer incidence; nonvascular death; total death |
4 |
| Leaf A (2005) [30] |
200/202 |
65.5 |
83.1 |
Implantable cardioverter defibrillator |
Fish oil |
Olive oil |
12 |
Nonvascular death; total death |
3 |
| Brouwer IA (2006) [15] |
273/273 |
61.5 |
84.5 |
Implantable cardioverter defibrillator |
2 g/d of fish oil |
Placebo |
12 |
Cancer incidence; nonvascular death; total death |
4 |
| JELIS Ivestigators (2007) [16] |
9326/9319 |
61.0 |
31.5 |
Total cholesterol of 6 · 5 mmol/L or greater |
EPA with statin |
Statin |
55.2 |
Cancer incidence; nonvascular death; total death |
5 |
| GISSI-HF investigators (2008) [31] |
3494/3481 |
67.0 |
NG |
Chronic heart failure |
N-3 PUFA 1 g daily |
Placebo |
46.8 |
Nonvascular death; total death |
5 |
| OMEGA Study Group (2010) [32] |
1919/1885 |
64.0 |
74.4 |
Recovered from myocardial infarction |
1 g omega-3 acid ethyl esters-90 |
Olive oil |
12 |
Nonvascular death; total death |
4 |
| Einvik G (2010) [33] |
282/281 |
70.1 |
100 |
Hypercholesterolemia (> 6.45 mmol/l) |
2.4 g n-3 PUFA |
Corn oil |
36 |
Nonvascular death; total death |
4 |
| SU.FOL.OM3 Collaborative Group (2010) [17] |
1253/1248 |
60.6 |
79.4 |
with a history of myocardial infarction, unstable angina, or ischaemic stroke |
Omega 3 fatty acids with or without vitamin B |
Placebo or vitamin B |
56.4 |
Cancer incidence; total death |
5 |
| Alpha Omega Trial Group (2010) [34] |
2404/2433 |
69.0 |
78.2 |
Recovered from myocardial infarction |
EPA and DHA with or without ALA |
ALA or placebo |
40.8 |
Nonvascular death; total death |
4 |
| The ORIGIN Trial Investigators (2012) [35] | 6281/6255 | 63.5 | 65.1 | High risk for cardiovascular events | N–3 fatty acids | Placebo | 74.4 | Nonvascular death; total death | 4 |
Data for the effect of omega-3 fatty acid supplementation on cancer incidence included 39,122 individuals and reported 1,039 cancer events. Overall, omega-3 fatty acid supplementation increased the risk of cancer incidence by 10%, but this increase was not statistically significant (RR, 1.10; 95% CI: 0.97–1.24; P = 0.12, without evidence of heterogeneity, Figure 2). A sensitivity analysis indicated that the results were not affected by sequential exclusion of any particular trial from all pooled analysis. Similarly, the non-significant response persisted after a cumulative meta-analysis for cancer incidence was conducted (Additional file 3: Figure S2).
Figure 2.
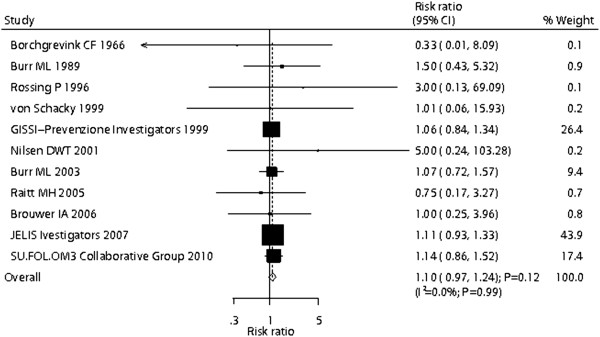
Effect of omega-3 fatty acid supplementation on the risk of cancer incidence.
Data for the effect of omega-3 fatty acid supplementation on nonvascular death included 66,204 individuals and 2,439 events of nonvascular death. There were no differences observed between participants receiving omega-3 fatty acid versus those receiving the placebo for nonvascular death (RR, 1.00; 95% CI: 0.93–1.08; P = 1.00, Figure 3), and no statistical heterogeneity was observed between trials (P = 0.94). After sequential exclusion of each trial from all pooled analysis, the results were not affected by exclusion of any specific trial. In a cumulative meta-analysis for nonvascular death (Additional file 4: Figure S3), the omega-3 fatty acid effect remained unchanged, i.e., not statistically significant.
Figure 3.
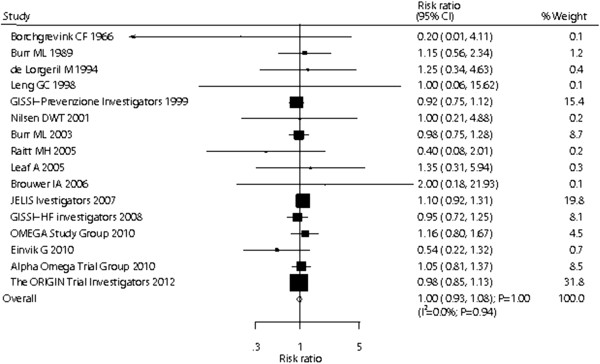
Effect of omega-3 fatty acid supplementation on the risk of nonvascular death.
Data for the effect of omega-3 fatty acid supplementation on total mortality included 68,705 individuals and reported 7,025 total mortality events. We noted that omega-3 fatty acid supplementation showed a 5% reduction in total mortality; however, there was no supporting evidence to show that omega-3 fatty acid protected against total mortality risk (RR, 0.95; 95% CI: 0.88–1.03; P = 0.24, Figure 4). Heterogeneity was observed in the magnitude of the effect across the trials (I2 = 46.3%; P = 0.02). However, after sequential exclusion of each trial from all pooled analysis, the conclusion was not affected by the exclusion of any specific trial. In a cumulative meta-analysis for total mortality (Additional file 5: Figure S4), the originally proposed significant omega-3 fatty acid effect was refuted by evidence accumulated up to 2003 and has continued to linger around a small effect and borderline statistical significance.
Figure 4.
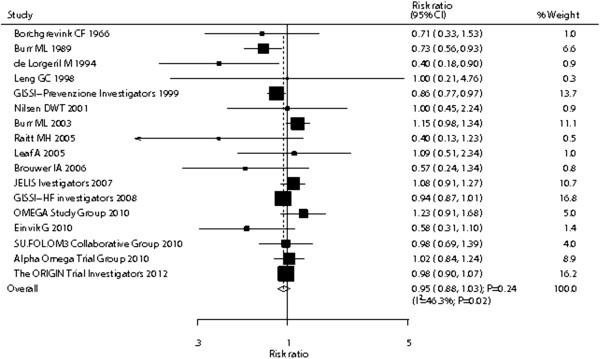
Effect of omega-3 fatty acid supplementation on the risk of total mortality.
Heterogeneity testing for the analysis showed a P > 0.10 for cancer incidence and nonvascular death. We concluded that heterogeneity is not significant in the overall analysis, suggesting that most variation was attributable to chance alone. However, substantial heterogeneity was observed in the magnitude of the effect for total mortality across the trials. We, therefore, conducted a meta-regression [24] analysis including sample size, mean age, percent men, and duration of the follow-up. However, these variables did not seem to be important factors contributing to the association between omega-3 fatty acid supplementation and total mortality risk (sample size, P = 0.302; mean age, P = 0.391; percent men, P = 0.804; and duration of follow-up, P = 0.786).
Subgroup analyses were also conducted for cancer incidence, nonvascular death, and total mortality to evaluate the effect of omega-3 fatty acid supplementation in a specific population. We noted that omega-3 fatty acid supplementation was associated with a reduction in total mortality risk if the trials were published before 2000, the number of individuals in the study was less than 1000, the proportion of men in the study was more than 80%, or participants received alpha-linolenic acid. No other significant differences were identified in pre-defined factors between those who took omega-3 fatty acid or placebo. Furthermore, there was no other significant difference in the effects of omega-3 fatty acid between the 2 subgroups by factors that could affect the treatment effects (Table 2).
Table 2.
Subgroup analysis for the effect of omega–3 fatty acid supplementation on cancer incidence, nonvascular death, and total mortality
| Outcomes | Group | Number of study | RR and 95% CI | P value | Heterogeneity (%) | P value for heterogeneity | P value for interaction test |
|---|---|---|---|---|---|---|---|
|
Cancer incidence |
Publication year |
||||||
| Before 2000 |
5 |
1.07 (0.85–1.34) |
0.56 |
0 |
0.87 |
0.789 |
|
| After 2000 |
6 |
1.11 (0.96–1.28) |
0.15 |
0 |
0.93 |
||
|
Number of patients | |||||||
| >1000 |
5 |
1.10 (0.97–1.24) |
0.12 |
0 |
0.98 |
0.878 |
|
| <1000 |
6 |
1.03 (0.45–2.38) |
0.94 |
0 |
0.83 |
||
|
Percent men (%) | |||||||
| >80 |
7 |
1.06 (0.87–1.28) |
0.58 |
0 |
0.98 |
0.608 |
|
| <80 |
4 |
1.13 (0.97–1.31) |
0.13 |
0 |
0.72 |
||
|
Mean age | |||||||
| >64 |
1 |
5.00 (0.24–103.28) |
0.30 |
– |
– |
0.328 |
|
| <64 |
10 |
1.10 (0.97–1.24) |
0.13 |
0 |
1.00 |
||
|
Intervention | |||||||
| Alpha–linolenic acid |
1 |
0.33 (0.01–8.09) |
0.50 |
– |
– |
0.481 |
|
| Long–chain n–3 PUFA |
10 |
1.10 (0.98–1.24) |
0.12 |
0 |
0.99 |
||
|
Primary or secondary prevention | |||||||
| Primary |
1 |
1.11 (0.93–1.33) |
0.26 |
– |
– |
0.882 |
|
| Secondary |
10 |
1.09 (0.93–1.28) |
0.29 |
0 |
0.98 |
||
|
Duration of the follow–up period (months) | |||||||
| >36 |
4 |
1.10 (0.97–1.24) |
0.14 |
0 |
0.98 |
0.883 |
|
| <36 |
7 |
1.16 (0.58–2.33) |
0.68 |
0 |
0.88 |
||
|
Jadad score | |||||||
| >4 |
6 |
1.11 (0.96–1.30) |
0.16 |
0 |
0.98 |
0.771 |
|
| <4 |
5 |
1.07 (0.88–1.30) |
0.49 |
0 |
0.77 |
||
|
Nonvascular death |
Publication year |
||||||
| Before 2000 |
5 |
0.93 (0.77–1.13) |
0.47 |
0 |
0.82 |
0.440 |
|
| After 2000 |
11 |
1.01 (0.93–1.10) |
0.75 |
0 |
0.86 |
||
|
Number of patients | |||||||
| >1000 |
8 |
1.01 (0.93–1.09) |
0.89 |
0 |
0.89 |
0.328 |
|
| <1000 |
8 |
0.77 (0.45–1.32) |
0.34 |
0 |
0.80 |
||
|
Percent men (%) | |||||||
| >80 |
9 |
0.93 (0.80–1.08) |
0.37 |
0 |
0.78 |
0.219 |
|
| <80 |
6 |
1.04 (0.94–1.14) |
0.47 |
0 |
0.92 |
||
|
Mean age | |||||||
| >64 |
7 |
1.01 (0.86–1.19) |
0.89 |
0 |
0.83 |
0.916 |
|
| <64 |
9 |
1.00 (0.91–1.09) |
0.94 |
0 |
0.78 |
||
|
Intervention | |||||||
| Alpha–linolenic acid |
3 |
0.95 (0.32–2.85) |
0.93 |
0 |
0.54 |
0.927 |
|
| Long–chain n–3 PUFA |
13 |
1.00 (0.93–1.08) |
1.00 |
0 |
0.89 |
||
|
Primary or secondary prevention | |||||||
| Primary |
3 |
1.01 (0.87–1.18) |
0.87 |
31.6 |
0.23 |
0.754 |
|
| Secondary |
13 |
0.98 (0.88–1.10) |
0.78 |
0 |
0.97 |
||
|
Duration of the follow–up period (months) | |||||||
| >36 |
7 |
0.99 (0.92–1.08) |
0.85 |
0 |
0.68 |
0.460 |
|
| <36 |
9 |
1.11 (0.83–1.49) |
0.49 |
0 |
0.92 |
||
|
Jadad score | |||||||
| >4 |
8 |
1.02 (0.93–1.11) |
0.71 |
0 |
0.62 |
0.427 |
|
| <4 |
8 |
0.95 (0.82–1.11) |
0.54 |
0 |
0.97 |
||
| Total mortality |
Publication year |
||||||
| Before 2000 |
5 |
0.79 (0.67–0.93) |
0.005 |
19 |
0.30 |
0.009 |
|
| After 2000 |
12 |
1.00 (0.94–1.08) |
0.90 |
29 |
0.16 |
||
|
Number of patients | |||||||
| >1000 |
9 |
0.98 (0.91–1.06) |
0.57 |
57 |
0.02 |
0.014 |
|
| <1000 |
8 |
0.67 (0.50–0.90) |
0.007 |
0 |
0.59 |
||
|
Percent men (%) | |||||||
| >80 |
9 |
0.80 (0.65–0.98) |
0.03 |
64 |
0.005 |
0.033 |
|
| <80 |
7 |
1.01 (0.95–1.08) |
0.71 |
0 |
0.84 |
||
|
Mean age | |||||||
| >64 |
7 |
0.96 (0.89–1.02) |
0.20 |
0 |
0.44 |
0.543 |
|
| <64 |
10 |
0.92 (0.82–1.04) |
0.18 |
62 |
0.004 |
||
|
Intervention | |||||||
| Alpha–linolenic acid |
3 |
0.58 (0.35–0.98) |
0.04 |
0 |
0.46 |
0.058 |
|
| Long–chain n–3 PUFA |
14 |
0.96 (0.89–1.04) |
0.36 |
47 |
0.03 |
||
|
Primary or secondary prevention | |||||||
| Primary |
3 |
0.99 (0.86–1.14) |
0.91 |
46 |
0.15 |
0.566 |
|
| Secondary |
14 |
0.94 (0.84–1.04) |
0.21 |
47 |
0.03 |
||
|
Duration of the follow–up period (months) | |||||||
| >36 |
8 |
0.98 (0.91–1.05) |
0.54 |
47 |
0.06 |
0.157 |
|
| <36 |
9 |
0.80 (0.61–1.05) |
0.11 |
43 |
0.08 |
||
|
Jadad score | |||||||
| >4 |
9 |
0.98 (0.91–1.06) |
0.65 |
30 |
0.18 |
0.320 | |
| <4 | 8 | 0.88 (0.72–1.07) | 0.19 | 59 | 0.02 | ||
A review of funnel plots could not rule out the potential for publication bias for lung cancer (Additional file 6: Figure S5). The Egger [26] and Begg test [27] results showed no evidence of publication bias for cancer incidence (Egger: P = 0.663; Begg: P = 0.876), nonvascular death (Egger: P = 0.519; Begg: P = 0.620), and total mortality (Egger: P = 0.255; Begg: P = 0.174).
Discussion
Several previous observational studies [1-3] have suggested that omega-3 fatty acid supplementation has a marked effect on cancer incidence. Kato and colleague [1] performed a prospective study of omega-3 fatty acid (fish or shellfish diet) and colorectal cancer in New York and Florida. This prospective study included 14724 individuals and found a 51% reduction in the risk of colorectal cancer with omega-3 fatty acid dietary consumption. Furthermore, Takezaki et al. [2] concluded that frequent fresh fish consumption, irrespective of the cooking method, might reduce the risk of lung cancer. However, the effect of omega-3 fatty acid supplementation in reducing the risk of incident cancer has not been confirmed by randomized controlled trials and meta-analysis.
Previous systematic review [36] have evaluated the impact of omega-3 fatty acid supplementation on cancer incidence on the basis of observational studies. These studies encompass a large body of literature spanning numerous cohorts, from many countries, with different demographic characteristics and do not provide any evidence to support a significant association between omega-3 fatty acid and cancer incidence. Our study was based solely on randomized controlled trials and explored all possible correlations between omega-3 fatty acid supplementation and the outcomes of cancer incidence, nonvascular death, and total mortality. This large quantitative study included 68,954 individuals from 19 trials with a broad range of baseline characteristics. The results of our meta-analysis suggest that omega-3 fatty acid supplementation has no effect on the incidence of cancer, nonvascular death, and total mortality.
Our main findings are inconsistent with the findings of previous epidemiologic research [1-3], and concluded that omega-3 fatty acid supplementation had no significant benefit or adverse effect on the risk of cancer incidence, nonvascular death or total mortality. The reason for this could be that observational studies may overestimate the effect of omega-3 supplementation.
There was no significant difference between omega-3 fatty acid supplementation and the placebo for the relative risk of cancer incidence. Omega-3 fatty acids do not seem to affect a mechanism of cancer development that is common across the different types of cancers evaluated in this study. Previous observational studies [1-3] suggested that risk reductions were observed for colorectal, lung, and prostate cancer. However, data on any specific cancer type were unavailable in this study; therefore, we did not identify the association between any specific type of cancer incidence and omega-3 fatty acid supplementation. According to our study, omega-3 fatty acid supplementation resulted in a 10% increase in the risk of cancer incidence, but this difference was not statistically significant. The possible reasons for this lack of significant effect are as follows: (1) the use of background omega-3 fatty acid supplementation might have impaired our ability to identify a treatment effect, and (2) trials included were designed to evaluate the effects of omega-3 fatty acid supplementation on cardiovascular outcomes, but not cancer-related outcomes, these results were derived from very few cases and should be regarded as preliminary results. Furthermore, high intake of omega-3 fatty acid might stimulate carcinogenesis by increasing oxidative DNA damage [37]. Finally, studies with an open, controlled design may have introduced behavioral differences which may have had an impact on the development of cancer. Therefore, although omega-3 fatty acid supplementation may have direct effects on cancer incidence, these effects may be balanced. Furthermore, subgroup analysis also supports that omega-3 fatty acid supplementation does not affect cancer incidence.
Omega-3 fatty acid supplementation might play an important role in reducing the risk of total mortality, due to the improved effects on cardiac death [38]. Our study suggests that omega-3 fatty acid produced a 5% reduction in total mortality; however, this reduction was not statistically significant. The reason for this absence of statistical difference could be that omega-3 fatty acids have no effect on the risk of nonvascular death. Hence, this effect may be reduced by nonvascular death. Subgroup analysis was also conducted on total mortal risk based on pre-defined factors. We noted that omega-3 fatty acid supplementation reduced the risk of total mortality only when we included trials published before 2000, the sample size was less than 1000, the proportion of men in the population was more than 80%, or participants received alpha-linolenic acid. The reason for these findings could be that several factors might affect the efficacy of treatment, which happened to occur more frequently in male patients, such as smoking status.
The limitations of our study are as follows: (1) relatively few events of cancer incidence were reported, which always contributed to broad confidence intervals, and restricted us from obtaining an intrinsic effect; (2) different types of supplements might provide a biased view of the study question; (3) data on any specific type of cancer were unavailable, due to which we could not identify the association between any specific type of cancer incidence and omega-3 fatty acid supplementation; and (4) since inherent assumptions are made for any meta-analysis, the analysis used pooled data, and individual patient data was not available, which restricted us from performing a more detailed relevant analysis and obtaining more comprehensive results.
Conclusions
The findings of this study suggest that omega-3 fatty acid supplementation has no significant effects on cancer incidence, nonvascular death, or total mortality. Future studies should focus on healthy individuals to analyze the primary prevention of cancer incidence. We suggest that ongoing trials should be improved in the following ways: (1) any specific type of cancer and total cancer incidents should be recorded and reported normatively, and it should be evaluated in any future trial, and (2) the role of intervention duration and dosage of supplementation should be taken into consideration before evaluating clinical outcomes.
Ethics
An ethics statement was not required for this work.
Competing interests
The authors declared that they have no competing interests.
Authors’ contributions
Conceived and designed the experiments: Z-YH. Performed the experiments: Z-YH, G-HF, H-AJ and Z-YF. Analyzed the data: Z-YH, and Z-YF. Contributed reagents/materials/analysis tools: Z-YH. Wrote the paper: Z-YH. All authors read and approved the final manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Supplementary Material
PRISMA Checklist.
PRISMA Flowchart.
Cumulative meta-analysis of the omega-3 fatty acid supplements for cancer incidence.
Cumulative meta-analysis of the omega-3 fatty acid supplements for nonvascular death.
Cumulative meta-analysis of the omega-3 fatty acid supplements for total mortality.
Funnel plot for cancer incidence, nonvascular death, and total mortality.
Contributor Information
Yu-Fei Zhang, Email: zhangyufeino7@126.com.
Hong-Fang Gao, Email: gaohongfangno7@126.com.
An-Ji Hou, Email: houanji_2012@126.com.
Yu-Hao Zhou, Email: zhou_ly@126.com.
Funding
No funding was received for this work.
References
- Kato I, Akhmedkhanov A, Koenig K, Toniolo PG, Shore RE. Prospective study of diet and female colorectal cancer: the New York University Women’s Health Study. Nutr Cancer. 1997;28:276–281. doi: 10.1080/01635589709514588. [DOI] [PubMed] [Google Scholar]
- Takezaki T, Inoue M, Kataoka H, Ikeda S, Yoshida M. Diet and lung cancer risk from a 14-year population-based prospective study in Japan: with special reference to fish consumption. Nutr Cancer. 2003;45:160–167. doi: 10.1207/S15327914NC4502_04. [DOI] [PubMed] [Google Scholar]
- Terry P, Bergkvist L, Holmberg L, Wolk A. No association between fat and fatty acids intake and risk of colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2001;10:913–914. [PubMed] [Google Scholar]
- Baronzio G, Freitas I, Griffini P, Bertone V, Pacini F. Omega-3 fatty acids can improve radioresponse modifying tumor interstitial pressure, blood rheology and membrane peroxidability. Anticancer Res. 1994;14:1145–1154. [PubMed] [Google Scholar]
- Avula CPR, Lawrence RA, Jolly CA, Fernandes G. Role of n-3 polyunsaturated fatty acids (PUFA) in autoimmunity, inflammation, carcinogenesis, and apoptosis. Recent Res Develop Lipids. 2000;4:303–319. [Google Scholar]
- Johnson IT. Anticarcinogenic effects of diet related apoptosis in the colorectal mucosa. Food Chem Toxicol. 2002;40:1171–1178. doi: 10.1016/S0278-6915(02)00051-0. [DOI] [PubMed] [Google Scholar]
- Borchgrevink CF, Skaga E, Berg KJ, Skjaeggestad O. Absence of prophylactic effect of linolenic acid in patients with coronary heart-disease. Lancet. 1966;2:187–189. doi: 10.1016/s0140-6736(66)92474-3. [DOI] [PubMed] [Google Scholar]
- Burr ML, Fehily AM, Gilbert JF, Rogers S, Holliday RM. Effects of changes in fat, fish, and fibre intakes on death and myocardial reinfarction: diet and reinfarction trial (DART) Lancet. 1989;2:757–761. doi: 10.1016/s0140-6736(89)90828-3. [DOI] [PubMed] [Google Scholar]
- Rossing P, Hansen BV, Nielsen FS, Myrup B, Holmer G. Fish oil in diabetic nephropathy. Diabetes Care. 1996;19:1214–1219. doi: 10.2337/diacare.19.11.1214. [DOI] [PubMed] [Google Scholar]
- von Schacky C, Angerer P, Kothny W, Theisen K, Mudra H. The effect of dietary omega-3 fatty acids on coronary atherosclerosis: a randomized, double-blind, placebo-controlled trial. Ann Intern Med. 1999;130:554–562. doi: 10.7326/0003-4819-130-7-199904060-00003. [DOI] [PubMed] [Google Scholar]
- GISSI-Prevenzione Investigators. Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Lancet. 1999;354:447–455. [PubMed] [Google Scholar]
- Nilsen DW, Albrektsen G, Landmark K, Moen S, Aarsland T. Effects of a high-dose concentrate of n-3 fatty acids or corn oil introduced early after an acute myocardial infarction on serum triacylglycerol and HDL cholesterol. Am J Clin Nutr. 2001;74:50–56. doi: 10.1093/ajcn/74.1.50. [DOI] [PubMed] [Google Scholar]
- Burr ML, Ashfield-Watt PA, Dunstan FD, Fehily AM, Breay P. Lack of benefit of dietary advice to men with angina: results of a controlled trial. Eur J Clin Nutr. 2003;57:193–200. doi: 10.1038/sj.ejcn.1601539. [DOI] [PubMed] [Google Scholar]
- Raitt MH, Connor WE, Morris C, Kron J, Halperin B. Fish oil supplementation and risk of ventricular tachycardia and ventricular fibrillation in patients with implantable defibrillators. A randomized controlled trial. JAMA. 2005;293:2884–2891. doi: 10.1001/jama.293.23.2884. [DOI] [PubMed] [Google Scholar]
- Brouwer IA, Zock PL, Camm AJ, Bocker D, Hauer RN. Effect of fish oil on ventricular tachyarrhythmia and death in patients with implantable cardioverter defibrillators: the study on omega-3 fatty acids and ventricular arrhythmia (SOFA) randomized trial. JAMA. 2006;295:2613–2619. doi: 10.1001/jama.295.22.2613. [DOI] [PubMed] [Google Scholar]
- Japan EPA lipid intervention study (JELIS) Investigators. Eff ects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised openlabel, blinded endpoint analysis. Lancet. 2007;369:1090–1098. doi: 10.1016/S0140-6736(07)60527-3. [DOI] [PubMed] [Google Scholar]
- SU.FOL.OM3 Collaborative Group. Effects of B vitamins and omega 3 fatty acids on cardiovascular diseases: a randomised placebo controlled trial. BMJ. 2010;341:c6273. doi: 10.1136/bmj.c6273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Plos Medicine. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jadad AR, Moore RA, Carroll D. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- Ades AE, Lu G, Higgins JP. The interpretation of random-effects metaanalysis in decision models. Med Decis Making. 2005;25:646–654. doi: 10.1177/0272989X05282643. [DOI] [PubMed] [Google Scholar]
- Deeks JJ, Higgins JPT, Altman DG. Analyzing data and undertaking meta-analyses. In: Higgins J, Green S, editor. Cochrane Handbook for Systematic Reviews of Interventions 5.0.1. Oxford, UK: The Cochrane Collaboration: chap 9; 2008. [Google Scholar]
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson SG, Higgins JP. How should meta-regression analyses be undertaken and interpreted? Stat Med. 2002;21:1559–1573. doi: 10.1002/sim.1187. [DOI] [PubMed] [Google Scholar]
- Altman DG, Bland JM. Interaction revisited: the difference between two estimates. BMJ. 2003;326:219. doi: 10.1136/bmj.326.7382.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. doi: 10.2307/2533446. [DOI] [PubMed] [Google Scholar]
- de Lorgeril M, Renaud S, Mamelle N, Sslen P, Martin JL. Mediterranean alpha-linolenic acid-rich diet in secondary prevention of coronary heart disease. Lancet. 1994;343:1454–1459. doi: 10.1016/S0140-6736(94)92580-1. [DOI] [PubMed] [Google Scholar]
- Leng GC, Lee AJ, Fowkes FG, Jepson RG, Lowe GD. Randomized controlled trial of gamma-linolenic acid eicosapentaenoic acid in peripheral arterial disease. Clin Nutr. 1998;17:265–271. doi: 10.1016/S0261-5614(98)80318-X. [DOI] [PubMed] [Google Scholar]
- Leaf A, Albert CM, Josephson M, Steinhaus D, Kluger J. Prevention of fatal arrhythmias in high-risk subjects by fish oil n-3 fatty acid intake. Circulation. 2005;112:2762–2768. doi: 10.1161/CIRCULATIONAHA.105.549527. [DOI] [PubMed] [Google Scholar]
- GISSI-HF investigators. Effect of n-3 polyunsaturated fatty acids in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial. Lancet. 2008;372:1223–1230. doi: 10.1016/S0140-6736(08)61239-8. [DOI] [PubMed] [Google Scholar]
- OMEGA Study Group. OMEGA, a randomized, placebo-controlled trial to test the effect of highly purified omega-3 fatty acids on top of modern guideline-adjusted therapy after myocardial infarction. Circulation. 2010;122:2152–2159. doi: 10.1161/CIRCULATIONAHA.110.948562. [DOI] [PubMed] [Google Scholar]
- Einvik G, Klemsdal TO, Sandvik L, Hjerkinn EM. A randomized clinical trial on n-3 polyunsaturated fatty acids supplementation and all-cause mortality in elderly men at high cardiovascular risk. Eur J Cardiovasc Prev Rehabil. 2010;17:588–592. doi: 10.1097/HJR.0b013e328339cc70. [DOI] [PubMed] [Google Scholar]
- Alpha Omega Trial Group. n–3 fatty acids and cardiovascular events after myocardial infarction. N Engl J Med. 2010;363:2015–26. doi: 10.1056/NEJMoa1003603. [DOI] [PubMed] [Google Scholar]
- The ORIGIN. Trial investigators: n–3 fatty acids and cardiovascular outcomes in patients with dysglycemia. N Engl J Med. 2012;367:309–318. doi: 10.1056/NEJMoa1203859. [DOI] [PubMed] [Google Scholar]
- MacLean CH, Newberry SJ, Mojica WA, Khanna P, Issa AM. Effects of omega-3 fatty acids on cancer risk a systematic review. JAMA. 2006;295:403–415. doi: 10.1001/jama.295.4.403. [DOI] [PubMed] [Google Scholar]
- Bartsch H, Nair J, Owen RW. Dietary polyunsaturated fatty acids and cancers of the breast and colorectum: emerging evidence for their role as risk modifiers. Carcinogenesis. 1999;20:2209–2218. doi: 10.1093/carcin/20.12.2209. [DOI] [PubMed] [Google Scholar]
- Leon H, Shibata MC, Sivakumaran S, Dorgan M, Chatterley T. Effect of fish oil on arrhythmias and mortality: systematic review. BMJ. 2009;338:a2931. doi: 10.1136/bmj.a2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PRISMA Checklist.
PRISMA Flowchart.
Cumulative meta-analysis of the omega-3 fatty acid supplements for cancer incidence.
Cumulative meta-analysis of the omega-3 fatty acid supplements for nonvascular death.
Cumulative meta-analysis of the omega-3 fatty acid supplements for total mortality.
Funnel plot for cancer incidence, nonvascular death, and total mortality.