Abstract
Objective
Rho GTPase proteins play a central role in regulating the dynamics of the platelet actin cytoskeleton. Yet, little is known regarding how Rho GTPase activation coordinates platelet activation and function. In this study, we aimed to characterize the role of the Rho GTPase effector p21 activated kinase (PAK) in platelet activation, lamellipodia formation and aggregate formation under shear.
Approach and Results
Stimulation of platelets with the GPVI agonist, collagen-related peptide (CRP), rapidly activated PAK in a time course preceding phosphorylation of PAK substrates LIMK1 and MEK and the subsequent activation of MAPKs and Akt. Pharmacological inhibitors of PAK blocked signaling events downstream of PAK and prevented platelet secretion as well as platelet aggregation in response to CRP. PAK inhibitors also prevented PAK activation and platelet spreading on collagen surfaces. PAK was also required for the formation of platelet aggregates and to maintain aggregate stability under physiological shear flow conditions.
Conclusions
These results suggest that PAK serves an orchestrator of platelet functional responses following activation downstream of the platelet collagen receptor, GPVI.
Keywords: platelet, cell signaling, signal transduction
Introduction
Platelets serve as the primary cellular mediators of hemostasis and thrombosis.1, 2 These circulating, anucleate fragments of megakaryocytic cells are optimally configured to detect a loss of vascular integrity, adhere to sites of vessel injury and aggregate to form thrombotic plugs. During the process of platelet activation, platelets undergo a dramatic change in shape from discs to small spheres with filopodial extensions and lamellipodial structures. This process is mediated by changes in the platelet actin cytoskeleton.3 Accordingly, an array of actin regulatory proteins including SCAR/WAVE,4 Arp2/3,5 cortactin,6 and the Rho GTPases7 play roles in regulating platelet morphology through the regulation of actin assembly and lamellipodia dynamics.3, 8, 9
Through the guanine nucleotide exchange factor (GEF)-mediated exchange of GDP for GTP, the ~21 kD Rho GTPases Rac1 and Cdc42 support the autocatalytic activation of the p21 activated kinases, or PAKs.10 The PAK family of serine/threonine protein kinases represent well characterized Rho GTPase effectors.11 As cells adhere to and spread on substrates, PAKs are recruited to focal adhesions and the actin leading edge to orchestrate actin dynamics, lamellipodia formation and cell motility. A system of PAK substrates, including the LIM domain kinase LIMK1,12 are thought to be major contributors to the PAK-based regulation of actin dynamics.11 Of the major PAK isoforms in mammalian cells, PAK2, represents the most ubiquitous and highly expressed PAK family member.11 While PAK2 was originally described as a kinase activated upon platelet stimulation,13 little is known regarding the role of PAKs in platelet biology. A number of platelet agonists, including GPVI agonists, have been demonstrated to stimulate PAK autophosphorylation in platelets.6, 14–16 These studies have shown that PAK activation occurs downstream of the Rho GTPases Rac1 and Cdc42 in platelets; however, a specific role for PAK in platelet function has not yet been defined.6, 14–18
Here, we demonstrate that PAK activity downstream of the platelet collagen receptor, GPVI, is necessary for platelet aggregation, secretion and lamellipodia formation as well as platelet aggregate stability under physiological conditions of shear. Platelet PAK activation occurs rapidly upon stimulation with the GPVI agonist, CRP. Through inhibition of platelet PAK, we show that PAK effectors LIMK1 and MEK are phosphorylated upon platelet activation in a PAK-dependent manner and that PAK activity is required for the coordination of MAPK and Akt signaling upstream of platelet lamellipodia formation, aggregation and the maintenance of platelet aggregates under shear. Together, these results support a role for PAK as a key regulator of platelet function.
Materials and Methods
Materials and Methods are available in online-only Supplement.
Results
PAK activation upon platelet stimulation with the GPVI agonist CRP
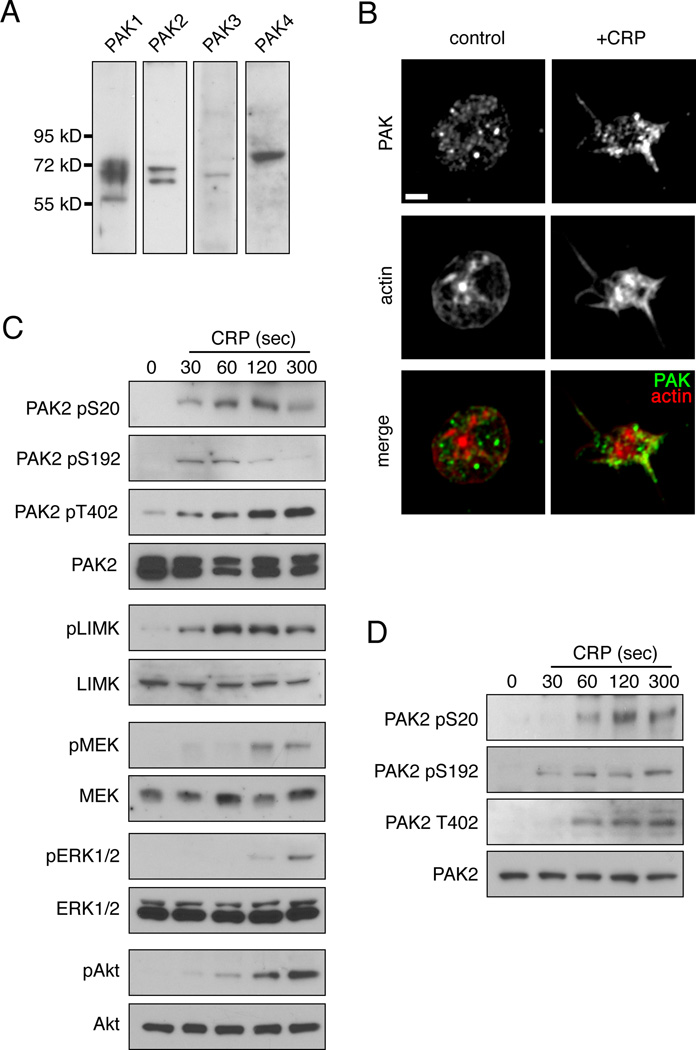
Previous studies have determined that the p21 activated kinase PAK is activated downstream of the Rho GTPases Rac1 and Cdc42 in platelets upon stimulation with GPVI agonists.6, 14–16 While the Rho GTPases Rac1 and Cdc42 have known functions in platelet filopodia and lamellipodia formation as well as platelet aggregation, secretion and thrombus formation, the role of PAK in platelet function has remained undetermined.7 To examine PAK isoform expression in human platelets, whole platelet lysates were analyzed by Western blot. As seen in Figure 1A, platelets express Group I PAKs PAK1 and PAK2 as well as the Group II PAK, PAK4 (Figure 1A). Low levels of PAK3 protein were also detectable by Western blot (Figure 1A). To better understand the role of PAK in actin reorganization in platelet activation mediated by GPVI, we next examined the localization of Group I PAKs in platelets treated with the GPVI-specific agonist collagen-related peptide (CRP). Replicate samples of quiescent and CRP-activated platelets were fixed in solution, permeabilized and stained for PAK1/2/3 and actin. As seen in Figure 1B, PAK shows a cytosolic distribution in platelets under basal conditions. Upon stimulation with CRP, PAK was found at the platelet periphery and in platelet filopodia (Figure 1B). To characterize the roles of PAK in platelet signaling downstream of stimulation with GPVI, we next examined the phosphorylation of PAK as well as the phosphorylation of PAK substrates and putative downstream signaling systems by Western blot over a time course of platelet activation by CRP. As shown in Figure 1C, treatment of platelets with CRP resulted in Serine and Threonine phosphorylation of a single PAK1/2/3-positive protein at residues conserved among the Group I PAKs, PAK1, PAK2 and PAK3. This phosphorylated Group I PAK protein also displayed immunoreactivity specific for PAK2 phosphorylated at Ser20, suggesting that PAK2 is a predominantly phosophorylated PAK species in platelets upon activation with CRP, but not ruling out the activation of other Group I PAK isoforms, PAK1 and PAK3. In addition to the autocatalytic phosphorylation of PAK2, the phosphorylation of the PAK substrate LIMK1 was also rapid and detectable within 30 sec of stimulation (Figure 1C). In nucleated cells, PAK plays a role in the activation of MAPK pathways through the phosphorylation of MEKs.11, 19, 20 PAK also has a role in coordinating the activation of Akt. In platelets, we found that CRP stimulation induced an activation of MEK and Akt following PAK activation (Figure 1C). ERK activation followed MEK activation. The PAK phosphorylation observed in platelets following CRP stimulation was found to be promoted by GPVI signaling and not secondary mediators such as ADP, TxA2 and integrin activation, as the presence of apyrase, indomethacin and eptifibatide did not hinder PAK phosphorylation in response to CRP (Figure 1D). Together, these results show that the PAK system is rapidly activated following stimulation with the platelet GPVI agonist CRP, that PAK activation occurs temporally before MAPK and ERK activation and that PAK redistributes to the platelet periphery and filopodia upon activation.
Figure 1. Platelet treatment with CRP stimulates PAK activation and the phosphorylation of PAK effectors.
(A) Replicate samples of human platelet lysates (50 µg total protein) were assayed for PAK isoform expression by Western blot. (B) Super resolution fluorescent microscopy analysis of PAK localization. Replicate samples of control and CRP stimulated human platelets were fixed and stained for PAK and actin. Scale bar = 1 µm. (C) Time course of platelet PAK phosphorylation and activation upon stimulation with CRP. Replicate samples of purified human platelets (5 × 108/ml) were treated with CRP (1 µg/ml) for 30, 60, 120 and 300 sec before lysis and Western blot analysis for PAK, LIMK, MEK, ERK and Akt phosphorylation. (D) Time course of platelet PAK phosphorylation in response to CRP stimulation in the presence of apyrase (2 U/ml), indomethacin (10 µM) and eptifibatide (20 µg/ml).
Activation of platelet PAK effectors and downstream signaling systems
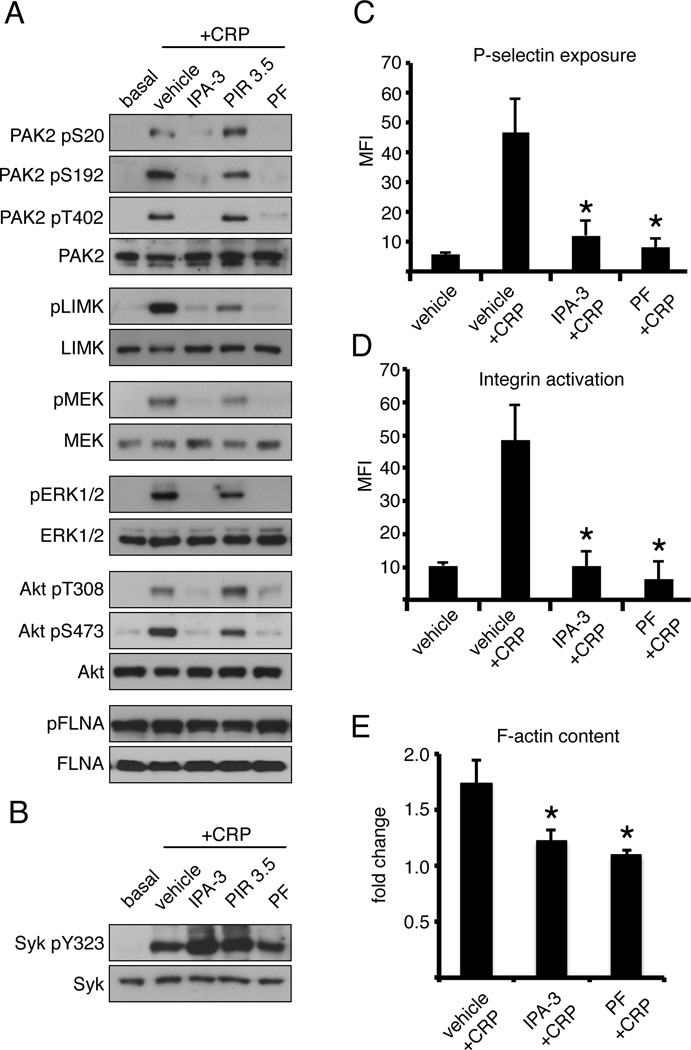
As PAK regulates actin dynamics in nucleated cell systems,11, 21 we hypothesized that PAK would likewise regulate platelet actin reorganization upon platelet activation. To investigate the function of PAK kinase activity in platelet signaling, we utilized two specific pharmacological inhibitors of PAKs. These included the inhibitor of PAK activation, IPA-3, an allosteric inhibitor that prevents activation of Group I PAKs (PAK1, 2 and 3).22, 23 IPA-3 covalently binds to PAK1 with a KD of 1.9 µM and inhibits the activation of PAK by Rho GTPases in a time- and dose-dependent manner with an in vitro IC50 of 2.5 µM.22, 23 In cellular systems, IPA-3 has been used to analyze PAK function at concentrations ranging from 5 to 25 µM in studies of cell biology,24 oncology,25 virology26 and neuroscience.27 PF-3758309, a competitive inhibitor of all PAK isoforms (PAK1–6), blocks tumor cell growth in vitro and promotes tumor regression and MAPK and Akt inhibition in vivo in mouse models of skin cancer.28, 29 As seen in ure 2A, treatment of platelets with 1 µg/ml CRP (in the presence of 2 U/ml apyrase and 20 µg/ml eptifibatide) readily activated PAK2, as evidenced by the autophosphorylation of PAK2 Ser20 as well as Ser192 and Thr402. PAK2 phosphorylation in response to CRP was completely inhibited following a 10 min incubation with PF-3758309, IPA-3 or FRAX-597,28 another pharmacologically distinct inhibitor of the Group I PAKs (Figure 2A and Supplemental Figure). An inactive PAK inhibitor relative compound, PIR 3.5,22, 23 had no effect on CRP-stimulated platelet PAK2 activation (Figure 2A). Inhibition of PAK had no effect on the Src-mediated phosphorylation of Syk Tyr323 which occurs upstream of PAK activation (Figure 2B).
Figure 2. PAK activity is required for platelet PAK effector phosphorylation, secretion, integrin activation and F-actin formation.
(A) Replicate samples of purified human platelets (5 × 108/ml) treated with vehicle (0.1% DMSO), IPA-3 (10 µM), PIR 3.5 (10 µM) or PF-3758309 (10 µM) for 10 min prior to stimulation with CRP (1 µg/ml, 300 sec) in the presence of apyrase (2 U/ml) and eptifibatide (20 µg/ml). After lysis, samples were analyzed for PAK2 Ser20, Ser192 and Thr402 autosphosphorylation, the phosphorylation PAK effectors LIMK1 (Thr508) and MEK1/2 (Ser217/221) and the activation of ERK and Akt signaling. Results representative of four experiments are shown. (B) Platelets were also examined for Src-mediated phosphorylation of Syk Tyr323. (C) Platelet P-selectin, (D) integrin αIIbβ3 activation and (E) F-actin content analyzed by flow cytometry following vehicle (DMSO), IPA-3 and PF-3758309 treatment and CRP stimulation. Data are represented as mean ± SEM. Significant results (p < 0.05) are indicated with an asterisk.
PAK organizes cytoskeletal dynamics through the phosphorylation of a complex set of substrates with known functions in actin regulation.10, 11 Such factors include LIMK1, a PAK-regulated kinase that mediates actin polymerization and microtubule disassembly in nucleated cellular systems.12 In addition to phosphorylating effectors like LIMK with specific functions in cytoskeletal organization, PAKs also support the activation of MAPK pathways and ERK signaling through the phosphorylation of MEK.19 As shown in Figure 2, PAK activation was found to be required for the PAK-mediated phosphorylation of platelet MEK1/2 Ser217/221 in response to CRP stimulation, as IPA-3 or PF-3758309 treatment inhibited PAK-mediated MEK phosphorylation in response to CRP. This loss of MEK phosphorylation activation was associated with the downstream blockade of MEK-mediated ERK phosphorylation in response to CRP (Figure 2A). In addition to activating MAPK pathways through MEK phosphorylation, PAKs also have a role coordinating the membrane recruitment and activation of Akt.30, 31 PAK activity was also required for complete activation of platelet Akt by CRP, as IPA-3 or PF-3758309 treatment blocked Akt Ser473 phosphorylation (Figure 2A) as well as Akt Thr308 phosphorylation which may be controlled through the PAK-mediated regulation of PDK1.30, 31 In nucleated cells, PAK regulates actin assembly in part through the phosphorylation of filamin A (FLNA) at Ser2152.32 In platelets, FLNA has a role in regulating platelet morphology.33 Interestingly, stimulation of platelets with CRP in solution led to no change in basal levels of FLNA Ser2152 phosphorylation which was also unaffected by PAK inhibition (Figure 2A). Together, these results show that in addition to activating a set of PAK-specific effectors, PAK also plays a role in regulating platelet MAPK and Akt activation in response to CRP.
PAK activity is required for platelet secretion and aggregation
To determine the role of PAK activation in platelet degranulation, we examined the surface exposure of P-selectin and the activation of the integrin αIIbβ3 by flow cytometry under vehicle and PAK-inhibited conditions. As seen in Figure 2B and 2C, treatment of platelets with 1 µg/ml CRP for 5 min effectively resulted in P-selectin exposure and integrin αIIbβ3 activation in 46.6% and 48.3% of platelets, respectively. Treatment of platelets with the PAK inhibitor PF-3758309 reduced CRP-induced granule release and integrin activation to 8.16% and 6.32%, respectively (Figure 2C & D). Similar inhibition was seen with IPA-3 treatment (Figure 2C & D) while the PAK inhibitor relative compound, PIR 3.5, did not significantly change platelet P-selectin exposure or PAC-1 binding (data not shown). Next, to examine the role of PAK activation in actin assembly in platelets, we assayed the fold-change in filamentous actin (Factin) content elicited by CRP stimulation under basal and PAK-inhibited conditions. As seen in Figure 2E, CRP promoted a 1.74±0.20 fold increase in total platelet F-actin content. Inhibition of PAK activity with IPA-3 or PF-3758309 significantly reduced this increase in F-actin content to a 1.24±0.10 and 1.10±0.05 fold-change, respectively.
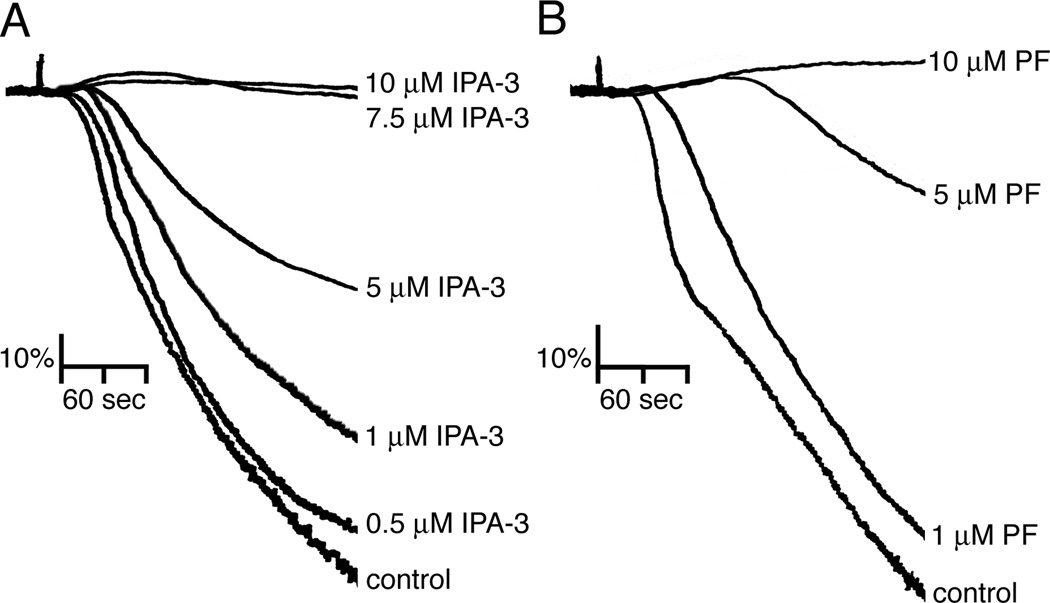
Platelet secretion, integrin activation and F-actin assembly are closely tied to the process of platelet aggregation. To next determine if PAK plays a role in platelet aggregation, the effects of PAK inhibition on platelet aggregation in solution were assayed using a Born aggregometer. As shown in Figure 3, purified human platelets readily aggregated in solution upon the addition of 1 µg/ml CRP. Pretreatment of platelets with IPA-3 concentration-dependently prevented the aggregation of platelets stimulated with CRP, while PIR 3.5 had no significant effects on aggregation relative to the vehicle-treated control (Figure 3A and data not shown). PF-3758309 as well as FRAX-597 similarly inhibited platelet aggregation in response to CRP in a concentration-dependent manner (Figure 3B and Supplemental Figure).
Figure 3. Inhibition of PAK blocks platelet aggregation in response to CRP.
Washed human platelets (2 × 108/ml) were incubated with vehicle (DMSO) or increasing concentrations of (A) IPA-3 or (B) PF-123758309 in the presence of 2 U/ml apyrase prior to stimulation with CRP (1 µg/ml) and the change in optical density indicative of platelet aggregation was recorded. Representative aggregation traces of four separate experiments are shown.
PAK is required for platelet lamellipodia formation
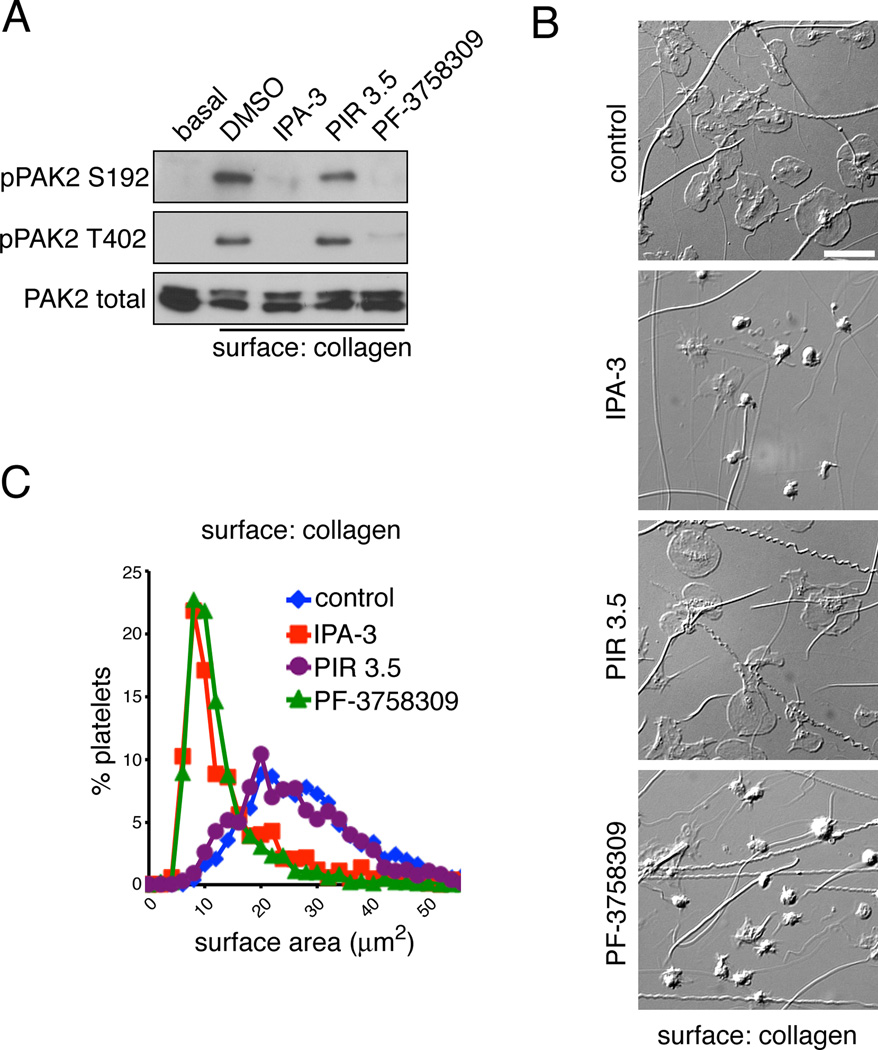
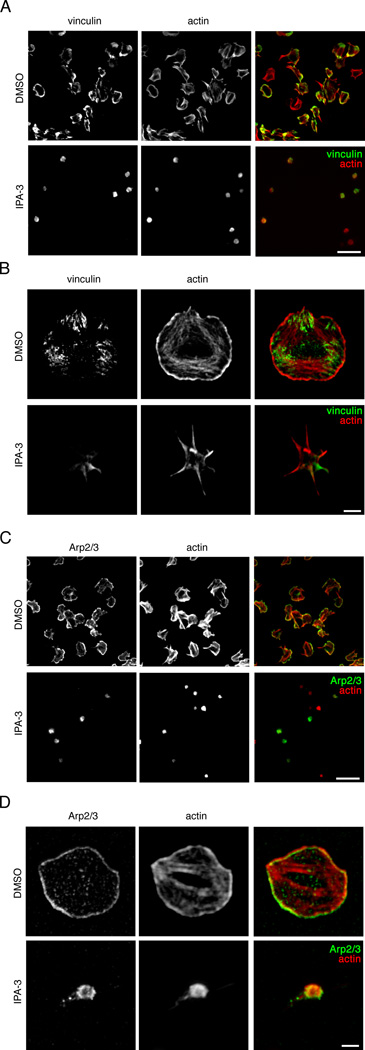
Upon binding to collagen, platelet activation downstream of GPVI stimulates the rapid reorganization of the actin cytoskeleton, resulting in the transformation of platelets from discoids to fully spread cells. To assay the effect of PAK inhibition on lamellipodia formation downstream of GPVI, platelets were pretreated with vehicle, PF-3758309, IPA-3 or PIR 3.5 for 10 min, then exposed to an immobilized surface of collagen for 45 min, before lysis into sample buffer and Western blot analysis. Collagen surfaces stimulated PAK activation in platelets as determined by Western blot for the phosphorylation of PAK2 Ser192 and Ser402 (Figure 4A). PAK activation by these substrate surfaces was readily blocked by the PAK inhibitors, IPA-3 and PF-3758309 (Figure 4A). To examine the role of PAK in platelet lamellipodia formation, platelets were fixed following spreading on collagen and visualized by DIC microscopy.34 As seen in Figure 4B and 4C, under basal conditions, platelets readily formed lamellipodia and spread out upon surfaces of collagen. Inhibition of PAK activation by PF-3758309, IPA-3 or FRAX- 597 treatment prevented lamellipodia formation and platelet spreading (Figure 4B & C and Supplemental Figure). To examine the architecture of platelet actin-rich adhesion structures under PAK-inhibited conditions, platelets were spread onto a surface of collagen, fixed, and stained for actin and the focal adhesion component vinculin. Conventional fluorescence microscopy and super resolution visualization by structured illumination microscopy revealed that platelets readily form actin-rich focal adhesion structures as they spread on collagen-coated substrates (Figure 5A & B). Treatment of platelets with IPA- 3 prior to exposure to a surface of collagen prevented spreading, focal adhesion formation and the efficiency of vinculin association with the actin cytoskeleton (Figure 5A & B). Similar results were observed with PF-3758309 (data not shown). Immunofluorescence microscopy experiments also confirmed that inhibition of PAK similarly blocked the formation of actin-rich lamellipodia, as platelets did not establish Arp2/3-positive lamellipodial structures while spread on collagen in PAK-inhibited conditions (Figure 5C & D). Together, these results show that PAK is activated by platelet exposure to collagen and has a role in platelet lamellipodia and actin-rich adhesion structure formation.
Figure 4. PAK activation is required for spreading on a surface of collagen.
(A) Replicate samples of purified human platelets (5 × 108/ml) were treated with vehicle (DMSO), IPA-3 (10 µM), PIR 3.5 (10 µM) or PF-3758309 (10 µM) for 10 min prior to exposure to a collagen surface in the presence of 2 U/ml apyrase. After 45 min, platelets were stimulated lysed and analyzed for PAK activation by Western blot for PAK2-pSer192 and PAK2-pThr402. (B) Replicate wells of collagen-coated coverglass were seeded with human platelets (2 × 107/ml) for 45 min after pretreatment with vehicle (DMSO), IPA-3 (10 µM), PIR 3.5 (10 µM) or PF-3758309 (10 µM). Platelets were fixed and analyzed by DIC microscopy. Images representative of five experiments are shown. Scale bar = 10 µm. (C) Quantification and distribution of platelet surface areas (n = 200 per condition) under control and PAK-inhibited conditions.
Figure 5. PAK activity regulates actin-rich adhesion and lamellipodia formation in platelets spread on collagen.
Replicate wells of collagen-coated coverglass were seeded with human platelets (2 × 107/ml) for 45 min after pretreatment with vehicle (DMSO), IPA-3 (10 µM), PIR 3.5 (10 µM) or PF-3758309 (10 µM). Platelets were fixed, stained for (A,B) vinculin and actin or (C,D) Arp2/3 and actin and analyzed by fluorescence microscopy. Images representative of five experiments are shown. (A,C) Scale bar = 10 µm. Super resolution microscopy analysis of (B) platelet focal adhesion formation and (D) lamellipodia formation of vehicle (DMSO) and IPA-3 (10 µM) treated platelets on a surface of collagen, visualized by SR-SIM of actin (red) and vinculin or Arp2/3 (green) staining. Scale bar = 2 µm.
PAK activation is required for platelet aggregate formation and aggregate stability under shear
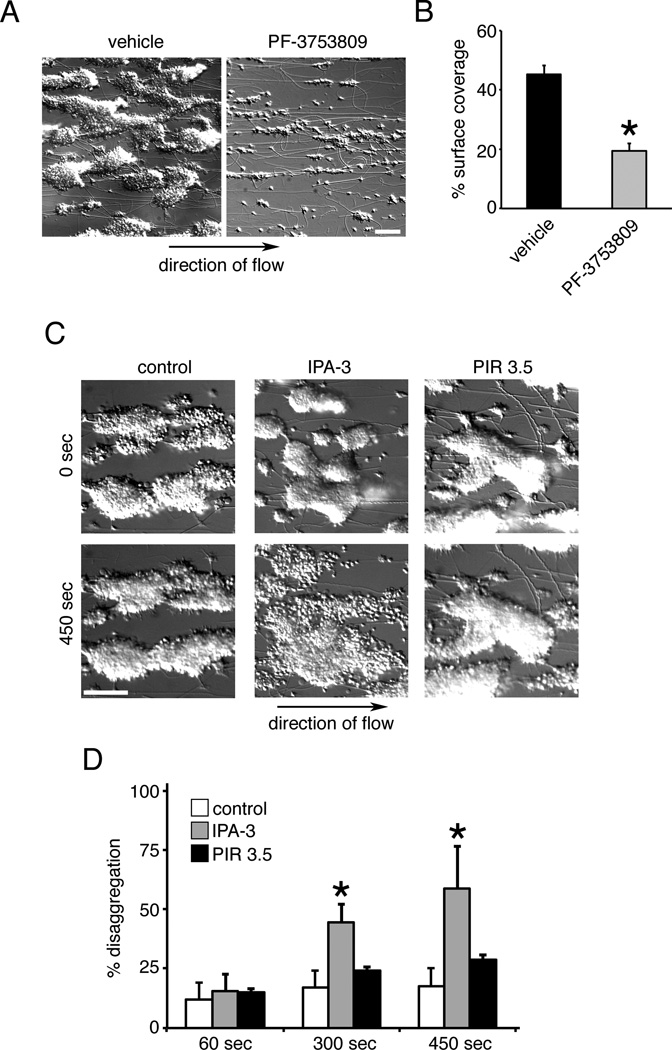
The effects of PAK inhibition on platelet aggregation and lamellipodia formation (Figures 3, 4) suggested that PAK may regulate platelet adhesion and aggregate formation under physiologically-relevant conditions of shear. To examine the role of PAK in platelet aggregate formation, human blood was treated with vehicle alone or PF-3758309, which is bioavailable in whole blood,29 for 10 min prior to flow over immobilized collagen. As seen in Figure 6A, under vehicle-treated conditions, platelets bound to and formed aggregates on collagen under shear. Treatment of blood with PF-3758309 prevented the formation of platelet aggregates (Figure 6A & B). In addition to platelet aggregate formation, signaling networks such as the mTOR,35, 36 Rho GTPase36, 37 and PI3K/Akt38 systems are also required to maintain platelet aggregate stability under physiological shear conditions. To determine if the PAK-mediated signaling is similarly required to maintain the integrity of platelet aggregates, we tested the ability of PAK inhibition to disrupt platelet aggregates formed under physiological shear flow. Platelet aggregates were formed through the perfusion of PPACK-anticoagulated blood over a surface of collagen (Figure 6C). Next, aggregate stability was assessed during a secondary perfusion of buffer containing physiological concentrations of fibrinogen. Under basal conditions, platelet aggregates remained stable (Figure 6C & D). Buffer containing fibrinogen and IPA-3, however, destabilized platelet aggregates under shear flow after 300 sec, with further instability at 450 sec (Figure 6B & C). Quantification of the surface area changes of the aggregates revealed a 44.6% increase in coverage after 300 sec, extending to 60.0% after 450 sec, in contrast to 17.4% and 17.8% for vehicle alone at 300 sec and 450 sec, respectively (Figure 6D). Similar results were observed with PF-3758309 (data not shown).
Figure 6. Platelet aggregate formation and stability under shear requires PAK.
(A) Human whole blood was treated with vehicle or PF-3758309 (20 µM) and perfused over collagen at a shear rate of 1500 s-1 to produce platelet aggregates. (B) Quantification of percentage of surface area coverage by platelet aggregates in the presence of vehicle or PF-3758309 (n = 3). (C) Platelet aggregates were formed following perfusion of PPACK-anticoagulated blood over collagen for 4 min at a wall shear rate of 1500 s-1. Following washing for 4 min with buffer containing fibrinogen (3 mg/ml), platelet aggregates were perfused with buffer containing fibrinogen and vehicle (DMSO), IPA-3 (10 µM) or PIR 3.5 (10 µM). Representative DIC images are shown. Scale bar = 100 µm. (D) Quantification of platelet disaggregation promoted by IPA-3 under flow (n = 3). Data are represented as mean ± SEM. Significant results (p < 0.05) are indicated with an asterisk.
Discussion
Here we report that in platelets, the PAK signaling system supports signaling processes that mediate platelet spreading, aggregation and platelet aggregate stability under conditions of physiological shear. Since their original discovery as serine/threonine protein kinases activated by GTP-bound Rac1 and Cdc42,39 the PAKs have been extensively characterized for roles in linking small GTPase activation to actin regulation, cell motility and cell growth.11 The Rho GTPases RhoA, Cdc42 and Rac1 orchestrate actin remodeling in platelets to regulate platelet activation, secretion, aggregation and thrombus stability.7 RhoA and Cdc42 have been shown to play roles in platelet contractility and secretion.6, 15, 40, 41 Platelet spreading is regulated by Rac1, which is required for platelet lamellipodia formation and the stability of platelet aggregates.36, 37 While some of the tyrosine kinase signaling events upstream of Rac1 activation in platelet thrombotic functions have been defined,3 it is not well understood how signals downstream of Rac1 are translated into changes in platelet actin structures. In addition to the WASP and formin proteins which support actin polymerization and reorganization, the PAKs are key candidates in Rac effectors in platelet function.7
PAK2, or γ-PAK, the most abundantly and ubiquitously expressed PAK family member,11 was originally characterized in platelets as a kinase activated upon platelet stimulation and associated with a signaling complex consisting of small GTPases and GAPs.13 While small GTPases have been an active subject of investigation in platelet biology for the past decade,7 a characterization of PAK in platelets has been absent. Early studies of actin dynamics in platelets have shown that Cdc42, Rac1 and PAK are activated downstream of tyrosine kinases.7, 16 Experiments with toxins that nonspecifically inhibit Rac and Cdc42 suggested that PAK activation occurs downstream of Rac signaling in platelets,6 but the specific effects of inhibiting Rac on PAK activation in platelets had not yet been determined. More recent studies with Cdc42-knockout mice have provided evidence that Cdc42 is required for platelet PAK activation, filopodia formation, aggregation and Akt activation;15 however, the role of Cdc42 in platelets is not straightforward.42 In this study, we show that platelet PAK activation temporally precedes MEK, ERK and Akt activation and that PAK localizes to platelet filopodia upon stimulation with the GPVI agonist CRP (Figure 1). Using two separate PAK-specific inhibitors, we show that inhibition of PAK activity blocks platelet secretion, integrin activation and aggregation. PAK inhibition also prevented platelets from forming lamellipodia or establishing actin-rich adhesions on an immobilized surface of collagen, as determined by a characterization of the platelet actin cytoskeleton by super resolution structured illumination fluorescence microscopy (SR-SIM) (Figure 5). A third pharmacologically distinct Group I PAK inhibitor, FRAX-597,28 also prevented platelet PAK phosphorylation, aggregation and lamellipodia formation in response to GPVI stimulation (Supplemental Figure).
Upon activation, PAKs phosphorylate a number of effector proteins with established roles in regulating the actin cytoskeleton in processes of cell migration and cell spreading.11 Of these targets, the LIM domain kinase LIMK1 may be the best characterized PAK substrate in connecting Rho GTPases and PAK to actin regulation.12 In nucleated cells, following phosphorylation by PAK, LIMK1 plays a role in actin polymerization through the phosphorylation of the actin binding protein cofilin.12 In platelets, LIMK1 phosphorylation is similarly hypothesized to regulate actin dynamics following its phosphorylation; however, the role of cofilin phosphorylation in platelet F-actin assembly is not clear.18 Here we show that inhibition of PAK activity blocks LIMK1 phosphorylation in response to CRP. LIMK1 is also phosphorylated by the RhoA activated kinase ROCK upon platelet activation.18 Platelet LIMK1 regulation may therefore be complex, as both ROCK and PAK may cooperate to regulate LIMK1 activity and actin reorganization at platelet focal adhesions. Intriguingly, time course experiments of platelet LIMK1 activation demonstrate that platelet LIMK1 is rapidly phosphorylated in response to CRP stimulation and may occur in a phase of signaling temporally distinct from Akt and MAPK activation (Figure 1).
In addition to LIMK1 Thr508 phosphorylation, PAK activation was also required for the complete phosphorylation of platelet MEK and the subsequent activation of ERKs. In nucleated cells, PAK plays a well-described role in MEK and ERK activation.11 In platelets, MEK1 activation is required for platelet aggregation in response to collagen and other agonists.43 While the exact roles of MEK and ERK in platelet function are not yet defined, MEK and ERK are associated with the platelet cytoskeleton and have reported roles in linking the signaling events of platelet activation to the cytoskeletal dynamics that mediate platelet function.44 In accordance with a role for platelet PAK upstream of MAPK activation, we found that platelet PAK activation temporally preceded platelet MEK activation (Figure 1) and that inhibition of PAK blocked MEK and ERK activation in platelets (Figure 2). PAK may therefore regulate platelet function through both signaling and cytoskeletal processes mediated through MAP kinase pathways and other cellular signaling systems with more classical roles in actin assembly.
The PAK-based signaling systems that regulate platelet aggregation in solution also have roles in platelet aggregate formation under physiological conditions of shear.45–47 Intriguingly, PAK was required not only for actin dynamics that guide activated platelets as they spread upon a surface, but also for platelet aggregation under flow (Figure 6). These results suggest that PAK signaling has key roles in platelet cytoskeletal processes in multiple aspects of platelet physiology. Moreover, like Rac, mTOR and PI3K/Akt,36–38 continuous PAK activity was also required to maintain the integrity of platelet aggregates formed under physiological conditions of shear (Figure 6). Our results showing that PAK regulates platelet aggregation (Figure 3) and aggregate stability (Figure 6) together with previous studies showing a PAK-associated shedding of microvesicles upon platelet activation17 support a potential physiological role for PAK in hemostasis. While we have shown that PAK effectors such as LIMK1 and MEK are phosphorylated in a PAK-dependent manner upstream of platelet aggregation, the exact roles of these proteins in the biology of platelets remains to be defined. Accordingly, the function of PAK as well as the system of PAK effectors in hemostasis, angiogenesis and vascular stability is certain to develop into an active area of future investigation.
Supplementary Material
Significance.
Platelets serve as the primary cellular mediators of thrombosis. While platelet activation is known to be regulated by Rho GTPases proteins such as Rac1, the signaling processes downstream of Rho GTPases in platelet physiology remain unexplored. In nucleated cells, the p21 activated kinases (PAKs) represent well-established Rho GTPase effectors in cytoskeletal dynamics and cell motility. This study shows for the first time that PAK activity is required for signaling events and cytoskeletal reorganization processes that underlie platelet activation, platelet aggregation, platelet spreading and platelet aggregate stability under physiological shear.
Acknowledgements
We thank S. Kaech Petrie and A. Snyder (OHSU Advanced Light Microscopy Core) for imaging assistance.
Sources of Funding
Super resolution microscopy studies were supported by the M. J. Murdock Charitable Trust. This work was supported by grants from the National Institutes of Health (R01CA142928 to J.C. and R01HL101972 to O.J.T.M.) and American Heart Association (13POST13730003 to J.E.A., 12PRE11930019 to G.W.T and 13EIA12630000 to O.J.T.M.). J.E.A is a 2012–2013 Fulbright Scholar. K.H.M. is an MRF Early Clinical Investigator. C.P.L. is a FV Leiden Scholar. A.I. is a Vertex Scholar.
Abbreviations
- CRP
collagen related peptide
- ERK
extracellular signal-regulated kinase
- GEF
guanine nucleotide exchange factor
- GPVI
glycoprotein receptor VI
- MEK
MAPK/ERK kinase
- PAK
p21 activated kinase
Footnotes
Disclosures
None.
References
- 1.Furie B, Furie BC. Thrombus formation in vivo. J Clin Invest. 2005;115:3355–3362. doi: 10.1172/JCI26987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jackson SP, Schoenwaelder SM. Antiplatelet therapy: in search of the 'magic bullet'. Nat Rev Drug Discov. 2003;2:775–789. doi: 10.1038/nrd1198. [DOI] [PubMed] [Google Scholar]
- 3.Watson SP, Auger JM, McCarty OJ, Pearce AC. GPVI and integrin alphaIIb beta3 signaling in platelets. J Thromb Haemost. 2005;3:1752–1762. doi: 10.1111/j.1538-7836.2005.01429.x. [DOI] [PubMed] [Google Scholar]
- 4.Calaminus SD, McCarty OJ, Auger JM, Pearce AC, Insall RH, Watson SP, Machesky LM. A major role for Scar/WAVE-1 downstream of GPVI in platelets. J Thromb Haemost. 2007;5:535–541. doi: 10.1111/j.1538-7836.2007.02377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li Z, Kim ES, Bearer EL. Arp2/3 complex is required for actin polymerization during platelet shape change. Blood. 2002;99:4466–4474. doi: 10.1182/blood.v99.12.4466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vidal C, Geny B, Melle J, Jandrot-Perrus M, Fontenay-Roupie M. Cdc42/Rac1-dependent activation of the p21-activated kinase (PAK) regulates human platelet lamellipodia spreading: implication of the cortical-actin binding protein cortactin. Blood. 2002;100:4462–4469. doi: 10.1182/blood.V100.13.4462. [DOI] [PubMed] [Google Scholar]
- 7.Aslan JE, McCarty OJ. Rho GTPases in platelet function. J Thromb Haemost. 2013;11:35–46. doi: 10.1111/jth.12051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Z, Delaney MK, O'Brien KA, Du X. Signaling during platelet adhesion and activation. Arterioscler Thromb Vasc Biol. 2010;30:2341–2349. doi: 10.1161/ATVBAHA.110.207522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Watson SP. Platelet activation by extracellular matrix proteins in haemostasis and thrombosis. Curr Pharm Des. 2009;15:1358–1372. doi: 10.2174/138161209787846702. [DOI] [PubMed] [Google Scholar]
- 10.Zhao ZS, Manser E. PAK family kinases: Physiological roles and regulation. Cell Logist. 2012;2:59–68. doi: 10.4161/cl.21912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arias-Romero LE, Chernoff J. A tale of two Paks. Biol Cell. 2008;100:97–108. doi: 10.1042/BC20070109. [DOI] [PubMed] [Google Scholar]
- 12.Bernard O. Lim kinases, regulators of actin dynamics. Int J Biochem Cell Biol. 2007;39:1071–1076. doi: 10.1016/j.biocel.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 13.Teo M, Manser E, Lim L. Identification and molecular cloning of a p21cdc42/rac1-activated serine/threonine kinase that is rapidly activated by thrombin in platelets. J Biol Chem. 1995;270:26690–26697. doi: 10.1074/jbc.270.44.26690. [DOI] [PubMed] [Google Scholar]
- 14.Akbar H, Kim J, Funk K, Cancelas JA, Shang X, Chen L, Johnson JF, Williams DA, Zheng Y. Genetic and pharmacologic evidence that Rac1 GTPase is involved in regulation of platelet secretion and aggregation. J Thromb Haemost. 2007;5:1747–1755. doi: 10.1111/j.1538-7836.2007.02646.x. [DOI] [PubMed] [Google Scholar]
- 15.Akbar H, Shang X, Perveen R, Berryman M, Funk K, Johnson JF, Tandon NN, Zheng Y. Gene targeting implicates Cdc42 GTPase in GPVI and non-GPVI mediated platelet filopodia formation, secretion and aggregation. PLoS One. 2011;6:e22117. doi: 10.1371/journal.pone.0022117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suzuki-Inoue K, Yatomi Y, Asazuma N, Kainoh M, Tanaka T, Satoh K, Ozaki Y. Rac, a small guanosine triphosphate-binding protein, and p21-activated kinase are activated during platelet spreading on collagen-coated surfaces: roles of integrin alpha(2)beta(1) Blood. 2001;98:3708–3716. doi: 10.1182/blood.v98.13.3708. [DOI] [PubMed] [Google Scholar]
- 17.Crespin M, Vidal C, Picard F, Lacombe C, Fontenay M. Activation of PAK1/2 during the shedding of platelet microvesicles. Blood Coagul Fibrinolysis. 2009;20:63–70. doi: 10.1097/MBC.0b013e32831bc310. [DOI] [PubMed] [Google Scholar]
- 18.Pandey D, Goyal P, Bamburg JR, Siess W. Regulation of LIM-kinase 1 and cofilin in thrombin-stimulated platelets. Blood. 2006;107:575–583. doi: 10.1182/blood-2004-11-4377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eblen ST, Slack JK, Weber MJ, Catling AD. Rac-PAK signaling stimulates extracellular signalregulated kinase (ERK) activation by regulating formation of MEK1-ERK complexes. Mol Cell Biol. 2002;22:6023–6033. doi: 10.1128/MCB.22.17.6023-6033.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park ER, Eblen ST, Catling AD. MEK1 activation by PAK: a novel mechanism. Cell Signal. 2007;19:1488–1496. doi: 10.1016/j.cellsig.2007.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sit ST, Manser E. Rho GTPases and their role in organizing the actin cytoskeleton. J Cell Sci. 2011;124:679–683. doi: 10.1242/jcs.064964. [DOI] [PubMed] [Google Scholar]
- 22.Deacon SW, Beeser A, Fukui JA, Rennefahrt UE, Myers C, Chernoff J, Peterson JR. An isoform-selective, small-molecule inhibitor targets the autoregulatory mechanism of p21-activated kinase. Chem Biol. 2008;15:322–331. doi: 10.1016/j.chembiol.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Viaud J, Peterson JR. An allosteric kinase inhibitor binds the p21-activated kinase autoregulatory domain covalently. Mol Cancer Ther. 2009;8:2559–2565. doi: 10.1158/1535-7163.MCT-09-0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li A, Ma Y, Yu X, Mort RL, Lindsay CR, Stevenson D, Strathdee D, Insall RH, Chernoff J, Snapper SB, Jackson IJ, Larue L, Sansom OJ, Machesky LM. Rac1 drives melanoblast organization during mouse development by orchestrating pseudopod- driven motility and cell-cycle progression. Dev Cell. 2011;21:722–734. doi: 10.1016/j.devcel.2011.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ong CC, Jubb AM, Haverty PM, Zhou W, Tran V, Truong T, Turley H, O'Brien T, Vucic D, Harris AL, Belvin M, Friedman LS, Blackwood EM, Koeppen H, Hoeflich KP. Targeting p21-activated kinase 1 (PAK1) to induce apoptosis of tumor cells. Proc Natl Acad Sci U S A. 2011;108:7177–7182. doi: 10.1073/pnas.1103350108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kalin S, Amstutz B, Gastaldelli M, Wolfrum N, Boucke K, Havenga M, DiGennaro F, Liska N, Hemmi S, Greber UF. Macropinocytotic uptake and infection of human epithelial cells with species B2 adenovirus type 35. J Virol. 2010;84:5336–5350. doi: 10.1128/JVI.02494-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grimsley-Myers CM, Sipe CW, Geleoc GS, Lu X. The small GTPase Rac1 regulates auditory hair cell morphogenesis. J Neurosci. 2009;29:15859–15869. doi: 10.1523/JNEUROSCI.3998-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chow HY, Jubb AM, Koch JN, Jaffer ZM, Stepanova D, Campbell DA, Duron SG, O'Farrell M, Cai KQ, Klein-Szanto AJ, Gutkind JS, Hoeflich KP, Chernoff J. p21-Activated Kinase 1 Is Required for Efficient Tumor Formation and Progression in a Ras-Mediated Skin Cancer Model. Cancer Res. 2012;72:5966–5975. doi: 10.1158/0008-5472.CAN-12-2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murray BW, Guo C, Piraino J, Westwick JK, Zhang C, Lamerdin J, Dagostino E, Knighton D, Loi CM, Zager M, Kraynov E, Popoff I, Christensen JG, Martinez R, Kephart SE, Marakovits J, Karlicek S, Bergqvist S, Smeal T. Small-molecule p21-activated kinase inhibitor PF-3758309 is a potent inhibitor of oncogenic signaling and tumor growth. Proc Natl Acad Sci U S A. 2010;107:9446–9451. doi: 10.1073/pnas.0911863107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mao K, Kobayashi S, Jaffer ZM, Huang Y, Volden P, Chernoff J, Liang Q. Regulation of Akt/PKB activity by P21-activated kinase in cardiomyocytes. J Mol Cell Cardiol. 2008;44:429–434. doi: 10.1016/j.yjmcc.2007.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Higuchi M, Onishi K, Kikuchi C, Gotoh Y. Scaffolding function of PAK in the PDK1-Akt pathway. Nat Cell Biol. 2008;10:1356–1364. doi: 10.1038/ncb1795. [DOI] [PubMed] [Google Scholar]
- 32.Vadlamudi RK, Li F, Adam L, Nguyen D, Ohta Y, Stossel TP, Kumar R. Filamin is essential in actin cytoskeletal assembly mediated by p21-activated kinase 1. Nat Cell Biol. 2002;4:681–690. doi: 10.1038/ncb838. [DOI] [PubMed] [Google Scholar]
- 33.Falet H, Pollitt AY, Begonja AJ, Weber SE, Duerschmied D, Wagner DD, Watson SP, Hartwig JH. A novel interaction between FlnA and Syk regulates platelet ITAM-mediated receptor signaling and function. J Exp Med. 2010;207:1967–1979. doi: 10.1084/jem.20100222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aslan JE, Itakura A, Gertz JM, McCarty OJ. Platelet shape change and spreading. Methods Mol Biol. 2012;788:91–100. doi: 10.1007/978-1-61779-307-3_7. [DOI] [PubMed] [Google Scholar]
- 35.Aslan JE, McCarty OJT. Regulation of the mTOR-Rac1 axis in platelet function. Small GTPases. 2012;3:16–19. doi: 10.4161/sgtp.19137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aslan JE, Tormoen GW, Loren CP, Pang J, McCarty OJ. S6K1 and mTOR regulate Rac1-driven platelet activation and aggregation. Blood. 2011;118:3129–3136. doi: 10.1182/blood-2011-02-331579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McCarty OJ, Larson MK, Auger JM, Kalia N, Atkinson BT, Pearce AC, Ruf S, Henderson RB, Tybulewicz VL, Machesky LM, Watson SP. Rac1 is essential for platelet lamellipodia formation and aggregate stability under flow. J Biol Chem. 2005;280:39474–39484. doi: 10.1074/jbc.M504672200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cosemans JM, Munnix IC, Wetzker R, Heller R, Jackson SP, Heemskerk JW. Continuous signaling via PI3K isoforms beta and gamma is required for platelet ADP receptor function in dynamic thrombus stabilization. Blood. 2006;108:3045–3052. doi: 10.1182/blood-2006-03-006338. [DOI] [PubMed] [Google Scholar]
- 39.Manser E, Leung T, Salihuddin H, Zhao ZS, Lim L. A brain serine/threonine protein kinase activated by Cdc42 and Rac1. Nature. 1994;367:40–46. doi: 10.1038/367040a0. [DOI] [PubMed] [Google Scholar]
- 40.Pleines I, Hagedorn I, Gupta S, May F, Chakarova L, van Hengel J, Offermanns S, Krohne G, Kleinschnitz C, Brakebusch C, Nieswandt B. Megakaryocyte-specific RhoA deficiency causes macrothrombocytopenia and defective platelet activation in hemostasis and thrombosis. Blood. 2012;119:1054–1063. doi: 10.1182/blood-2011-08-372193. [DOI] [PubMed] [Google Scholar]
- 41.Schoenwaelder SM, Hughan SC, Boniface K, Fernando S, Holdsworth M, Thompson PE, Salem HH, Jackson SP. RhoA sustains integrin alpha IIbbeta 3 adhesion contacts under high shear. J Biol Chem. 2002;277:14738–14746. doi: 10.1074/jbc.M200661200. [DOI] [PubMed] [Google Scholar]
- 42.Pleines I, Eckly A, Elvers M, Hagedorn I, Eliautou S, Bender M, Wu X, Lanza F, Gachet C, Brakebusch C, Nieswandt B. Multiple alterations of platelet functions dominated by increased secretion in mice lacking Cdc42 in platelets. Blood. 2010;115:3364–3373. doi: 10.1182/blood-2009-09-242271. [DOI] [PubMed] [Google Scholar]
- 43.McNicol A, Philpott CL, Shibou TS, Israels SJ. Effects of the mitogen-activated protein (MAP) kinase kinase inhibitor 2-(2'-amino-3'-methoxyphenyl)-oxanaphthalen-4-one (PD98059) on human platelet activation. Biochem Pharmacol. 1998;55:1759–1767. doi: 10.1016/s0006-2952(97)00632-1. [DOI] [PubMed] [Google Scholar]
- 44.McNicol A, Shibou TS, Pampolina C, Israels SJ. Incorporation of map kinases into the platelet cytoskeleton. Thromb Res. 2001;103:25–34. doi: 10.1016/s0049-3848(01)00271-7. [DOI] [PubMed] [Google Scholar]
- 45.Li Z, Xi X, Du X. A mitogen-activated protein kinase-dependent signaling pathway in the activation of platelet integrin alpha IIbbeta3. J Biol Chem. 2001;276:42226–42232. doi: 10.1074/jbc.M106129200. [DOI] [PubMed] [Google Scholar]
- 46.Garcia A, Quinton TM, Dorsam RT, Kunapuli SP. Src family kinase-mediated and Erk-mediated thromboxane A2 generation are essential for VWF/GPIb-induced fibrinogen receptor activation in human platelets. Blood. 2005;106:3410–3414. doi: 10.1182/blood-2005-05-1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mazharian A, Roger S, Maurice P, Berrou E, Popoff MR, Hoylaerts MF, Fauvel-Lafeve F, Bonnefoy A, Bryckaert M. Differential Involvement of ERK2 and p38 in platelet adhesion to collagen. J Biol Chem. 2005;280:26002–26010. doi: 10.1074/jbc.M414083200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.