Abstract
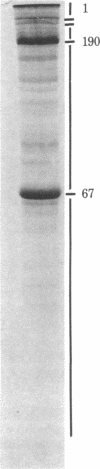
Circumstantial evidence suggests that nucleocytoplasmic exchange or transport is an active process involving the nuclear pore complex of the nuclear envelope. To test this hypothesis, antibodies were generated against nuclear envelope components from a highly enriched pore complex fraction from Spisula solidissima oocytes. Some of these antibodies inhibited ATP-dependent ribonucleoprotein release from prelabeled, isolated rat nuclei and inhibited nucleoside triphosphatase activity essential in nucleocytoplasmic transport. Inhibition of both functions by lectins indicated that the antigen was a glycoprotein. It was identified as lamin B, a major component of the nuclear envelope and nuclear matrix. This glycoprotein may not only be a structural nuclear protein but also may have nucleoside triphosphatase activity. We speculate that lamin B represents the solid support for ribonucleoprotein transport. This protein is expected to be highly conserved if active transport in and out of the nucleus is essential in the eukaryotic system.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agutter P. S., McArdle H. J., McCaldin B. Evidence for involvement of nuclear envelope nucleoside triphosphatase in nucleocytoplasmic translocation of ribonucleoprotein. Nature. 1976 Sep 9;263(5573):165–167. doi: 10.1038/263165a0. [DOI] [PubMed] [Google Scholar]
- Blobel G., Potter V. R. Nuclei from rat liver: isolation method that combines purity with high yield. Science. 1966 Dec 30;154(3757):1662–1665. doi: 10.1126/science.154.3757.1662. [DOI] [PubMed] [Google Scholar]
- Bornens M., Kasper C. B. Fractionation and partial characterization of proteins of the bileaflet nuclear membrane from rat liver. J Biol Chem. 1973 Jan 25;248(2):571–579. [PubMed] [Google Scholar]
- Ciejek E. M., Nordstrom J. L., Tsai M. J., O'Malley B. W. Ribonucleic acid precursors are associated with the chick oviduct nuclear matrix. Biochemistry. 1982 Sep 28;21(20):4945–4953. doi: 10.1021/bi00263a018. [DOI] [PubMed] [Google Scholar]
- Clawson G. A., James J., Woo C. H., Friend D. S., Moody D., Smuckler E. A. Pertinence of nuclear envelope nucleoside triphosphatase activity to ribonucleic acid transport. Biochemistry. 1980 Jun 10;19(12):2748–2756. doi: 10.1021/bi00553a033. [DOI] [PubMed] [Google Scholar]
- FLEISCHMAN J. B., PORTER R. R., PRESS E. M. THE ARRANGEMENT OF THE PEPTIDE CHAINS IN GAMMA-GLOBULIN. Biochem J. 1963 Aug;88:220–228. doi: 10.1042/bj0880220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher P. A., Berrios M., Blobel G. Isolation and characterization of a proteinaceous subnuclear fraction composed of nuclear matrix, peripheral lamina, and nuclear pore complexes from embryos of Drosophila melanogaster. J Cell Biol. 1982 Mar;92(3):674–686. doi: 10.1083/jcb.92.3.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller G. M., Brinkley B. R., Boughter J. M. Immunofluorescence of mitotic spindles by using monospecific antibody against bovine brain tubulin. Science. 1975 Mar 14;187(4180):948–950. doi: 10.1126/science.1096300. [DOI] [PubMed] [Google Scholar]
- Gerace L., Blobel G. The nuclear envelope lamina is reversibly depolymerized during mitosis. Cell. 1980 Jan;19(1):277–287. doi: 10.1016/0092-8674(80)90409-2. [DOI] [PubMed] [Google Scholar]
- Gerace L., Blum A., Blobel G. Immunocytochemical localization of the major polypeptides of the nuclear pore complex-lamina fraction. Interphase and mitotic distribution. J Cell Biol. 1978 Nov;79(2 Pt 1):546–566. doi: 10.1083/jcb.79.2.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashnig D. M., Kasper C. B. Isolation, morphology, and composition of the nuclear membrane from rat liver. J Biol Chem. 1969 Jul 25;244(14):3786–3792. [PubMed] [Google Scholar]
- Kaufmann S. H., Coffey D. S., Shaper J. H. Considerations in the isolation of rat liver nuclear matrix, nuclear envelope, and pore complex lamina. Exp Cell Res. 1981 Mar;132(1):105–123. doi: 10.1016/0014-4827(81)90088-4. [DOI] [PubMed] [Google Scholar]
- Kletzien R. F. Nucleocytoplasmic transport of RNA. The effect of 3'-deoxyadenosine triphosphate on RNA release from isolated nuclei. Biochem J. 1980 Nov 15;192(2):753–759. doi: 10.1042/bj1920753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krohne G., Franke W. W., Ely S., D'Arcy A., Jost E. Localization of a nuclear envelope-associated protein by indirect immunofluorescence microscopy using antibodies against a major polypeptide from rat liver fractions enriched in nuclear envelope-associated material. Cytobiologie. 1978 Oct;18(1):22–38. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lam K. S., Kasper C. B. Selective phosphorylation of a nuclear envelope polypeptide by an endogenous protein kinase. Biochemistry. 1979 Jan 23;18(2):307–311. doi: 10.1021/bi00569a012. [DOI] [PubMed] [Google Scholar]
- Maul G. G., Avdalović N. Nuclear envelope proteins from Spisula solidissima germinal vesicles. Exp Cell Res. 1980 Nov;130(1):229–240. doi: 10.1016/0014-4827(80)90059-2. [DOI] [PubMed] [Google Scholar]
- Maul G. G. Determination of newly synthesized and phosphorylated nuclear proteins in mass-isolated germinal vesicle of Spisula solidissima. Exp Cell Res. 1980 Oct;129(2):431–440. doi: 10.1016/0014-4827(80)90512-1. [DOI] [PubMed] [Google Scholar]
- Maul G. G. The nuclear and the cytoplasmic pore complex: structure, dynamics, distribution, and evolution. Int Rev Cytol Suppl. 1977;(6):75–186. [PubMed] [Google Scholar]
- McDonald J. R., Agutter P. S. The relationship between polyribonucleotide binding and the phosphorylation and dephosphorylation of nuclear envelope protein. FEBS Lett. 1980 Jul 28;116(2):145–148. doi: 10.1016/0014-5793(80)80629-6. [DOI] [PubMed] [Google Scholar]
- Mills K. I., Bell L. G. Protein migration from transplanted nuclei in Amoeba proteus. II. An electrophoretic study. Exp Cell Res. 1981 Dec;136(2):469–473. doi: 10.1016/0014-4827(81)90029-x. [DOI] [PubMed] [Google Scholar]
- Paine P. L., Moore L. C., Horowitz S. B. Nuclear envelope permeability. Nature. 1975 Mar 13;254(5496):109–114. doi: 10.1038/254109a0. [DOI] [PubMed] [Google Scholar]
- Schumm D. E., Webb T. E. Modified messenger ribonucleic acid release from isolated hepatic nuclei after inhibition of polyadenylate formation. Biochem J. 1974 Apr;139(1):191–196. doi: 10.1042/bj1390191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz A., Allen J. C., Harigaya S. Possible involvement of cardiac Na+, K+-adenosine triphosphatase in the mechanism of action of cardiac glycosides. J Pharmacol Exp Ther. 1969 Jul;168(1):31–41. [PubMed] [Google Scholar]
- Shelton K. R., Guthrie V. H., Cochran D. L. Oligomeric structure of the major nuclear envelope protein lamin B. J Biol Chem. 1982 Apr 25;257(8):4328–4332. [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stuart S. E., Clawson G. A., Rottman F. M., Patterson R. J. RNA transport in isolated myeloma nuclei. Transport from membrane-denuded nuclei. J Cell Biol. 1977 Jan;72(1):57–66. doi: 10.1083/jcb.72.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]