Abstract
Objectives
To quantify the prevalence of frailty in adult patients of all ages undergoing chronic hemodialysis, its relationship to comorbidity and disability, and its association with adverse outcomes of mortality and hospitalization.
Design
Prospective cohort study.
Setting
Single hemodialysis center in Baltimore, Maryland.
Participants
146 prevalent hemodialysis patients enrolled between January 2009 and March 2010 and followed through August 2012.
Measurements
Frailty, comorbidity, and disability on enrollment into the study and subsequent mortality and hospitalizations.
Results
At enrollment, 50.0% of older (age≥65) and 35.4% of younger (age<65) hemodialysis patients were frail; 35.9% and 29.3% were intermediately frail, respectively. The 3-year mortality was 16.2% for non frail, 34.4% for intermediately frail, and 40.2% for frail participants. Intermediate frailty and frailty were associated with a 2.68-fold (95% CI: 1.02-7.07, P=0.046) and 2.60-fold (95%CI: 1.04-6.49, P=0.041) higher risk of death independent of age, sex, comorbidity, and disability. In the year after enrollment, median number of hospitalizations was 1 (IQR 0-3). The proportion with 2 or more hospitalizations was 28.2% for non frail, 25.5% for intermediately frail, and 42.6% for frail participants. While intermediate frailty was not associated with the number of hospitalizations (RR=0.76, 95%CI:0.49-1.16, P=0.21), frailty was associated with a 1.43-fold (95%CI:1.00-2.03, P=0.049) higher number of hospitalizations independent of age, sex, comorbidity, and disability. The association of frailty with mortality and hospitalizations did not differ between older and younger participants (Interaction P=0.64 and P=0.14, respectively).
Conclusions
Adults of all ages undergoing hemodialysis have a very high prevalence of frailty, more than 5-fold higher than community dwelling older adults. In this population, regardless of age, frailtyis a strong, independent predictor of mortality and number of hospitalizations.
Keywords: Frailty, hemodialysis, mortality, hospitalization
INTRODUCTION
Frailty is a state of decreased physiological reserve and multi-system dysregulation associated with increased vulnerability to stressors.1Frailty was described, and has been predominantly studied, in older adults, where it has been classified as a clinical phenotype and risk predictor independent of comorbidity and disability. In community dwelling older adults, the prevalence of frailty is 7%.1 Frail older adults, regardless of their comorbidity and disability status, are at twice the risk of mortality and hospitalization,1-5as well as other adverse outcomes including falls, decreased mobility, physical limitations, respiratory impairment, and cognitive decline.1,6-9
There are over 500,000 patients with end-stage renal disease (ESRD) undergoing hemodialysis, of whom over half are over the age of 65; this population is at high risk of mortality as well as hospitalization, an important measure of morbidity that leads to poor outcomes and results in one-third of total ESRD expenditures.10 Accurate risk prediction in ESRD could lead to better patient education, resource allocation, and targeted interventions. However, there is currently limited ability to predict hospitalization and mortality in this population.11-14While comorbidity and disability are associated with mortality and hospitalization in ESRD,15-18 the high prevalence of these risk factors limits their ability to inform risk prediction.19-24Metrics of aging, like frailty, may be better suited for prediction of mortality and hospitalization than traditional risk factors, because these patients are thought to experience a physiologic decline similar to that seen in aging. Similar associations have been described in HIV,25,26 a chronic condition that, like ESRD, is also characterized by physiologic changes resembling aging.27-31
For these reasons, we hypothesized that frailty, as described and validated in older adults, might be applicable to ESRD patients of all ages. The primary goals of this study were to quantify the prevalence of frailty and explore the relationship of frailty as a domain independent of comorbidity and disability in a prospective cohort of patients undergoing chronic hemodialysis.
METHODS
Study Design
This was a prospective study of prevalent hemodialysis patients from a single dialysis center in Baltimore, Maryland, recruited between January 2009 and March 2010. The Johns Hopkins Institutional Review Board approved the study. At enrollment, a trained research assistant collected medical information including date of birth, gender, race, education, tobacco use, time on hemodialysis, and comorbidities from participant interviews and medical record review. Frailty and disability were also directly measured at this time as described below.
Frailty
The main exposure of interest was frailty as defined and validated by Fried et al.1,32-41 At enrollment, the 5 components of this frailty scale were measured: shrinking (self-report of unintentional weight loss of more than 10 lbs in the past year based on dry weight); weakness (grip-strength below an established cutoff based on gender and BMI);1 exhaustion (self-report); low activity (Kcals/week below an established cutoff);1 and slowed walking speed (walking time of 15 feet below an established cutoff by gender and height).1 A score of 1 was given to those with the presence of each measured component. The aggregate frailty score was calculated as the sum of the component scores (range 0-5) and empirically categorized as nonfrail (0-1), intermediately frail (2), and frail (3-5). This categorization maintained Fried’s definition of frailty, but expanded the definition of nonfrail to include a score of 1; this empiric decision was important because very few (7%) participants had a frailty score of 0.
Comorbidity
Comorbidity was defined as 4 or more of the conditions considered in Fried’s landmark frailty study:1 peripheral vascular disease (PVD), rheumatoid arthritis (RA), cancer, hypertension, chronic obstructive pulmonary disease (COPD), diabetes, congestive heart failure (CHF), angina, and myocardial infarction (MI). All comorbidities were abstracted from the medical charts.
Disability
Activities of daily living (ADL)were measured at enrollment as previously published.42 Participants reported the need for assistance with each of the 6ADL domains at enrollment (feeding, dressing, ambulation, grooming, using a toilet, and bathing). Disability was defined as the inability to perform at least 2 of the domains without assistance.
Outcomes
Participants were followed until August 31, 2012. Vital status and date of death (where applicable) were obtained from the dialysis center; deaths were augmented by linkage to the National Death Index. Hospitalizations in the year after enrollment were ascertained from the dialysis center as well as medical record review.
Statistical Analysis
Cox proportional hazards models were used to estimate the association between frailty and mortality. Participants were censored at kidney transplantation or end of study. Proportional hazards assumptions were confirmed by visual inspection of the complimentary log-log plots. Poisson regression models were used to estimate the association between frailty and the number of hospitalizations in 1 year. The final models for mortality and number of hospitalization were adjusted for age, sex, comorbidity, and disability. Weighted survival analysis was performed to weight each participant in our regression model by their relative representation in the US dialysis population as a whole. All analyses were performed using STATA 12.1/SE (College Station, Texas).
RESULTS
Study Population
Among 146 study participants, the mean age was 60.6 years ± 13.6, 43.8% were older adults (age≥65), 46.6% were female, and 84.3% were African American. The median follow-up time was 3.0 years (IQR: 2.4-3.1 and range: 0.2-3.2 years). Comorbidity (more than 4 conditions specified in the methods) and disability were observed in 28.8% and 19.2% of participants.
Frailty
At enrollment, 26.0% were nonfrail, 32.2% were intermediately frail, and 41.8% were frail (Figure 1). Participants who were frail and those who were intermediately frail were more likely to be older (62.9 and 62.1 vs. 55.1 years, P=0.01) compared to nonfrail participants (Table 1). However, both intermediate frailty and frailty were present in a high proportion of younger (29.3% and 35.4% in those under 65) as well as older (35.9% and 50.0% in those 65 or older) participants. Consistent with other populations, there was overlap between comorbidity, disability, and frailty (Figure 2): 20.5% were frail without comorbidity or disability, 10.3% were frail withcomorbidity but no disability, and 5.5% were frail with disability but no comorbidity.
Figure 1. Distribution of Frailty Score in Study Population.
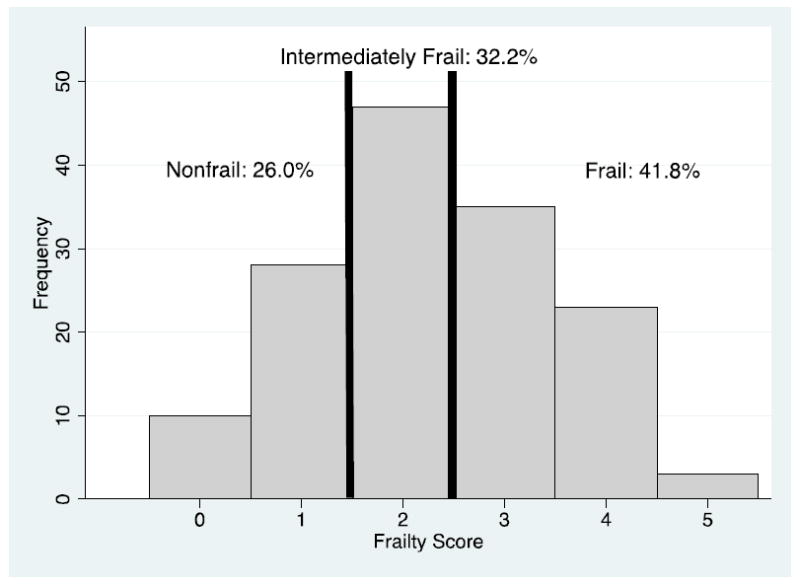
The percentage of nonfrail, intermediately frail, and frail participants are listed above the cutoffs.
Table 1.
Characteristics of Study Population, by Frailty
| Nonfrail (n=38) | Intermediately Frail (n=47) | Frail (n=61) | P value | |
|---|---|---|---|---|
|
| ||||
| Female sex | 42.1 | 40.4 | 54.1 | 0.30 |
| Age | 55.1 ± 13.4 | 62.1 ± 13.7 | 62.9 ± 12.9 | 0.01 |
| Black race | 89.5 | 85.1 | 80.3 | 0.50 |
| Enrollment body mass index (kg/m2) | 28.9 ±8.8 | 28.4 ± 7.8 | 28.7 ± 7.8 | 0.97 |
| Pre-dialysis body mass index (kg/m2) | 28.6 ± 8.9 | 29.3 ± 8.6 | 31.6±9.6 | 0.23 |
| Tobacco use (history of smoking) | 15.8 | 19.2 | 26.2 | 0.49 |
| Time on hemodialysis (years)* | 4.5 [4.4] | 3.7 [4.4] | 3.3 [5.3] | 0.78 |
| High school or higher education Comorbidities | 79.0 | 85.1 | 80.3 | 0.74 |
| Peripheral vascular disease | 10.5 | 29.8 | 42.6 | 0.002 |
| Rheumatoid arthritis | 5.3 | 6.4 | 8.2 | 0.92 |
| History of cancer | 15.8 | 23.4 | 16.4 | 0.59 |
| Hypertension | 94.7 | 85.1 | 88.5 | 0.37 |
| Chronic obstructive pulmonary disease | 7.9 | 23.4 | 23.0 | 0.12 |
| Diabetes | 44.7 | 70.2 | 75.4 | 0.01 |
| Congestive heart failure | 47.4 | 34.0 | 37.7 | 0.45 |
| Angina | 7.9 | 4.3 | 3.3 | 0.55 |
| Myocardial infarction | 18.4 | 10.6 | 19.7 | 0.44 |
| Number of comorbidities | 2.4 ±1.3 | 2.8 ± 1.5 | 3.0 ±1.5 | 0.13 |
| Comorbidity** | 15.8 | 29.8 | 36.1 | 0.09 |
| Disability** | 15.8 | 12.8 | 26.2 | 0.19 |
Mean ±standard deviations are reported for continuous variables.
Median and interquartile range provided.
As defined by Fried et al;1 see methods.
Figure 2. Overlap of Frailty, Comorbidity, and Disability.
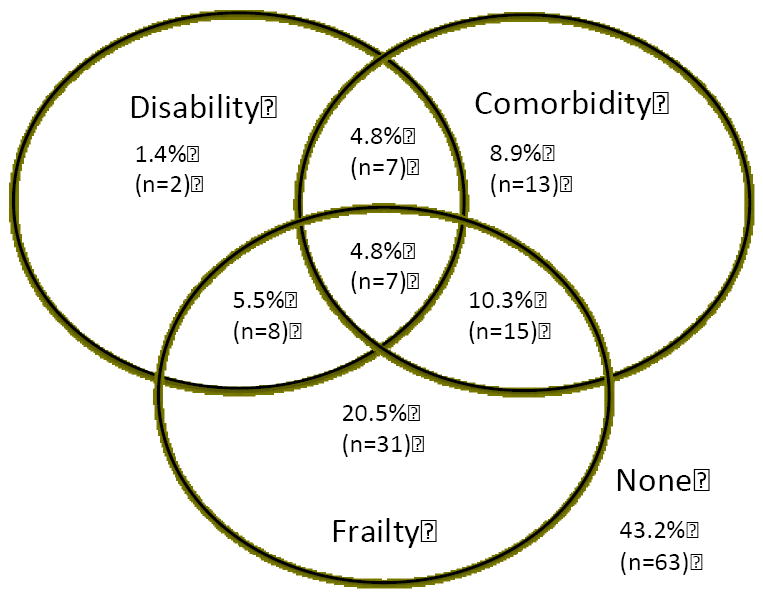
Frailty is defined as 3 or more components as defined by Fried. Disability is defined as the need for assistance in 2 or more activities of daily living categories. Comorbidity is defined as 4 or more conditions as specified in the methods. The total represents 166 study participants on hemodialysis. The n of each subgroup indicated in parentheses.
Mortality
The mortality rate was58 per 1000 person-years for those who were nonfrail, 154 per 1000 person-years for those who were intermediately frail (P=0.03), and 163 per 1000 person-years for those who were frail (P=0.02). Similarly, 3-year mortality was 16.2% for nonfrail, 34.4% for intermediately frail (p=0.03), and 40.2% for frail (p=0.02) (Figure 3). Adjusting for age, sex, comorbidity, and disability, intermediate frailty was independently associated with a 2.68-fold (95% CI: 1.02-7.07, P=0.046) higher risk of death and frailty was associated with a 2.60-fold (95% CI: 1.04-6.49, P=0.041) higher risk of death(Table 2); this relationship was not modified by age (interaction between age and frailty: HR=1.07, 95% CI: 0.61-1.88, P=0.64). The association did not differ after adjusting for time on dialysis (Intermediately Frail: HR=2.65, 95% CI: 1.05-6.67; Frail: HR=2.87, 95% CI: 1.17-7.03) or access type (Intermediately Frail: HR=2.67, 95% CI: 1.06-6.73; Frail: HR=2.78, 95% CI: 1.12-6.88). Furthermore, the association was similar after reweighting the cohort to represent the national dialysis population (Intermediately Frail: HR=2.99, 95% CI: 1.04-8.57; Frail: HR=2.75, 95% CI: 1.04-7.27).
Figure 3. Estimated Cumulative Incidence of Mortality, by Frailty.
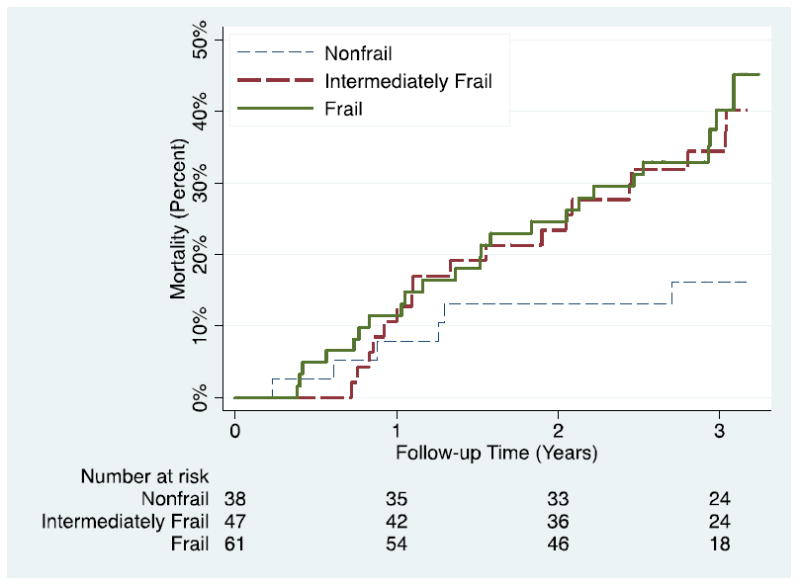
Kaplan-Meier method was used to estimate curves; the log-rank test was statistically significant (P=0.047).
Table 2.
Mortality and Hospitalization, by Frailty
| Nonfrail | Intermediately Frail | Frail | |
|---|---|---|---|
|
| |||
| Hazard ratio of Mortality | |||
| Unadjusted | Reference | 2.67 (1.06, 6.73) | 2.90 (1.18, 7.11) |
| Adjusted for age, sex, comorbidity, and disability | Reference | 2.68 (1.02, 7.07) | 2.60 (1.04, 6.49) |
| Incident Rate Ratio of Hospitalization | |||
| Unadjusted | Reference | 0.74 (0.49, 1.12) | 1.48 (1.05, 2.07) |
| Adjusted for age, sex, comorbidity, and disability | Reference | 0.76 (0.49, 1.16) | 1.43 (1.00, 2.03) |
Hospitalization
In the year after enrollment, 51.7% of participants had 1 or more hospitalizations, 33.3% had 2 or more hospitalizations, and median (IQR) number of hospitalizations was 1 (0-3); the maximum number of hospitalizations was 9. The proportion with 1 or more hospitalizations was 46.2% for nonfrail, 44.7% for intermediately frail, and 60.7% for frail patients; similarly, proportion with 2 or more hospitalizations was 28.2% for non frail, 25.5% for intermediately frail, and 42.6% for frail participants (p=0.20 for intermediately frail and p=0.02 for frail). Adjusting for age, sex, comorbidity, and disability, intermediate frailty was not associated with number of hospitalizations (RR=0.76, 95% CI: 0.49-1.16, P=0.21) but frailty was associated with a 1.43-fold (95% CI: 1.00-2.03, P=0.049) higher number of hospitalizations (Table 2);this relationship was not modified by age (interaction between age and frailty: RR=0.81, 95% CI: 0.62-1.07, P=0.14). The association did not differ after adjusting for time on dialysis (Intermediately Frail: RR=0.74, 95% CI: 0.49-1.11; Frail: RR=1.47, 95% CI: 1.05-2.06) or access type (Intermediately Frail: RR=0.74, 95% CI: 0.49, 1.12; Frail: RR=1.44, 95% CI: 1.02, 2.03).
DISCUSSION
In this prospective study of adults undergoing hemodialysis, frailty had a prevalence of 41.8% and was associated with a 2.60-foldhigher risk of mortality (95% CI: 1.04-6.49, P=0.041) and 1.43-fold higher number of hospitalizations (95% CI: 1.00-2.03, P=0.049), independent of age, sex, comorbidity, and disability. This study proposes a standardized measure of frailty that can be applied to more than 500,000 ESRD patients, extending a construct from gerontology to a population of adults of all ages suffering from the physiologic decline resulting from a chronic condition. Additionally, the findings support the hypothesis that frailty is a domain independent of comorbidity and disability, even in this novel population.
To our knowledge, this is the first study that can directly compare frailty measured in ESRD patients with the same measure of frailty in other populations. Interestingly, the prevalence of frailty among ESRD patients in our study was much (five to seven-fold) higher than in community dwelling older adults.1Compared with frailty prevalence of 7% in adults aged 65 or older in the Cardiovascular Health Study, 50.0% of our study participants aged 65 or older, and also 35.4% of our study participants under 65, were frail.
Our findings are consistent with subjective approximations of physiologic decline in ESRD patients as captured in registry data based on self-reported limitation in activities, self-reported fatigue, and administratively coded cachexia.43,44 In the United States Renal Data System (USRDS) Wave 2, mortality risk for hemodialysis initiates was a 2.24-fold (95% CI 1.60-3.15) higher risk in those with physiologic decline approximated using the registry data.44Additionally, physiologic decline was associated with a 1.26-fold (95% CI 1.09-1.45) higher risk of hospitalization in the USRDS Comprehensive Dialysis Study (CDS) using a similar approximation.43 However, these studies were limited by their retrospective study design, use of data not designed for studying physiologic decline, limited ability to adjust for comorbidity, inability to adjust for disability, and the absence of objective measures of frailty including weakness, weight loss, and slowed walking speed.
Strengths of this study include prospective measurement of a validated, objective construct of frailty, granular ascertainment of comorbidities using medical records abstraction, and use of a validated measure of disability. The main limitation was the prevalent sampling strategy; in other words, presence or absence of frailty was not established prior to hemodialysis initiation. As such, survivor bias limits inferences from our finding that frailty was not associated with time on hemodialysis. Our study also has the limitations inherent in a single-center study of 146 participants, both in terms of generalizability and statistical power to detect subtle subgroup effects.
This study is the first evidence of the frailty phenotype as defined by Fried et al in patients undergoing hemodialysis and thus, there may be concerns that not all components of frailty were contributing to the mortality effect. However, we performed analyses to assess whether frailty was truly a syndrome in adults undergoing hemodialysis. We observed that 1) the variance inflation factor was approximately 1 for all components, suggesting that there is no co linearity between the components, and 2) Akaike’s Information Criterion of the full frailty phenotype (a measure of goodness-of-fit) was better than the individual components. In adults undergoing hemodialysis, frailty is a syndrome that is consistent with the phenotype Fried documented in older adults.
In this first study of a validated construct of frailty prospectively measured in ESRD patients, frailty was highly prevalent and associated with mortality and hospitalization, independent of comorbidity and disability, in adults of all ages. This finding has important implications for researchers, patients and providers in the ESRD field, potentially improving counseling, clinical decision-making, and even targeting at-risk individuals for targeted interventions. Furthermore, our findings illustrate that metrics derived from gerontology can be both prevalent and predictive of adverse outcomes in non-geriatric patients with chronic conditions such as ESRD, regardless of their age, comorbidity, or disability status.
Acknowledgments
We thank the study participants, as well as the staff at the Renal Dialysis Program at Good Samaritan, including Colleen Reft, for their dedication to this study.
This study was supported by a Clinical Scientist Development Award from the Doris Duke Charitable Foundation (Segev, PI). Megan Salter was supported by T32AG000247 from the National Institute on Aging.
Sponsor’s Role: The sponsor had no role in the design, methods, subject recruitment, data collection, analysis or preparation of paper.
Footnotes
Author Contributions: All authors contributed: 1) to the conception and design or acquisition of data or analysis and interpretation of the data; 2) drafting the article or revising it critically for important intellectual content; and 3) final approval of the version to be published.
No authors have a conflict of interest to report.
References
- 1.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: Evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 2.Diaz de Leon Gonzalez E, Tamez Perez HE, Gutierrez Hermosillo H, et al. Frailty and its association with mortality, hospitalization and functional dependence in Mexicans aged 60-years or older. Medicina Clinica. 2012;138:468–474. doi: 10.1016/j.medcli.2011.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Puts MT, Monette J, Girre V, et al. Does frailty predict hospitalization, emergency department visits, and visits to the general practitioner in older newly-diagnosed cancer patients? Results of a prospective pilot study. Crit Rev Oncol/Hematol. 2010;76:142–151. doi: 10.1016/j.critrevonc.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 4.Bouillon K, Sabia S, Jokela M, et al. Validating a widely used measure of frailty: Are all sub-components necessary? Evidence from the Whitehall II cohort study. Age (Disord) 2012 Jul 8; doi: 10.1007/s11357-012-9446-2. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Avila-Funes JA, Helmer C, Amieva H, et al. Frailty among community-dwelling elderly people in France: The three-city study. J Gerontol A Biol Sci Med Sci. 2008;63:1089–1096. doi: 10.1093/gerona/63.10.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boyd CM, Xue QL, Simpson CF, et al. Frailty, hospitalization, and progression of disability in a cohort of disabled older women. Am J Med. 2005;118:1225–1231. doi: 10.1016/j.amjmed.2005.01.062. [DOI] [PubMed] [Google Scholar]
- 7.Mitnitski A, Fallah N, Rockwood MR, et al. Transitions in cognitive status in relation to frailty in older adults: A comparison of three frailty measures. J Nutr Health Aging. 2011;15:863–867. doi: 10.1007/s12603-011-0066-9. [DOI] [PubMed] [Google Scholar]
- 8.Vaz Fragoso CA, Enright PL, McAvay G, et al. Frailty and respiratory impairment in older persons. Am J Med. 2012;125:79–86. doi: 10.1016/j.amjmed.2011.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Woo J, Leung J, Morley JE. Comparison of frailty indicators based on clinical phenotype and the multiple deficit approach in predicting mortality and physical limitation. J Am Geriatr Soc. 2012;60:1478–1486. doi: 10.1111/j.1532-5415.2012.04074.x. [DOI] [PubMed] [Google Scholar]
- 10.United States Renal Data System, 2011 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. Bethesda: National Institute of Diabetes and Digestive and Kidney Diseases; 2011. [DOI] [PubMed] [Google Scholar]
- 11.Bazeley J, Bieber B, Li Y, et al. C-reactive protein and prediction of 1-year mortality in prevalent hemodialysis patients. Clin J Am Soc Nephrol. 2011;6:2452–2461. doi: 10.2215/CJN.00710111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Quinn RR, Laupacis A, Hux JE, et al. Predicting the risk of 1-year mortality in incident dialysis patients: Accounting for case-mix severity in studies using administrative data. Med Care. 2011;49:257–266. doi: 10.1097/MLR.0b013e318202aa0b. [DOI] [PubMed] [Google Scholar]
- 13.Wagner M, Ansell D, Kent DM, et al. Predicting mortality in incident dialysis patients: An analysis of the United Kingdom Renal Registry. Am J Kidney Dis. 2011;57:894–902. doi: 10.1053/j.ajkd.2010.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miskulin D, Bragg-Gresham J, Gillespie BW, et al. Key comorbid conditions that are predictive of survival among hemodialysis patients. Clin J Am Soc Nephrol. 2009;4:1818–1826. doi: 10.2215/CJN.00640109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pan Y, Xu XD, Guo LL, et al. Association of early versus late initiation of dialysis with mortality: systematic review and meta-analysis. Nephron Clin Pract. 2012;120:c121–131. doi: 10.1159/000337572. [DOI] [PubMed] [Google Scholar]
- 16.Chang TI, Paik J, Greene T, et al. Updated comorbidity assessments and outcomes in prevalent hemodialysis patients. Hemodial Int. 2010;14:478–485. doi: 10.1111/j.1542-4758.2010.00468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vaiciuniene R, Kuzminskis V, Ziginskiene E, et al. Adherence to treatment and hospitalization risk in hemodialysis patients. J Nephrol. 2012;25:672–678. doi: 10.5301/jn.5000038. [DOI] [PubMed] [Google Scholar]
- 18.McAdams-Demarco MA, Law A, Garonzik-Wang JM, et al. Activity of daily living disability and dialysis mortality: Better prediction using metrics of aging. J Am Geriatr Soc. 2012;60:1981–1982. doi: 10.1111/j.1532-5415.2012.04161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roberts TL, Foley RN, Weinhandl ED, et al. Anaemia and mortality in haemodialysis patients: Interaction of propensity score for predicted anaemia and actual haemoglobin levels. Nephrol Dial Transplant. 2006;21:1652–1662. doi: 10.1093/ndt/gfk095. [DOI] [PubMed] [Google Scholar]
- 20.Carrero JJ, de Mutsert R, Axelsson J, et al. Sex differences in the impact of diabetes on mortality in chronic dialysis patients. Nephrol Dial Transplant. 2011;26:270–276. doi: 10.1093/ndt/gfq386. [DOI] [PubMed] [Google Scholar]
- 21.Kalantar-Zadeh K, Block G, Humphreys MH, et al. Reverse epidemiology of cardiovascular risk factors in maintenance dialysis patients. Kid Int. 2003;63:793–808. doi: 10.1046/j.1523-1755.2003.00803.x. [DOI] [PubMed] [Google Scholar]
- 22.Port FK, Hulbert-Shearon TE, Wolfe RA, et al. Predialysis blood pressure and mortality risk in a national sample of maintenance hemodialysis patients. Am J Kid Dis. 1999;33:507–517. doi: 10.1016/s0272-6386(99)70188-5. [DOI] [PubMed] [Google Scholar]
- 23.Longenecker JC, Coresh J, Powe NR, et al. Traditional cardiovascular disease risk factors in dialysis patients compared with the general population: The CHOICE Study. J Am Soc Nephrol. 2002;13:1918–1927. doi: 10.1097/01.asn.0000019641.41496.1e. [DOI] [PubMed] [Google Scholar]
- 24.Beto JA, Bansal VK, Gohlke NP, et al. Using the hemodialysis prognostic nutrition index and urea reduction ratio to predict morbidity and mortality: A pilot study of the 1995 council on renal nutrition national research question. J Renal Nutr. 1998;8:21–24. doi: 10.1016/s1051-2276(98)90033-4. [DOI] [PubMed] [Google Scholar]
- 25.Desquilbet L, Jacobson LP, Fried LP, et al. A frailty-related phenotype before HAART initiation as an independent risk factor for AIDS or death after HAART among HIV-infected men. J Gerontol A Biol Sci Med Sci. 2011;66:1030–1038. doi: 10.1093/gerona/glr097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Desquilbet L, Margolick JB, Fried LP, et al. Relationship between a frailty-related phenotype and progressive deterioration of the immune system in HIV-infected men. J Acquir Immune Defic Syndr. 2009;50:299–306. doi: 10.1097/QAI.0b013e3181945eb0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bjorntorp P. Neuroendocrine ageing. J Intern Med. 1995;238:401–404. doi: 10.1111/j.1365-2796.1995.tb01217.x. [DOI] [PubMed] [Google Scholar]
- 28.Bhavan KP, Kampalath VN, Overton ET. The aging of the HIV epidemic. Curr HIV/AIDS Rep. 2008;5:150–158. doi: 10.1007/s11904-008-0023-3. [DOI] [PubMed] [Google Scholar]
- 29.Martin J, Volberding P. HIV and premature aging: A field still in its infancy. Ann Intern Med. 2010;153:477–479. doi: 10.7326/0003-4819-153-7-201010050-00013. [DOI] [PubMed] [Google Scholar]
- 30.Effros RB, Fletcher CV, Gebo K, et al. Aging and infectious diseases: Workshop on HIV infection and aging: What is known and future research directions. Clin Infect Dis. 2008;47:542–553. doi: 10.1086/590150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Deeks SG, Phillips AN. HIV infection, antiretroviral treatment, ageing, and non-AIDS related morbidity. BMJ. 2009;338:a3172. doi: 10.1136/bmj.a3172. [DOI] [PubMed] [Google Scholar]
- 32.Bandeen-Roche K, Xue QL, Ferrucci L, et al. Phenotype of frailty: Characterization in the women’s health and aging studies. J Gerontol A Biol Sci Med Sci. 2006;61:262–266. doi: 10.1093/gerona/61.3.262. [DOI] [PubMed] [Google Scholar]
- 33.Barzilay JI, Blaum C, Moore T, et al. Insulin resistance and inflammation as precursors of frailty: The Cardiovascular Health Study. Arch Intern Med. 2007;167:635–6341. doi: 10.1001/archinte.167.7.635. [DOI] [PubMed] [Google Scholar]
- 34.Cappola AR, Xue QL, Fried LP. Multiple hormonal deficiencies in anabolic hormones are found in frail older women: The Women’s Health and Aging studies. J Gerontol A Biol Sci Med Sci. 2009;64:243–248. doi: 10.1093/gerona/gln026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leng SX, Hung W, Cappola AR, et al. White blood cell counts, insulin-like growth factor-1 levels, and frailty in community-dwelling older women. J Gerontol A Biol Sci Med Sci. 2009;64:499–502. doi: 10.1093/gerona/gln047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leng SX, Xue QL, Tian J, et al. Inflammation and frailty in older women. J Am Geriatr Soc. 2007;55:864–871. doi: 10.1111/j.1532-5415.2007.01186.x. [DOI] [PubMed] [Google Scholar]
- 37.Newman AB, Gottdiener JS, McBurnie MA, et al. Associations of subclinical cardiovascular disease with frailty. J Gerontol A Biol Sci Med Sci. 2001;56:M158–166. doi: 10.1093/gerona/56.3.m158. [DOI] [PubMed] [Google Scholar]
- 38.Walston J, McBurnie MA, Newman A, et al. Frailty and activation of the inflammation and coagulation systems with and without clinical comorbidities: Results from the Cardiovascular Health Study. Arch Intern Med. 2002;162:2333–2341. doi: 10.1001/archinte.162.20.2333. [DOI] [PubMed] [Google Scholar]
- 39.Xue QL, Bandeen-Roche K, Varadhan R, et al. Initial manifestations of frailty criteria and the development of frailty phenotype in the Women’s Health and Aging Study II. J Gerontol A Biol Sci Med Sci. 2008;63:984–990. doi: 10.1093/gerona/63.9.984. [DOI] [PubMed] [Google Scholar]
- 40.Chang SS, Weiss CO, Xue QL, et al. Association between inflammatory-related disease burden and frailty: Results from the Women’s Health and Aging Studies (WHAS) I and II. Arch Gerontol Geriatr. 2012;54:9–15. doi: 10.1016/j.archger.2011.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chang SS, Weiss CO, Xue QL, et al. Patterns of comorbid inflammatory diseases in frail older women: The Women’s Health and Aging Studies I and II. J Gerontol A Biol Sci Med Sci. 2010;65:407–413. doi: 10.1093/gerona/glp181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Katz S, Akpom CA. A measure of primary sociobiological functions. Int J Health Serv. 1976;6:493–508. doi: 10.2190/UURL-2RYU-WRYD-EY3K. [DOI] [PubMed] [Google Scholar]
- 43.Bao Y, Dalrymple L, Chertow GM, et al. Frailty, dialysis initiation, and mortality in end-stage renal disease. Arch Intern Med. 2012;172:1071–1077. doi: 10.1001/archinternmed.2012.3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Johansen KL, Chertow GM, Jin C, et al. Significance of frailty among dialysis patients. J Am Soc Nephrol. 2007;18:2960–2967. doi: 10.1681/ASN.2007020221. [DOI] [PubMed] [Google Scholar]


