Abstract
Objective
To examine medication safety in two ICUs and to assess the complexity of medication errors and adverse drug events (ADEs) in ICUs across the stages of the medication-management process.
Methods
Four trained nurse data collectors gathered data on medication errors and ADEs between October 2006 and March 2007. Patient care documents (e.g., medication order sheets, notes) and incident reports were used to identify medication errors and ADEs in a 24-bed adult medical/surgical ICU and an 18-bed cardiac ICU in a tertiary care, community teaching hospital. In this cross-sectional study, a total of 630 consecutive ICU patient admissions were assessed to produce data on the number, rates and types of potential and preventable ADEs across stages of the medication-management process.
Results
An average of 2.9 preventable or potential ADEs occurred in each admission, i.e., 0.4 events per patient-day. Preventable or potential ADEs occurred in 2.6% of the medication orders. The rate of potential ADEs per 1,000 patient-days was 276, whereas the rate of preventable ADEs per 1,000 patient-days was 9.2. Most medication errors occur at the ordering (32%) and administration stages (39%). In 16–24% of potential and preventable ADEs, clusters of errors occurred either as sequence of errors (e.g., delay in medication dispensing leading to delay in medication administration) or grouped errors (e.g., route and frequency errors in the order for a medication). Many of the sequences led to administration errors that were caused by errors earlier in the medication-management process.
Conclusions
Understanding the complexity of the vulnerabilities of the medication-management process is important to devise solutions to improve patient safety. Electronic health record technology with computerized physician order entry may be one step necessary to improve medication safety in ICUs. Solutions that target multiple stages of the medication-management process are necessary to address sequential errors.
Keywords: medication safety, medication errors, adverse drug events, intensive care unit, human factors engineering
INTRODUCTION
Medication safety is a central problem in health care, and particularly in intensive care units (ICUs). Along with preventable nosocomial infections, medication errors were the most frequent patient safety events in a medical ICU and a coronary care unit; medication errors occurred in 13% of patient-days.1 This is likely related to illness severity and the sizeable number and type of medications used in the ICU.2,3 In ICUs, potential adverse drug events (ADEs) – medication errors that do not harm the patient but have the potential to– range from 3.38 to 116.8 potential ADEs per 1,000 patient-days1,4,5 and preventable ADEs – medication errors that lead to patient harm – range from 0.21 to 20.3 per 1000 patient-days.1,4,6–9 The wide variation in the rates of medications and ADEs most likely reflect the variety of methods used in these studies10,11 to define and detect medication errors.12–14 Most only documented one ADE per patient.2 The study contexts also differed substantially, such as technology and human resources, which contributed to the wide variation in the findings.
The complexity of the medication-management process is mirrored in the complexity of assessing medication errors and ADEs in ICUs. Prior research has neither accounted for nor considered this complexity in assessing medication safety in ICUs. Sub-processes in the medication-management process may occur in series or parallel,2,15,16 and errors at different stages of the process can interact and accumulate.16 Therefore, it is important to describe and examine clustering of medication errors.
According to human factors and systems engineering, errors in the medication-management process can be temporally connected.17 Errors at different stages of the process may not occur in isolation, but are related to each other. First, errors may occur as a group, which includes errors related to the same medication order that occur in the same stage of the process. For instance, an order for a medication may include many errors, such as missing information on the frequency and route. Second, errors frequently lead to additional errors, such as a delay in dispensing medications leading to delays in medication administration. In contrast to grouped errors, sequential errors typically span multiple stages of the medication-management process. These error sequences and grouped errors have not been examined in the literature and are a focus of this investigation. In addition to understanding the specific content and temporal connection of medication errors, we collected detailed data on the work system contextual factors; this allowed a more precise and specific categorization of error types.
This study examines medication safety in two ICUs and assesses the complexity of medication errors and ADEs in ICUs across the stages of the medication-management process. In particular, we specifically assess grouped and sequential errors. We build upon a combination of methods used by Bates and colleagues8 to collect and analyze medication safety event data, while adding to their methods through a human factors and systems engineering perspective17 on medication error. We specifically considered how errors propagated throughout the entire medication-management process.
METHODS
Study setting and participants
This research is part of a larger study evaluating the impact of an electronic health record (EHR) on quality and safety in ICUs (http://cqpi.engr.wisc.edu/cpoe_home). This cross-sectional study reports medication safety data prior to EHR implementation in two ICUs at a tertiary care, community teaching hospital in the Northeastern US. The Adult Intensive Care Unit (AICU) is a 24-bed unit specializing in critical care, trauma and non-cardiac post-surgical care. The average ICU length of stay is 7.1 days. All non-trauma patients are managed by the critical care (medical) teaching service with surgical consultation or co-management as warranted. The Cardiac Intensive Care Unit (CICU) is an 18-bed unit specializing in cardiothoracic surgery and cardiovascular care as well as solid-organ transplantation and pulmonary/critical care overflow. The average ICU length of stay is 3.6 days. In the CICU, the medical staff consists of cardiologists working with cardiology fellows and cardiothoracic and transplant surgeons working with physician assistants.
The study examines data from 630 consecutive ICU patient admissions: 304 AICU admissions between October 2006 and February 2007 and 326 CICU admissions between January and March 2007. Data were collected for each day the patient was in the ICU. In each unit, the data collection period ended one week after the final study patient was admitted to the ICU. We stopped reviewing patient records to identify medication errors and ADEs at the end of the data collection period. If a patient remained in the ICU after the end of data collection, we later reviewed his records to find out when he left the ICU. Six percent of patient admissions had multiple periods in the ICU during the same hospital admission.* Patients were excluded from the study if they were younger than 18 years old, prisoners, or spent less than 4 hours in an ICU. The sample size in each ICU was determined through power analyses to detect a 20% decrease in potential and preventable ADEs after EHR implementation, considering the medication order volume and average length of stay in each ICU. IRB approval was obtained from University of Wisconsin-Madison and the study site.
Description of the medication-management process
An overview of the medication management process can be found in a box on page 9. The typical ICU medication-management process began when physicians and physician assistants (ordering providers) wrote orders into a paper chart (ordering). Pre-printed order sets existed for admission orders, antibiotic orders and initial insulin and heparin drip orders; otherwise orders were written on generic order forms that created carbon copies. Verbal orders were given infrequently. Charts with newly written orders were flagged and placed in the unit desk clerk’s pending order rack. Ordering providers were expected to communicate directly with the nurse about orders that should be administered immediately (STAT orders). Nursing staff or the unit desk clerk would either give a carbon copy of the order to the ICU pharmacist on the unit (6 am – 3 pm) or fax a copy to the central pharmacy. Pharmacists reviewed all medication orders and performed therapeutic substitutions by protocol.* Other medication order changes required contacting the ordering provider. When a pharmacist clarified a medication order, an orange colored order form with the changed order was placed in the patient chart. Hospital policy required antibiotics orders to be renewed every five days and intravenous fluids and continuous-drip medications every three days.* Nurses left sticky notes on the chart to remind physicians about the need to renew an order for a medication.
Transcription began when the pharmacist entered medication orders into the pharmacy computer system (BDM RxTFC ®, BDM Information Systems, Ltd., Saskatoon, SK), which performed drug-allergy and drug-drug interaction checks, and checked for duplicate orders. Medication administration record (MAR) labels were printed in the central hospital pharmacy and delivered with the first dose of the medication. A nurse or unit desk clerk hand transcribed each medication order onto the MAR, either the scheduled medication MAR or one-time dose MAR, from the paper order sheet, entered an administration time in accordance with hospital standards and added the printed MAR label when available. Another nurse double-checked the MAR transcription for accuracy. Nightly at midnight, computer-generated MARs were printed for scheduled medications for each patient for the next day. Nurses would review the previous day’s MAR with the computer generated MAR, confirm the accuracy of all current orders, resolve any discrepancies (by contacting physicians or pharmacy, if needed), place the computer-generated MAR in a book stored in the medication room and place the previous day’s MAR in the patient chart.
Pharmacy technicians in the central pharmacy used the MAR labels to prepare and dispense the medication. Only a small number of medications required preparation; these were all prepared in the central pharmacy (preparation). ICU nurses do not prepare medications in this hospital. Pharmacy technicians prioritized the orders and dispensed STAT medications first (dispensing). Hospital policy was to have STAT medications delivered to the unit within one hour of ordering. Pharmacists double-checked each medication and MAR label; then both were delivered to the ICU via the tubing system or robotic dispenser. An automated dispensing machine (AccuDose-® ™, McKesson Corporation, San Francisco, CA) was used by ICU nurses to directly self-dispense a small number of medications. On arrival at the unit, pharmacy-dispensed medications were placed in the medication room by a pharmacist, nurse or unit desk clerk.
Administration began when the nurse reviewed the patient’s paper MAR, which was kept in the MAR book in the medication room. The nurse administered the medication to the patient, recorded the administration time in the MAR and initialed the record. Nurses tended to record administration times on the quarter-hour rather than exact times for routine medications. STAT orders were administered as soon as possible; routine orders were administered at the scheduled time. When a medication dose was not available, the nurse filled out a missing dose form and faxed it to the pharmacy or called the pharmacy when urgent. If a dose was held or omitted, the administration time was circled and a comment made next to it describing the reason. Respiratory therapists administered and documented inhaled medications.
Clinicians routinely monitored their patients for therapeutic and unintended effects of medication. For example, nurses monitored their patients after medication administration and informed providers of adverse effects related to medications. Pharmacists routinely monitored the pharmacokinetics of vancomycin, aminoglycosides and warfarin while participating in dose adjustments.
Data collection and identification of medication errors and ADEs
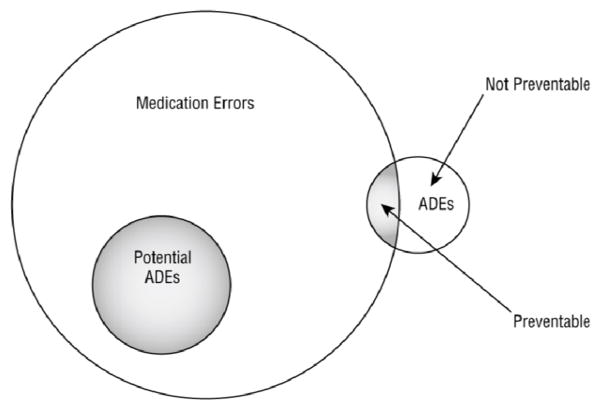
Data on medication safety events (i.e., medication errors and ADEs) were collected by four trained nurse data collectors (NDCs) using a protocol adapted from Bates and colleagues.18 The protocol involved review of each ICU medication order through stages of the medication-management process and identifying all errors and ADEs related to the order. The errors and ADEs for a specific order are a single case within the dataset, which we call an event. The outcome measures of interest were: (1) potential ADE events in which one or more medication errors occurred and the patient did not suffer harm but could have, (2) preventable ADE events in which one or more medication errors occurred and the patient suffered harm, and (3) non-preventable ADE events in which the patient suffered harm from medication use but no error occurred (see Figure 1).
Figure 1.
Relationship between medication errors, potential adverse drug events (ADEs), and ADEs (Kaushal et al., 2001)
The NDCs reviewed all paper medication order sheets, including pharmacist clarifications of orders that were compared to the transcribed medication orders on the MAR, and documented medication administrations. They also reviewed physician, nursing and respiratory care notes, the nursing flowsheet, procedure and code notes, other clinical documentation with medication or symptom information, and laboratory results. Clinicians were also invited to report adverse events to the NDCs in person or anonymously via paper report.
All ICU orders were reviewed for each day the patient was in the unit, beginning with the orders for admission or transfer to the ICU* and ending prior to the orders to transfer the patient out of the ICU. Orders written prior to admission or transfer were also reviewed if they were implemented in the ICU. Emergency Department, post-anesthesia, operating room and hospital discharge orders were excluded. Orders for intravenous fluids, albumin and hetastarch were considered to be medication orders; orders for other blood products, nitric oxide and inhaled helium were not included because they were not handled like medications per hospital policy. A method was developed for the NDCs to count medication orders, which considered medication order processing for the CPOE system to be implemented shortly after the conclusion of this study (available at http://cqpi.engr.wisc.edu/system/files/MedSafetyMedOrder.docx).
Specifically, the NDCs sought:
Incomplete orders that lacked the medication name, dose, rate, route, or frequency, and dose formulation or start/stop times when relevant. Continuous-infusion orders only needed to contain the medication’s name and the infusion rate.
Order modifications or pharmacy clarifications that could potentially indicate an error in the original order.
Duplicate orders.
Illegible orders. Illegibility was determined by the nurse data collector and verified by the research team.
Unapproved abbreviations.
Medications to which the patient had a documented allergy.
Late or omitted administration. Based on hospital practices, medications were considered late if a STAT dose was administered over one hour after the order time or a routine or scheduled dose was administered more than two hours after the order or scheduled time.
Orders for or use of antidotes that could indicate an ADE.
Patient symptoms and out-of-range laboratory values that could be associated with an error or ADE.19
References to medication errors or adverse drug events in physician, nurse, pharmacist or respiratory therapist notes.
Although hospital policy dictated that paper orders needed to be dated and signed, many did not list the time of the order. The absence of an order time was not considered to be an error unless it contributed to other medication errors with the order, such as a late administration. If the NDC identified a serious medication error or ADE that may have had serious consequences for the patient and had not been recognized by the patient care team, the NDC verbally reported it to the physician or nurse caring for the patient.
For each possible error, NDCs noted the following: stage of process where error started, at which stage(s) error was present, whether error reached the patient and any patient harm that occurred consequent to the medication error over the next seven days. After detecting a possible ADE, NDCs collected the following information: the event trigger (see list above), patient condition and extent of symptoms, any medications that the patient received that could account for the reaction including dates and doses, and any information about when and how the healthcare team recognized and addressed the error or ADE.
Similar to the protocol of Bates and colleagues, the NDCs prepared a case summary for any potential ADE or medication error; the case summaries were reviewed by a physician (TBW) and if necessary, additional information on the event was gathered. Events were then processed through double adjudication for error type and harm.
Data analysis
Two researchers (PC, TBW) developed a list of medication error types based on the literature (see Appendix).5,19–22 Researchers reviewed each event (medication order with one or more related errors or ADE) and determined the type(s) of medication error, the stage of medication-management at which the error occurred, and the error-recovery processes (if any). For events with more than one error, the errors were categorized as grouped when they occurred during the same medication-management stage or sequential when one error led to others. Whenever there was adjudication uncertainty, both researchers reviewed the data and mutually agreed upon the classification. To assess inter-rater reliability, researchers independently adjudicated a random sample of 146 events. All Cohen’s kappa scores were 0.97 or higher for whether an error occurred, categorizing medication error types, categorizing events into single, grouped or sequential error events and categorizing errors at the stage of the medication-management process.
Adverse drug events and potential ADEs were reviewed independently by two critical-care physicians (RK, SK) to determine (1) if the patient suffered harm due to a medication and (2) the severity of the harm or potential harm.18 The severity of harm was categorized23 as (1) fatal (for ADEs only), (2) life-threatening (e.g., readmission to the ICU, respiratory failure, anaphylaxis, or severe mental-status deterioration), (3) serious (e.g., intestinal bleeding, altered mental status, excessive sedation, acute kidney injury, symptomatic hypotension, allergic reaction less serious than anaphylaxis and more serious than a rash, or fever), and (4) significant (e.g., diarrhea, mild thrombocytopenia, nausea, vomiting or rash.) For grouped errors, the severity of harm related to all of the errors was adjudicated, while for sequential errors, the harm of the final error in the sequence was assessed. Inter-rater reliability (Cohen’s kappa) was 0.81 for ADE occurrence, 0.39 for the severity of ADE-associated harm and 0.34 for the severity of potential harm. Although low, these reliabilities are typical for these studies,2 justifying a third adjudicator for disagreements occurring between the first two adjudicators. A third critical-care physician (MJ) reviewed and resolved disagreements.
RESULTS
Table 1 describes the admissions, patients and medication orders included in this study. In the rest of the paper, data for AICU and CICU admissions are combined.
Table 1.
Description of patients and medication orders
| AICU | CICU | Total | |
|---|---|---|---|
| Number of admissions | 304 | 326 | 630 |
| Number of unique patients | 294 | 322 | 610 |
| Patient age in years: mean, ±SD | 59 ± 18 | 64 ± 13 | 61 ± 16 |
| Patient gender: % female | 44% | 43% | 43% |
| Patient ethnicity: % white | 94% | 96% | 95% |
| Days in the ICU: mean ±SD; range | 9 ± 9; 1–67 | 5 ± 4; 1–35 | 7 ± 7; 1–67 |
| Study period in days | 119 | 72 | 191 |
| Patient-days | 2643 | 1504 | 4147 |
| Medication orders | 27817 | 17841 | 45658 |
| Orders per patient-day: mean, ±SD | 11.4 ± 4.4 | 12.4 ± 5.0 | 11.9 ± 4.8 |
| Orders per admission: mean, ±SD | 91.5 ± 88.0 | 54.7 ± 46.8 | 72.5 ± 72.1 |
Medication errors and ADEs
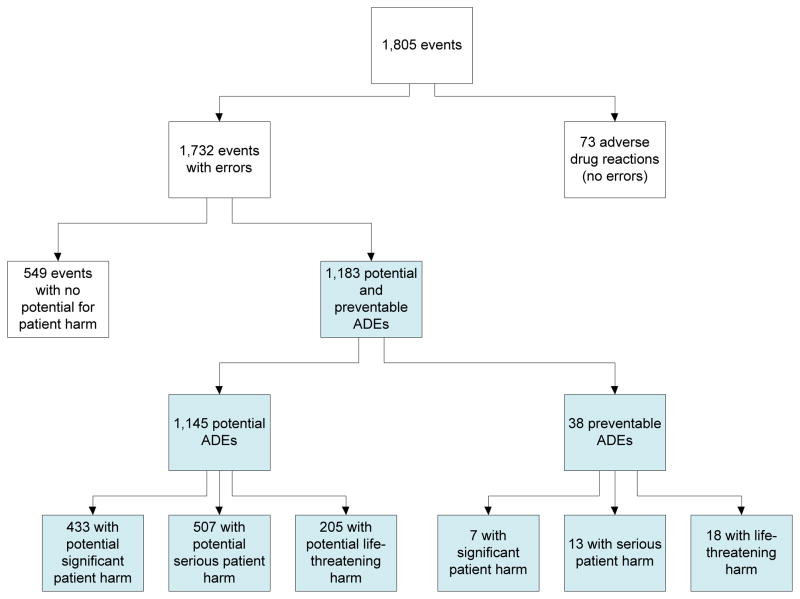
A total of 1,805 medication-related events were discovered, including 73 non-preventable ADEs (see Figure 2). Among the 1,732 events that involved medication errors, 38 (2.2%) were preventable ADEs. Thirty-two percent of the events involved medication errors with no potential harm. The remaining 1,145 (66%) events are potential ADEs (see Table 2), of which 433 could have produced significant harm (38%), 507 could have produced serious harm (44%) and 205 could have produced life-threatening harm (18%). Of the 38 preventable ADEs, 7 produced significant harm (18%), 13 produced serious harm (34%) and 18 produced life-threatening harm (47%). No medication safety events led to a patient death.
Figure 2.
Breakdown of medication safety events
Table 2.
Level of patient harm for medication error events and ADEs
| EVENTS | Level of potential or actual patient harm | ||||||
|---|---|---|---|---|---|---|---|
| No harm | Significant | Serious | Life threatening | Fatal | Total | ||
Potential ADEs (
 ) and medication errors with no potential for harm ) and medication errors with no potential for harm |
Single errors | 470 (33%) | 370 (26%) | 421 (29%) | 175 (12%) | - | 1436 (100%) |
| Group of errors | 15 (19%) | 18 (22%) | 40(50%) | 7 (9%) | - | 80 (100%) | |
| Sequential errors | 64 (36%) | 45 (25%) | 46(26%) | 23 (13%) | - | 178 (100%) | |
| Total | 549 (32%) | 433 (26%) | 507(30%) | 205 (12%) | - | 1694 (100%) | |
| ADEs | Preventable ADEs | - | 7 (18%) | 13(34%) | 18 (47%) | 0 (0%) | 38 (100%) |
| Non-preventable ADEs | - | 34 (46%) | 29(40%) | 10 (14%) | 0 (0%) | 73 (100%) | |
| Total | - | 41 (37%) | 42(38%) | 28 (25%) | 0 (0%) | 111 (100%) | |
| Total | 549 (30%) | 474 (26%) | 549(30%) | 233 (13%) | 0 (0%) | 1805 (100%) | |
An example of a preventable life-threatening ADE is a patient developing severe, recurrent hypoglycemia from receiving a 100-fold overdose of intravenous insulin. An example of a potential life-threatening ADE is a patient being ordered a 10-fold overdose of phenylephrine for hypotension related to sepsis. An example of a significant potential ADE was the potential for patient agitation when nighttime dose of risperidone was initially unavailable and delivered shortly after nurse request and administered.
An average of 2.9 preventable or potential ADEs occurred in each admission, which represented 0.4 events per patient-day; preventable and potential ADEs occurred in 2.6% of medication orders (data not shown). In 31% of admissions, no events took place. One or two events occurred in 33% of admissions, 3–5 events occurred in 20%, 6–10 events in 11% and 11–34 events in 5%.
Events were primarily discovered by review of the paper chart or electronic notes (51%), review of missing dose reports (22%), review of the MAR (19%), staff report to the NDCs (5%), and the hospital’s event reporting system (2%). In all, 934 events (52%) were discovered using more than one mode of discovery.
In the rest of the paper, we focus the data analysis on the 1,183 potential and preventable ADEs (see shaded boxes in figure 2). Medications most involved in potential ADEs were antibiotics (25%) and electrolyte concentrates (12%) (data not shown). Nearly half of preventable ADEs involved antibiotics (26%) or diabetes medications (20%).
Single, grouped and sequential medication errors in potential and preventable ADEs
Each medication safety event could involve a single medication error, a group of errors or a sequence of errors. The 1,183 potential and preventable ADEs represented a total of 1,404 medication errors. Events with sequential and grouped errors ranged from 2 to 4 errors per event. The majority of potential and preventable ADEs involved single medication errors (84% for potential ADEs and 82% for preventable ADEs). An example of a single medication error was an order written for “Clopidogrel 75mg QD”. QD, meaning daily, is an unapproved abbreviation at the research hospital because it could be mistaken for QID, which means four times per day. The order was transcribed correctly in the pharmacy as “Clopidogrel 75mg daily.”
Events with sequential errors represented 10% of potential ADEs and 15% of preventable ADEs, and events with grouped errors represented 6% and 3% of potential and preventable ADEs respectively. Almost all of the potential ADEs with grouped errors involved errors at the ordering stage (98%), such as a physician writing a medication order without providing enough information on dose, route or frequency. An example was an order written for 480 micrograms of intravenous filgrastim to be administered once per day; the route and dose were incorrect. The order was changed by the pharmacist to 480 milligrams administered subcutaneously once daily.
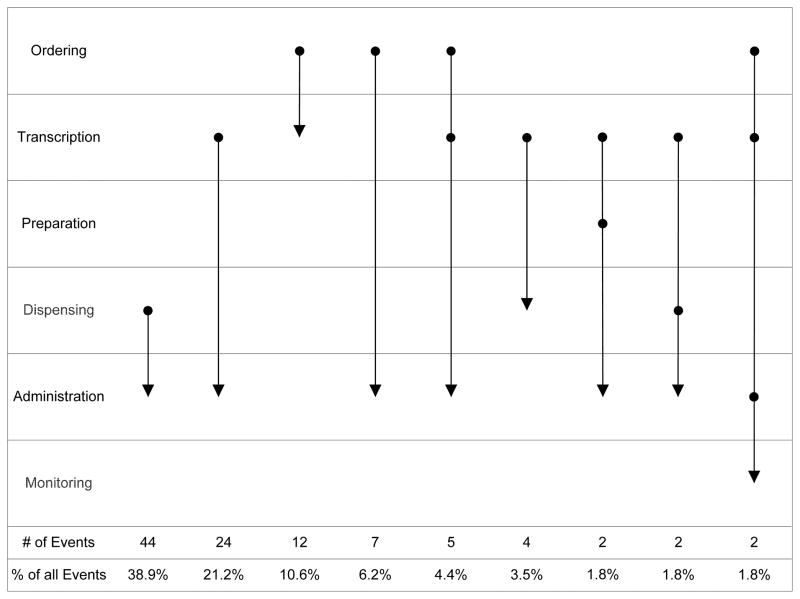
We identified a total of 21 different types of sequences for potential and preventable ADEs. Figure 3 shows the 9 sequences with 2 or more events for potential ADEs; the other sequences for potential and preventable ADEs are in Appendix. Many medication safety events with sequential errors (38%) involved an error at the dispensing stage followed by a medication-administration error. This often occurred when a nurse was unable to administer a medication because it was not found in the medication room. The nurse would subsequently send a missing dose report to pharmacy, which often led to a delay in medication administration or an omitted administration. A specific example of this sequence occurred when one dose of piperacillin and tazobactam was not dispensed by pharmacy and was not administered to the patient. The nurse then sent a report to pharmacy indicating that a dose was missing but did not receive the dose in time to administer it. The second most frequent sequence of errors (21%) was an administration error preceded by a transcription error. This sequence frequently occurred when an order was not transcribed onto the MAR and subsequently not administered or administered late. An example of this sequence occurred when metoclopramide was ordered but the order was not transcribed for 20 hours, which resulted in three doses not administered. Other common sequences of errors involved an ordering error leading to a transcription error (10%), an ordering error leading to an administration error (7%) or a combination of ordering error leading to a transcription error and then leading to an administration error (6%).
Figure 3.
Patterns of sequential errors for potential ADEs (common sequences; see Appendix for the other sequences)
Medication error types for potential and preventable ADEs
The analysis of medication errors across stages of the medication-management process showed that, for potential and preventable ADEs, most errors occurred at the ordering stage (21% and 32% respectively) or the administration stage (60% and 38%) (data not shown). Table 3 displays the frequency of error types for potential and preventable ADEs (additional information on error types of the medication-management process is in Appendix). Most frequent error types in potential ADEs were late administrations, late or not dispensed medications, wrong or inappropriate information (e.g., information on dose/rate, route, or frequency/duration), omitted administrations, omitted information, overdose and underdose. An example of late administration was when hydrocortisone was ordered “now” for a patient with sepsis but was administered 4 hours late. For preventable ADEs, the most frequent error types were late administrations, other errors such as failures to discontinue a medication order, overdose, wrong or inappropriate information, and omitted administrations.
Table 3.
Medication error types in potential and preventable potential ADEs
| Error types | Potential ADEs | Preventable ADEs |
|---|---|---|
| Late administration | 363 (27%) | 14 (29%) |
| Not dispensed or dispensed late | 300 (22%) | 1 (2%) |
| Wrong or inappropriate information | 136 (10%) | 5 (10%) |
| Omitted administration | 105 (8%) | 5 (10%) |
| Omitted information | 98 (7%) | 1 (2%) |
| Overdose | 59 (4%) | 5 (10%) |
| Error prone abbreviations | 52 (4%) | |
| Underdose | 45 (3%) | |
| Duplicate | 30 (2%) | |
| Other (e.g., failure to discontinue medication) | 24 (2%) | 7 (15%) |
| Wrong drug | 21 (2%) | 1 (2%) |
| Not transcribed | 19 (1%) | 1 (2%) |
| Allergy | 18 (1%) | 2 (4%) |
| Wrong patient | 16 (1%) | |
| Illegible order | 16 (1%) | |
| Failure to renew | 15 (1%) | 1 (2%) |
| Administered but not documented | 12 (1%) | 1 (2%) |
| Incomplete or no documentation | 7 (1%) | |
| Administered without order | 7 (1%) | |
| Late transcription | 5 (1%) | |
| Transcribed without order | 4 (1%) | |
| Failure to act | 3 (1%) | 3 (6%) |
| Drug-drug interaction | 1 (1%) | 1 (2%) |
| Total # of errors | 1,356 (100%) | 48 (100%) |
DISCUSSION
In this study of medication errors and adverse drug events among 630 patient admissions in two ICUs, we identified a total of 1,733 events with medication errors, consisting of 549 events with medication errors with no potential for patient harm and 1,184 potential and preventable ADEs. Among the 1,145 potential ADEs, percentages for significant, serious and life threatening harm were 38%, 44% and 18% respectively. Corresponding percentages for the same categories in the 39 preventable ADEs were 18%, 33% and 49% respectively. For each patient admission, 2.9 preventable or potential ADEs occurred in 2.6% of medication orders. These results confirm that hazardous medication safety problems continue to occur in ICUs.
In our study, the rates of potential and preventable ADEs per 1,000 patient-days were 276 and 9.2 respectively, which are higher than those reported by Cullen et al.2 (13.8 and 5.2 respectively). Methodological differences between Cullen2 and our study may explain the large difference in rates of potential ADEs per patient-day. Whereas both studies used the Bates and colleagues’ protocol,8 Cullen et al.2 included only the first ADE of each admission and therefore underestimated the rate of preventable and potential ADEs. In our study, we included all potential and preventable ADEs for every patient admission, finding a rate of 2.87 preventable or potential ADEs per admission. Consistent with the Cullen study, our study shows that most medication errors in ICUs occur at the ordering stage (32% in our study; 38% in Cullen) and at the administration stage (39% in our study; 44% in Cullen). A significant number of medication errors also occurred in the dispensing stage (23% in our study; 10% in Cullen et al.). We may have found more dispensing errors because of the additional availability of supplemental reports at the study hospital (e.g., missing dose reports).
Different technologies have been suggested to improve medication safety. For instance, CPOE technology is seen as a major intervention for reducing ordering errors.24 The following types of medication errors, which occurred in this study at the ordering and transcription stages may be reduced or eliminated with CPOE with Clinical Decision Support: overdose (53), underdose (41), omitted information (99), wrong or inappropriate information (132), and error-prone abbreviations (52) [total=376 or 27% of errors]. Transcription errors can also be eliminated with CPOE technology (91 errors or 6.5%). However, other solutions are needed to eliminate or mitigate medication errors at other stages of the medication-management process as well as to address complex error patterns such as sequential errors.25
Our study begins to unveil the complexity of medication safety by examining clusters of errors: groups and sequences of errors. Whereas the majority of medication safety events involve single medication errors (84% of potential ADEs and 76% of preventable ADEs), many events involve grouped errors (6% of potential ADEs and 5% of preventable ADEs) and sequential errors (10% of potential ADEs and 18% of preventable ADEs). The 114 potential ADEs with sequential errors included a total of 248 medication errors and 20 different sequences. The majority of sequences culminated in an error at the administration stage (88 events), but most frequently began at ordering (29 events), transcription (35 events) or dispensing (45 events). Research on medication errors and ADEs often report errors most proximate to the patient, such as errors at administration; but this method obscures the fact that administration errors are often influenced by breakdowns in other stages of the process. It is important to consider the complexity of medication safety to devise appropriate and effective solutions. This information about temporal sequences or coupling of errors is important for system redesign. The final error in the sequence is the closest one to reaching the patient; however, the initial error is the one that begins the sequence of errors and, whenever possible, should be eliminated. Technological solutions such as bar coding medication administration and smart infusion pumps have been proposed to reduce medication errors at the administration stage. However, given our data on sequential errors, these technologies focus on a single stage of the medication-management process and may not be sufficient to address errors that occur earlier in the process but manifest themselves during administration.
Information on error types is also important for system and process redesign. According to our data, medications are often dispensed late (27% of potential ADEs and 29% of preventable ADEs) or not at all (22% of potential ADEs and 2% of preventable ADEs). EHR technology may reduce these errors by accelerating information flow and allowing pharmacy to dispense more efficiently. Auto-populating an electronic MAR in a well-integrated, well-implemented EHR may help clinicians reduce errors by making information rapidly and reliably available to the patient’s care team (including physicians, unit clerks, nurses, pharmacists and others). Other solutions, e.g., automated alerting of nurses and unit clerks when medication is sent from central pharmacy to the unit or automated dispensing cabinets, may be required to improve medication dispensing. Solutions need to address multiple stages of the medication-management process.
Study limitations include reliance on NDCs to collect data. Significant time and resources were invested in the process of training NDCs and monitoring their performance, which helped to ensure that NDCs identified a large number of medication errors. In addition, we used other approaches (e.g., hospital’s event reporting system and clinician report to NDCs) to identify potential medication errors. The data were collected in 2006–2007, but have unique characteristics with details on error types and temporal patterns (grouped and sequential) so that we can begin to understand the complexity of medication safety, in particular in ICUs.
CONCLUSION
Medication errors and preventable adverse drug events are common in ICUs: 2.87 potential or preventable ADEs occur for every admission. Medication errors reaching and harming patients represent a small proportion (about 3%) of errors, which indicate the ability of clinicians to detect and recover from errors and the resilience of patients. Errors often occur at the ordering and administration stages, but also occur concomitantly or sequentially with other errors. Understanding this temporal complexity of the vulnerabilities of the medication-management process is important to devise appropriate solutions to improve patient safety. EHR technology with CPOE may be necessary to improve medication safety, but not sufficient. Other solutions are necessary to address errors that occur at specific stages and across multiple stages of the medication-management process.
Supplementary Material
Overview of Medication Management Process.
Ordering
physician wrote order in paper chart
Transcription
pharmacist entered order into the pharmacy computer system
nurse or clerk hand copied order into medication administration record (MAR)
Preparation
pharmacy technician in central pharmacy prepared the medication, if needed
Dispensing
pharmacy technician located the medication in the pharmacy and brought it to the pharmacist
pharmacist checked the medication against a computer generated MAR label
The medication and label are delivered to ICU by robot or tube system
Administration
nurse administered medication to patient, recorded the administration time in the MAR and initialed the record
Monitoring
nurses (and sometimes physicians and pharmacists) monitor the patient for therapeutic and unintended effects of the medication
Acknowledgments
This research was made possible by funding from the Agency for Healthcare Research and Quality (AHRQ). Grant Number: R01 HS15274. Principal Investigator: Pascale Carayon, PhD; co-PI: Kenneth Wood, DO. This publication was also supported by the Clinical and Translational Science Award (CTSA) program, previously through the National Center for Research Resources (NCRR) grant 1UL1RR025011, and now by the National Center for Advancing Translational Sciences (NCATS), grant 9U54TR000021. Dr. Wetterneck’s involvement in the study was partially supported by a NIH/NCCR Clinical Scholar Research Award (K12 RR017614-01). Bonnie Paris’ Ph.D. studies are partially funded by the University of Wisconsin-Madison Graduate Engineering Research Scholars (GERS) program, AHRQ T32 Training Grant 5 T32 HS000083-10 [PI: M. Smith], and NIH grant R25 GM083252 for the TEAM-Science program of the UW Center for Women’s Health Research [PI: M. Carnes]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or AHRQ.
Role of the sponsor: The sponsor had no role in the design and conduct of the study, in the collection, analysis, and interpretation of the data, or in the preparation, review, or approval of the manuscript.
Footnotes
Twenty patients had more than one hospital admission during the study period: nine with two admissions to the AICU, four with two admissions to the CICU and seven with one admission each in the AICU and the CICU.
In US hospitals, protocols are developed by a committee of physicians and pharmacists to indicate when and how a specific medication should be replaced by an alternate medication. The hospital’s physicians then agree that pharmacists can follow the protocol in making the specified changes to medication orders without consulting the ordering physician.
At the research hospital, antibiotics and intravenous drip orders end five or three days, respectively, after being ordered. The physician must write a new order at that time to continue to provide the medication to the patient.
In the research hospital, all existing orders are ended when the patient is transferred to the ICU. New orders are then written by a critical care physician.
Conflict of interest disclosures: No conflict of interest.
References
- 1.Rothschild JM, Landrigan CP, Cronin JW, et al. The Critical Care Safety Study: The incidence and nature of adverse events and serious medical errors in intensive care. Crit Care Med. 2005;33:1694–700. doi: 10.1097/01.ccm.0000171609.91035.bd. [DOI] [PubMed] [Google Scholar]
- 2.Cullen DJ, Sweitzer BJ, Bates DW, et al. Preventable adverse drug events in hospitalized patients: A comparative study of intensive care and general care units. Crit Care Med. 1997;25(8):1289–97. doi: 10.1097/00003246-199708000-00014. [DOI] [PubMed] [Google Scholar]
- 3.Kane-Gill SL, Kirisci L, Verrico MM, et al. Analysis of risk factors for adverse drug events in critically ill patients*. Crit Care Med. 2012;40(3):823–8. doi: 10.1097/CCM.0b013e318236f473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harris CB, Krauss MJ, Coopersmith CM, et al. Patient safety event reporting in critical care: a study of three intensive care units. Crit Care Med. 2007;35(4):1068–76. doi: 10.1097/01.CCM.0000259384.76515.83. [DOI] [PubMed] [Google Scholar]
- 5.Bates DW, Leape LL, Cullen DJ, et al. Effect of computerized physician order entry and a team intervention on prevention of serious medication errors. JAMA. 1998;280(15):1311–16. doi: 10.1001/jama.280.15.1311. [DOI] [PubMed] [Google Scholar]
- 6.Kane-Gill S, Rea RS, Verrico MM, et al. Adverse-drug-event rates for high-cost and high-use drugs in the intensive care unit. Am J Health Syst Pharm. 2006;63(19):1876–81. doi: 10.2146/ajhp060045. [DOI] [PubMed] [Google Scholar]
- 7.Resar RK, Rozich JD, Simmonds T, et al. A trigger tool to identify adverse events in the intensive care unit. Jt Comm J Qual Patient Saf. 2006;32(10):585–90. doi: 10.1016/s1553-7250(06)32076-4. [DOI] [PubMed] [Google Scholar]
- 8.Bates DW, Leape LL, Petrycki S. Incidence and preventability of adverse drug events in hospitalized adults. J Gen Int Med. 1993;8(6):289–94. doi: 10.1007/BF02600138. [DOI] [PubMed] [Google Scholar]
- 9.Leape LL, Cullen DJ, Clapps MD, et al. Pharmacist participation on physician rounds and adverse drug events in the intensive care unit. JAMA. 1999;282(3):267–70. doi: 10.1001/jama.282.3.267. [DOI] [PubMed] [Google Scholar]
- 10.Moyen E, Camire E, Stelfox HT. Clinical review: medication errors in critical care. Crit Care. 2008;12(2):208. doi: 10.1186/cc6813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kane-Gill S, Weber RJ. Principles and practices of medication safety in the ICU. Crit Care Clin. 2006;22(2):273–90. doi: 10.1016/j.ccc.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 12.Wilmer A, Louie K, Dodek P, et al. Incidence of medication errors and adverse drug events in the ICU: A systematic review. Quality & safety in health care. 2010;19(5):e7. doi: 10.1136/qshc.2008.030783. [DOI] [PubMed] [Google Scholar]
- 13.Pintor-Mármol A, Baena MI, Fajardo PC, et al. Terms used in patient safety related to medication: a literature review. Pharmacoepidem Dr S. 2012;21(8):799–809. doi: 10.1002/pds.3296. [DOI] [PubMed] [Google Scholar]
- 14.Kane-Gill SL, Chapman TR, Dasta JF. Drug-related hazardous conditions to prevent injury and defining injury is also important. Pharmacoepidem Dr S. 2012;21(11):1247–48. doi: 10.1002/pds.3347. [DOI] [PubMed] [Google Scholar]
- 15.Beuscart-Zephir MC, Pelayo S, Anceaux F, et al. Impact of CPOE on doctor-nurse cooperation for the medication ordering and administration process. Int J Med Inf. 2005;74(7–8):629–41. doi: 10.1016/j.ijmedinf.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 16.Horsky J, Kuperman G, Patel VL. Comprehensive analysis of a medication dosing error related to CPOE. J Am Med Inform Assn. 2005;12(4):377–82. doi: 10.1197/jamia.M1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carayon P, Hundt AS, Karsh B-T, et al. Work system design for patient safety: The SEIPS model. Quality & Safety in Health Care. 2006;15(Supplement I):i50–i58. doi: 10.1136/qshc.2005.015842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bates DW, Cullen DJ, Laird N, et al. Incidence of adverse drug events and potential adverse drug events: Implications for prevention. JAMA. 1995;274(1):29–34. [PubMed] [Google Scholar]
- 19.Lesar T, Briceland L, Stein D. Factors related to errors in medication prescribing. JAMA. 1997;277:312–317. [PubMed] [Google Scholar]
- 20.Dean B, Barber N, Schachter M. What is a prescribing error? Qual Health Care. 2000;9:232–37. doi: 10.1136/qhc.9.4.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.National Coordinating Council of Medication. NCC MERP Taxonomy of Medication Errors. 1998. Error Reporting and Prevention Taxonomy of Medication Errors. [Google Scholar]
- 22.Bobb A, Gleason K, Husch M, et al. The epidemiology of prescribing errors. Arch Int Med. 2004;164(7):785–92. doi: 10.1001/archinte.164.7.785. [DOI] [PubMed] [Google Scholar]
- 23.Bates DW, Boyle DL, Vander Vliet M, et al. Relationship between medication errors and adverse drug events. J Gen Int Med. 1995;10:199–205. doi: 10.1007/BF02600255. [DOI] [PubMed] [Google Scholar]
- 24.Bates DW, Cohen M, Leape LL, et al. Reducing the frequency of errors in medicine using information technology. J Am Med Inform Assn. 2001;8(4):299–308. doi: 10.1136/jamia.2001.0080299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Manias E, Williams A, Liew D. Interventions to reduce medication errors in adult intensive care: A systematic review. Brit J Clin Pharmaco. 2012;74(3):411–23. doi: 10.1111/j.1365-2125.2012.04220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.