Abstract
Context
Many studies have reported that blacks survive longer on dialysis than whites. This observation is paradoxical given racial disparities in access to and quality of care, and is inconsistent with observed lower survival among blacks with chronic kidney disease. We hypothesized that age and the competing risk of transplantation modify survival differences by race.
Objectives
To estimate death on dialysis by race, accounting for age as an effect modifier and kidney transplantation as a competing risk.
Design, Setting, and Participants
An observational cohort study of 1,330,007 incident end-stage renal disease patients as captured in the United States Renal Data System between January 1, 1995 and September 28, 2009 (median potential follow-up time = 6.7 years, range 1 day-14.8 years). Multivariate age-stratified Cox proportional hazards and competing risk models were constructed to examine death on dialysis.
Main Outcome Measures
Death on dialysis in blacks versus whites
Results
Similar to previous studies, blacks had a lower death rate on dialysis compared with whites (232,361 deaths (57.1% mortality) for blacks versus 585,792 deaths (63.5% mortality) for whites, adjusted hazard ratio (aHR) 0.84, 95% CI: 0.83-0.84, p<0.001). However, stratifying by age and treating kidney transplantation as a competing risk, blacks had significantly higher mortality than their white counterparts at ages 18-30 (27.6% mortality versus 14.2%, aHR 1.93, 95% CI: 1.84-2.03), 31-40 (37.5% versus 26.8%, aHR 1.46, 95% CI: 1.41-1.50), and 41-50 (44.8% versus 38.0%, aHR 1.12, 95% CI: 1.10-1.14, p <0.001 for interaction terms between race and each prior age category), as opposed to those 51-60 (51.5% versus 50.9%, aHR 0.93, 95% CI: 0.92-0.94), 61-70 (64.9% versus 67.2%, aHR 0.87, 95% CI: 0.86-0.88), 71-80 (76.1% versus 79.7%, aHR 0.85, 95% CI: 0.84-0.86), and > 80 (82.4% versus 83.6%, aHR 0.87, 95% CI: 0.85-0.88).
Conclusions
Overall, among dialysis patients in the United States, there was a lower risk of death for black patients compared to their white counterparts. However, the commonly cited dialysis survival advantage for blacks applies only to older adults, and those under the age of 50 have a higher risk of death.
Keywords: survival advantage, ESRD, transplant, African-American
INTRODUCTION
Blacks are significantly overrepresented in the end-stage renal disease (ESRD) population. Of more than half a million persons suffering from ESRD in the United States, nearly one-third are black, and the relative incidence of ESRD among blacks is 3.6-times higher than that among whites.1 Moreover, racial disparities in quality of and access to care for patients with kidney disease are well documented.2-4 Compared with whites, fewer black patients with chronic kidney disease (CKD) are under the care of a nephrologist, and their rates of referral for peritoneal dialysis and kidney transplantation are significantly lower.5,6 Once on dialysis, blacks are less likely to receive an adequate dialysis dose,7,8 have a fistula placed,9,10 and achieve target hemoglobin levels, all metrics associated with decreased dialysis survival.11
Despite the disparity in care, current thinking – supported by over 30 previous studies – is that blacks survive longer on dialysis than whites.2,3,8,12-42 Blacks with ESRD are reported to have 13-45% lower mortality on dialysis than their white counterparts, a finding that persists in both unadjusted analysis and after adjustment for comorbidities and socioeconomic status. Varying postulations for this counterintuitive observation have included differential sensitivity to dialysis dose,8 racial differences in nutritional status, or racial differences in inflammation,39 the biological or sociological mechanisms for which remain unclear. Moreover, the perception of enhanced dialysis survival seems to have affected clinical decision-making and engendered complacency about the low rates of transplantation among blacks.2,43 Although kidney transplantation is the preferred form of renal replacement therapy regardless of race, blacks have lower rates of transplant referral and longer dialysis vintage at referral than their white counterparts.5,44-49
The paradox of enhanced dialysis survival in the setting of decreased access to care may be in part a byproduct of study design. Population-based studies of dialysis survival can mask important subgroup effects, particularly given that the majority of dialysis patients are over the age of 65.1 As such, studies of the dialysis population as a whole are dominated by effects among older adults,50 where disparities in access to care are attenuated by Medicare eligibility. In fact, racial disparities in CKD mortality occur almost exclusively among younger age groups,51 as do inequities in access to transplantation.52 With the hypothesis that disparities in socioeconomic status and insurance coverage are likely greatest in younger ESRD patients, and that these disparities are manifest both in rates of transplantation and dialysis mortality, the goals of this study were (1) to examine age as an effect modifier of the racial disparities in dialysis survival, and (2) to determine if differential rates of kidney transplantation modify the risk of death on dialysis.
METHODS
Study Design
The study cohort included 1,330,007 adults, identified as either African American/black (n=407,140) or Caucasian/white (n=922,867), as indicated on the 2728 Medical Evidence Form, signed and filed for each patient by the supervising physician within 45 days of dialysis initiation or transplant. All patients initiated dialysis or received a pre-dialysis transplant for the first time between January 1, 1995 and September 28, 2009. Data were drawn from the United States Renal Data System (USRDS), a national registry of all ESRD patients in the United States. Patients were followed from ESRD diagnosis until death, which was ascertained via linkage to the Social Security Master Death File. The presence or absence of 18 major comorbidities and 2 physical impairments was captured via a checklist on the 2728 Medical Evidence Form. All patients were followed until (1) death, (2) kidney transplantation, or (3) end-of-study (September 28 2009). Comorbidity status was ascertained at ESRD-onset; only joining the waitlist, receiving a kidney transplant and death were measured longitudinally. Rates of death and transplantation are high in this population; as such outcomes for many patients occurred soon after ESRD-onset and the median follow-up time (time from ESRD-onset to first noted event) was 21.5 months (range 1 day-14.8 years). However, the median potential follow-up time (time from ESRD-onset to end of study) was 6.7 years. This study was reviewed by the institutional review board at Johns Hopkins School of Medicine and determined to qualify for an exemption under 45 CFR 46.101(b) as study participants cannot be identified directly or through linked identifiers.
Population-Based Survival Analysis
In order to replicate previous studies, a multivariate Cox proportional hazards model including patients of all ages was adjusted for the following factors: age, race (black versus white), sex, insurance type at ESRD-onset, BMI, cause of ESRD, pre-dialysis or transplant erythropoietin (epo) administration (a surrogate for having nephrology care prior to ESRD-onset), atherosclerotic heart disease, cardiac failure, peripheral vascular disease (PVD), cerebrovascular disease (CVD), hypertension, diabetes, smoking, immobility, malignant neoplasm/cancer, chronic obstructive pulmonary disease (COPD), drug dependence, alcohol dependence, and dialysis type (peritoneal or hemodialysis). Patients were censored at transplantation or end-of-study.
Age as an Effect Modifier
The cohort was then stratified by age, and survival on dialysis among blacks and whites was compared using Cox proportional hazards models (adjusted for the factors listed above). As above, patients were censored at transplantation or end-of-study. To confirm differences between age groups were statistically significant, an additional model was built including interaction terms for each age category and black race.
To account for age-stratified differences in transplant rates between blacks and whites, the same analyses were repeated using competing-risk regression according to the methods of Fine and Gray.53 These models, where transplantation was treated as a competing risk and end-of-study as administrative censoring, provided an estimate of the risk that those who start dialysis will die while on dialysis. In other words, while the Cox model better estimated the risk of death if blacks and whites were transplanted at equal rates(adjusted hazard ratio, aHR), the competing-risk model better estimated the risk of death on dialysis for a patient given the current disparities in access to transplantation (adjusted subhazard ratio, aSHR).
Subgroup Analysis
To further explore those patients where disparities of the highest magnitude were identified, dialysis survival among additional subgroups within 18-30 year-olds were explored by sex, insurance, BMI, type of dialysis, epo use, and reported primary cause of ESRD to see whether racial disparities persisted across all or were specific to certain subgroups of younger patients.
Statistical Analysis
All analyses were performed by using multiprocessor Stata version 11.0/MP for Linux (Stata-Corp, College Station, Texas). All hypothesis tests were 2-sided, with statistical significance defined as having a p-value < 0.05.
RESULTS
Population-Based Analyses
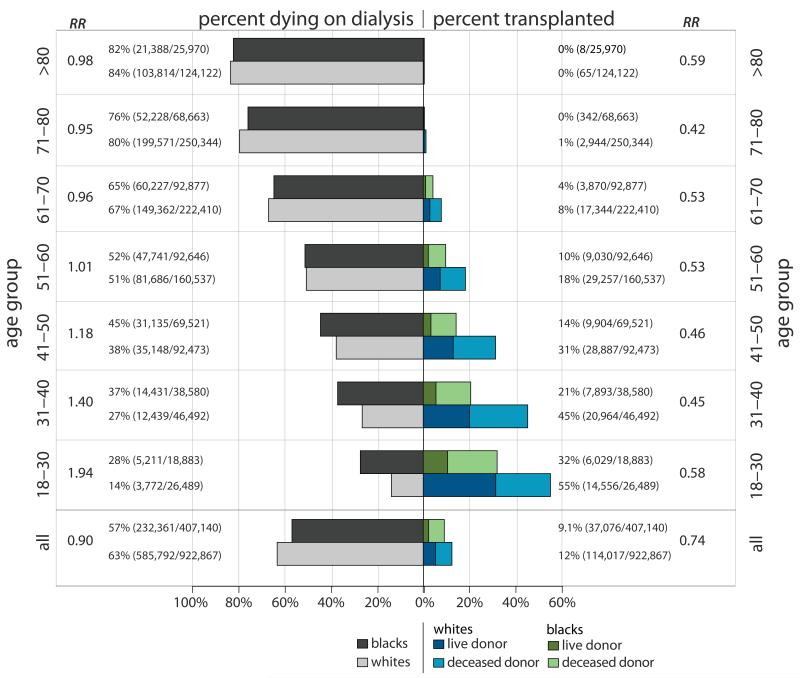
Among 1,330,007 incident ESRD patients between 1995-2009, blacks were on average younger (58.1 vs. 64.5 years). While blacks age 18-30 had rates of comorbid conditions similar to whites, those > 30 were less likely to have atherosclerotic heart disease (6.7% vs. 10.9% for 31-50; 20.6% vs. 34.1% for those >50), and peripheral vascular disease (4.7% vs. 8.4% for 31-50; 12.3% vs. 18.6% for those > 50), and those > 50 were less likely to have cardiac failure (33.5% vs. 38.8%) and COPD (6.2% vs. 11.3%), and more likely to have hypertension (88.6% vs. 81.8%, Table 1). Of incident ESRD patients of all ages entering the study, 57.1% of blacks and 63.5% of whites died on dialysis, and 9.1% of blacks and 12.4% of whites received kidney transplants, with 25.7% of transplants in black patients from live donors compared to 42.8% in whites (Figure 1, first set). Adjusting for differences in demographics and comorbidities, and censoring for transplantation, blacks had a lower risk of death on dialysis (57% of blacks died and 63% of whites died on dialysis, aHR 0.84, 95% CI: 0.83-0.84, p <0.001).
Table 1. Clinical and Demographic Characteristics Incident ESRD Patients, by Age and Race.
Cells include # (%) unless otherwise specified. SD= Standard Deviation; EPO = Erythropoietin; ASHD = Atherosclerotic Heart Disease; PVD = Peripheral Vascular Disease; CVD = Cerebrovascular Disease
| Age 18-30 | Age 31-50 | Age >50 | ||||
|---|---|---|---|---|---|---|
| White | Black | White | Black | White | Black | |
| (n=26,489) | (n=18,883) | (n=138,965) | (n=108,101) | (n=757, 413) | (n=280,156) | |
| Age in Years (SD) | 25.1 (3.6) | 25.5 (3.5) | 42.7 (5.5) | 42.4 (5.5) | 69.9 (10.0) | 66.3 (9.8) |
| Female | 11,370 (42.9) | 9,040 (47.9) | 54,871 (39.5) | 45,516 (42.1) | 331,228 (43.7) | 150,520 (53.7) |
| Insurance Type | ||||||
| Medicare | 3,802 (14.8) | 1,976 (10.8) | 26, 426 (19.6) | 15,910 (15.2) | 418,662 (57.1) | 108,879 (40.1) |
| Private | 8,677 (33.8) | 4,037 (22.1) | 53,657 (39.9) | 29,050 (27.7) | 164,770 (22.5) | 56,703 (20.9) |
| Medicaid/None | 13,177 (51.4) | 12,255 (67.1) | 54,481 (40.5) | 59,895 (57.1) | 149,615 (20.4) | 105,779 (39.0) |
| BMI | ||||||
| <18 | 1,505 (6.0) | 984 (5.6) | 4,947 (3.8) | 4,488 (4.4) | 28,985 (4.0) | 13,630 (5.1) |
| 18-24.9 | 12,558 (51.1) | 6,931 (39.8) | 46,748 (36.4) | 33,425 (33.2) | 268,641 (38.0) | 91,609 (34.8) |
| 25-29.9 | 5690 (23.1) | 4,059 (23.3) | 34,955 (27.2) | 25,704 (25.5) | 208,534 (29.5) | 73,226 (27.8) |
| 30-34.9 | 2,651 (10.8) | 2,404 (13.8) | 20,563 (16.0) | 16,710 (16.4) | 111,098 (15.7) | 44,267 (16.8) |
| ≥ 35 | 2,139 (9.2) | 3,034 (18.4) | 21,050 (17.0) | 20,189 (21.0) | 88,262 (13.0) | 39,649 (15.8) |
| Cause of ESRD | ||||||
| Glomerulonephritis | 8,108 (30.9) | 4,393 (23.4) | 20,443 (14.8) | 12,763 (11.9) | 52,096 (7.0) | 11,737 (4.2) |
| Diabetes | 5,131 (19.6) | 3,226 (17.2) | 63,260 (46.0) | 32,965 (30.6) | 338,101 (45.3) | 139,369 (50.3) |
| Hypertension | 2,945 (11.2) | 5,192 (27.6) | 16,744 (12.2) | 38,760 (36.1) | 207,740 (27.7) | 96,652 (34.8) |
| Polycystic Kidney | 536 (2.0) | 85 (0.5) | 9,807 (7.1) | 1,753 (1.6) | 16,859 (2.3) | 2,445 (0.9) |
| Urologic | 1,542 (5.9) | 251 (1.3) | 4,223 (3.1) | 1,064 (1.0) | 21,238 (2.8) | 3,239 (1.2) |
| Other | 6,056 (23.1) | 4,830 (25.7) | 17,811 (12.9) | 16,625 (15.5) | 78,590 (10.5) | 16,217 (5.9) |
| Unknown | 1,924 (7.3) | 808 (4.3) | 5,322 (3.9) | 3,558 (3.3) | 32,533 (4.4) | 7,612 (2.7) |
| Dialysis Type | ||||||
| Hemodialysis | 22,465 (86.1) | 16,981( 91.3) | 118,360 (86.5) | 98,546 (92.6) | 686,295 (92.6) | 262,463 (95.5) |
| Peritoneal | 3,632 (13.9) | 1,610 (8.7) | 18,496 (13.5) | 7,887 (7.4) | 55,220 (7.4) | 12,435 (4.5) |
| Use of EPO | 7,200 (30.1) | 3,682 (21.4) | 40,030 (32.2) | 22,515 (23.0) | 225,933 (33.5) | 73,894 (29.5) |
| Cardiac Failure | 1,733 (6.7) | 1,839 (9.9) | 21,652 (15.9) | 19,551 (18.4) | 287,483 (38.8) | 92,048 (33.5) |
| ASHD | 500 (1.9) | 280 (1.5) | 14,795 (10.9) | 7,079 (6.7) | 252,708 (34.1) | 56,626 (20.6) |
| PVD | 421 (1.6) | 322 (1.7) | 11,381 (8.4) | 5,035 (4.7) | 138,068 (18.6) | 33,912 (12.3) |
| CVD | 303 (1.2) | 319 (1.7) | 5,549 (4.1) | 4,913 (4.6) | 78,887 (10.6) | 32,109 (11.7) |
| Hypertension | 17,951 (69.0) | 14,681 (78.9) | 104,976 (77.0) | 90,944 (85.2) | 607,863 (81.8) | 244,300 (88.6) |
| Diabetes | 5,725 (22.0) | 3,902 (21.0) | 70,133 (51.3) | 40,729 (38.2) | 411,954 (55.4) | 171,147 (62.0) |
| Smoker | 1,620 (6.2) | 900 (4.9) | 12,659 (9.3) | 9,772 (9.2) | 35,740 (4.8) | 14,086 (5.1) |
| Immobile | 383 (1.5) | 248 (1.3) | 3,722 (2.7) | 2,238 (2.1) | 43,682 (5.9) | 17,340 (6.3) |
| Cancer | 267 (1.0) | 99 (0.5) | 3,514 (2.6) | 1,639 (1.5) | 62,422 (8.4) | 15,807 (5.8) |
| COPD | 138 (0.5) | 114 (0.6) | 4,111 (3.0) | 2,269 (2.1) | 83,747 (11.3) | 17,013 (6.2) |
| Alcohol Abuse | 214 (0.8) | 168 (0.9) | 3,422 (2.5) | 3,609 (3.4) | 7,637 (1.0) | 4,842 (1.8) |
| Drug Abuse | 511 (2.0) | 497 (2.7) | 2,771 (2.0) | 7,026 (6.6) | 1,408 (0.2) | 3,346 (1.2) |
Figure 1. Percent of Incident ESRD Patients Who Died on Dialysis (Left Panel) or Received a Transplant (Right Panel) During the Study Period, by Race and Age Category.
RR = Relative Risk (unadjusted) of dying on dialysis (left panel) or receiving a transplant (right panel) for blacks compared to whites
Age-Stratified Analyses
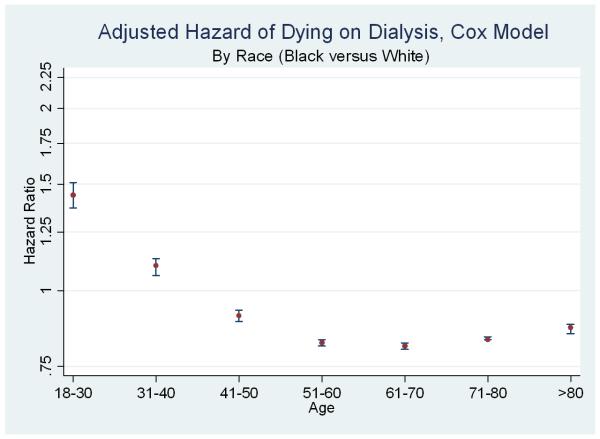
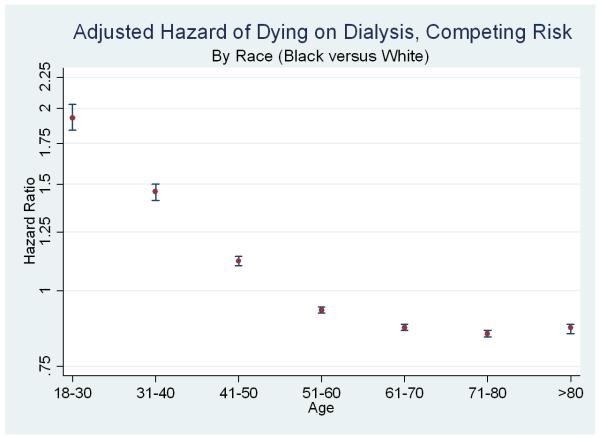
However, the relationships between race, dialysis survival, and transplantation were substantially modified by patient age, with opposite inferences in the younger age groups. Among incident 18-30 year-olds entering the study, 27.6% of blacks and 14.2% of whites died on dialysis, and 31.9% of blacks and 54.9% of whites received transplants (Figure 1, second set). In adjusted models specific to this age group, blacks had a higher risk of death on dialysis censoring for transplantation (aHR 1.44, 95% CI: 1.37-1.51) (Figure 2, upper panel) and treating transplantation as acompeting event (aSHR 1.93, 95% CI: 1.84-2.03) (Figure 2, lower panel).
Figure 2. Relative Adjusted Hazard of Death (Black versus White) on Dialysis, by Age.
Each point and 95% confidence interval represents results from one multivariate model (adjusting for demographics and comorbidities). Upper panel represents Cox models (hazard ratios, log scale) and lower panel represents Fine & Gray models (subhazard ratios, log scale). The line at 1.0 represents parity; estimates above the line indicate a higher risk of death for black dialysis patients (compared to similar white dialysis patients); estimates below the line indicate a lower risk of death for black dialysis patients.
Similarly, among incident 31-40 year-olds entering the study, 37.4% of blacks and 26.8% of whites died on dialysis, and 20.5% of blacks and 45.1% of whites received transplants (Figure 1, third set). In adjusted models specific to this age group, blacks had a higher risk of death on dialysis censoring for transplantation (aHR 1.10, 95% CI: 1.06-1.13) and treating transplantation as a competing event (aSHR 1.46, 95% CI: 1.41-1.50) (Figure 2).
Subgroup estimates specific to older patients were very similar to population-based estimates previously reported in the literature. For example, among patients ages 61-70 entering the study, 64.9% of blacks and 67.2% of whites died on dialysis, and 4.2% of blacks and 7.8% of whites received transplants (Figure 1, sixth set). In adjusted models specific to this age group, blacks had a lower risk of death on dialysis censoring for transplantation (aHR 0.81, 95% CI: 0.80-0.82) and treating transplantation was treated as a competing event (aSHR 0.87, 95% CI: 0.86-0.88) (Figure 2). In a model including interaction terms for race and each age category, all interaction terms were statistically significant, confirming differences in the race disparity between age subgroups (p<0.05 for all).
Subgroup Analyses in 18-30 Year-Olds
The age subgroup with the highest disparity in risk of death between black and white dialysis patients was that of 18-30 year-olds. As such, further exploration of this subgroup was performed to better understand potential mechanisms for the marked difference in racial disparities among younger patients. In the 18-30 year-old subgroup, blacks were less likely to have private insurance (22.1% versus 33.8%), more likely to have Medicaid or no insurance (67.1% versus 51.4%), more likely to have hypertension as the primary cause of renal failure (27.6% of blacks versus 11.2% of whites), and less likely to receive erythropoietin (21.4% of blacks versus 30.1% of whites). Rates of other comorbidities were similar by race (Table 1). Further stratification of 18-30 year olds by clinically important subgroups showed that the disparity for blacks persists across all with the exception of those with a BMI >35 (aHR 1.09, 95% CI 0.94-1.26) and those with diabetes as the primary cause of ESRD (aHR 1.02, 95% CI 0.93-1.11). In a competing risk analysis a substantial survival disparity persisted across all subgroups (Table 2).
Table 2. Relative Adjusted Hazard of Death (Black versus White) Among 18-30 Year Dialysis Patients.
Each row represents results from one multivariate model (adjusting for demographics and comorbidities). Left columns represent Cox models (hazard ratios) and right columns represent Fine & Gray models (subhazard ratios).
| Stratum | Whites deaths/total |
Blacks deaths/total |
Censored HR (95% CI) |
Competing Risks SHR (95% CI) |
|---|---|---|---|---|
| Sex | ||||
| Male | 1,899/15,119 | 2,539/9,843 | 1.54 (1.44-1.65) | 2.10 (1.96-2.25) |
| Female | 1,873/11,370 | 2,672/9,040 | 1.34 (1.25-1.43) | 1.77 (1.65-1.90) |
| Insurance | ||||
| Medicare/VA | 524/3,802 | 480/1,976 | 1.35 (1.17-1.55) | 1.95 (1.69-2.24) |
| Private | 631/8,677 | 631/4,037 | 1.47 (1.39-1.56) | 1.85 (1.74-1.95) |
| Medicaid/None | 2,469/13,177 | 3,841/12,255 | 1.38 (1.22-1.57) | 2.40 (2.11-2.73) |
| BMI | ||||
| <18 | 318/1,505 | 385/984 | 1.55 (1.32-1.83) | 1.93 (1.63-2.28) |
| 18-24.9 | 1,787/12,558 | 2,166/4,765 | 1.59 (1.49-1.71) | 2.13 (1.99-2.29) |
| 25-29.9 | 619/5,690 | 971/4,059 | 1.43 (1.28-1.59) | 2.03 (1.82-2.27) |
| 30-34.9 | 316/2,651 | 465/2,404 | 1.12 (0.96-1.31) | 1.48 (1.26-1.74) |
| >35 | 321/2,139 | 657/3,034 | 1.09 (0.94-1.26) | 1.38 (1.19-1.61) |
| Use of Erythropoietin | ||||
| No | 2,492/16,692 | 3,933/13,501 | 1.51 (1.43-1.59) | 1.97 (1.86-2.09) |
| Yes | 933/7,200 | 877/3,682 | 1.22 (1.11-1.36) | 1.79 (1.61-1.99) |
| Cause of ESRD | ||||
| Diabetes | 1,561/5,131 | 1,231/3,226 | 1.02 (0.93-1.11) | 1.35 (1.24-1.48) |
| Hypertension | 2,438/17,951 | 1,057/5,192 | 1.33 (1.16-1.54) | 1.65 (1.43-1.90) |
| Glomerulonephritis | 619/8,108 | 670/4,393 | 1.21 (1.07-1.37) | 1.71 (1.51-1.95) |
| Urologic | 196/1,542 | 77/251 | 1.63 (1.21-2.18) | 2.32 (1.74-3.10) |
| Other | 862/6,056 | 1,975/4,830 | 2.18 (1.99-2.38) | 2.88 (2.63-3.15) |
| Unknown | 168/1,924 | 169/808 | 1.64 (1.28-2.12) | 2.06 (1.58-2.69) |
| Dialysis Type | ||||
| Hemodialysis | 3,232/22,465 | 4,689/16,981 | 1.45 (1.38-1.53) | 1.93 (1.83-2.03) |
| Peritoneal Dialysis | 467/3,632 | 389/1,610 | 1.29 (1.10-1.52) | 1.94 (1.65-2.28) |
DISCUSSION
In this national study of dialysis survival among over 1.3 million incident ESRD patients, we have shown that the commonly cited survival advantage for blacks on dialysis applies only to those over the age of 50. In marked contrast, younger blacks have up to twice the hazard of death on dialysis compared with their white counterparts, even after adjusting for many demographic factors and comorbidities. Additionally, the disparity in younger patients widens when the differential rates of kidney transplantation for blacks and whites are considered in a competing risk analysis.
Our population-based results are consistent with many earlier studies,2,3,8,12-42 supporting face validity of our study cohort and analytical approach. However, the demonstration of significant age-based effect modification of the racial differences in dialysis survival is novel, challenging conventional wisdom and identifying a significant disparity among younger blacks that needs to be addressed. The finding of increased mortality among younger black ESRD patients is consistent with previous findings among the general population and in those with chronic but not yet end-stage renal disease.3,5
The majority of dialysis patients are over 65, so inferences from population-based models are driven by this subgroup and may not be generalizable to patients of all ages. Younger patients may be fundamentally different from older patients in the prevalence of comorbidities, SES, and the underlying biology of disease. Furthermore, the influence of comorbidities and SES disparities likely influence outcomes of younger and older adults differentially. Previous studies have shown that age modifies race and sex disparities in transplant.50,52,54 Likewise, this study has shown that inferences about survival on dialysis drawn from the entire population, in this case that blacks do better than whites, do not apply to younger patients. Assuming that population-based inferences are generalizable to subgroups within the population has the potential to mislead clinical decision-making. Furthermore, while older black patients have better survival on dialysis compared to older whites, it is important to note that patients of all ages and races derive a survival benefit from transplantation versus remaining on dialysis.55
Additionally, accounting for transplant as a competing risk brings to light an even greater disparity in death on dialysis in younger age groups where transplant is most common and racial disparities in access are greatest.52 Use of this method allows us to infer that the two-fold increased hazard of death on dialysis in younger blacks is composed of two distinct components: one of differential rates of transplantation and one of biology (or some interaction between biology and socioeconomic factors). Blacks are much less likely to receive a transplant from a live donor; as such interventions to reduce transplant disparities should prioritize the improvement of live donation rates for blacks.
One potential contributor to the significant racial disparity among younger adults, but not older adults, is insurance coverage: young blacks are more likely to be uninsured (or Medicaid insured) than young whites, while all older adults are Medicare eligible. As hypothesized, disparity in insurance is greater among the younger ESRD population; however, even among those with private insurance, young blacks had a higher risk of death on dialysis than young whites on dialysis. And even among young patients with previous erythropoietin stimulating agent use, a proxy for medical care during CKD progression, young blacks experienced a higher risk of death on dialysis. It is likely, then, that any socioeconomic contributors to this disparity remain uncharacterized or unmeasured.
Several limitations merit consideration. First, only patients who survived long enough to develop ESRD were captured, and therefore the impact of differential mortality for blacks in earlier stages of CKD on dialysis survival could not be quantified. Blacks with CKD die at a higher rate than whites, possibly causing a survivor bias where the black patients who survive to ESRD are healthier than whites. Without linkage of CKD data to the USRDS registry, we were unable to quantify the effects of this survival bias. Second, comorbidity data captured in the 2728 form is coded as presence or absence of disease, and nuances of severity of comorbidities could not be considered. However, the 2728 form does provide a rich source of comorbidity information, and this comorbidity ascertainment was adequate to demonstrate consistency between our initial population-based analyses and previous literature.
In summary, this study challenges the widespread notion that blacks survive longer than whites on dialysis. Despite a survival advantage in older age groups, blacks under the age of 50 are at substantially increased risk of death on dialysis. Inequities in dialysis survival are compounded by inequities in access to transplantation. Determining why younger blacks are at increased risk of death on dialysis is critical in order to improve clinical decision-making and inform policies aimed at achieving equity in ESRD care.
ACKNOWLEDGEMENTS
Segev and Kucirka had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. This work was supported by grant number R21DK085409 from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) and by a Paul Beeson Career Development Award (co-funded by National Institute of Aging K23AG032885 and American Federation for Aging Research) (DLS).
Footnotes
No authors have any relevant financial conflicts of interest.
REFERENCES
- 1.USRDS . United States Renal Data System Annual Data Report 2010: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda: 2010. [Google Scholar]
- 2.Reddan DN, Szczech LA, Klassen PS, Owen WF., Jr Racial inequity in America’s ESRD program. Semin Dial. 2000 Nov-Dec;13(6):399–403. doi: 10.1046/j.1525-139x.2000.00109.x. [DOI] [PubMed] [Google Scholar]
- 3.Kalantar-Zadeh K, Kovesdy CP, Derose SF, Horwich TB, Fonarow GC. Racial and survival paradoxes in chronic kidney disease. Nat Clin Pract Nephrol. 2007 Sep;3(9):493–506. doi: 10.1038/ncpneph0570. [DOI] [PubMed] [Google Scholar]
- 4.Powe NR, Melamed ML. Racial disparities in the optimal delivery of chronic kidney disease care. Med Clin North Am. 2005 May;89(3):475–488. doi: 10.1016/j.mcna.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 5.Epstein AM, Ayanian JZ, Keogh JH, et al. Racial disparities in access to renal transplantation--clinically appropriate or due to underuse or overuse? N Engl J Med. 2000 Nov 23;343(21):1537–1544. 1532. doi: 10.1056/NEJM200011233432106. p preceding 1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Winkelmayer WC, Glynn RJ, Levin R, Owen W, Jr., Avorn J. Late referral and modality choice in end-stage renal disease. Kidney Int. 2001 Oct;60(4):1547–1554. doi: 10.1046/j.1523-1755.2001.00958.x. [DOI] [PubMed] [Google Scholar]
- 7.Leonard MB, Stablein DM, Ho M, Jabs K, Feldman HI. Racial and center differences in hemodialysis adequacy in children treated at pediatric centers: a North American Pediatric Renal Transplant Cooperative Study (NAPRTCS) report. J Am Soc Nephrol. 2004 Nov;15(11):2923–2932. doi: 10.1097/01.ASN.0000143475.39388.DE. [DOI] [PubMed] [Google Scholar]
- 8.Owen WF, Jr., Chertow GM, Lazarus JM, Lowrie EG. Dose of hemodialysis and survival: differences by race and sex. JAMA. 1998 Nov 25;280(20):1764–1768. doi: 10.1001/jama.280.20.1764. [DOI] [PubMed] [Google Scholar]
- 9.Hopson S, Frankenfield D, Rocco M, McClellan W. Variability in reasons for hemodialysis catheter use by race, sex, and geography: findings from the ESRD Clinical Performance Measures Project. Am J Kidney Dis. 2008 Oct;52(4):753–760. doi: 10.1053/j.ajkd.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 10.Reddan D, Klassen P, Frankenfield DL, et al. National profile of practice patterns for hemodialysis vascular access in the United States. J Am Soc Nephrol. 2002 Aug;13(8):2117–2124. doi: 10.1097/01.asn.0000022422.79790.a8. [DOI] [PubMed] [Google Scholar]
- 11.Gadegbeku C, Freeman M, Agodoa L. Racial disparities in renal replacement therapy. J Natl Med Assoc. 2002 Aug;94(8 Suppl):45S–54S. [PMC free article] [PubMed] [Google Scholar]
- 12.Abbott KC, Glanton CW, Trespalacios FC, et al. Body mass index, dialysis modality, and survival: analysis of the United States Renal Data System Dialysis Morbidity and Mortality Wave II Study. Kidney Int. 2004 Feb;65(2):597–605. doi: 10.1111/j.1523-1755.2004.00385.x. [DOI] [PubMed] [Google Scholar]
- 13.Agodoa L. Racial disparities in kidney health: the puzzle to solve. Am J Kidney Dis. 2002 Dec;40(6):1337–1339. doi: 10.1053/ajkd.2002.37395. [DOI] [PubMed] [Google Scholar]
- 14.Agodoa L, Eggers P. Racial and ethnic disparities in end-stage kidney failure-survival paradoxes in African-Americans. Semin Dial. 2007 Nov-Dec;20(6):577–585. doi: 10.1111/j.1525-139X.2007.00350.x. [DOI] [PubMed] [Google Scholar]
- 15.Bleyer AJ. Race and dialysis survival. Arch Intern Med. 1992 Apr;152(4):879–880. [PubMed] [Google Scholar]
- 16.Bleyer AJ, Tell GS, Evans GW, Ettinger WH, Jr., Burkart JM. Survival of patients undergoing renal replacement therapy in one center with special emphasis on racial differences. Am J Kidney Dis. 1996 Jul;28(1):72–81. doi: 10.1016/s0272-6386(96)90133-x. [DOI] [PubMed] [Google Scholar]
- 17.Bloembergen WE, Port FK, Mauger EA, Wolfe RA. Causes of death in dialysis patients: racial and gender differences. J Am Soc Nephrol. 1994 Nov;5(5):1231–1242. doi: 10.1681/ASN.V551231. [DOI] [PubMed] [Google Scholar]
- 18.Buckalew VM, Jr., Freedman BI. Reappraisal of the impact of race on survival in patients on dialysis. Am J Kidney Dis. Jun;55(6):1102–1110. doi: 10.1053/j.ajkd.2009.10.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cowie CC, Port FK, Rust KF, Harris MI. Differences in survival between black and white patients with diabetic end-stage renal disease. Diabetes Care. 1994 Jul;17(7):681–687. doi: 10.2337/diacare.17.7.681. [DOI] [PubMed] [Google Scholar]
- 20.Eisenstein EL, Sun JL, Anstrom KJ, et al. Do income level and race influence survival in patients receiving hemodialysis? Am J Med. 2009 Feb;122(2):170–180. doi: 10.1016/j.amjmed.2008.08.025. [DOI] [PubMed] [Google Scholar]
- 21.Frankenfield DL, Rocco MV, Frederick PR, Pugh J, McClellan WM, Owen WF., Jr. Racial/ethnic analysis of selected intermediate outcomes for hemodialysis patients: results from the 1997 ESRD Core Indicators Project. Am J Kidney Dis. 1999 Oct;34(4):721–730. doi: 10.1016/s0272-6386(99)70399-9. [DOI] [PubMed] [Google Scholar]
- 22.Held PJ, Pauly MV, Diamond L. Survival analysis of patients undergoing dialysis. JAMA. 1987 Feb 6;257(5):645–650. [PubMed] [Google Scholar]
- 23.Kalantar-Zadeh K, Miller JE, Kovesdy CP, et al. Impact of race on hyperparathyroidism, mineral disarrays, administered vitamin D mimetic, and survival in hemodialysis patients. J Bone Miner Res. Dec;25(12):2448–2458. doi: 10.1002/jbmr.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kjellstrand CM, Logan GM. Racial, sexual and age inequalities in chronic dialysis. Nephron. 1987;45(4):257–263. doi: 10.1159/000184160. [DOI] [PubMed] [Google Scholar]
- 25.Lowrie EG, Lew NL, Huang WH. Race and diabetes as death risk predictors in hemodialysis patients. Kidney Int Suppl. 1992 Oct;38:S22–31. [PubMed] [Google Scholar]
- 26.Medina RA, Pugh JA, Monterrosa A, Cornell J. Minority advantage in diabetic end-stage renal disease survival on hemodialysis: due to different proportions of diabetic type? Am J Kidney Dis. 1996 Aug;28(2):226–234. doi: 10.1016/s0272-6386(96)90306-6. [DOI] [PubMed] [Google Scholar]
- 27.Mesler DE, McCarthy EP, Byrne-Logan S, Ash AS, Moskowitz MA. Does the survival advantage of nonwhite dialysis patients persist after case mix adjustment? Am J Med. 1999 Mar;106(3):300–306. doi: 10.1016/s0002-9343(99)00020-0. [DOI] [PubMed] [Google Scholar]
- 28.Morris D, Samore MH, Pappas LM, Ramkumar N, Beddhu S. Nutrition and racial differences in cardiovascular events and survival in elderly dialysis patients. Am J Med. 2005 Jun;118(6):671–675. doi: 10.1016/j.amjmed.2005.02.023. [DOI] [PubMed] [Google Scholar]
- 29.Myers OB, Adams C, Rohrscheib MR, et al. Age, race, diabetes, blood pressure, and mortality among hemodialysis patients. J Am Soc Nephrol. Nov;21(11):1970–1978. doi: 10.1681/ASN.2010010125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Norris KC, Agodoa LY. Unraveling the racial disparities associated with kidney disease. Kidney Int. 2005 Sep;68(3):914–924. doi: 10.1111/j.1523-1755.2005.00485.x. [DOI] [PubMed] [Google Scholar]
- 31.Owen WF., Jr Racial differences in incidence, outcome, and quality of life for African-Americans on hemodialysis. Blood Purif. 1996;14(4):278–285. doi: 10.1159/000170274. [DOI] [PubMed] [Google Scholar]
- 32.Pei YP, Greenwood CM, Chery AL, Wu GG. Racial differences in survival of patients on dialysis. Kidney Int. 2000 Sep;58(3):1293–1299. doi: 10.1046/j.1523-1755.2000.00285.x. [DOI] [PubMed] [Google Scholar]
- 33.Price DA, Owen WF., Jr African-Americans on maintenance dialysis: a review of racial differences in incidence, treatment, and survival. Adv Ren Replace Ther. 1997 Jan;4(1):3–12. doi: 10.1016/s1073-4449(97)70011-6. [DOI] [PubMed] [Google Scholar]
- 34.Pugh JA, Tuley MR, Basu S. Survival among Mexican-Americans, non-Hispanic whites, and African-Americans with end-stage renal disease: the emergence of a minority pattern of increased incidence and prolonged survival. Am J Kidney Dis. 1994 Jun;23(6):803–807. doi: 10.1016/s0272-6386(12)80133-8. [DOI] [PubMed] [Google Scholar]
- 35.Robinson BM, Joffe MM, Pisoni RL, Port FK, Feldman HI. Revisiting survival differences by race and ethnicity among hemodialysis patients: the Dialysis Outcomes and Practice Patterns Study. J Am Soc Nephrol. 2006 Oct;17(10):2910–2918. doi: 10.1681/ASN.2005101078. [DOI] [PubMed] [Google Scholar]
- 36.Roderick P, Byrne C, Casula A, et al. Survival of patients from South Asian and Black populations starting renal replacement therapy in England and Wales. Nephrol Dial Transplant. 2009 Dec;24(12):3774–3782. doi: 10.1093/ndt/gfp348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Salem M. End-stage renal disease survival in blacks and whites. Am J Med Sci. 2002 Feb;323(2):100–101. doi: 10.1097/00000441-200202000-00008. [DOI] [PubMed] [Google Scholar]
- 38.Sehgal AR. Outcomes of renal replacement therapy among blacks and women. Am J Kidney Dis. 2000 Apr;35(4 Suppl 1):S148–152. doi: 10.1016/s0272-6386(00)70242-3. [DOI] [PubMed] [Google Scholar]
- 39.Streja E, Kovesdy CP, Molnar MZ, et al. Role of Nutritional Status and Inflammation in Higher Survival of African American and Hispanic Hemodialysis Patients. Am J Kidney Dis. 2011 Jan 14; doi: 10.1053/j.ajkd.2010.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Unruh M, Miskulin D, Yan G, et al. Racial differences in health-related quality of life among hemodialysis patients. Kidney Int. 2004 Apr;65(4):1482–1491. doi: 10.1111/j.1523-1755.2004.00529.x. [DOI] [PubMed] [Google Scholar]
- 41.Waikar SS, Curhan GC, Ayanian JZ, Chertow GM. Race and mortality after acute renal failure. J Am Soc Nephrol. 2007 Oct;18(10):2740–2748. doi: 10.1681/ASN.2006091060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tanna MM, Vonesh EF, Korbet SM. Patient survival among incident peritoneal dialysis and hemodialysis patients in an urban setting. Am J Kidney Dis. 2000 Dec;36(6):1175–1182. doi: 10.1053/ajkd.2000.19832. [DOI] [PubMed] [Google Scholar]
- 43.Young CJ, Gaston RS. Renal transplantation in black Americans. N Engl J Med. 2000 Nov 23;343(21):1545–1552. doi: 10.1056/NEJM200011233432107. [DOI] [PubMed] [Google Scholar]
- 44.Ellison MD, Breen TJ, Guo TG, Cunningham PR, Daily OP. Blacks and whites on the UNOS renal waiting list: waiting times and patient demographics compared. Transplant Proc. 1993 Aug;25(4):2462–2466. [PubMed] [Google Scholar]
- 45.Alexander GC, Sehgal AR. Barriers to cadaveric renal transplantation among blacks, women, and the poor. JAMA. 1998 Oct 7;280(13):1148–1152. doi: 10.1001/jama.280.13.1148. [DOI] [PubMed] [Google Scholar]
- 46.Ayanian JZ, Cleary PD, Weissman JS, Epstein AM. The effect of patients’ preferences on racial differences in access to renal transplantation. N Engl J Med. 1999 Nov 25;341(22):1661–1669. doi: 10.1056/NEJM199911253412206. [DOI] [PubMed] [Google Scholar]
- 47.Ozminkowski RJ, White AJ, Hassol A, Murphy M. Minimizing racial disparity regarding receipt of a cadaver kidney transplant. Am J Kidney Dis. 1997 Dec;30(6):749–759. doi: 10.1016/s0272-6386(97)90078-0. [DOI] [PubMed] [Google Scholar]
- 48.Sanfilippo FP, Vaughn WK, Peters TG, et al. Factors affecting the waiting time of cadaveric kidney transplant candidates in the United States. JAMA. 1992 Jan 8;267(2):247–252. [PubMed] [Google Scholar]
- 49.Hata Y, Cecka JM, Takemoto S, Ozawa M, Cho YW, Terasaki PI. Effects of changes in the criteria for nationally shared kidney transplants for HLA-matched patients. Transplantation. 1998 Jan 27;65(2):208–212. doi: 10.1097/00007890-199801270-00011. [DOI] [PubMed] [Google Scholar]
- 50.Segev DL, Kucirka LM, Oberai PC, et al. Age and comorbidities are effect modifiers of gender disparities in renal transplantation. J Am Soc Nephrol. 2009 Mar;20(3):621–628. doi: 10.1681/ASN.2008060591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mehrotra R, Kermah D, Fried L, Adler S, Norris K. Racial differences in mortality among those with CKD. J Am Soc Nephrol. 2008 Jul;19(7):1403–1410. doi: 10.1681/ASN.2007070747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Garg PP, Diener-West M, Powe NR. Reducing racial disparities in transplant activation: whom should we target? Am J Kidney Dis. 2001 May;37(5):921–931. doi: 10.1016/s0272-6386(05)80007-1. [DOI] [PubMed] [Google Scholar]
- 53.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 54.Schold JD, Srinivas TR, Braun WE, Shoskes DA, Nurko S, Poggio ED. The relative risk of overall graft loss and acute rejection among African American renal transplant recipients is attenuated with advancing age. Clin Transplant. 2010 Oct 22; doi: 10.1111/j.1399-0012.2010.01343.x. [DOI] [PubMed] [Google Scholar]
- 55.Port FK, Wolfe RA, Mauger EA, Berling DP, Jiang K. Comparison of survival probabilities for dialysis patients vs cadaveric renal transplant recipients. JAMA. 1993 Sep 15;270(11):1339–1343. [PubMed] [Google Scholar]