Dear Sir
The hemophilia B dog colony maintained at the Francis Owen Blood Laboratory at UNC-Chapel Hill serves as an invaluable model for coagulation factor and gene therapy studies. These animals have severe hemophilia B that closely mirrors the human disease, including spontaneous joint and soft tissue bleeds. Numerous published studies have reported absence of detectable canine factor IX (F.IX) antigen in the circulation of these animals using ELISA or RIA techniques (1–6). The genetic defect in these hemophilic dogs has been defined as a missense mutation within the sequence of the F.IX gene encoding the catalytic domain (1). Molecular modeling studies suggest that the change from a glycine to a glutamic acid residue does not allow the protein to fold properly which likely results in intracellular degradation of the protein and accounts for the absence of circulating protein (1).
In a recently published letter by Chao and Walsh, the authors present evidence that the hemophilia B dogs at UNC have circulating F.IX antigen that can be detected by Western blot of dilute plasma samples (7). Moreover, F.IX antigen levels by this assay were found to be identical in normal and hemophilia B dogs. Western blot analysis on crude plasma samples generally must be viewed with caution, because of the enormous number of proteins in the sample and potential cross-reactivity of antibodies. Here, we demonstrate that Western blot results published by Chao and Walsh do not allow the conclusion that the hemophilic dogs have circulating F.IX antigen. Rather, Western blots performed in our laboratories strongly support previously published and unpublished results from several laboratories, i.e. absence of F.IX antigen in plasma of these animals.
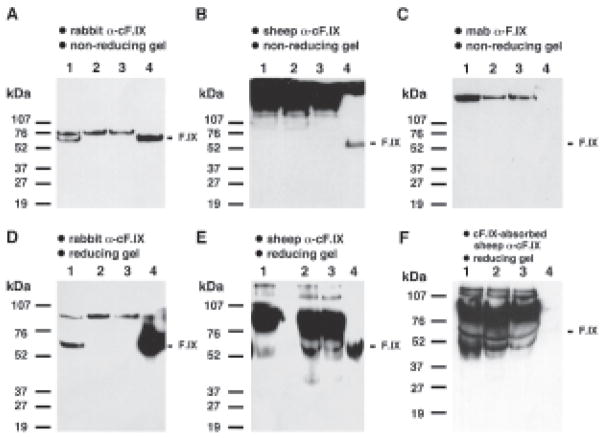
In Western blots summarized in Fig. 1, we tested pooled normal dog plasma (NDP) and plasma samples from two different hemophilia B dogs (canine hemophilia B plasma, HBP) for the presence of canine F.IX (cF.IX) antigen. Plasma samples where diluted 1:20 in PBS (that is twice as concentrated as the lowest dilution chosen by Chao and Walsh). The diluted samples were mixed with an equal volume of loading dye (10 μl of sample per lane), heat-denatured, and separated by SDS-PAGE under non-reducing or reducing conditions. We also included a lane loaded with 25 ng of purified plasma derived cF.IX protein (prepared in collaboration with Enzyme Research Laboratories, South Bend, Indiana) as a control for each blot. Published data suggest a cF.IX antigen concentration of 5–11.5 μg/ml in NDP (normal human FIX levels are 5 μg/ml). Assuming a concentration of cF.IX twice as high as human F.IX in plasma (2 × 5 μg/ml = 10 μg per ml), a 1:20 dilution of canine plasma with 10 μl loaded onto the gel would result in 5 ng cFI.X per lane, and therefore 5-times less than in the control lane (purified cF.IX protein). In this study, all primary and secondary antibodies were applied at a 1:1000 dilution. We first used a rabbit anti-cF.IX (Affinity Biologicals, Hamilton, Ontario, Canada) as the primary antibody followed by a swine anti-rabbit coupled to horseradish peroxidase (Dako Corporation, Carpinteria, California). The secondary antibody does not cross-react with canine plasma on a Western blot (data not shown). As shown in Fig. 1A and D, the rabbit antibody binds to a ~ 60 kDa band in NDP that is identical in size to cF.IX (lanes 1 and 4). In addition, it recognizes a band > 80 KDa in NDP and hemophilia B dog plasma (HBP, lanes 1–3. Cross-reactivity of a polyclonal antibody with other plasma proteins is not unusual for an antibody raised against a plasma-derived protein.). The intensity of the cF.IX band in NDP is as expected proportionally weaker than the band in the lane containing 25 ng of purified cF.IX protein. Results were identical under reducing and non-reducing conditions, as one would expect for F.IX (Fig. 1A and D). (The reducing gel in Fig. 1D was run longer to allow for better separation and size determination than in Fig. 1A.) Note the complete absence of a band corresponding in size to cF.IX in HBP samples. The rabbit anti-cF.IX is used in our laboratory for immunofluorescence staining and as the detecting antibody in the cF.IX ELISA because of its consistently background-free and reproducible results.
Fig. 1.
Western blot analysis of canine plasma. Lane 1: normal dog plasma. Lanes 2 and 3: hemophilia B dog plasma from two different animals. Lane 4: purified plasma-derived canine F.IX (25 ng). A–C: Nonreducing SDS-PAGE. D–E: Reducing SDS-PAGE, A, D: Primary antibody: rabbit anti-canine F.IX, secondary antibody: swine anti-rabbit immunoglobulins, B, E, F: Primary antibody; sheep anti-canine F.IX with horseardish peroxidase label. F: Antibody absorbed with purified cF.IX prior to incubation with membrane. C: Primary antibody: mouse monoclonal anti-human F.IX (clone FX008), secondary antibody: goat anti-mouse IgG (both antibodies from Boehringer Mannheim). Molecular size markers indicated for each blot were from Bio-Rad (Hercules, CA)
Next, we tested the sheep anti-cF.IX labeled with horseradish peroxidase, the antibody used by Chao and Walsh. This antibody was some what less sensitive in detecting purified cF.IX and failed to detect the 60 kDa cF.IX band in NDP (Fig. 1B, lanes 1 and 4.) when used at a 1:1,000 dilution. Interestingly, under non-reducing conditions, the antibody gave a strong and broad signal of >100 kDa. Under reducing conditions, the antibody yields at least six distinct bands, with the strongest reactivity to a protein of an apparent size of 80–90 kDa (Fig. 1E, lanes 1–3). This band is considerably more intense than the 25 ng cFIX band, and is equally strong in NDP and HBP. When compared with the molecular size marker and the 60 kDa cF.IX band, this slower migrating band clearly represents a protein that is approximately 20–30 kDa larger than cF.IX. These results are in direct disagreement with the size indicated for this band in the Western blot presented by Chao and Walsh, who labeled this band as 61 kDa. In the range of 60 kDa, we found a doublet of fainter bands (Fig. 1F, lanes 1–3) that was indicated as 50 kDa by Chao and Walsh (pre-absorption of the antibody with serum albumin results in disappearance of one of these bands, data not shown). Given the fact that the plasma bands in Fig. 1B and E disagree with the band for purified cF.IX and published data on F.IX in size, band intensity, and migration patterns in reducing vs. non-reducing gel conditions, we must conclude that none of the plasma protein bands recognized by the sheep antibody in Fig. 1B and E represents cF.IX. This point is particularly well illustrated in Fig. 1B which shows the failure of the sheep antibody to detect cF.IX in NDP even at plasma dilutions much more concentrated than those presented by Chao and Walsh. Furthermore, in both our laboratories, the sheep anti-cFIX is not used in ELISA because of high background, low sensitivity, and lack of reproducible results. Yet, Chao and Walsh claim to be able to detect cF.IX at a 1:640 dilution of NDP or HBP using a Western blot without having ruled out cross-reactivity of the polyclonal antibody with other plasma proteins. When used for IF staining, we were only able to obtain F.IX-specific results when this antibody was pre-absorbed in serum of a F.IX-deficient dog. As indicated above, the rabbit antibody does not detect the Western blot bands that are recognized by the sheep antibody in canine plasma (compare Fig. 1A and B and Fig. 1D and E), again illustrating strong affinity of the sheep antibody to proteins other than F.IX. To confirm that plasma protein bands seen with the sheep antibody under reducing conditions do not represent F.IX, we absorbed the antibody with purified cF.IX for 2 h at room temperature prior to incubation with the membrane. As shown in Fig. 1F, c.F.IX in the control lane 4 is now only detectable as a faint band if the blot is overexposed, while the intensities of the bands in normal and hemophilic plasma remain unchanged (lanes 1–3).
In an additional effort to demonstrate absence of cF.IX in HBP, we separated NDP and HBP by two-dimensional gel electrophoresis followed by Western blot and incubation with the rabbit anti-cF.IX. This analysis reveals cF.IX in NDP as a protein of approximately 60 kDa found at a pI between 6.4 and 6.5 (Fig. 2, right panel). No signal in this range was obtained for HBP (Fig. 2, left panel).
Fig. 2.
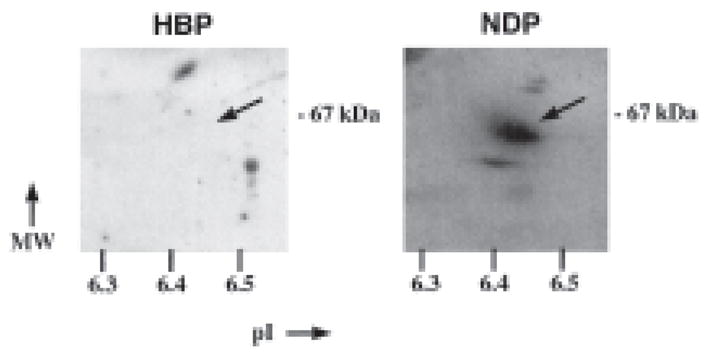
Western analysis of 2D-IEF/SDS-PAGE hemophilia B dog plasma (HBP, left panel) and normal dog plasma (NDP, right panel). Isoelectric focusing utilized a 20% pH 3–10, 80% 5–7 ampholine mixture according to previously described methods (10), while SDS-PAGE was performed in 10% acrylamide gels. Plasma (0.5 ml) was electrophoresed on each gel. Western blot analysis was performed with the rabbit anti-cF.IX (Affinity Biologicals) with enhanced chemiluminescence detection with modifications detailed by White et al.(11)
Chao and Walsh published as data not shown that the mouse monoclonal antibody FX008, originally raised against human F.IX, gives bands identical to those seen with the sheep antibody. This monoclonal antibody cross-reacts with cF.IX and is useful as a capture antibody in an ELISA or for purification of cFIX (8). As shown in Fig. 1C, this antibody failed to detect purified cF.IX and cF.IX in NDP at least at the low amount of sample loaded (≤25 ng). The higher band (> 100 kDa) in all plasma samples likely represents canine IgG which is detected due to cross-reactivity of the secondary anti-mouse IgG. These bands are also obtained if the secondary antibody is used alone (data not shown). Note that Figure 1C represents a non-reducing gel. Under the reducing conditions, IgG migrates at ~50 kDa, and therefore appears similar in size to F.IX.
In conclusion, our Western blot results support absence of cF.IX antigen in plasma of the Chapel-Hill strain of hemophilia B dogs and strongly suggest the bands shown by Chao and Walsh do not represent F.IX, but rather were obtained due to lack of specificity of the antibody used in their study. In support of this conclusion, it should be noted that the hemophilia B data base reports a mutation at the identical position in human F.IX (residue 381, glycine to alanine), and this is associated with very low levels of circulating protein (9). Finally, we have introduced the identical mutation, glycine to glutamic acid at amino acid residue 381, into the human F.IX sequence. Transient transfection assays of this construct demonstrate synthesis of intracellular mutant F.IX protein at levels comparable to wild-type F.IX, whereas very little mutant F.IX is secreted into the conditioned media of transfected cells (Fitzgerald, Pleimes, and Herzog, unpublished results). These results directly confirm the published interpretation that this particular amino acid change results in a gene product that is translated but not efficiently secreted. Since these results were obtained with an ELISA, the argument by Chao and Walsh that Western blot analysis will detect a mutant F.IX protein that cannot be detected by ELISA is likely not valid. Last, in light of the data presented here, conclusions by Chao and Walsh about tolerance to F.IX in the hemophilia B dogs arising from the presence of F.IX in the circulation are likely incorrect.
This is a commentary on article Chao H, Walsh CE. Endogenous canine FIX antigen exists in Chapel Hill strain hemophilia B canine. Thromb Haemost. 1999;82(4):1378.
References
- 1.Evans JP, Brinkhous KM, Brayer GD, Reisner HW, High KA. Canine hemophilia B resulting from a point mutation with unusual consequences. Proc Natl Acad Aci USA. 1989;86:10095–99. doi: 10.1073/pnas.86.24.10095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kay MA, Rothenberg S, Landen CN, Bellinger DA, Leland F, Toman C, Finegold M, Thompson AR, Read MS, Brinkous KM, Woo SLC. In vivo gene therapy of hemophilia B: sustained partial correction in factor IX-deficient dogs. Science. 1993;262:117–9. doi: 10.1126/science.8211118. [DOI] [PubMed] [Google Scholar]
- 3.Kay MA, Landen CN, Rothenberg SR, Taylor LA, Leland F, Wiehle S, Fang B, Bellinger D, Finegold M, Thompson AR, et al. In vivo hepatic gene therapy: complete albeit transient correction of factor IX deficiency in hemophilia B dogs. Proc Natl Acad Sci USA. 1994;91:2353–7. doi: 10.1073/pnas.91.6.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fang B, Wang H, Gordon G, Bellinger DA, Read MS, Brinkhous KM, Woo SLC, Eisensmith RC. Lack of persistent of E1- recombinant adenoviral vectors containing a temperature-sensitive E2A mutation in immunocompetent mice and hemophilia B dog. Gene Ther. 1996;3:217–22. [PubMed] [Google Scholar]
- 5.Herzog RW, Yang EY, Couto LB, Hagstrom JN, Elwell D, Fields PA, Burton M, Bellinger DA, Read MS, Brinkhous KM, Podsakoff GM, Nichols TC, Kurtzman GJ, High KA. Long-term correction of canine hemophilia B by gene transfer of blood coagulation factor IX mediated by adeno-associated viral vector. Nat Med. 1999;5:56–63. doi: 10.1038/4743. [DOI] [PubMed] [Google Scholar]
- 6.Synder RO, Miao C, Meuse L, Tubb J, Donahue BA, Lin H-F, Stafford DW, Patel S, Thompson AR, Nichols T, Read MS, Bellinger DA, Brinkhous KM, Kay MA. Correction of hemophilia B in canine and murine models using recombinant adeno-associated viral vectors. Nat Med. 1999;5:64–70. doi: 10.1038/4751. [DOI] [PubMed] [Google Scholar]
- 7.Chao H, Walsh CE. Endogenous FIX antigen exists in Chapel Hill strain hemophilia B canine. Thromb Haemost. 1999;82:1378. [PubMed] [Google Scholar]
- 8.Sugahara Y, Catelfamo J, Brooks M, Hitomi E, Bajaj SP, Kurachi K. Isolation and characterization of canine factor IX. Thromb Haemost. 1996;75:450–55. [PubMed] [Google Scholar]
- 9.Green PM, et al. Haemophilia B: database of point mutations and short additions and delitions—v9.0. 1999 doi: 10.1093/nar/18.14.4053. http://www.umds.acuk/molgen. [DOI] [PMC free article] [PubMed]
- 10.O’Farrell P. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975;250:4007–21. [PMC free article] [PubMed] [Google Scholar]
- 11.White T, Lacal J, Reep B, Fischer T, Lapetina E, White G. Thrombolamban, the 22 kilodalton platelet substrate of cAMP-dependent protein kinase, is immunologically homologous with the ras family of GTP-binding proteins. Proc Natl Acad Sci USA. 1990;87:758–62. doi: 10.1073/pnas.87.2.758. [DOI] [PMC free article] [PubMed] [Google Scholar]