Key Points
The prevalence of MBL among blood donors is much higher than previously reported.
Although uncommon, higher-risk MBL phenotypes and genotypes were observed.
Abstract
Circulating monoclonal B cells may be detected in healthy adults, a condition called monoclonal B-cell lymphocytosis (MBL). MBL has also been identified in donated blood, but no systematic study of blood donors has been reported. Using sensitive and specific laboratory methods, we detected MBL in 149 (7.1%; 95% confidence interval, 6.0% to 8.3%) of 2098 unique donors ages 45 years or older in a Midwestern US regional blood center between 2010 and 2011. Most of the 149 donors had low-count MBL, including 99 chronic lymphocytic leukemia–like (66.4%), 22 atypical (14.8%), and 19 CD5– (12.8%) immunophenotypes. However, 5 donors (3.4%) had B-cell clonal counts above 500 cells per µL, including 3 with 1693 to 2887 cells per µL; the clone accounted for nearly all their circulating B cells. Four donors (2.7%) had 2 distinct MBL clones. Of 51 MBL samples in which immunoglobulin heavy chain (IGH)V-D-J genotypes could be determined, 71% and 29% used IGHV3- and IGHV4-family genes, respectively. Sequencing revealed 82% with somatic hypermutation, whereas 18% had >98% germ-line identity, including 5 with entirely germ-line sequences. In conclusion, MBL prevalence is much higher in blood donors than previously reported, and although uncommon, the presence of high-count MBL warrants further investigations to define the biological fate of the transfused cells in recipients.
Introduction
Older adults in apparent good health may have small numbers of monoclonal B cells detectable in their peripheral blood,1-7 a condition called monoclonal B-cell lymphocytosis (MBL).8 MBL is an essential precursor to chronic lymphocytic leukemia (CLL)9 and is variably associated with other B-cell malignancies.5,10 The reported prevalence of MBL ranges from <1%4,5 to 18%,7 depending on the detection methods and populations tested.11 Most MBL clones have an immunophenotype resembling typical CLL and represent a small number of circulating B cells,12 referred to as low-count MBL.1 This MBL variant is considered quiescent with low risk of progression to CLL.1 However, some CLL-like MBL clones are present in much higher numbers in blood and progress to symptomatic CLL at a rate of 1% to 2% per year.13,14 Other MBL clones have less common immunophenotypes that do not resemble typical CLL.12 The natural history of these variants is not as well understood, but they may have a higher risk of progression to other B-cell malignancies.5,10
MBL has been detected in donated blood,4 and a recent meta-analysis suggests that blood transfusions may be associated with an increased risk for developing B-cell malignancies.15 However, a systematic study of MBL prevalence in blood donors using sensitive and specific laboratory methods is lacking.
We conducted the first such study to obtain stable estimates of age- and sex-specific MBL prevalence, ensuring exclusion of repeat donors. The study revealed a much higher prevalence of MBL in blood donors than previously reported.4 The predominant immunophenotype was low-count CLL-like MBL, but high-count (clinical) MBL was also observed, warranting further investigations aimed at defining the biological fate of the transfused cells in the recipients.
Materials and methods
Study population and sample collection
The study base population comprised individuals age 45 years or older who voluntarily donated whole blood to the Community Blood Center of Greater Kansas City, Missouri, between May 2010 and November 2011. On 2 to 3 days weekly during the 18-month study period, we collected residual blood from the diversion pouch of the whole blood unit donated by each individual sampled from the base population. The blood specimens in sodium heparin tubes were maintained at room temperature and sent to the flow cytometry laboratory of St. Luke’s Hospital within 24 hours of collection. We obtained the following information from donor history forms routinely filled out by the blood center during the donor screening: age, gender, date of most recent donation, history of transfusion within the past 12 months, and history of any cancer. Family history of cancer was not available. We also reviewed the results of routine screening tests for hepatitis B virus, hepatitis C virus (HCV), and HIV for individuals who donated blood at a site and on a date when samples were being collected for the study. We unlinked the donor identity from the study results by using separate identification numbers for the blood specimens and the study data collection form that are different from the original donor identification number. A master identification number linking the blood specimen and the data collection form was kept by the study principal investigator for the data analysis.
To ensure that no donor was sampled more than once, we excluded donors who had previously donated at a site on a day when study samples had been collected at that site. We also reviewed all the immunophenotype patterns and immunoglobulin gene analyses we obtained to exclude any possibility of duplicate specimens from the same donors with MBL. The study protocol was approved by the Institutional Review Board of the US Centers for Disease Control and Prevention.
Flow cytometry
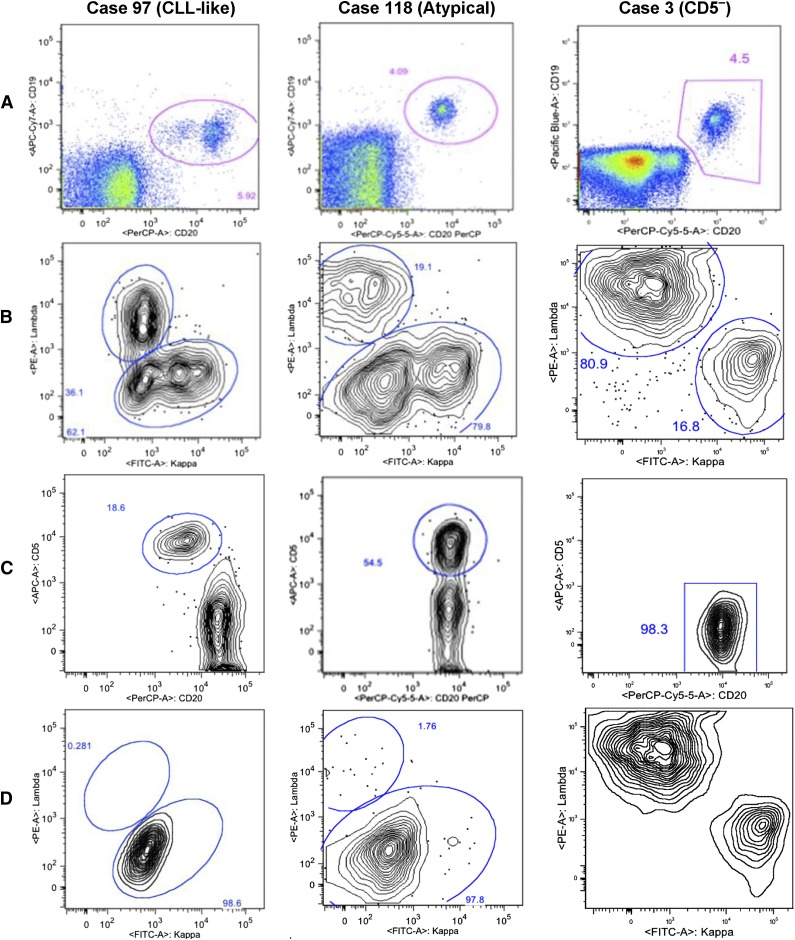
Samples were initially screened by flow cytometry at St. Luke’s Hospital using a 6-color antibody panel (BD Biosciences) containing CD19-PerCP-Cy5.5, CD20-allophycocyanin, CD5-V450, CD45-V500, κ-phycoerythrin, and λ–fluorescein isothiocyanate. At least 500 000 events were collected on a BD FACS Canto II flow cytometer. The data analysis method was similar to that described in previous MBL studies.16,17 Briefly, CD45+ lymphocytes were first gated on CD45 vs side scatter (SSC), and then singlet cells were isolated using forward scatter (FSC) height vs FSC area. Debris and monocytes were excluded by gating on FSC and SSC, and B cells were isolated by gating on CD20 vs CD19. B cells were examined on a CD20 vs CD5 plot. The CD20dim B-cell populations were interrogated for intensity of CD5 expression and for light-chain exclusion by κ/λ analysis. When possible, CD5+ and CD5– B cells were gated separately for κ/λ analysis. Samples were classified as positive for MBL if they contained a phenotypic cluster of at least 50 clonal B cells. MBL subtypes were determined as previously described16,17 (see Figure 1).
Figure 1.
Gating strategy used for the detection of 3 distinct immunophenotypes of MBL. Representative immunophenotypes are shown for the 3 categories: CLL-like (CD19+CD5+CD20dim), atypical (CD19+CD5+CD20bright), and CD5ˉ (CD19+CD5ˉ) MBL.16,17 (A) Two parameter histograms of CD20 peridinin chlorophyll protein complex (PerCP) on x-axis and CD19 (allophycocyanin Cy7 or Pacific Blue) on y-axis were based on initial CD45 expression and SSC signal. B cells are gated for subsequent analysis. (B) κ (FITC) λ (PE) analysis on gated B cells. Cases 97 and 118 have bimodal κ populations showing dim clone and bright polyclonal κ cells. Case 3 shows 80.9% λ population. (C) Gated B cells are displayed on a CD20 vs CD5 plot. Case 97 is subgated on CD20dim and CD5+,bright B cells. Case 118 is subgated on CD5+ B cells. In case 3, all the B cells are CD5ˉ. (D) Subgated B cells from panel C are displayed on a κ vs λ plot. Case 97 shows 98.6% κ+ clone on κ-λ analysis on gated CD20dim/CD5+ B cells. Case 118 shows κ-λ analysis on gated CD5+ B cells showing 97.8% κ+ clone. Case 3 shows a skewed κ-λ analysis on gated CD5ˉ B cells showing 81.9% λ+ cells. FITC, fluorescein isothiocyanate; PE, phycoerythrin.
Complete blood counts were obtained with a Sysmex XE2100. Absolute B-cell counts were calculated from the flow cytometry and complete blood count data. For CLL-like MBL, absolute monoclonal B-cell counts were obtained from the percentage of the gated CD5+ B-cell population showing light-chain restriction. For atypical MBL and CD5– MBL, absolute monoclonal B-cell counts were estimated from the skewness of the light-chain distribution in the gated population.
Donor samples classified as screen positive or inconclusive for MBL were shipped overnight to the flow cytometry laboratory at the Food and Drug Administration for confirmative immunophenotpying. The interlaboratory review resulted in complete agreement for all cases of CLL-like MBL, including monotypic light-chain expression. For a small number of atypical and CD5– MBL cases where discrepancy existed concerning the level of CD5 expression and light-chain expression, consensus diagnoses were reached by the 2 laboratories. There was also unanimous agreement on the cases with biclonal expression.
PCR amplification of IGHV-D-J rearrangements and sequence analysis
DNA was extracted utilizing the QIAmp blood kit (Qiagen, Hilden, Germany). An aliquot of the DNA samples was stored at −80°C and sent to the Istituto Scientifico San Raffaele in Milano for polymerase chain reaction (PCR) amplification and nucleotide sequence analysis. PCR amplification of the immunoglobulin heavy chain region (IGH)V-D-J rearrangements was performed as previously described using a seminested PCR approach (consensus framework region [FR]1 or FR2 primers plus 2 consensus IGHJ primers).3 Monoclonal PCR products were purified from low-melting-point agarose gels and sequenced on an automated ABI sequencer directly. In 2 cases, because of the limited size of the clone, sequencing was performed after cloning, using the TA Cloning kit (Invitrogen, Carlsbad, Germany), and at least 10 colonies were analyzed for each PCR amplification product.
Sequence data were analyzed using the international ImMunoGeneTics information system database and tools.18 A cutoff value of 98% germ-line identity was used to discriminate between mutated and unmutated rearrangements.19,20 Rearrangements involving the IGHV4-59 and IGHV4-61 genes were considered together because the use of an FR2 primer for PCR amplification hampered the possibility of clearly assigning the sequences to either one of the IGHV genes as a result of the high identity between the 2 genes except in the heavy framework region 1/heavy complementarity determining region 1.3 Our sequences were aligned to a previously reported series of CLL rearrangements21 to exclude stereotypy within the HCDR3 sequences.
Statistical methods
Demographic characteristics and laboratory findings of the blood donors are summarized as counts, proportions, percentiles, means, and ranges. We calculated age- and gender-specific prevalence estimates of MBL with the 95% confidence interval (CI). Age was categorized into 45-54 years, 55-64 years, and >64 years. Multivariate log-binomial regression analyses22 were used to calculate prevalence ratios (PRs) for MBL after adjusting for the effects of covariates. We examined the association between MBL subtype and donor characteristics by using Fisher’s exact test or Kruskal-Wallis test.23 All analyses were performed using SAS software, Version 9.3 (SAS Institute Inc., Cary, NC).
Results
During the 18-month study period, we collected blood samples from 2098 unique donors of the 35 752 total donors in the study base population. The median age of the 2098 donors was 57 years (range 45-91 years), and 54% were men. The race of the individual donors was not known, but ∼94% of the overall donor population was white. The median cell counts of white blood cells, absolute lymphocytes, and absolute B-cells were 5700/µL (range 2450-16 920/µL), 1760/µL (range 490-5210/µL), and 171/µL (range 14-2925/µL), respectively.
A history of cancer was reported in 114 (5.4%) of the 2098 donors. HCV was positive in 4 individuals who donated blood at a site and on a date when study samples were collected. No donors tested positive for hepatitis B virus or HIV.
Prevalence of MBL
MBL was confirmed by both laboratories in 149 of the 2098 donors, yielding the overall prevalence of 7.1% (95% CI, 6.0% to 8.3%) (Table 1). The prevalence was higher in men than in women and increased with age in both genders. The higher prevalence in men was consistent across all age groups. There was an ∼1.4-fold increase for every 10 years in the MBL prevalence among men, starting from 6.0% in the 45-54 years group and reaching 12.2% in the 65 years or older group. Among women, the prevalence was 1.7%, 7.6%, and 8.8% in the 45-54, 55-64, and 65 years or older age groups, respectively.
Table 1.
Age- and gender-specific prevalence of MBL among 2098 blood donors age 45 years or older in a Midwestern US regional blood center between 2010 and 2011
| Age | Women | Men | All* | |||
|---|---|---|---|---|---|---|
| N† | Prevalence estimate‡ (95% CI) | N† | Prevalence estimate‡ (95% CI) | N† | Prevalence estimate‡ (95% CI) | |
| 45-54 y | 7/406 | 1.7 (0.7-3.5) | 24/398 | 6.0 (3.9-8.8) | 31/805 | 3.9 (2.6-5.4) |
| 55-64 y | 27/357 | 7.6 (5.0-10.8) | 38/440 | 8.6 (6.2-11.7) | 65/798 | 8.1 (6.3-10.3) |
| 65 y or older | 18/205 | 8.8 (5.3-13.5) | 35/288 | 12.2 (8.6-16.5) | 53/495 | 10.7 (8.1-13.8) |
| All ages | 52/968 | 5.4 (4.0-7.0) | 97/1126 | 8.6 (7.0-10.4) | 149/2098 | 7.1 (6.0-8.3) |
The denominators in this column include a total of 4 donors whose gender was unreported.
Number of donors with MBL/number of all donors in the category.
Prevalence estimate per 100 persons.
In multivariate analyses, age and gender were independent risk factors for MBL (Table 2). The age-adjusted PR for gender (men/women) was 1.5 (95% CI, 1.1-2.1; P = .01). Compared with the youngest age group (45-54 years), the gender-adjusted PR was 2.1 (95% CI, 1.4-3.1; P = .0007) for the 55-64 age group and 2.7 (95% CI, 1.7-4.1; P < .0001) for the 65 years or older age group. MBL was more prevalent among donors who had a history of any cancer (11.4% of 114) than donors who did not (6.8% of 1983); however, this difference was not significant after adjusting for the effects of age and gender. None of the donors with MBL were positive for HCV.
Table 2.
Adjusted PRs of MBL
| Independent risk factor | Adjusted PR | 95% CI | P value |
|---|---|---|---|
| Gender | |||
| Men | 1.5* | 1.1-2.1 | .01 |
| Women | 1 (ref) | ||
| Age | |||
| >64 y | 2.7† | 1.7-4.1 | <.0001 |
| 55-64 y | 2.1† | 1.4-3.1 | .0007 |
| 45-54 y | 1 (ref) |
Adjusted for age.
Adjusted for gender.
MBL subtype and clonal B-cell count
CLL-like MBL was the most prevalent subtype (101 or 67.8%) among the 149 MBL cases, followed by atypical (23 or 15.4%) and CD5– (21 or 14.1%) MBL subtypes. Four donors (2.7%) had more than 1 clone. Donors with CD5– immunophenotype were significantly older (median 67 years) than donors with CLL-like (median 61 years; P = .002) or atypical CLL-like (median 62 years; P = .04) immunophenotypes; whereas there was no significant age difference between CLL-like and atypical immunophenotypes. Gender distribution was also different between the MBL subtypes. The majority of donors with atypical immunophenotype were men (91%) compared with 57% in CLL-like immunophenotype (P = .002). However, gender distribution of donors with CD5– phenotype was not significantly different from those with CLL-like or atypical immunophenotypes.
The absolute B-cell count ranged from 16/µL to 2925/µL (median 177/µL) among all 149 MBL cases, including 3 cases whose counts were above 1000/µL. In 45% of all MBL cases, the B-cell count was below the median values of non-MBL donors of the same age. There were no significant differences in the absolute B-cell counts between MBL subtypes.
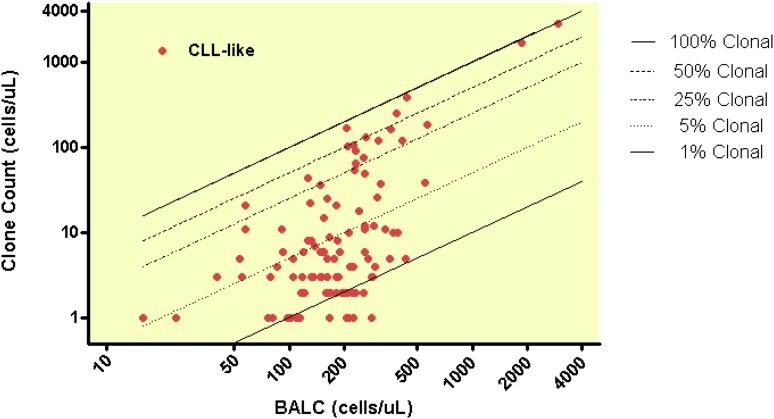
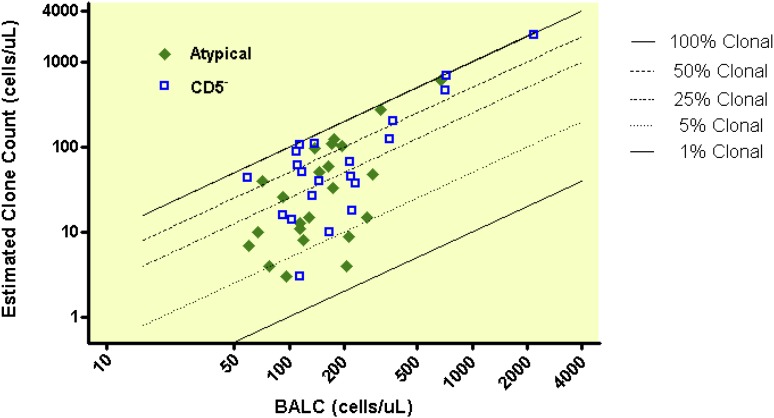
The relationship between absolute B-cell count and clonal B-cell count is shown for CLL-like phenotype (Figure 2) and for atypical and CD5– immunophenotypes (Figure 3). Clonal B-cell count was low in most MBL cases (median 10/µL), with 75% of all MBL cases having a clonal count ≤51/µL. However, 13 MBL cases (9% of 149) had a clonal B-cell count above 165/µL, including 5 cases that had a count above 500/µL. The clonal B-cell count differed significantly between the MBL subtypes (<.0001); the median clonal B-cell counts were 5/µL, 26/µL, and 51/µL for CLL-like, atypical, and CD5– immunophenotypes, respectively.
Figure 2.
Clonal B-cell counts among 101 CLL-like MBL cases.
Figure 3.
Clonal B-cell counts among 23 atypical and 21 CD5– MBL cases.
Immunoglobulin gene repertoire and mutational status of MBL cases
Of the 149 MBL cases, 147 were PCR-amplified and examined for the presence of monoclonal IGHV-D-J rearrangements. A total of 53 IGHV-D-J rearrangements were detected by either direct sequencing (51) or cloning (2): 32 CLL-like MBL, 8 atypical MBL, 11 CD5– MBL, and 2 biclonal MBL cases. The absolute clonal B-cell counts were much higher (median 44/µL) among these 53 cases than in the remaining 94 cases where IGHV genes could not be sequenced (median 6/µL).
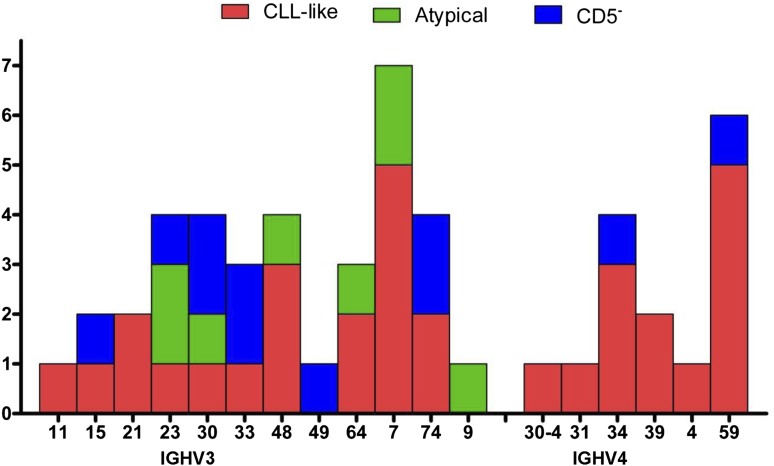
Excluding 2 biclonal cases, all detected IGHV genes belonged to the IGHV3 and IGHV4 families (Figure 4). Among these, as expected, IGHV3 family genes were more prevalent in all MBL subtypes, although the frequency was very different: 59.4% (19/32), 100% (8/8), and 81.8% (9/11) of CLL-like, atypical, and CD5– immunophenotypes, respectively (Figure 4). The most frequent IGHV genes among CLL-like MBL were IGHV3-7 (5/32) and IGHV4-59 (5/32). In the sequence analysis, 41 MBL (82% of 50) cases were identified as having mutated sequences, using the 98% germ-line identity cut-point value (Table 3). Of the 9 unmutated cases, 5 had 100% homology to the corresponding germ-line sequences: 4 CLL-like and 1 atypical MBL. The remaining 4 included 2 CLL-like and 2 CD5– immunophenotypes. None of the IGHV-D-J rearrangements detected showed any features of stereotypy in the HCDR3 region including the unmutated MBL cases. Table 4 shows the IGHV-D-J rearrangements and mutational status of 13 MBL cases with a clonal B-cell count above 165/µL. Of these, 3 cases were unmutated, including 2 cases with 100% homology to the corresponding germ-line sequences. The B-cell clonal count was well above 1000/µL in 3 of the 13 cases, and 1 of them had 100% homology to the corresponding germ-line sequence.
Figure 4.
IGHV gene repertoires in 51 MBL cases.
Table 3.
Germ-line and somatic hypermutation status of IGHV gene in 50 MBL cases
| MBL subtype | Mutated N (%) | Unmutated* N (%) | Total N (%) |
|---|---|---|---|
| CLL-like | 25 (80.6) | 6 (19.4) | 31 (100) |
| Atypical | 7 (87.5) | 1 (12.5) | 8 (100) |
| CD5– | 9 (81.8) | 2 (18.2) | 11 (100) |
| Total | 41 (82.0) | 9 (18.0) | 50 (100) |
Having a ≥98% germ-line identity. Includes 5 cases with 100% homology to the corresponding germ-line sequence: 4 CLL-like (2 IGHV3-74, 1 IGHV3-48, and 1 IGHV4-59) and 1 atypical MBL (IGHV3-23).
Table 4.
IGHV-D-J rearrangements and IGHV mutational status in MBL cases that had a clonal B-cell count above 165/µL
| ID | MBL subtype | Age | History cancer* | WBC† | ALC† | BALC† | Clonal B cell† | κ/λ‡ | IGHV | IGHD | IGHJ | IGHD RF | HCDR3 length | Mutation§ (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | CLL-like | 57 | No | 5080 | 1840 | 357 | 166 | 0.02 | n/a | n/a | n/a | n/a | n/a | n/a |
| 2 | CLL-like | 63 | No | 6090 | 2080 | 206 | 169 | 96.00 | IGHV3-33 | IGHD3-22*01 | IGHJ3*02 | 2 | 23 | Mutated (97.6) |
| 3 | CLL-like | 53 | No | 10 300 | 2940 | 567 | 183 | 31.67 | IGHV4-59 | IGHD6-19*01 | IGHJ4*01 | 1 | 13 | Mutated (93.7) |
| 4 | CLL-like | 74 | Yes | 3650 | 1170 | 385 | 251 | 79.00 | n/a | n/a | n/a | n/a | n/a | n/a |
| 5 | CLL-like | 52 | Yes | 7390 | 2590 | 438 | 385 | 99.00 | IGHV3-74 | IGHD6-19*01 | IGHJ6*02 | 1 | 22 | Unmutated (100) |
| 6 | CLL-like | 56 | No | 6610 | 3260 | 1871 | 1693 | 60.00 | IGHV4-34 | IGHD3-10*01 | IGHJ5*01 | 2 | 23 | Mutated (91.3) |
| 7 | CLL-like | 75 | No | 9110 | 5140 | 2925 | 2887 | 99.00 | IGHV3-48 | IGHD4-23*01 | IGHJ5*01 | 2 | 18 | Unmutated (100) |
| 8 | Atypical | 73 | No | 5470 | 2180 | 318 | 280 | 18.80 | IGHV3-64 | IGHD2-21*02 | IGHJ6*03 | 2 | 16 | Mutated (88.3) |
| 9 | Atypical | 71 | Yes | 6360 | 2320 | 670 | 621 | 98.00 | IGHV3-23 | IGHD6-13*01 | IGHJ4*01 | 1 | 14 | Mutated (97.0) |
| 10 | CD5– | 71 | No | 6740 | 2260 | 368 | 201 | 18.80 | IGHV3-15 | IGHD3-10*01 | IGHJ6*02 | 3 | 17 | Unmutated (98.2) |
| 11 | CD5– | 53 | No | 9480 | 3190 | 711 | 464 | 32.33 | IGHV3-23 | IGHD2-21*02 | IGHJ4*01 | 3 | 16 | Mutated (93.2) |
| 12 | CD5– | 58 | No | 6090 | 1970 | 727 | 704 | 47.00 | n/a | n/a | n/a | n/a | n/a | n/a |
| 13 | CD5– | 67 | No | 8910 | 4720 | 2185 | 2065 | 49.00 | IGHV3-49 | IGHD5-12*01 | IGHJ4*01 | 2 | 14 | Mutated (95.3) |
ALC, absolute lymphocyte count; BALC, absolute B-cell lymphocyte count; IGHD RF, IGHD reading frame; n/a, data not available; WBC, white blood cell count.
History of any cancer.
Absolute cell count per µL.
Percent of κ-expressing cells divided by percent of λ-expressing cells, derived from analysis of the identified MBL population.
Somatic hypermutation status of IGHV gene (percent homology to the corresponding germ-line sequences).
Discussion
This is the first study to characterize MBL in blood donors using sensitive and specific laboratory methods. Our study population was sufficiently large and detection methods were sufficiently sensitive to observe the expected predominance of low-count MBL. Specificity was strengthened by independent analysis in a separate laboratory of samples initially screened as positive for MBL, the first cross-laboratory verification to be conducted in an MBL prevalence study. We employed rigorous procedures to exclude duplicate blood specimens from the same donors, ensuring validity of the prevalence estimates.
Our observed overall MBL prevalence of 7.1% is much higher than previous observations of 0.12% in blood donors, which used less sensitive laboratory methods and was limited to a subset of donors with higher B-cell counts or coexpression of CD19 and CD5 with light-chain restriction.4 On the other hand, our prevalence is lower than the 14.3% observed in the healthy volunteers from a primary health care population in Salamanca, Spain.2 This difference is likely attributable to the greater sensitivity provided by immunophenotyping a larger number of lymphocytes,24 10-fold more than our study. Our MBL prevalence is also lower than the 13.6%6 and 18.2%7 observed in unaffected first-degree relatives of CLL patients, a difference attributable to the known familial risk of lymphoproliferative diseases.25,26 However, it is definitely in line with the 7.4% observed in the similar age group of healthy volunteers living near Milan, Italy,3 using laboratory methods with comparable sensitivity to this study. It is indeed notable that the high median age of donors, with the oldest being 91, closely mirrors the age distribution of the Italian study population.
Our observations of the higher MBL prevalence in men than in women and increasing prevalence with increasing age confirms the findings of previous MBL studies.11 The higher prevalence in men than in women was consistent across all age groups, and the increasing prevalence with age was consistent in both genders, which parallels observations of CLL incidence.27 Biological factors attributable to these differences in MBL prevalence or CLL incidence are not clearly understood. In our multivariate log-binomial regression analysis, men were 50% (P = .01) more likely to have MBL than women, after taking into account the effects of age; the probability of having MBL was 110% higher in the 55-64 years age group (P = .0007) and 170% higher in the 65 years or older age group (P < .0001) than the 45-54 years age group, after taking into account the effects of gender.
The biological characteristics of our MBL case series were very similar to those reported in previous studies. The most prevalent MBL subtype was the CLL-like phenotype (CD5+CD20dim) with normal absolute B-cell counts and low clonal B-cell counts, followed by atypical and CD5– subtypes, as observed in general population MBL cases.2,3 Four donors had more than 1 clone. These observations confirm the previous reports of phenotypic heterogeneity of MBL.2,3,10,16,28
The most frequently identified IGHV genes in our CLL-like MBL cases were IGHV3-7 and IGHV4-59, showing an immunoglobulin gene repertoire similar to that in the normal aging population.1,3 In contrast, we did not observe immunoglobulin genes commonly expressed in CLL (eg, IGHV1-69, IGHV3-07), nor stereotypy in the HCDR3 region, which is common in CLL, confirming observations of biased usage specific in MBL cases with low-count clonal B-cells.1,3 Somatic hypermutation status of IGHV genes is an independent prognostic factor in CLL patients, with unmutated IGHV genes being associated with more aggressive forms of CLL.19,20 In our CLL-like MBL cases, 80.6% had mutated IGHV genes, slightly higher than 70% reported in a general population MBL case series.3 The predominance of somatic hypermutation in the IGHV genes was also noted in our atypical and CD5– MBL cases, as previously reported.2,9 The immunoglobulin gene repertoire expressed by low-count MBL in this study may indeed represent a biased sample of cases with a concentration of monoclonal B cells above the detection threshold of the technique. The possibility therefore exists that cases with very low-count MBL for which no immunoglobulin gene sequences could be obtained may carry a different immunoglobulin gene repertoire. In the near future, enrichment of MBL clones by flow cytometric sorting and use of next-generation sequencing may be helpful to clarify this issue.
Although the majority of cases in our MBL series had low absolute counts of B cells and clonal B cells, we also observed some MBL cases with clonal B cells above 500/µL, a proposed cutoff to distinguish CLL-like MBL cases based on their potential to progress to CLL.1 In particular, 1 CLL-like MBL case had an absolute B-cell count of 2925/µL and a clonal count of 2887/µL. This case also demonstrated an unmutated status of IGHV genes, with 100% homology to the corresponding germ-line sequence.
Our study was limited by design to testing samples drawn at 1 time point. In theory, low-count MBL might regress toward normal or progress to high-count MBL and to a B-cell malignancy. Current data to determine this potential have been derived from a study that followed a cohort of MBL cases detected in a general population.29 Although low-count MBL clones persisted over time in 54 of 60 CLL-like MBL cases, none of them developed clinically relevant lymphocytosis nor became high-count MBL during the follow-up period (median 34 months, range 11-50 months).29 Approximately 50% of MBL cases with atypical or CD5– phenotypes became undetectable, but this observation was based on a small number of cases.29
MBL transfer from donor to recipient has been reported in the setting of allogeneic stem cell transplant for CLL.30,31 To date, however, MBL has not been reported in any recipients following blood transfusion. Indeed, no MBL studies have been conducted in transfusion recipients. An association between allogeneic blood transfusions and non-Hodgkin lymphoma (NHL) was investigated in a number of studies with mixed results. A meta-analysis summarizing these reports found an increased risk for NHL in cohort studies.15 The risk was significantly higher for CLL/small lymphocytic lymphoma than other NHL subtypes (relative risk = 1.66; 95% CI, 1.08-2.56).15 Based on these findings, the investigators recommended a conservative approach to blood transfusion needs.15 Knowledge of the potential of monoclonal B-cells to persist or proliferate in transfusion recipients is lacking, but transfused leukocytes may persist for decades in recipients with severe trauma.32
In conclusion, we demonstrated a much higher prevalence of MBL in blood donors than previously reported. Most MBL cases detected in our donor population have low-count CLL-like MBL that is considered to have little, if any, increased risk for progression to a lymphoid malignancy for the donor, let alone for a transfusion recipient. However, because we found that a proportion of donated blood does indeed contain high absolute counts of monoclonal B-cells with less indolent phenotypes, this evidence indicates that a conservative approach toward blood transfusions may be prudent.15 In addition, our findings warrant further investigations aimed at defining the biological fate of the transfused cells in the recipients. Future studies on the natural history of MBL need to involve longer follow-up periods with larger numbers of low-count MBL cases, including all 3 subtypes. Such studies could explore risk factors for MBL progression, including biological markers and environmental exposures, which may shed light on MBL natural history in blood donors and recipients.
Acknowledgments
We thank Dr Kostas Stamatopoulos for his help in analyzing potential stereotypy of the IGHV-D-J rearrangements and Mr Spencer Jones for database management and technical assistance.
This project was supported in part by the Agency for Toxic Substances and Disease Registry and in part by the Associazione Italiana per la Ricerca sul Cancro AIRC (Investigator Grant and Special Program Molecular Clinical Oncology – 5 per mille #9965), Ricerca Finalizzata 2010, Ministero della salute, Roma; Progetti di Ricerca di Interesse Nazionale – Ministero Istruzione, Università e Ricerca, Roma, Italy.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention/Agency for Toxic Substances and Disease Registry or the US Food and Drug Administration.
Footnotes
Presented in poster form in part at the 53rd annual meeting of the American Society of Hematology, San Diego, CA, December 2011.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: All authors contributed to the study design, data analysis, and manuscript writing; Y.K.S., J.K., and R.F.V. oversaw the overall conduct of data collection and performed final data analysis; J.M.R., J.B., G.E.M., F.V.P., H.D., and F.A. oversaw and performed immunophenotyping; P.G. and A.D. oversaw and performed genotyping; and G.V. and J.E.M. oversaw blood donor specimen and data collection.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The publisher acknowledges the right of the US government to retain a nonexclusive royalty-free license in and to any copyright covering the article.
Correspondence: Youn K. Shim, Agency for Toxic Substances and Disease Registry, Division of Toxicology and Human Health Sciences, 4770 Buford Highway, Atlanta, GA 30341-3717; e-mail: yshim@cdc.gov.
References
- 1.Vardi A, Dagklis A, Scarfò L, et al. Immunogenetics shows that not all MBL are equal: the larger the clone, the more similar to CLL. Blood. 2013;121(22):4521–4528. doi: 10.1182/blood-2012-12-471698. [DOI] [PubMed] [Google Scholar]
- 2.Nieto WG, Almeida J, Romero A, et al. Increased frequency (12%) of circulating chronic lymphocytic leukemia–like B-cell clones in healthy subjects using a highly sensitive multicolor flow cytometry approach. Blood. 2009;114(1):33–37. doi: 10.1182/blood-2009-01-197368. [DOI] [PubMed] [Google Scholar]
- 3.Dagklis A, Fazi C, Sala C, et al. The immunoglobulin gene repertoire of low-count chronic lymphocytic leukemia (CLL)–like monoclonal B lymphocytosis is different from CLL: diagnostic implications for clinical monitoring. Blood. 2009;114(1):26–32. doi: 10.1182/blood-2008-09-176933. [DOI] [PubMed] [Google Scholar]
- 4.Rachel JM, Zucker ML, Fox CM, et al. Monoclonal B-cell lymphocytosis in blood donors. Br J Haematol. 2007;139(5):832–836. doi: 10.1111/j.1365-2141.2007.06870.x. [DOI] [PubMed] [Google Scholar]
- 5.Shim YK, Vogt RF, Middleton D, et al. Prevalence and natural history of monoclonal and polyclonal B-cell lymphocytosis in a residential adult population. Cytometry B Clin Cytom. 2007;72B(5):344–353. doi: 10.1002/cyto.b.20174. [DOI] [PubMed] [Google Scholar]
- 6.Rawstron AC, Yuille MR, Fuller J, et al. Inherited predisposition to CLL is detectable as subclinical monoclonal B-lymphocyte expansion. Blood. 2002;100(7):2289–2290. doi: 10.1182/blood-2002-03-0892. [DOI] [PubMed] [Google Scholar]
- 7.Marti GE, Carter P, Abbasi F, et al. B-cell monoclonal lymphocytosis and B-cell abnormalities in the setting of familial B-cell chronic lymphocytic leukemia. Cytometry B Clin Cytom. 2003;52B(1):1–12. doi: 10.1002/cyto.b.10013. [DOI] [PubMed] [Google Scholar]
- 8.Marti GE, Rawstron AC, Ghia P, et al. International Familial CLL Consortium. Diagnostic criteria for monoclonal B-cell lymphocytosis. Br J Haematol. 2005;130(3):325–332. doi: 10.1111/j.1365-2141.2005.05550.x. [DOI] [PubMed] [Google Scholar]
- 9.Landgren O, Albitar M, Ma W, et al. B-cell clones as early markers for chronic lymphocytic leukemia. N Engl J Med. 2009;360(7):659–667. doi: 10.1056/NEJMoa0806122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lanasa MC, Allgood SD, Slager SL, et al. Immunophenotypic and gene expression analysis of monoclonal B-cell lymphocytosis shows biologic characteristics associated with good prognosis CLL. Leukemia. 2011;25(9):1459–1466. doi: 10.1038/leu.2011.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shim YK, Middleton DC, Caporaso NE, et al. Prevalence of monoclonal B-cell lymphocytosis: a systematic review. Cytometry B Clin Cytom. 2010;78B(S1):S10–S18. doi: 10.1002/cyto.b.20538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marti GE, Abbasi F, Raveche E, et al. Overview of monoclonal B-cell lymphocytosis. Br J Haematol. 2007;139(5):701–708. doi: 10.1111/j.1365-2141.2007.06865.x. [DOI] [PubMed] [Google Scholar]
- 13.Rawstron AC, Bennett FL, O’Connor SJ, et al. Monoclonal B-cell lymphocytosis and chronic lymphocytic leukemia. N Engl J Med. 2008;359(6):575–583. doi: 10.1056/NEJMoa075290. [DOI] [PubMed] [Google Scholar]
- 14.Shanafelt TD, Kay NE, Rabe KG, et al. Brief report: natural history of individuals with clinically recognized monoclonal B-cell lymphocytosis compared with patients with Rai 0 chronic lymphocytic leukemia. J Clin Oncol. 2009;27(24):3959–3963. doi: 10.1200/JCO.2008.21.2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Castillo JJ, Dalia S, Pascual SK. Association between red blood cell transfusions and development of non-Hodgkin lymphoma: a meta-analysis of observational studies. Blood. 2010;116(16):2897–2907. doi: 10.1182/blood-2010-03-276683. [DOI] [PubMed] [Google Scholar]
- 16.Ghia P, Prato G, Scielzo C, et al. Monoclonal CD5+ and CD5- B-lymphocyte expansions are frequent in the peripheral blood of the elderly. Blood. 2004;103(6):2337–2342. doi: 10.1182/blood-2003-09-3277. [DOI] [PubMed] [Google Scholar]
- 17.Rawstron AC, Kennedy B, Evans PA, et al. Quantitation of minimal disease levels in chronic lymphocytic leukemia using a sensitive flow cytometric assay improves the prediction of outcome and can be used to optimize therapy. Blood. 2001;98(1):29–35. doi: 10.1182/blood.v98.1.29. [DOI] [PubMed] [Google Scholar]
- 18.Giudicelli V, Chaume D, Lefranc MP. IMGT/V-QUEST, an integrated software program for immunoglobulin and T cell receptor V-J and V-D-J rearrangement analysis. Nucleic Acids Res. 2004;32(suppl 2):W435–W440. doi: 10.1093/nar/gkh412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hamblin TJ, Davis Z, Gardiner A, Oscier DG, Stevenson FK. Unmutated Ig V(H) genes are associated with a more aggressive form of chronic lymphocytic leukemia. Blood. 1999;94(6):1848–1854. [PubMed] [Google Scholar]
- 20.Damle RN, Wasil T, Fais F, et al. Ig V gene mutation status and CD38 expression as novel prognostic indicators in chronic lymphocytic leukemia. Blood. 1999;94(6):1840–1847. [PubMed] [Google Scholar]
- 21.Agathangelidis A, Darzentas N, Hadzidimitriou A, et al. Stereotyped B-cell receptors in one-third of chronic lymphocytic leukemia: a molecular classification with implications for targeted therapies. Blood. 2012;119(19):4467–4475. doi: 10.1182/blood-2011-11-393694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spiegelman D, Hertzmark E. Easy SAS calculations for risk or prevalence ratios and differences. Am J Epidemiol. 2005;162(3):199–200. doi: 10.1093/aje/kwi188. [DOI] [PubMed] [Google Scholar]
- 23.Hollander M, Wolfe DA. 2nd ed. New York, NY: John Wiley & Sons; 1999. Nonparametric Statistical Methods. [Google Scholar]
- 24.Champion PD. A computer simulation for exploring the detection of monoclonal B-cell lymphocytosis by flow cytometry. Cytometry B Clin Cytom. 2010;78B(S1):S110–S114. doi: 10.1002/cyto.b.20557. [DOI] [PubMed] [Google Scholar]
- 25.Goldin LR, Pfeiffer RM, Li X, Hemminki K. Familial risk of lymphoproliferative tumors in families of patients with chronic lymphocytic leukemia: results from the Swedish Family-Cancer Database. Blood. 2004;104(6):1850–1854. doi: 10.1182/blood-2004-01-0341. [DOI] [PubMed] [Google Scholar]
- 26.Crowther-Swanepoel D, Corre T, Lloyd A, et al. Inherited genetic susceptibility to monoclonal B-cell lymphocytosis [published correction appears in Blood. 2011;117(12):3477]. Blood. 2010;116(26):5957–5960. doi: 10.1182/blood-2010-07-294975. [DOI] [PubMed] [Google Scholar]
- 27.Howlader N, Noone AM, Krapcho M, et al. eds. SEER Cancer Statistics Review, 1975-2010. National Cancer Institute, Bethesda, MD. http://seer.cancer.gov/csr/1975_2010/. Based on November 2012 SEER data submission, posted to the SEER Web site, April 2013.
- 28.Lanasa MC, Allgood SD, Volkheimer AD, et al. Single-cell analysis reveals oligoclonality among ‘low-count’ monoclonal B-cell lymphocytosis. Leukemia. 2010;24(1):133–140. doi: 10.1038/leu.2009.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fazi C, Scarfò L, Pecciarini L, et al. General population low-count CLL-like MBL persists over time without clinical progression, although carrying the same cytogenetic abnormalities of CLL. Blood. 2011;118(25):6618–6625. doi: 10.1182/blood-2011-05-357251. [DOI] [PubMed] [Google Scholar]
- 30.Pavletic SZ, Zhou G, Sobocinski K, et al. Center for International Blood and Marrow Transplant Research (CIBMTR), Medical College of Wisconsin, Milwaukee. Genetically identical twin transplantation for chronic lymphocytic leukemia. Leukemia. 2007;21(12):2452–2455. doi: 10.1038/sj.leu.2404928. [DOI] [PubMed] [Google Scholar]
- 31.Herishanu Y, Eshel R, Kay S, et al. Unexpected detection of monoclonal B-cell lymphocytosis in a HLA-matched sibling donor on the day of allogeneic stem cell transplantation for a patient with chronic lymphocytic leukaemia: clinical outcome [Correspondence]. Br J Haematol. 2010;149(6):905–907. doi: 10.1111/j.1365-2141.2010.08133.x. [DOI] [PubMed] [Google Scholar]
- 32.Utter GH, Lee TH, Rivers RM, et al. Microchimerism decades after transfusion among combat-injured US veterans from the Vietnam, Korean, and World War II conflicts. Transfusion. 2008;48(8):1609–1615. doi: 10.1111/j.1537-2995.2008.01758.x. [DOI] [PubMed] [Google Scholar]