Key Points
Washing older blood before transfusion reduces plasma iron, improving outcomes from established infection in canines.
In contrast, washing fresh blood before transfusion increases in vivo plasma CFH release, worsening outcomes.
Abstract
In a randomized controlled blinded trial, 2-year-old purpose-bred beagles (n = 24), with Staphylococcus aureus pneumonia, were exchanged-transfused with either 7- or 42-day-old washed or unwashed canine universal donor blood (80 mL/kg in 4 divided doses). Washing red cells (RBC) before transfusion had a significantly different effect on canine survival, multiple organ injury, plasma iron, and cell-free hemoglobin (CFH) levels depending on the age of stored blood (all, P < .05 for interactions). Washing older units of blood improved survival rates, shock score, lung injury, cardiac performance and liver function, and reduced levels of non-transferrin bound iron and plasma labile iron. In contrast, washing fresh blood worsened all these same clinical parameters and increased CFH levels. Our data indicate that transfusion of fresh blood, which results in less hemolysis, CFH, and iron release, is less toxic than transfusion of older blood in critically ill infected subjects. However, washing older blood prevented elevations in plasma circulating iron and improved survival and multiple organ injury in animals with an established pulmonary infection. Our data suggest that fresh blood should not be washed routinely because, in a setting of established infection, washed RBC are prone to release CFH and result in worsened clinical outcomes.
Introduction
Transfusion of older stored canine universal donor blood in a canine model of experimental Staphylococcus aureus pneumonia results in markedly increased lung injury and mortality rates.1 Transfusion with older blood is also associated with increased levels of cell-free hemoglobin (CFH), transferrin bound iron (TBI), non-TBI (NTBI) and plasma labile iron (PLI). NTBI represents iron excess bound to proteins that do not normally handle circulating iron, and PLI is the toxic iron moiety in plasma. Whereas increased nitric oxide scavenging by CFH causing vasoconstriction and vascular injury and increased available iron promoting bacterial growth represent 2 candidate mechanisms of injury, multiple other biological changes have been documented with increasing blood storage interval.2,3 Some of these changes involve the release into the plasma of biologically active proteins, microvesicles, potassium, acid, and plasticizer, all of which can be reduced by means of standard red cell (RBC) washing procedures.4-10 The clinical effect(s) of washing on the RBC storage lesion has not been studied.
RBC washing has long been performed to reduce potassium levels in stored blood transfused to neonates, debris from RBCs recovered during surgery, cryoprotectant glycerol from cryopreserved RBCs, and plasma proteins from blood intended for patients who have been sensitized to those proteins.11-13 Automated cell washers capable of removing more than 90% of plasma solute have been available for more than 40 years. Washing has been shown to improve the in vitro characteristics of stored RBCs.4-10,14-17 Earlier generations of cells washers were labor intensive and considered “open systems,” which limited the shelf life of washed cells to 24 hours. Current microprocessor-driven, closed-system cell-washers can be programmed to provide extensive cell washing and an approved shelf life of 14 days. These instruments effectively reduce supernatant potassium and plasma proteins while maintaining low levels of hemolysis and acceptable in vivo RBC survival.5,8,9
Transfusion of older units of RBCs reportedly increases morbidity and mortality in a variety of clinical situations.18 Currently, 5 randomized controlled trials are being conducted in Canada, the United States, and Australia to confirm or refute these reports.19-23 Strategies to improve the quality of stored RBC by limiting the storage period and licensing improved preservative solutions have already been introduced in the United States and in Europe.24 Washing blood is another practical approach to improving RBC quality by removing substances that accumulate progressively during the 6 weeks of refrigerated storage.
We conducted a blinded randomized controlled study of RBC washing in our canine model of transfusion injury. We hypothesized that washing older units of blood before transfusion would improve clinical outcomes by removing CFH and iron, whereas washing fresher units would have no effect on outcome. We found that washing improved clinical outcomes of canines receiving older blood transfusions but unexpectedly worsened morbidity and mortality in animals that received fresh, washed blood.
Materials and methods
Study design
Synopsis.
Twenty-four purpose-bred beagles (12 to 28 months old, 9 to 12.5 kg) were studied using a 2 × 2 factorial study design comparing transfusion of 42- vs 7-day-old stored canine universal donor blood that was 1) washed using a commercially available automated blood cell processing system just before transfusion or 2) not washed. Four animals each study week for 6 sequential weeks were given an intrabronchial challenge of S aureus (1.5 × 109 CFU/kg). Four hours later, animals were randomized to undergo an exchange-transfusion (80 mL/kg) in 4 divided doses (20 mL/kg) given sequentially over 3 hours each with either washed or unwashed old or washed or unwashed fresh universal donor canine blood. Except for the 4 different treatments of blood used for transfusion, animals were treated identically. All animals were given standard intensive care ancillary therapies including mechanical ventilation, fluids, vasopressors, and antibiotics. The animals were continuously monitored and cared for by a clinician or trained technician blinded to animal treatment groups throughout the 96-hour study. The National Institutes of Health Clinical Center Institutional Animal Care and Use Committee approved all studies.
Full study design.
This model of pneumonia using S aureus and performing exchange-transfusions was previously described.1 Briefly, anesthetized animals day 0 were instrumented (external jugular vein catheter, femoral artery catheter, and urinary catheter) followed with placement of a balloon-tipped pulmonary arterial thermodilution catheter and a tracheostomy performed as described.1,25 Following these procedures, animals were weaned off inhalation anesthesia, and continuous sedation (fentanyl, midazolam, and medetomidine) and mechanical ventilation via a tracheostomy tube were initiated. At time 0 hours (T0), S aureus isolates were prepared and administered via bronchoscope into the right lower lobe of the lung.25 The dose of S aureus (1.5 × 109 CFU/kg suspended in 1 mL of PBS), based on previous studies, produced 100% lethality with unwashed older stored canine blood and 30% mortality with fresher blood. The bacterial dose was determined spectrophotometrically and confirmed turbidometrically as previously described.1 At 4 hours, antibiotic treatment with Oxacillin (30 mg/kg IV), demonstrated to be effective against this strain of S aureus, was initiated and administered every 4 hours until 96 hours or death. Following antibiotic therapy at 4 hours, an exchange transfusion was initiated with each animal, randomized in a blinded fashion kept by the statistician (J.S.) to receive either: 7-day-old unwashed blood, 42-day-old unwashed blood, 7-day-old washed blood, and 42-day-old washed blood. At 0 hours and before each transfusion (4, 7, 10, and 13 hours), intravascular hemodynamic measures and blood samples were obtained including arterial blood gases, complete blood counts, serum chemistries, plasma CFH, haptoglobin, NTBI, PLI and TBI (see the supplemental Data available on the Blood Web site). All animals were provided conventional intensive care unit support, including prophylaxis for gastrointestinal ulcer (famotidine 1 mg/kg iv, every 12 hours) and deep venous thrombosis (heparin 3000 IU, sq, every 8 hours) as well as procedures to prevent pressure ulcers (regular position change). Mechanical ventilation, fluid support, and vasopressor therapy were also used and titrated using physiological and laboratory end points using previously described algorithms1,25 (see the supplemental Methods). Animals alive at 96 hours were considered survivors and were killed while still sedated (Beuthanol; 75 mg/kg IV).
A detailed description of the statistical methods and the cell washing procedure is provided in the supplemental Methods.
Results
Survival
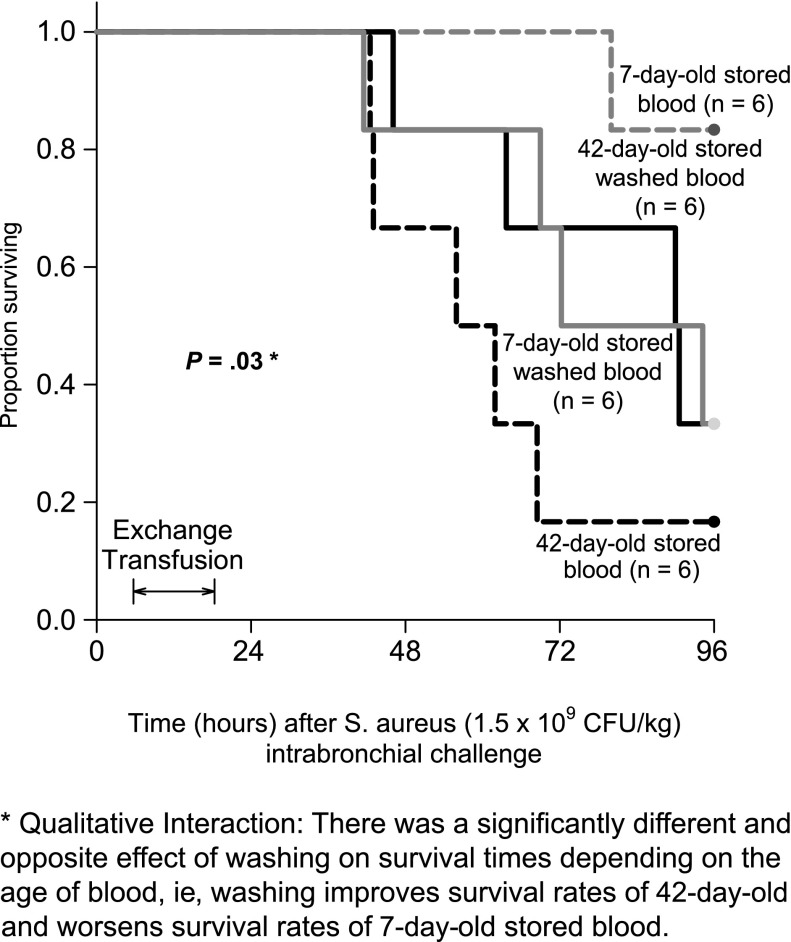
Washing RBC before exchange transfusion in animals with experimental S aureus pneumonia had a significantly different and opposite effect on survival rates depending on the age of the stored blood (qualitative interaction, P = .03, Figure 1). In animals transfused with 42-day stored blood, washing the RBC improved survival time. In contrast, with 7-day-old stored blood, washing the RBC worsened survival time.
Figure 1.
Survival curves. Kaplan-Meier plots throughout the 96-hour study comparing the 4 types of transfused blood are represented. The different types of blood are represented by different line patterns as follows: dashed black line (42-day-old unwashed blood), solid black line (42-day-old washed blood), solid gray line (7-day-old washed blood), and dashed gray line (7-day-old unwashed blood). There is a qualitative interaction between washing and the age of stored blood (P = .03).
Shock reversal score and lung injury score
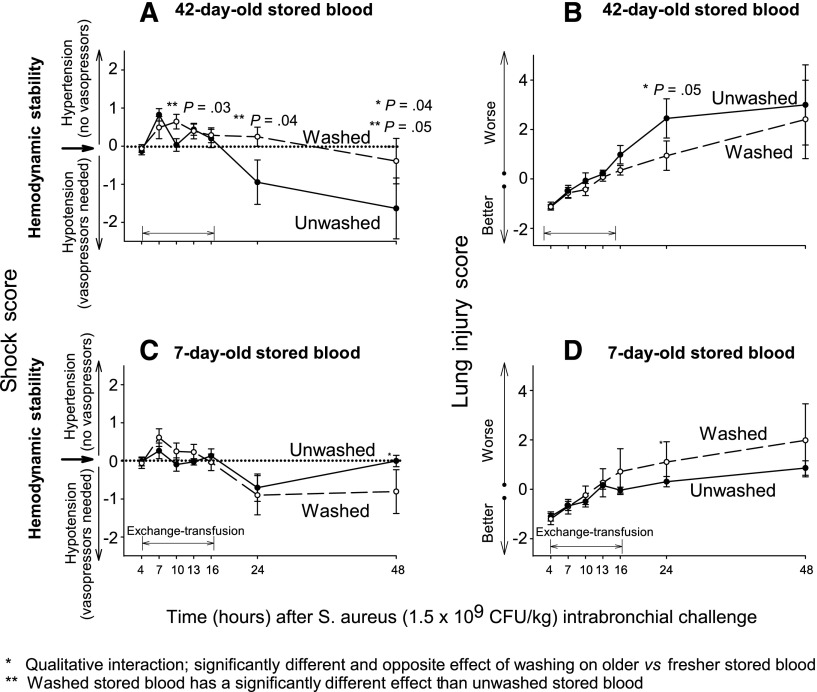
The shock reversal score takes into account the level of vasopressor support (norepinephrine) needed to maintain the mean arterial pressure at a preset normal level for canines (80-100 mm Hg).1 The lung injury score (LIS) takes into account 5 parameters of lung function (mPAP, arterial alveolar oxygen gradient, plateau pressures, breathing rates, and oxygen saturation based on pulse oximetry), and its use has been previously validated to increase the ability to detect abnormalities in the lung.1 Similar to the effect on survival, washing had significantly different and opposite effects on the level of shock reversal score (48 hours) and LIS (24 hours) (qualitative interaction, P = .04 and P = .05 respectively, Figure 2). Washing older RBCs improved the LIS and the shock reversal score (ie, less hypotension and vasopressor use and less severe lung injury respectively). In contrast, at these same time points washing fresher RBCs worsened both the LIS and the shock score. For completeness the effect of washing on the different components of the shock and LISs are provided in the supplemental Data (supplemental Figures 1 and 2).
Figure 2.
Mean (±SE) shock scores and LIS at serial time points. (A-D) The shock score accounts for the level of vasopressor support (norepinephrine) needed to maintain the mean arterial pressure at a preset normal level for canines (mean 80 mm Hg).1,28,29 (A,C) the shock score is plotted through time (x-axis) for animals receiving 42-day-old blood (A) or 7-day-old blood (C). The LIS in a previously published scoring system increases our ability to find the abnormalities in the lungs, which includes mean pulmonary artery pressure, alveolar-arterial oxygen gradient, plateau pressure, O2 saturation, and respiratory rate.1,28,29 (B,D) The LIS is plotted in the same fashion as the shock score for old and fresh blood. In each of these panels, unwashed blood is represented by a solid line and washed blood by a dashed line. The P value in each panel indicates differences between washed and unwashed blood (**) and the presence of qualitative interactions between age of blood and washing (*).
Cardiac performance
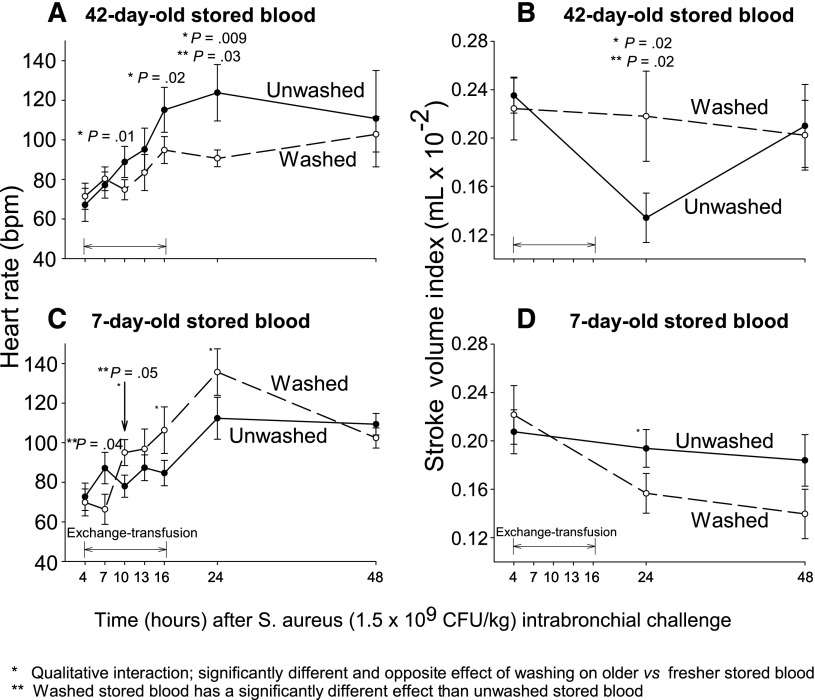
To investigate the cardiac response to these acute changes in lung injury with washing (Figure 2 and supplemental Figure 1), we examined the effect of washing older or fresher RBCs on the components of cardiac output, heart rate, and stroke volume. Similar significant qualitative interactions were found for heart rate and stroke volume at 24 hours after infection (P = .009 and P = .02, respectively, Figure 3) as were found for survival, shock, and LIS scores. The washing of older red blood cells lowered (normalized) heart rate and improved stroke volume index (SVI). In contrast, at the same time, washing fresher RBCs increased the heart rate causing marked tachycardia while decreasing SVI. Using cardiac echo, the size of the right ventricle was estimated at 24 hours in comparison with the left ventricle in a 4-chambers view. Right ventricle dilation was considered present if the right ventricle was greater than ⅔ the size of the left ventricle read blinded to allocation groups. Examining all available studies, animals receiving older unwashed red blood cells had nominally more right ventricular dilation (¾ [75%]) compared with washed cells (⅖ [40%]), and animals receiving washed fresher RBC had nominally more right ventricular dilatation (¼ [25%]) than those receiving unwashed fresh RBCs (⅕ [20%]). These data taken together are most consistent with the notion that washing older RBCs either directly or indirectly lessened severe lung injury, lowering mPAP and right-side afterload causing stroke volume to increase and heart rate to decline or normalize. Washing fresher RBCs had the opposite effect.
Figure 3.
Mean (±SE) heart rate and SVI at serial time points. This figure uses the same format as Figure 2, except now heart rate (A,C) or SVI (B,D) are plotted on the y-axis. Similarly, P values are denoted by asterisks in each panel and are explained below the figures.
Liver function
To investigate the effect of washing on liver function, we examined serial mean levels of ALT and total bilirubin. At 48 hours after S. aureus challenge, we found, similar to the survival, shock, LIS, and cardiovascular data, a significantly different and opposite effect of washing on ALT and total bilirubin levels depending on the age of transfused blood (qualitative interaction, P = .03 and P = .02 for ALT and bilirubin, respectively, supplemental Figure 3A-B). This effect was mainly driven by a marked decrease with washing of both markers of liver dysfunction in the animals transfused with older blood.
Serial mean plasma levels of NTBI, PLI, and TBI
We measured serial indicators of excess plasma iron after transfusion with assays for NTBI and PLI. After 75% of the blood was transfused, at 13 hours after bacterial challenge, we found a significantly different and opposite effect of washing red blood cells on plasma iron levels depending on the age of blood storage (qualitative interaction, P = .009 and P = .02 for NTBI and PLI, respectively, Figure 4). Washing older RBCs just before transfusion significantly lowered the plasma levels of both NTBI and PLI found after transfusion, and washing fresher RBCs just before transfusion raised, if minimally, the plasma levels after transfusion. TBI levels were significantly lower after fresh blood transfusion compared with older blood, but there were no differences between washed and unwashed blood of each age of blood group.
Figure 4.
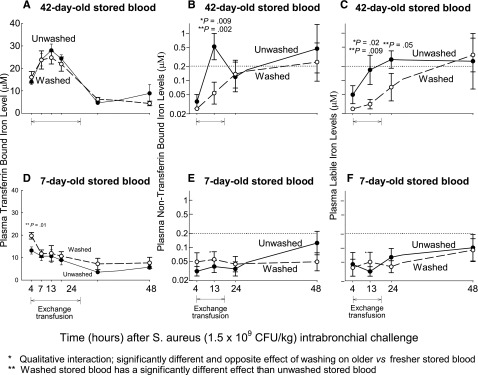
Mean (±SE) levels of NTBI, PLI, and TBI at serial time points. The format is similar to Figure 2, except now plasma levels of NTBI (A,D), PLI (B,E) and TBI (C,F) are plotted on the y-axis. P values are denoted by asterisks and explained below the figures.
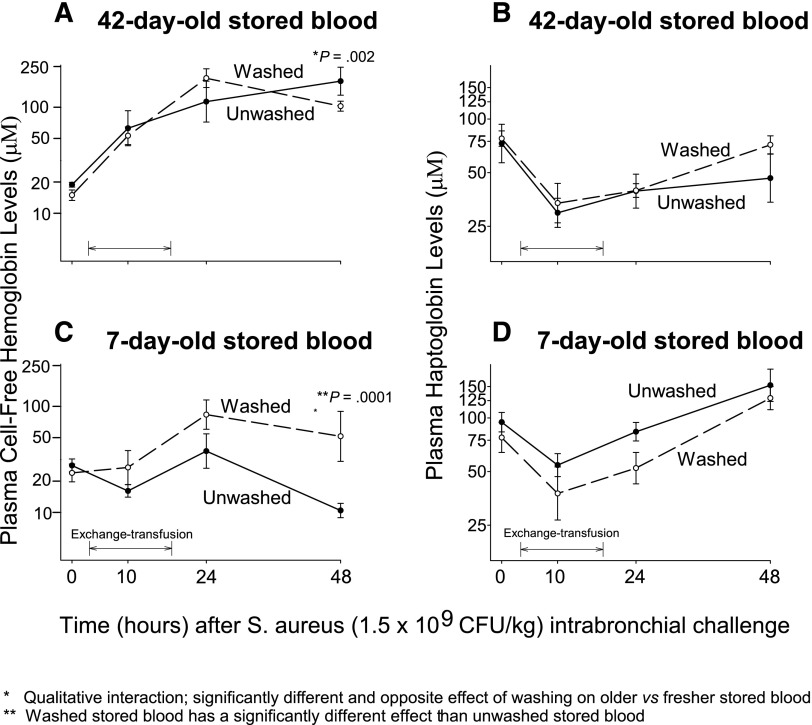
Serial mean levels of plasma CFH and haptoglobin
We also measured serial plasma CFH, the levels of which are known to be increased after older stored blood transfusion and also to be increased with severe bacterial infections26,27 At 48 hours after bacterial challenge and 34 hours after transfusion, washing RBCs produced a significantly different and opposite effect on plasma levels of CFH depending on the age of the stored blood (qualitative interaction, P = .002). Washing older red blood cells decreased CFH-plasma levels 34 hours after transfusion (Figure 5A) whereas washing fresher red blood cells increased CFH levels at 34 hours after transfusion (Figure 5C). Plasma levels of haptoglobin, a high-affinity hemoglobin scavenger, significantly decreased during transfusion in the 4 treatment groups and slowly recovered to reach near baseline values 10 to 34 hours after transfusion (Figure 5B,D).
Figure 5.
Mean (±SE) levels of plasma CFH and plasma haptoglobin at serial time points. The format is similar to Figure 2, except now plasma levels of CFH (A,C) and haptoglobin (B,D) are plotted on the y-axis. P values are denoted by asterisks and explained below the figures.
Other laboratory values
For completeness, commonly measured laboratory parameters are shown in supplemental Tables 1 and 2. None of these measurements show marked changes as a result of RBC washing nor were any differences noted that might not be expected by chance.
In vitro studies: effects of washing stored RBC units
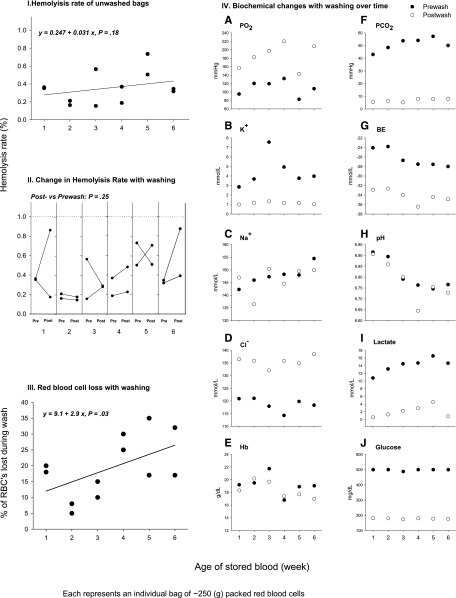
To examine the changes occurring over a 6-week storage period of canine universal donor blood, as well as the effects of washing, 2 units of canine stored RBCs were washed each week during the storage period (12 bags of canine universal donor stored blood DEA 1.1 ABRINT, Dixon, CA, were used to complete this series of in vitro studies). Each unit was sampled before and after washing. First, we examined the hemolysis rate throughout time and the effect of washing on the hemolysis rate (Figure 6I-II). During the 6-week storage period, all the units maintained an hemolysis rate below 1% as required by the FDA (Figure 6I). There were no significant increments of hemolysis rate through time, with the average hemolysis rate ranging between 0.3% and 0.4% throughout the whole 6-week storage period (Figure 6II). We then examined the RBC loss induced by washing (Figure 6III). Canine units lose more RBCs with washing as storage age increases (y = 9.1 + 2.9x, P = .03 for slope). Seven-day-old blood loses 12% and 42-day-old blood 26% of the RBCs with washing. We also investigated the biochemical effects of washing over the storage period (Figure 6IVA-J). Washing decreased the levels of potassium, pCO2, lactate, base deficit, and glucose throughout the whole 6-week storage period. Minimal effects on pH, which decreases throughout storage time, were found after washing the units.
Figure 6.
Hemolysis rate, red blood cells loss, and biochemical changes with washing of canine blood during the 6-week storage period. Serial changes in stored blood components during 6 weeks. Serial values of (I) percent hemolysis rate (calculated by dividing the supernatant hemoglobin levels by the total sample hemoglobin levels and multiplying by 100 minus the hematocrit value); (II) the change in hemolysis rate with washing; (III) red blood cells loss with washing and (IVA-J) biochemical changes with washing of canine blood of 1 through 6 weeks of storage period are shown. Two bags of canine universal donor leukoreduced blood were sampled each week before and after washing. In panels I-III, each dark circle represents an individual bag of ∼250 g of packed red blood cells. In panel IV (A-J) individual mean values (2 bags) of the different biochemical parameters before washing are represented as dark circles and after washing as white circles.
An additional 3 units of canine blood were stored for 6 weeks and were sampled weekly for analysis of iron and CFH levels. During the 6-week storage period, NTBI as well as CFH levels increased significantly with time (P < .001 for both trends, Figure 7A-C). Six additional canine RBC units were sampled at week 1 (n = 3), before and after washing, and week 6 (n = 3), before and after washing. Washing efficiently lowered NTBI, PLI, and CFH levels at both extremes of the storage period (Figure 7D-F).
Figure 7.
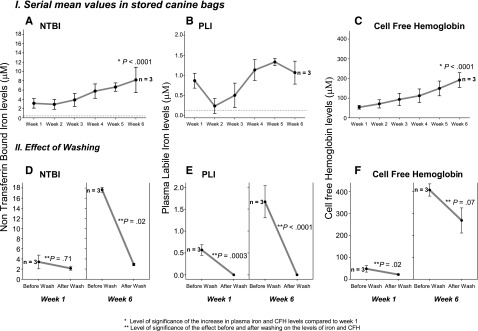
Mean (±SE) levels of NTBI, PLI, and CFH and effect of washing in canine stored blood during 6-week storage period. Mean levels of NTBI (IA), PLI (IB), and CFH (IC) sampled from 3 bags of canine universal donor leukoreduced blood are plotted throughout time of storage. (IID-F) mean levels of NTBI, PLI, and CFH, respectively, are represented before and after washing 3 additional bags of 1-week and 6-week stored blood.
Discussion
We conducted a blinded randomized controlled trial to determine the effect of an FDA-licensed washing procedure on canine universal donor blood used to examine the RBC storage lesion in a transfusion model of experimental canine S aureus pneumonia. We found a significant interaction for survival rates between washing and the age of blood transfused. Transfusing washed 42-day-old stored blood improved survival rates when compared with unwashed stored blood of the same age, whereas transfusing washed 7-day-old stored blood had an unexpectedly different and opposite effect that worsened survival rates (Figure 1). Similar significant findings regarding multiple organ injury and physiological abnormalities appear to corroborate the survival results. Transfusing 42-day-old washed stored blood lessened the degree of lung and liver injury when compared with unwashed controls, decreased vasopressor requirements (reversed shock), and improved cardiac performance (Figures 2 and 3).28,29 In contrast, transfusing washed fresher blood had a different and opposite effect that made these same parameters worse when compared with unwashed controls.
Previous studies in this model have suggested that substances released into the plasma both during RBC storage and as a result of hemolysis after transfusion are responsible for the morbidity and mortality associated with stored RBCs. Iron released during transfusion can enhance tissue injury by promoting bacterial growth. CFH released during storage and transfusion may cause injury both by scavenging nitric oxide and by providing an additional source of iron. We used an automated cell washing procedure to study the effects of removing these substances and determine whether washing might provide a strategy to interdict the clinical changes seen in this model and attributed to the RBC storage defect(s). We found significant interactions between washing, the age of stored RBCs, and the plasma levels of iron and CFH. Washing older blood prevented significant elevations of plasma iron in the form of NTBI and PLI found during and after transfusion of unwashed older blood (Figure 4). This effect was significantly different when fresh blood was washed. Fresh blood washed or unwashed resulted in NTBI and PLI plasma levels that were very low throughout the study, and below levels believed to be potentially injurious (0.20 µM). However, washing fresh blood significantly raised levels of CFH after transfusion compared with unwashed, and this effect was significantly different and opposite from washing older blood (Figure 5).
Older blood, whether washed or not, produces much higher levels of CFH than fresher blood, washed or not. RBCs lose membrane during the storage interval and are progressively more susceptible to lysis. We found that approximately 12% to 26% of the RBCs are lost during the washing process. Mechanical shear stress associated with washing has been shown previously to cause a sublethal injury to RBCs and increased in vivo hemolysis after transfusion with subsequent release of CFH and iron.30,31 Although exposure to saline during the wash may contribute to increased cell fragility, the washed cells are immediately resuspended in standard preservative solution. We postulate that the “older” more fragile cells are lost during the washing process and the cells that remain are more susceptible to lysis than before washing, especially after 42 days of storage. If this is true, more “fragile” RBCs will be transfused after washing fresh blood, and more hemolysis should occur during the several hours after transfusion with washed cells than with unwashed cells. After 42 days of storage, more “very fragile” cells should be lysed and the cellular debris washed away by the washing process. This is consistent with our current observations and previous studies. Washing has the added benefit of removing iron-containing proteins including CFH from the storage bag. Washing fresh RBCs should have far less effect, since there are far fewer cells present with increased susceptibility to hemolysis. However washing fresh red blood cells with saline solution introduces some membrane damage, making RBCs more prone to in vivo hemolysis (as noted earlier) than are fresh unwashed cells. The effect overall is the opposite of that observed with older blood: an increase in CFH levels with washing but much lower levels than seen with the unwashed blood that still contains very fragile but intact old RBCs.
The opposite effects on outcome that we found with washing different ages of stored blood can be explained by the observation that older stored blood has an increased propensity to hemolyze and release iron in the form of NTBI and PLI, which is not seen with fresher blood. Washing older blood removes this iron, which is no longer available after transfusion to promote bacterial growth and worsen outcome. In addition, washing older blood removes CFH and may also be beneficial because of the removal of older cells that are prone to in vivo hemolysis and can cause nitric oxide scavenging and vascular injury, and potentially raise iron levels and promote bacterial growth. The effect of washing fresher blood appears to be intermediate; washed fresh cells increase morbidity and mortality when compared with unwashed fresh blood, similar to the effects of washed older blood, and significantly less than unwashed old blood. The increased in vivo CFH with washing fresh blood may worsen outcome, either through scavenging nitric oxide, causing vasoconstriction and vascular injury, or by providing an additional source of iron for promoting bacterial growth.
To examine the changes in RBC during storage, as well as the effects of washing, we performed serial in vitro studies weekly on canine RBC units over a 6-week storage interval. Canine stored blood shows no significant increases in hemolysis rate during the 6 weeks of storage and levels are always lower than 1%, and washing does not significantly change the hemolysis rate in the storage bag (Figure 6I-II). We found about 12% of 1-week RBCs and 26% of 6-week RBCs are lost during washing (Figure 6III). Significantly more blood is lost with washing as stored blood ages, consistent with the notion that the older RBCs are removed preferentially. Washing lowers the base deficit, lactate, glucose, bicarbonate, and PCo2 levels in the storage bag during the 6 weeks, but has little effect on pH of the storage solution, which decreases steadily over the 6-week storage time (supplementary Figure 6IV). Throughout a 6-week storage period, canine stored blood accumulates CFH and NTBI significantly and progressively in the supernatant of the bags, reaching a mean of 190 µM of CFH and of 8 µM of NTBI at the end of the 42-day period (P < .0001, Figure 7A-C). Washing efficiently lowers iron and CFH in the stored bags at both extremes of the storage period (1 and 6 week, Figure 7D-F).
Our study demonstrates that washing removes 2 sources of iron: 1) iron in the form of NTBI as well as CFH, which accumulates over time in the storage bag,32-34 and 2) older cells that are more likely to hemolyze in vivo after transfusion and release iron. Several previous studies have shown that human stored blood also accumulates CFH and NTBI over time, producing levels of CFH from 80 to 90 μM (26) and NTBI from 5 to 15 μM.33,34 Our study of canine stored blood showed that CFH and NTBI increased over the storage period, reaching mean levels of CFH of 190 μM and of NBTI of 8 μM at 6 weeks. The possibility exists that iron might increase the risk of transfusion by promoting bacterial growth, inducing inflammation, or both, as has been proposed previously.35,36 Although the role of NTBI in promoting bacterial growth is well documented, its role in inflammation and the effect of washing are less clear. Noninfectious murine models are contradictory; some studies report that washed older blood induces a significantly lower inflammatory cytokine response, whereas others show that washing had no effect on this response.35,37 Iron has also been shown to inhibit cellular immune function, mainly through monocyte effector pathways,38,39 and to impair neutrophil function,40 affecting the host´s response to bacteria and possibly worsening the infection and outcome in our study. In addition, iron participates in the formation of toxic reactive oxygen species, which contribute directly to the pathogenesis of endothelial dysfunction and tissue injury and worsen the effect of infection in our model.39,41
Findings in this canine model should be extended to human transfusions with caution. The hemolysis rate we found in the storage bags, although low (<1%), could indicate that canine blood may not store as well as human blood. We have implicated 2 possible mechanisms of toxicity. We cannot exclude the possibility that other changes that occur in RBCs during storage are equally important in producing the clinical outcomes. We used a standardized model of lethal bacterial pneumonia. Results may vary in a different infectious model, a noninfectious model, or in a model of pneumonia that uses a different organism. Lastly, although we tried to mimic as closely as possible standard human intensive care and blood banking conditions, differences in clinical practice remain.
In conclusion, older blood releases iron during storage and after transfusion, has an increased propensity for hemolysis with release of CFH and iron, and increases the risk of transfusion in subjects with established infection. In critically ill subjects with infection, our study showed a favorable risk-to-benefit ratio for washing older blood. However, our data show that fresh blood is still superior to older blood, whether washed or not, in critically ill subjects with infection; fresh blood results in less in vivo hemolysis and less accumulation of CFH, NTBI, and PLI after transfusion. We unexpectedly found that fresh blood should be washed with caution in a setting of established infection; RBC treated with extensive saline washing may undergo increased hemolysis and iron release and may result in increased morbidity and mortality when compared with unwashed fresh blood controls.
Supplementary Material
Acknowledgments
Haemonetics (Braintree, MA) provided the ACP215 (automated cell processing system) as well as the disposable kits (RBC De-Glycerol Set 325 mL BMB Ref.236) used throughout the experiments.
This work was supported by Intramural National Institutes of Health, National Heart, Lung and Blood Institute funds and National Institutes of Health, National Heart, Lung and Blood Institute external grants HL058091 and HL098032.
The work by the authors was done as part of U.S. government–funded research; however, the opinions expressed are not necessarily those of the National Institutes of Health.
Footnotes
The online version of this article contains a data supplement.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: I.C.-P. performed experiments and wrote the first draft of the manuscript; D.W. and S.B.S. performed experiments and helped to write first draft of the manuscript; J.S. contributed to the study design and statistical analysis; M.F., J.F., and K.E.R. performed experiments and laboratory analysis; T.K., L.B., D.S., and A.P. contributed to laboratory analysis; M.A.S. performed echocardiograms; W.E.K. performed laboratory tests and set up assays; M.A.P. helped to train in blood washing techniques; M.T.G. provided laboratory support; D.B.K.-S. provided laboratory support and edited the manuscript; H.G.K. set up blood-banking procedures and editing of the manuscript; and C.N. contributed to study conception and design, wrote the first draft, and edited the manuscript.
Conflict-of-interest disclosure: D.B.K.-S. and M.T.G. are listed as coauthors on a patent application on methods of treating hemolysis and on patent applications related to development of blood substitutes using recombinant human neuroglobin. The work by the authors was done as part of US government-funded research, however, the opinions expressed are not necessarily those of the National Institutes of Health. The remaining authors declare no competing financial interests.
Correspondence: Irene Cortés-Puch, Critical Care Medicine Department, NIH Clinical Center, 10 Center Dr, Room 2C145, NIH, Bethesda, MD 20892; e-mail: irene.cortespuch@nih.gov.
References
- 1.Solomon SB, Wang D, Sun J, et al. Mortality increases after massive exchange transfusion with older stored blood in canines with experimental pneumonia. Blood. 2013;121(9):1663–1672. doi: 10.1182/blood-2012-10-462945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hess JR. Red cell storage. J Proteomics. 2010;73(3):368–373. doi: 10.1016/j.jprot.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 3.Tinmouth A, Fergusson D, Yee IC, Hébert PC ABLE Investigators; Canadian Critical Care Trials Group. Clinical consequences of red cell storage in the critically ill. Transfusion. 2006;46(11):2014–2027. doi: 10.1111/j.1537-2995.2006.01026.x. [DOI] [PubMed] [Google Scholar]
- 4.Bansal I, Calhoun BW, Joseph C, Pothiawala M, Baron BW. A comparative study of reducing the extracellular potassium concentration in red blood cells by washing and by reduction of additive solution. Transfusion. 2007;47(2):248–250. doi: 10.1111/j.1537-2995.2007.01095.x. [DOI] [PubMed] [Google Scholar]
- 5.Weisbach V, Riego W, Strasser E, et al. The in vitro quality of washed, prestorage leucocyte-depleted red blood cell concentrates. Vox Sang. 2004;87(1):19–26. doi: 10.1111/j.1423-0410.2004.00526.x. [DOI] [PubMed] [Google Scholar]
- 6.Melo A, Serrick CJ, Scholz M, Singh O, Noel D. Quality of red blood cells using the Dideco Electa autotransfusion device. J Extra Corpor Technol. 2005;37(1):58–59. [PubMed] [Google Scholar]
- 7.Westphal-Varghese B, Erren M, Westphal M, et al. Processing of stored packed red blood cells using autotransfusion devices decreases potassium and microaggregates: a prospective, randomized, single-blinded in vitro study. Transfus Med. 2007;17(2):89–95. doi: 10.1111/j.1365-3148.2007.00732.x. [DOI] [PubMed] [Google Scholar]
- 8.Hansen A, Yi QL, Acker JP. Quality of red blood cells washed using the ACP 215 cell processor: assessment of optimal pre- and postwash storage times and conditions. Transfusion. 2013;53(8):1772–1779. doi: 10.1111/trf.12170. [DOI] [PubMed] [Google Scholar]
- 9.Grabmer C, Holmberg J, Popovsky M, et al. Up to 21-day banked red blood cells collected by apheresis and stored for 14 days after automated wash at different times of storage. Vox Sang. 2006;90(1):40–44. doi: 10.1111/j.1423-0410.2005.00719.x. [DOI] [PubMed] [Google Scholar]
- 10.de Vroege R, Wildevuur WR, Muradin JA, Graves D, van Oeveren W. Washing of stored red blood cells by an autotransfusion device before transfusion. Vox Sang. 2007;92(2):130–135. doi: 10.1111/j.1423-0410.2006.00852.x. [DOI] [PubMed] [Google Scholar]
- 11.AABB. Standards for Blood Banks and Transfusion Services. 28th ed. Bethesda, MD: American Association of Blood Banks; 2012. [Google Scholar]
- 12.11th ed. Strasbourg, France: 2005. Guide to Preparation, Use, and Quality Assurance of Blood Components. [Google Scholar]
- 13.Canadian Standards Association. CAN/CSA Z902-10 Blood and Blood Components. 2010. edition. Mississauga (ON): CSA. [Google Scholar]
- 14.Burman JF, Westlake AS, Davidson SJ, et al. Study of five cell salvage machines in coronary artery surgery. Transfus Med. 2002;12(3):173–179. doi: 10.1046/j.1365-3148.2002.00369.x. [DOI] [PubMed] [Google Scholar]
- 15.Gueye PM, Bertrand F, Duportail G, Lessinger JM. Extracellular haemoglobin, oxidative stress and quality of red blood cells relative to perioperative blood salvage. Clin Chem Lab Med. 2010;48(5):677–683. doi: 10.1515/CCLM.2010.106. [DOI] [PubMed] [Google Scholar]
- 16.Gruber M, Breu A, Frauendorf M, Seyfried T, Hansen E. Washing of banked blood by three different blood salvage devices. Transfusion. 2013;53(5):1001–1009. doi: 10.1111/j.1537-2995.2012.03853.x. [DOI] [PubMed] [Google Scholar]
- 17.Biffl WL, Moore EE, Offner PJ, Ciesla DJ, Gonzalez RJ, Silliman CC. Plasma from aged stored red blood cells delays neutrophil apoptosis and primes for cytotoxicity: abrogation by poststorage washing but not prestorage leukoreduction. J Trauma. 2001;50(3):426–431, discussion 432. doi: 10.1097/00005373-200103000-00005. [DOI] [PubMed] [Google Scholar]
- 18.Wang D, Sun J, Solomon SB, Klein HG, Natanson C. Transfusion of older stored blood and risk of death: a meta-analysis. Transfusion. 2012;52(6):1184–1195. doi: 10.1111/j.1537-2995.2011.03466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lacroix J, Hébert P, Fergusson D, et al. ABLE study group. The Age of Blood Evaluation (ABLE) randomized controlled trial: study design. Transfus Med Rev. 2011;25(3):197–205. doi: 10.1016/j.tmrv.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 20.Heddle NM, Cook RJ, Arnold DM, et al. The effect of blood storage duration on in-hospital mortality: a randomized controlled pilot feasibility trial. Transfusion. 2012;52(6):1203–1212. doi: 10.1111/j.1537-2995.2011.03521.x. [DOI] [PubMed] [Google Scholar]
- 21.Australian and New Zealand Intensive Care Research Centre. Standard issue transfusion versus fresher red cell use in intensive care: a randomized controlled trial (TRANSFUSE). http://clinicaltrials.gov/ct2/show/NCT01638416. Accessed December 17, 2013.
- 22.Steiner ME, Assmann SF, Levy JH, et al. Addressing the question of the effect of RBC storage on clinical outcomes: the Red Cell Storage Duration Study (RECESS) (Section 7). Transfus Apheresis Sci. 2010;43(1):107–116. doi: 10.1016/j.transci.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Canadian Critical Care Trials Group. Age of Blood in Children PICU - http://www.ccctg.ca/Programs/ABC-PICU.aspx. Accessed December 17, 2013.
- 24.Hess JR. An update on solutions for red cell storage. Vox Sang. 2006;91(1):13–19. doi: 10.1111/j.1423-0410.2006.00778.x. [DOI] [PubMed] [Google Scholar]
- 25.Minneci PC, Deans KJ, Hansen B, et al. A canine model of septic shock: balancing animal welfare and scientific relevance. Am J Physiol Heart Circ Physiol. 2007;293(4):H2487–H2500. doi: 10.1152/ajpheart.00589.2007. [DOI] [PubMed] [Google Scholar]
- 26.Janz DR, Bastarache JA, Peterson JF, et al. Association between cell-free hemoglobin, acetaminophen, and mortality in patients with sepsis: an observational study. Crit Care Med. 2013;41(3):784–790. doi: 10.1097/CCM.0b013e3182741a54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Larsen R, Gozzelino R, Jeney V, et al. A central role for free heme in the pathogenesis of severe sepsis. Sci Transl Med. 2010;2(51):51ra71. doi: 10.1126/scitranslmed.3001118. [DOI] [PubMed] [Google Scholar]
- 28.Hicks CW, Sweeney DA, Danner RL, et al. Efficacy of selective mineralocorticoid and glucocorticoid agonists in canine septic shock. Crit Care Med. 2012;40(1):199–207. doi: 10.1097/CCM.0b013e31822efa14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hicks CW, Sweeney DA, Danner RL, et al. Beneficial effects of stress-dose corticosteroid therapy in canines depend on the severity of staphylococcal pneumonia. Intensive Care Med. 2012;38(12):2063–2071. doi: 10.1007/s00134-012-2735-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harm SK, Raval JS, Cramer J, Waters JH, Yazer MH. Haemolysis and sublethal injury of RBCs after routine blood bank manipulations. Transfus Med. 2012;22(3):181–185. doi: 10.1111/j.1365-3148.2011.01127.x. [DOI] [PubMed] [Google Scholar]
- 31.Ley JT, Yazer MH, Waters JH. Hemolysis and red blood cell mechanical fragility in shed blood after total knee arthroplasty. Transfusion. 2012;52(1):34–38. doi: 10.1111/j.1537-2995.2011.03217.x. [DOI] [PubMed] [Google Scholar]
- 32.Donadee C, Raat NJ, Kanias T, et al. Nitric oxide scavenging by red blood cell microparticles and cell-free hemoglobin as a mechanism for the red cell storage lesion. Circulation. 2011;124(4):465–476. doi: 10.1161/CIRCULATIONAHA.110.008698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marwah SS, Blann A, Harrison P, et al. Increased non-transferrin bound iron in plasma-depleted SAG-M red blood cell units. Vox Sang. 2002;82(3):122–126. doi: 10.1046/j.1423-0410.2002.00153.x. [DOI] [PubMed] [Google Scholar]
- 34.Collard K, White D, Copplestone A. The effect of maximum storage on iron status, oxidative stress and antioxidant protection in paediatric packed cell units. Blood Transfus. 2013;11(3):419–425. doi: 10.2450/2012.0046-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hod EA, Zhang N, Sokol SA, et al. Transfusion of red blood cells after prolonged storage produces harmful effects that are mediated by iron and inflammation. Blood. 2010;115(21):4284–4292. doi: 10.1182/blood-2009-10-245001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hod EA, Brittenham GM, Billote GB, et al. Transfusion of human volunteers with older, stored red blood cells produces extravascular hemolysis and circulating non-transferrin-bound iron. Blood. 2011;118(25):6675–6682. doi: 10.1182/blood-2011-08-371849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Belizaire RM, Makley AT, Campion EM, et al. Resuscitation with washed aged packed red blood cell units decreases the proinflammatory response in mice after hemorrhage. J Trauma Acute Care Surg. 2012;73(2 Suppl 1):S128–S133. doi: 10.1097/TA.0b013e3182606301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sonnweber T, Theurl I, Seifert M, et al. Impact of iron treatment on immune effector function and cellular iron status of circulating monocytes in dialysis patients. Nephrol Dial Transplant. 2011;26(3):977–987. doi: 10.1093/ndt/gfq483. [DOI] [PubMed] [Google Scholar]
- 39.Weiss G, Goodnough LT. Anemia of chronic disease. N Engl J Med. 2005;352(10):1011–1023. doi: 10.1056/NEJMra041809. [DOI] [PubMed] [Google Scholar]
- 40.Patruta SI, Edlinger R, Sunder-Plassmann G, Hörl WH. Neutrophil impairment associated with iron therapy in hemodialysis patients with functional iron deficiency. J Am Soc Nephrol. 1998;9(4):655–663. doi: 10.1681/ASN.V94655. [DOI] [PubMed] [Google Scholar]
- 41.Meroño T, Gómez L, Sorroche P, Boero L, Arbelbide J, Brites F. High risk of cardiovascular disease in iron overload patients. Eur J Clin Invest. 2011;41(5):479–486. doi: 10.1111/j.1365-2362.2010.02429.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.