Abstract
YAP and its paralog protein TAZ are downstream effectors of the Hippo pathway. Both are amplified in many human cancers and promote cell proliferation and epithelial–mesenchymal transition. Little is known about the status of the Hippo pathway in cutaneous melanoma. We profiled Hippo pathway component expression in a panel of human melanoma cell lines and melanocytic lesions, and characterized the capacity of YAP and TAZ to control melanoma cell behavior. YAP and TAZ immuno-staining in human samples revealed mixed cytoplasmic and nuclear staining for both proteins in benign nevi and superficial spreading melanoma. TAZ was expressed at higher levels than YAP1/2 in all cell lines and in those with high invasive potential. Stable YAP or TAZ knockdown dramatically reduced the expression of the classical Hippo target CCN2/connective-tissue growth factor (CTGF), as well as anchorage-independent growth, capacity to invade Matrigel, and ability form lung metastases in mice following tail-vein injection. YAP knockdown also reduced invasion in a model of skin reconstruct. Inversely, YAP overexpression increased melanoma cell invasiveness, associated with increased TEA domain–dependent transcription and CCN2/CTGF expression. Together, these results demonstrate that both YAP and TAZ contribute to the invasive and metastatic capacity of melanoma cells and may represent worthy targets for therapeutic intervention.
Introduction
Components of the Hippo pathway were initially identified in Drosophila in which a strong overgrowth phenotype was shared by pathway mutants (Staley and Irvine, 2012). Hippo pathway components are highly conserved and are often found mutated or expressed at lower levels in a variety of cancers (Zhao et al., 2008a; Bao et al., 2011; Chan et al., 2011; Yin and Zhang, 2011; Varelas and Wrana, 2012). The Hippo suppressor pathway consists of a cascade of kinases, MST and LATS in mammals, that phosphorylate the effector proteins YAP and TAZ, thereby controlling their nucleo-cytoplasmic localization and functions. MST1/2 phosphorylate and activate the kinases LATS1/2. The latter phosphorylate YAP and TAZ, leading to their cytoplasmic retention by 14-3-3 proteins (Mauviel et al., 2012). Inactivation of the MST and LATS kinases results in nuclear accumulation of YAP and TAZ and subsequent activation of target genes, many of them involved in cell proliferation. Complex cell membrane-associated signals upstream of the Hippo kinases involve numerous proteins such as Merlin and Kibra, the protocadherin FAT4, the cell polarity-associated Crumbs complex (review in Grusche et al. (2010)), and the adherens junction protein α-catenin (Schlegelmilch et al., 2011).
There are two main YAP isoforms, YAP1 and YAP2, also called YAP1-1 and YAP1-2, each existing as four isoforms, and one TAZ molecule. All three contain WW domain(s) and an SH3 domain-binding motif, as well as a PDZ-binding motif in their C-terminus (Gaffney et al., 2012). YAP1 and YAP2 contain a proline-rich N-terminal sequence not present in TAZ and differ from each other by their number of WW domains: YAP1, like TAZ, contains one WW domain, whereas YAP2 has two. WW domain proteins have the property to bind other partners that exhibit proline-rich modules named PY motifs (reviewed in Sudol et al. (2001)).
The primary partners of YAP and TAZ in the cell nucleus are TEA domain (TEAD) transcription factors, for which they function as transcriptional co-activators. Knockdown experiments in mice have shown that a number of phenotypes associated with disruption of the Hippo pathway may be recapitulated by alteration of TEAD function. A classical target gene associated with YAP/TAZ-regulated TEAD-dependent transcription is CCN2, which encodes connective-tissue growth factor (CTGF), a secreted factor implicated in tumor progression (reviewed in de Winter et al. (2008) and Dhar and Ray (2010)). YAP/TAZ interact with other transcription factors, such as SMADs, RUNX2, and p73 (for review, see Sudol (2010) and Mauviel et al. (2012)).
Alterations in the expression and function of upstream components of the Hippo pathway in human cancers, together with functional studies in vitro and in mice, point to a tumor suppressor role of the Hippo pathway. For example, overexpression in mammary epithelial cells of YAP/TAZ mutants that cannot be phosphorylated, and are therefore exclusively nuclear, leads to cell transformation and acquisition of tumorigenic potential associated with their binding to TEAD transcription factors (Chan et al., 2011; Yin and Zhang, 2011). Conditional and simultaneous knockout of Mst1/2 and YAP overexpression in mouse liver both result in the development of hepatocarcinomas (Liu et al., 2012). YAP and TAZ are expressed at high levels with strong nuclear localization in a number of cancers, and their oncogenic potential is often associated with their interactions with TEADs and WW domain-binding proteins (reviewed in Zeng and Hong (2008); Pan (2010); and Sudol et al. (2012)). Conversely, expression of LATS and MST is often reduced in cancer, possibly via hypermethylation of their gene promoters (reviewed in Hergovich (2012)). Noteworthy, both TAZ and YAP expression in mammary epithelial tumor cells contribute to their metastatic properties associated with expression of epithelial-to-mesenchymal transition (markers; Overholtzer et al., 2006; Chan et al., 2008).
To date, little is known about the role of Hippo signaling and its effectors YAP and TAZ in cutaneous melanoma biology. In this work, using both cell-based assays and tumor xenograft experiments in mice, we undertook an in-depth analysis of the expression, activation status, and function of YAP and TAZ in melanoma.
Results
Expression profiles of Hippo pathway members in human melanoma cell lines
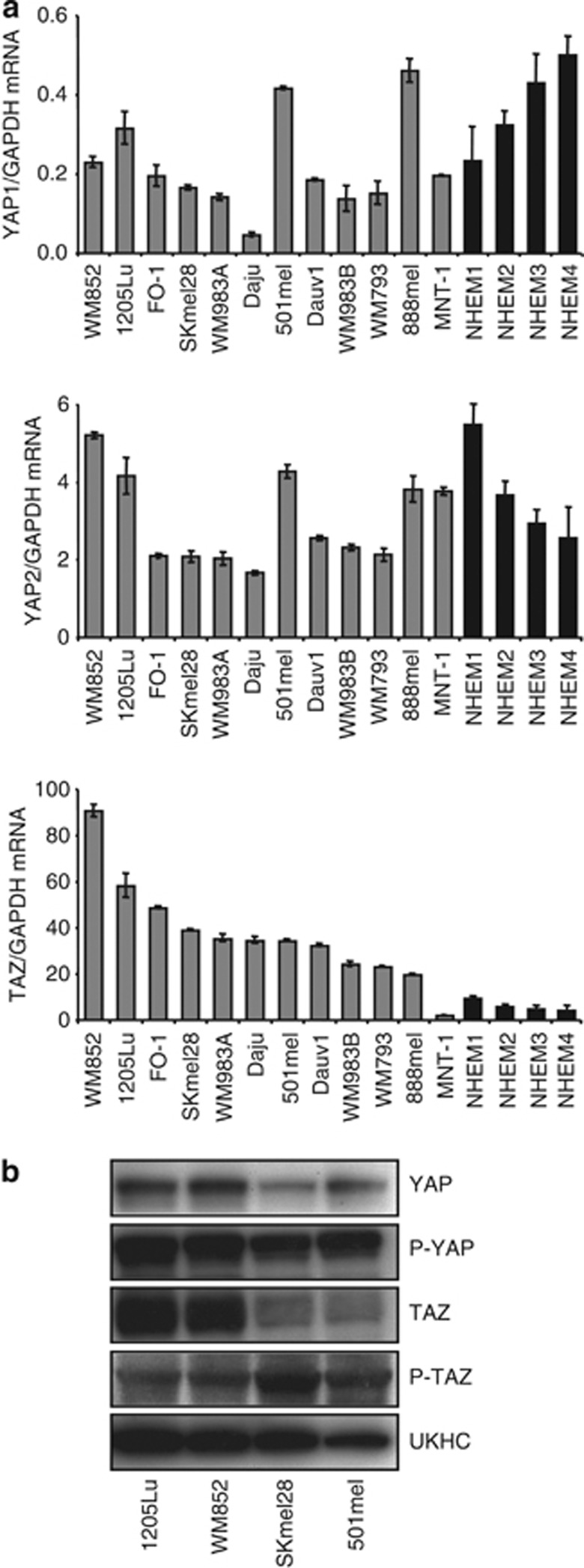
The expression of Hippo pathway effector molecules YAP1, YAP2, and TAZ in a panel of 12 human melanoma cell lines and 4 distinct normal human melanocyte cultures was measured by quantitative reverse transcriptase–PCR (Figure 1a). Levels of these three transcripts were highly variable across the panel. TAZ was expressed at levels 5–10 times higher than those for YAP2, itself at levels 10–20 times higher than YAP1. YAP1 and YAP2 were expressed at similar low levels in both normal human epidermal melanocytes and melanoma cell lines. Noticeably, expression of TAZ was higher in all melanoma cell lines compared with melanocytes, except in the MNT1 cell line that expressed the lowest levels of TAZ among melanoma lines. MNT1 cells are non-tumorigenic after sub-cutaneous injection in mice under conditions whereby all other cell lines form tumors (Moore et al., 2004).
Figure 1.
YAP and TAZ expression in a series of human melanoma cell lines and normal human neonatal melanocytes (NHEMs). (a) Quantitative reverse transcriptase–PCR analysis of YAP1, YAP2, and TAZ expression in a panel of 12 human melanoma cell lines (gray) and normal human neonatal melanocyte (black) cultures from four distinct donors, normalized against GAPDH. (b) Western analysis of YAP, TAZ, and phospho-YAP/TAZ protein levels in 1205Lu, WM852, SKmel28, and 501mel human melanoma cell lines. Note that anti-P-TAZ detection required film exposure 5 × longer than for other antibodies.
Four melanoma cell lines were selected for subsequent determination of YAP and TAZ protein levels. 1205Lu and WM852 were the two cell lines of the panel with the highest expression of TAZ, whereas the SKmel28 and 501mel cell lines express TAZ at lower levels. 1205Lu and WM852 cell lines are the more aggressive, when compared in either Matrigel invasion assays or in a model of experimental bone metastasis in mice (Javelaud et al., 2007; Alexaki et al., 2010; Mohammad et al., 2011). Western analysis revealed higher TAZ and YAP protein levels in 1205Lu and WM852 cells compared with SKmel28 and 501mel cells (Figure 1b), consistent with the gene expression profiles. Phosphorylated TAZ and YAP were detected in all cell lines, with slightly higher phospho-TAZ levels in SKmel28 and 501mel cells compared with 1205Lu and WM852 cells.
Consistent with the presence of P-YAP/TAZ in melanoma cell lines, Hippo pathway kinases MST and LATS were expressed in all cell lines: MST1 and MST2 were expressed at similar levels within a given cell line, yet at variable levels and degree of phosphorylation across cell lines (Supplementary Figures S1A and S1B online, respectively). LATS2 was expressed at levels 5–10 times higher than LATS1. Merlin/NF2 expression was also highly variable across cell lines (Supplementary Figure S1A online).
Immunofluorescent staining of proliferating 1205Lu melanoma cells confirmed that most YAP and TAZ protein is nuclear, whereas a small proportion is cytoplasmic Supplementary Figure S1C online). As expected, P-YAP/TAZ were exclusively cytoplasmic (Supplementary Figure S1C online), consistent with a partial constitutive activation of the pathway, as already suggested by P-YAP/TAZ levels detected by western blotting (see above). As all melanoma cell lines except WM852 (11/12) carry an activating mutation on the BRAF gene, we conclude that the expression pattern of Hippo pathway members is independent from the BRAF mutational status.
YAP and TAZ expression in human melanocytic lesions
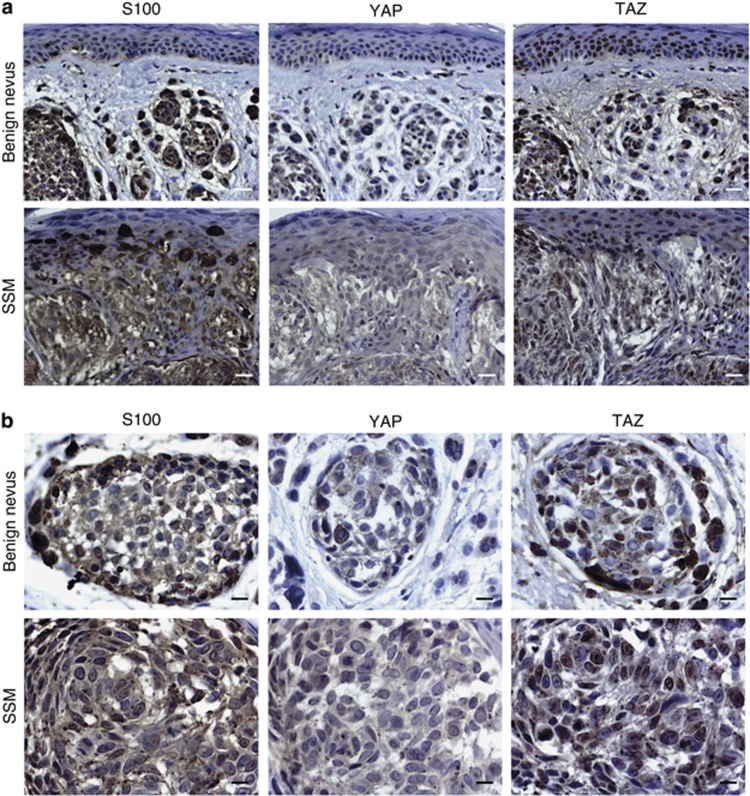
To our knowledge, there has been no analysis of Hippo signaling effector molecules in human melanocytic lesions published to date. We thus examined the expression of YAP and TAZ by immunohistochemistry in a series of clinical samples, including benign nevi (n=8) and superficial spreading melanoma (n=29). Representative images are shown in Figure 2. Blinded analysis by a professional dermato-pathologist found no significant differences in the staining of TAZ and YAP between benign and malignant melanocytic lesions. We did not find any trend in the positivity or distribution of both antibodies according to Breslow thickness. When positive in melanocytes, all other cells including keratinocytes and stromal cells stained positive for both antibodies as well. TAZ and YAP were distributed either in the nucleus or cytoplasm or both, but YAP was more often distributed in the cytoplasm.
Figure 2.
Detection of YAP and TAZ proteins in human melanocytic lesions by immunohistochemistry. Representative staining for YAP and TAZ by immunohistochemistry in a series of benign nevi and superficial spreading melanoma (SSM). S100 staining of an adjacent slide served to identify cells of melanocytic origin in tissue. Note the presence of both cytoplasmic and nuclear YAP and TAZ in both types of melanocytic lesions. (a) Bar=24 μm, (b) bar=12 μm.
YAP and TAZ knockdown specifically inhibit melanoma cell tumorigenicity and invasiveness, not proliferation, in vitro
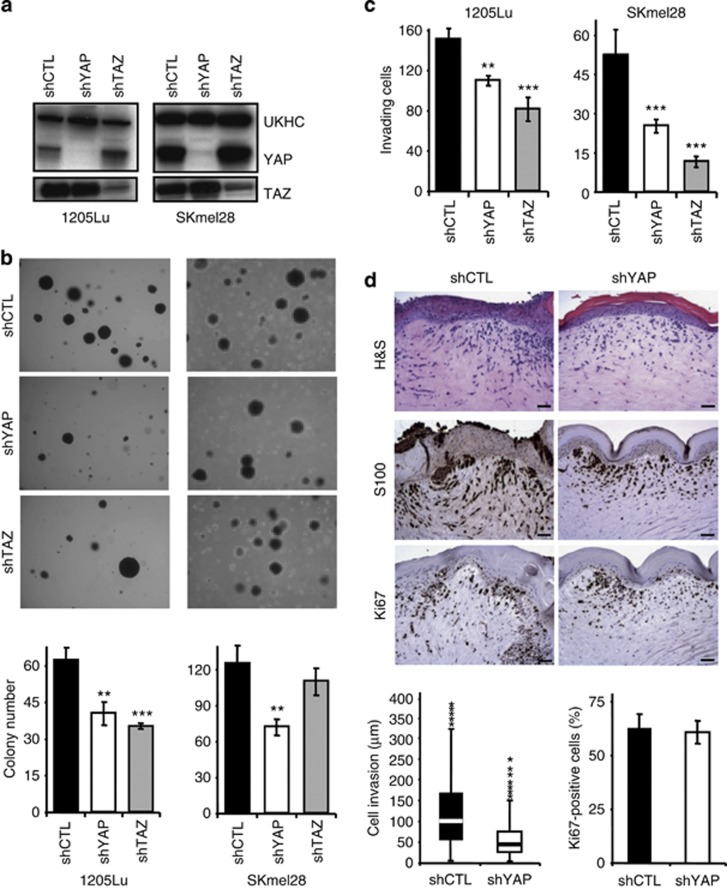
To determine whether there is a causal relationship between YAP/TAZ expression and melanoma cell behavior, we performed lentivirus-driven, small hairpin RNA-mediated stable knockdown of YAP1/2 or TAZ in 1205Lu, WM852, SKmel28, and 501mel melanoma cell lines. Efficacy and specificity of each knockdown was verified by quantitative PCR (Supplementary Figure S2A online) and western blotting (Figure 3a and Supplementary Figure S2B online). No obvious modification of cell morphology or population doubling times was observed after either knockdown when compared with mock-infected cells (Supplementary Figure S2C and S2D online, respectively). Cell cycle analysis determined by flow cytometry after BrdU incorporation showed no changes in the relative proportion of cells in G1, S, and G2/M phases upon either YAP or TAZ knockdown (not shown). Likewise, small interfering RNA (siRNA)-mediated YAP or TAZ knockdown showed high efficacy and specificity in 1205Lu and SK28 melanoma cells (Supplementary Figures S3A and S3B online), without affecting cell proliferation (Supplementary Figure S3C online).
Figure 3.
YAP1/2 or TAZ knockdown inhibits melanoma cell tumorigenicity in vitro. 1205Lu and SKmel28 melanoma cell lines were infected with lentiviruses expressing either YAP1/2 or TAZ small hairpin RNA. (a) Western analysis of YAP and TAZ protein levels in shCTL-, shYAP-, and shTAZ-transduced 1205Lu (left) and SKmel28 (right) melanoma cell lines. (b) Representative fields of a clonogenic assay on soft agar. (c) Matrigel invasion assay. Results, expressed as number of invading cells, are the mean±SEM of three independent experiments. *P<0.05, **P<0.001, ***P<0.0001. (d) shCTL- and shYAP-transduced 1205Lu melanoma cells were incorporated into skin reconstructs (see Materials and Methods). At day 18, the latter were harvested and embedded into paraffin. 5 μm parallel sections were stained with hematoxylin and eosin, S-100 antibody, and Ki67. Representative images (upper panel) are shown. Bar=50 μm. Invasion depth (lower left panel) was measured in eight random fields of each of the skin reconstructs using Image J software, with the dermo-epidermal junction as a starting point. Data are presented as whisker plots. The median value is indicated as horizontal bar surrounded by upper and lower quartiles. Outliers are indicated by asterisks. The percentage of KI67-positive melanoma cells (lower right panel) was calculated in eight random fields, in relation to S100 positivity in parallel histological sections.
To determine whether YAP and TAZ contribute to the tumorigenicity of melanoma cells, we first performed anchorage-independent growth assays on soft agar. As shown in Figure 3b (left panels and corresponding histograms), both YAP and TAZ knockdown reduced the number of colonies formed by 1205Lu cells by 30–50%. In SKmel28 cells, YAP knockdown had a strong inhibitory activity, whereas TAZ knockdown had limited effects (Figure 3b, right panels and histograms).
Both YAP and TAZ stable knockdown efficiently reduced the propensity of 1205Lu (Figure 3c, left panel) and SKmel28 (right panel) cells to invade Matrigel. Similar results were obtained after siRNA-mediated YAP and TAZ knockdown (Supplementary Figure S3D online, left panels). Simultaneous YAP/TAZ siRNA knockdown was not more efficient than either single knockdown (Supplementary Figure S3D online, right panels).
In a skin reconstruct model, shYAP-transduced 1205Lu melanoma cells, identified as S100-positive cells in 6-μm thick histological sections, did not migrate as deep into the dermis as control shRNA (shCTL)-transduced cells (Figure 3d online). Also, the number of cells that transmigrated from the epidermal compartment to the dermis was dramatically lower after YAP knockdown.
YAP and TAZ knockdown inhibits melanoma lung metastasis in mice
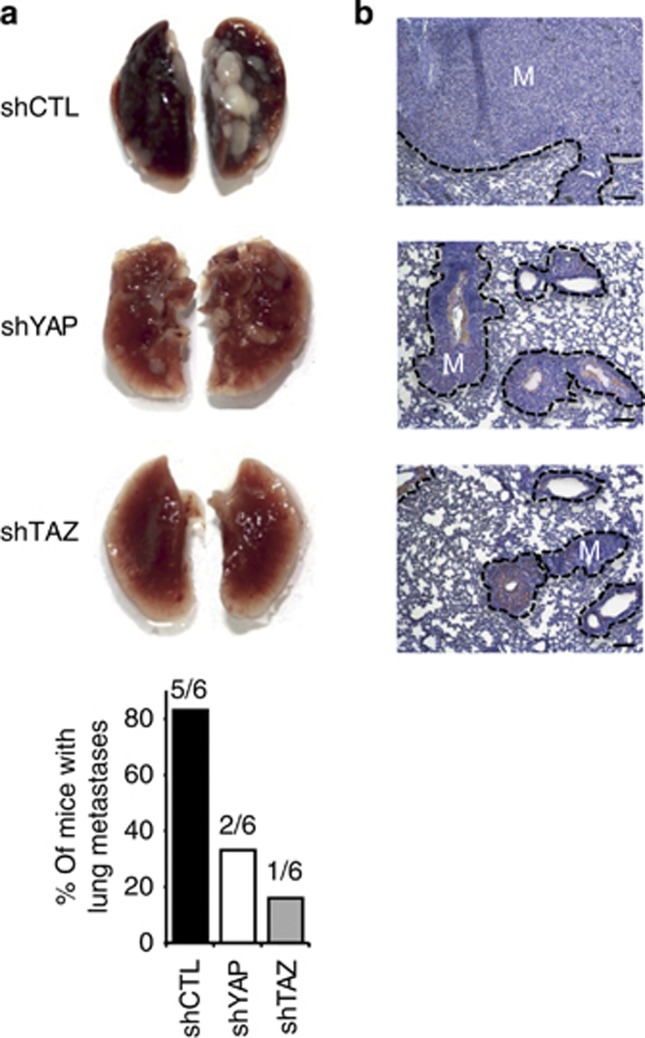
Next, we determined whether YAP and TAZ are implicated in the capacity of 1205Lu melanoma cells to form lung metastases following tail-vein injection in mice. Forty-five days later, mice were killed when the first signs of distress were noticed in shCTL-injected group. Macroscopic examination of lungs revealed massive development of metastases in five out of six mice from the shCTL group, whereas metastases were found in the lungs of only two out of six and one out of six mice inoculated with shYAP and shTAZ cells, respectively (Figure 4a). The latter was dramatically smaller in size than those observed in the two former groups. Histological analysis confirmed these post-autopsy observations and indicated a drastic reduction in total metastasis area per lung in shYAP and shTAZ groups compared with the shCTL group (Figure 4b). Thus, YAP and TAZ knockdown reduce both the incidence and the growth of lung metastases by intravenously inoculated 1205Lu melanoma cells.
Figure 4.
YAP and TAZ knockdown inhibit lung metastasis following tail-vein injection in nude mice. (a) Representative macroscopic images of lungs from 6-week-old female nude mice, 45 days after tail-vein injection of 4 × 105 mock-, shYAP-, or shTAZ-1205Lu cells. Histograms represent the % of mice bearing metastases in each group. (b) Representative lung sections. Bar=125 μm. M, metastasis.
YAP overexpression enhances the clonogenic potential and invasive capacity of melanoma cells in vitro
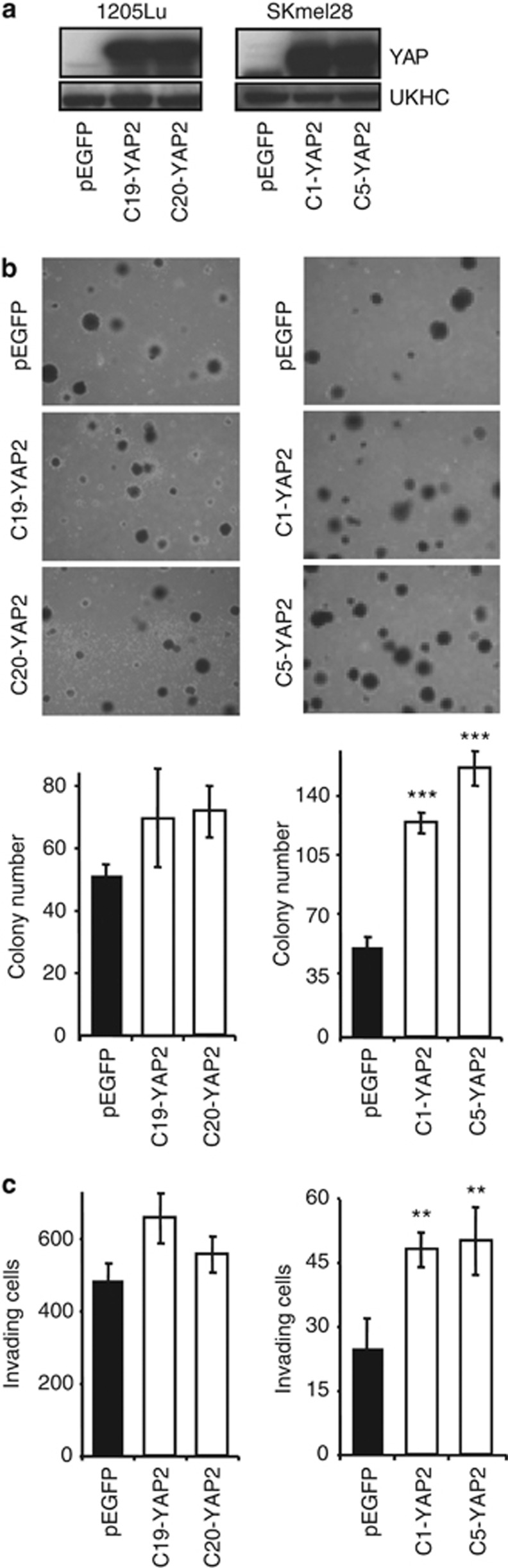
We next generated clones of 1205Lu and SKmel28 melanoma cells overexpressing YAP, as verified by quantitative PCR (Supplementary Figure S4A online) and western blotting (Figure 5a). No modification of cell morphology (Supplementary Figure S4B online) or growth rate was observed in either cell line upon YAP overexpression (Supplementary Figure S4C online), consistent with the knockdown data, which also did not show modifications of cell growth (see Supplementary Figure S2C and D online).
Figure 5.
YAP2 overexpression in 1205Lu and SKmel28 melanoma cell lines enhances their clonogenic and invasive potential in vitro. 1205Lu (left) or SKmel28 (right) melanoma cells were stably transfected with either empty pEGFP or YAP2 expression vectors. (a) Western blot validation of YAP overexpression in melanoma cell clones. (b) Soft agar clonogenic assay. Representative fields are shown. Results are expressed as mean±SEM of three independent experiments, each performed with triplicate dishes. (c) Matrigel invasion assay. The number of invading cells was counted using bright-field microscopy after staining colonies with crystal violet, 24 hours (1205Lu) or 48 hours (SKmel28) later. Results are expressed as mean±SEM of three independent experiments. **P<0.001, ***P<0.0001.
YAP overexpression did not significantly increase the capacity of 1205Lu cells to grow as clones on agar (Figure 5b, left panels and histograms), whereas markedly increasing SKmel28 anchorage-independent growth (Figure 5b, right panels and histograms). It is possible that 1205Lu cells, which express a lot more TAZ than SKmel28 cells (see Figure 1), may be less responsive to forced YAP overexpression, as these proteins have been known to be functionally redundant in certain situations (Mauviel et al., 2012). In fact, although both cells lines exhibited increased invasive capacity in a Matrigel assay upon YAP overexpression, the effect was markedly higher in SKmel28 compared with 1205Lu cells (Figure 5c right versus left panel, respectively), consistent with their lower levels of endogenous YAP/TAZ.
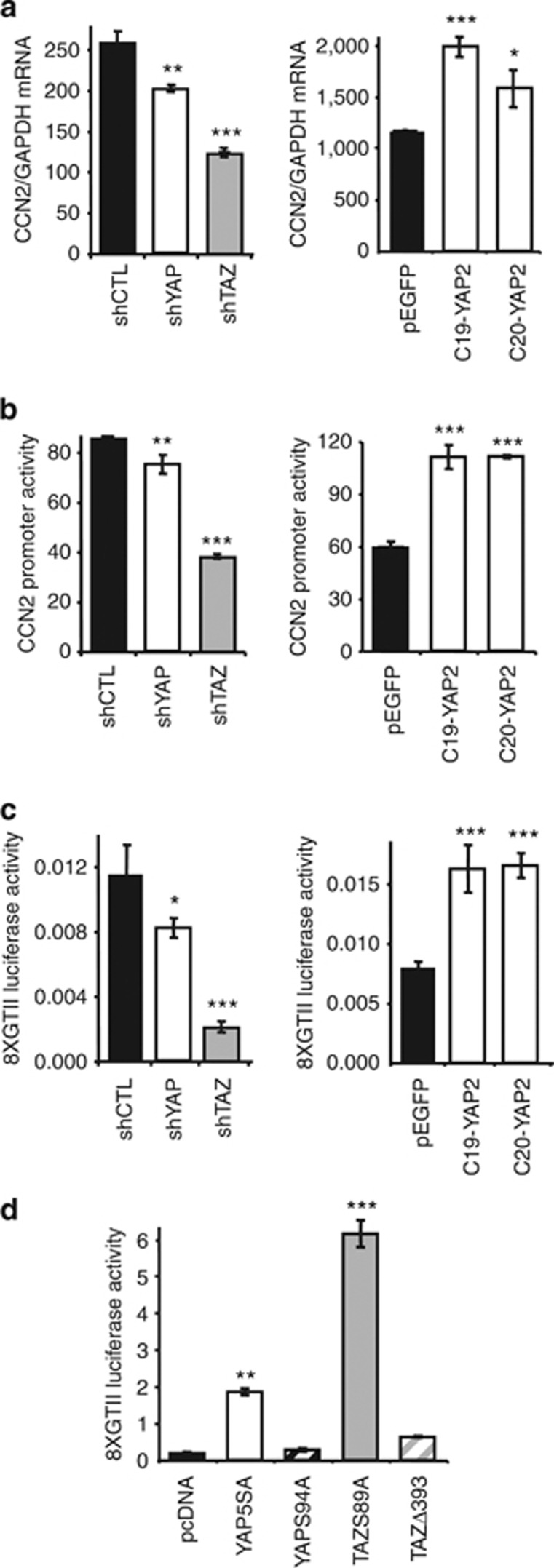
Altering YAP and TAZ levels modifies TEAD-dependent transcription and CCN2/CTGF expression in 1205Lu melanoma cells
To gain insight into the molecular mechanisms underlying forced alteration of YAP and TAZ expression, we focused our attention on the modulation of CTGF, a canonical YAP/TAZ target (Zhang et al., 2009) that contributes to metastasis both in melanoma and breast cancer (Kang et al., 2003; Mohammad et al., 2011). CTGF is encoded by CCN2, a TEAD transcription factor gene target (Zhang et al., 2009) for which YAP and TAZ function as transcriptional co-activators (Vassilev et al., 2001). Stable YAP and TAZ knockdown in 1205Lu cells reduced CCN2 mRNA steady-state levels (Figure 6a, left panel), whereas YAP overexpression did the opposite (right panel). CCN2 gene modulation was transcriptional as CCN2 promoter activity showed parallel modulation upon YAP/TAZ knockdown or YAP overexpression (Figure 6b). TEAD-specific transcription followed the same modulation (Figure 6c). TAZ knockdown was consistently more efficient than YAP knockdown in regulating CCN2 expression and TEAD-dependent transcription, likely reflecting the higher expression levels of TAZ versus YAP in melanoma cells (see Figure 1). Also, YAP and TAZ mutants with an exclusive nuclear localization (YAP5SA and TAZS89A) efficiently enhanced TEAD-specific transcription, whereas YAPS94A, incapable of binding TEADs, and the exclusively cytoplasmic mutant TAZΔ393 did not (Figure 6d).
Figure 6.
CCN2 expression and TEAD-dependent transcription levels are proportional to YAP and TAZ levels in 1205Lu melanoma cells. Quantitative reverse transcriptase–PCR analysis of CCN2 expression normalized against GAPDH (a), CCN2 promoter activity (b), and TEAD-specific transcription (c) in 1205Lu melanoma cells after either stable knockdown (left panels) of YAP or TAZ or YAP overexpression (right panels). (d) Effects of various YAP and TAZ mutants on TEAD-specific transcription in 1205Lu melanoma cells: YAP5SA and TAZS89A are exclusively nuclear; TAZΔ393 is exclusively cytoplasmic; YAPS94A is unable to bind TEADs. In panels b–d, Renilla was used as a control for transfection efficiency. Results are mean±SEM of three independent experiments using triplicate dishes. *P<0.05, **P<0.001, ***P<0.0001. TEAD, TEA domain.
In SKmel28 cells, YAP and TAZ knockdown had little effect on CCN2 expression, promoter activity, and TEAD-specific transcription (Supplementary Figure S5A–C online), whereas YAP overexpression increased CCN2 expression and promoter activity, as well as TEAD-specific transcription (Supplementary Figure S5D–F online). Overexpression of nuclear mutants of YAP and TAZ (YAP5SA and TAZS89A) dramatically increased TEAD-dependent transcription, whereas cytoplasmic mutants YAPS94A and TAZΔ393 did not (Supplementary Figure S5G online). Reduced CCN2 expression upon siRNA-mediated knockdown of YAP and TAZ, either alone or in combination, occurred in 1205Lu, not SKmel28 melanoma cells (Supplementary Figure S6 online), consistent with the lower levels of expression of YAP and TAZ in SKmel28 compared with 1205Lu cells.
These data provide direct evidence for the dependence of TEAD-dependent transcription and target gene expression with YAP and TAZ expression levels in melanoma cells. How this relates to their invasive and metastatic capacity remains to be determined.
Discussion
Overexpression of YAP and TAZ induces cell transformation and tumor-forming ability in mammary epithelial cells (Overholtzer et al., 2006; Dong et al., 2007; Chan et al., 2008), because of their capacity to interact with, and function as transcriptional co-activators of, the TEAD family of transcription factors (Zhao et al., 2008b; Chan et al., 2009; Zhang et al., 2009). YAP overexpression in mouse livers results in hepatocellular carcinomas (Zhou et al., 2009; Lu et al., 2010; Song et al., 2010), and a wide range of cancers displays high levels of TAZ or YAP expression (reviewed in Zhao et al. (2010)). In this report, we provide evidence for active Hippo signaling in both benign and malignant melanocytic lesions and in melanoma cell lines, with both proteins localized both in the cytoplasm and in the nucleus.
We also provide evidence that endogenous YAP/TAZ contribute to the metastatic behavior of melanoma cells, as specific knockdown of either of these Hippo effectors lead to reduced clonogenic and invasive capacity in vitro, and reduced their propensity to metastasize to lungs following tail-vein injection in nude mice. Inversely, YAP/TAZ overexpression increased anchorage-independent growth and invasion into Matrigel, thus identifying a direct role for endogenous YAP and TAZ in controlling melanoma cell metastatic potential. Consistent with our observations, it was recently found that overexpression of a mutant YAP with exclusive nuclear localization enhances the metastatic potential of the 4T1 mammary carcinoma cell line, as well as that of the A375 melanoma cell line (Lamar et al., 2012). In the latter study, based solely on overexpression approaches, the authors concluded that YAP–TEAD interactions were necessary for the pro-metastatic activity of YAP. Our data are consistent with their conclusion, yet bring important information about the role of Hippo effectors in melanoma biology. Although Lamar et al. (2012) only examined the effects of overexpression of mutant forms of YAP with altered functional domains in one melanoma cell line and one breast carcinoma cell line, they did not examine TAZ function. We chose a broader approach, which consisted in profiling human melanocytic lesions and melanoma cell lines for endogenous YAP and TAZ expression, followed by thorough mechanistic analysis of the respective roles played by endogenous YAP or TAZ in melanoma cell behavior. As TAZ is expressed at much higher levels than both YAP isoforms in melanoma cells (see Figure 1), TAZ knockdown was more potent in inhibiting melanoma cell invasive and metastatic capacities than YAP knockdown (Figures 3 and 4). Yet, in agreement with the data by Lamar et al. (2012), overexpression of wild-type YAP enhanced the invasive capacity of melanoma cells, associated with increased TEAD-dependent transcription and CCN2 expression (Figures 5 and 6). Thus, despite active Hippo signaling, as evidenced by the detection of cytoplasmic, phosphorylated YAP and TAZ in melanoma cells, the nuclear functions of YAP and TAZ are enhanced by overexpression of wild-type proteins. Our knockdown approach allowed, to our knowledge previously unreported demonstration for a role of endogenous YAP/TAZ in melanoma cell behavior, and suggest that YAP and TAZ may be important targets for therapeutic intervention. From our data herein and those by Lamar et al. (2012), it appears that disrupting or antagonizing their capacity to enhance TEAD transcriptional activity, and possibly that of other signaling pathways known to promote melanoma metastasis, including transforming growth factor-β and WNT/β-catenin signaling (Ferrigno et al., 2002; Javelaud et al., 2007, 2008; Mohammad et al., 2011; Mauviel et al., 2012), is likely to negatively affect melanoma cell invasiveness and capacity to metastasize. In this respect, the aromatic heterocyclic small-molecule verteporfin was recently found to disrupt YAP-TEAD interactions (Liu-Chittenden et al., 2012). This molecule, known commercially as Visudyne (Novartis International AG, Basel, Switzerland), is approved by the FDA for photodynamic therapy of neovascular macular degeneration (Michels and Schmidt-Erfurth, 2001). Based upon these findings, it will be interesting to test the capacity of verteporfin to interfere with melanoma cell invasive properties.
We did not observe any significant effect of the modulation of YAP/TAZ expression on the proliferative capacity of melanoma cells, either after knockdown or overexpression. All melanoma cell lines used in our studies carry the BRAFV600E mutation, except for the WM852 cell line that carries the NRASQ61K mutation. These oncogenic mutations, found in a large percentage of melanoma tumors (Chin et al., 2006), result in active MEK/ERK signaling and have an important role in promoting melanoma cell proliferation (Chin et al., 2006). Our data suggest that the roles played by BRAFV600E and NRASQ61K as drivers of melanoma cell growth are not significantly affected by YAP/TAZ levels.
To conclude, this report provides comprehensive profiling of YAP/TAZ expression and function in melanoma: YAP and TAZ were detected in both benign nevi and metastatic melanoma with similar distribution and expression levels. Thus, we could not establish any prognostic value for either YAP or TAZ. Yet, from our functional studies, it appears that YAP and TAZ Hippo effector molecules are important players controlling the invasive and metastatic potential of melanoma cells.
Materials and Methods
Cell culture
All human melanoma cell lines have been described previously (Rodeck et al., 1999; Javelaud et al., 2007; Alexaki et al., 2008, 2010; Javelaud et al., 2011). They all carry an activating mutation (V600E) of the BRAF gene, except for the WM852 cell line, which carries an activating mutation of NRAS (Q61K). They all were grown in RPMI 1640 (Invitrogen, Carlsbad, CA) supplemented with 10% fetal calf serum and antibiotics, at 37 °C, 5% CO2 in a humidified atmosphere. Primary human melanocytes from neonatal foreskins were obtained from four distinct Caucasian individuals, and grown in modified MCDB medium (GIBCO, Invitrogen), as described previously (Busca et al., 2005).
Immunohistochemistry and human tissues
Paraffin-embedded pathological samples from human melanocytic lesions were obtained from the pathology archives of the National Health Services (West Hertfordshire Hospitals NHS Trust, Herts, UK). All specimens were re-evaluated by an expert pathologist. Tissues were obtained according to local ethical guidelines and approved by the local ethics committee. Five-μm sections were de-waxed and rehydrated through graded alcohol baths. After antigen retrieval (20 minutes at 95 °C) in citrate buffer, pH 6.0, tissue sections were preincubated for 30 minutes with 20% horse serum, then incubated with antibodies directed against YAP (sc-15407, 1:200, 1 hour at room temperature), TAZ (sc-17130, 1:200, overnight at 4 °C), or S100 (Dako France SAS, Trappes, France, Z0311 1:1000, 1 hour at room temperature). A Vectastain ABC kit (Vector Laboratories, Burlingame, CA) was used as biotinylated secondary antibody. Counterstaining was performed with hematoxylin.
Cell proliferation assays
Human melanoma cells were seeded in six-well culture plates. Every other day, cells were trypsinized and enumerated using a Coulter Counter (Beckman Coulter, Fullerton, CA).
Cells transfections
For reporter assays, melanoma cells were seeded in 24-well plates and transfected at 70–80% confluency in fresh medium with the polycationic compound FuGENE (Roche Diagnostics, Indianapolis, IN). Cells were transfected with 200 ng of firefly luciferase promoter reporter construct, CCN2-luc or (8XGTII)2-luc ((Ota and Sasaki, 2008; Zhang et al., 2009), kind gifts from Kun-Liang Guan, UCSD, La Jolla, CA, and Hiroshi Sasaki, Kumamoto University, Japan, respectively), together with 50 ng of expression vectors, pcDNA, YAP5SA, YAPS94A ((Zhang et al., 2009), gifts from K.-L. Guan), TAZS89A or TAZD393 ((Varelas et al., 2008), gifts from Jeffrey Wrana, University of Toronto, ON, Canada). Fifty nanogram of Renilla luciferase expression vector was co-transfected to estimate transfection efficiencies. After incubation, luciferase activities were determined with a Dual-Glo luciferase assay kit (Promega, Madison, WI) using a Fluoroskan Ascent FL (Thermo Labsystems, Courtaboeuf, France). All experiments were performed at least three times.
For stable YAP overexpression, melanoma cells in 100-mm diameter culture dishes were transfected with 10 μg of either empty pEGFP or the same vector-expressing YAP (Basu et al., 2003). Three days later, G418 (Sigma-Aldrich, St Louis, MO, 0.7 g ml−1) was added to the culture medium. Selection of stably transfected clones occurred over a 3-week period. For transient, acute YAP and TAZ silencing, subconfluent melanoma cells were transfected with two distinct siRNAs targeting either YAP or TAZ (Sigma-Aldrich human YAP or TAZ Mission siRNAs 1 and 2, respectively) using HiperFect (Qiagen, Courtaboeuf, France). Two non-targeting siRNAs (Sigma-Aldrich Mission Universal Negative control siRNAs 1 and 2) were used as controls. For stable YAP or TAZ gene silencing, subconfluent human melanoma cells were infected in 96-well dishes with lentiviral particles that expressed a control, non-targeting, scrambled small hairpin RNA sequence or three distinct target-specific constructs that encode 19–25 nt (plus hairpin) small hairpin RNA to knock down YAP1/2 or TAZ expression (Santa Cruz Biotechnology, Santa-Cruz, CA, sc-108080, sc-38637-V and sc-38568-V, respectively), in the presence of 4 μg ml−1 polybrene. Stably transduced cell populations were selected with puromycin (2 μg ml−1). Reverse transcriptase–PCR was used to verify YAP expression after each passage and before new experiments.
RNA extraction and gene expression analysis
Total RNA was isolated from cell cultures using a NucleoSpin RNA kit (Macherey-Nagel, Düren, Germany). Genomic DNA contamination was eliminated by DNase I treatment. One microgram of RNA was reverse transcribed using a Thermoscript kit (Invitrogen). cDNAs were processed for real-time reverse transcriptase–PCR using SYBR Green technology in a 7900 Real-Time PCR System (Applied Biosystems, Foster City, CA). Reactions were carried out for 40 cycles (95 °C for 15 seconds and 60 °C for 1 minute) after an initial 10-minute incubation at 95 °C. Data were analyzed using Applied Biosystems Sequence Detection Software (version 1.2.1) and normalized to GAPDH expression. Experiments were performed in triplicate to validate gene expression data in each cell line. Primers are: GAPDH, forward (F): 5′-TGGGTGTGAACCATGAGAAGTATG-3′, reverse (R), 5′-GGTGCAGGAGGCATTGCT-3′ MERLIN, F: 5′-TGAACGCACGAGGGATGAGTTG-3′, R: 5′-GCCTTTTCAGCCAACAGGTCAG-3′ LATS1, F: 5′-CACTGGCTTCAGATGGACACAC-3′, R: 5′-GGCTTCAGTCTGTCTCCACATC-3′ LATS2, F: 5′-GTTCTTCATGGAGCAGCACGTG-3′, R: 5′-CTGGTAGAGGATCTTCCGCATC-3′ MST1, F: 5′-CTGTGTAGCAGACATCTGGTCC-3′, R: 5′-CTGGTTTTCGGAATGTGGGAGG-3′ MST2 F: 5′-GGCAGATTTTGGAGTGGCTGGT-3′, R: 5′-AATGCCAAGGGACCAGATGTCG-3′ YAP1, F: 5′-GTGAGCCCACAGGAGTTAGC-3′, R: 5′-CTCGAGAGTGATAGGTGCCA-3′ YAP2, F: 5′-TCTTCCTGATGGATGGGAAC-3′, R: 5′-GGCTGTTTCACTGGAGCACT-3′ TAZ, F: 5′-GTATCCCAGCCAAATCTCG-3′, R: 5′-TTCTGAGTGGGGTGGTTC-3′ CCN2, F: 5′-TGCACCGCCAAAGATGGT-3′, R: 5′-GACTCTCCGCTGCGGTACAC-3′.
Western blotting
Whole-cell extracts were prepared on ice in 50 mM Tris pH 7.5, 150 mM NaCl, 0.5% NP40, 20 μM phenylmethylsulfonyl fluoride and protease inhibitor cocktail for 10 minutes. Debris were removed by centrifugation. Protein concentration was assayed with a one-step colorimetric method (Bio-Rad protein reagent; Bio-Rad, Hercules, CA), and 35 μg of protein were resolved by SDS-PAGE and transferred to Hybond ECL nitrocellulose filters (Amersham Biosciences, Glattburgg, Switzerland). Filters were placed in blocking solution (1 × Tris-buffered saline, 5% nonfat milk) for 1 hour and immunoblotted with either rabbit anti-YAP (Santa Cruz Biotechnology, sc-15407), rabbit anti-phospho-YAP (Cell Signaling Technology, Danvers, MA, #4911), rabbit anti-TAZ (Cell Signaling Technology, #4883) rabbit anti-phospho-TAZ (sc-17610), rabbit anti-MERLIN (sc-332) rabbit anti-LATS1 (sc-28223), rabbit anti-phospho-LATS1 (Cell Signaling Technology, #9157), rabbit anti-MST2 (Cell Signaling Technology, #3952), rabbit anti-phospho-MST1/2 (Cell Signaling Technology, #3681), at a 1:1,000 dilution in 1 × Tris-buffered saline, 0.1% Tween-20, and 5% nonfat milk, overnight at 4 °C. A goat anti-UKHC (sc-13356) antibody was used as a control. For detection of phosphorylated proteins, BSA replaced nonfat milk. Filters were then washed and incubated with horseradish peroxidase-conjugated secondary antibodies (rabbit or goat, Santa Cruz Biotechnology) for 2 hours. Filters were washed, developed, and revealed according to chemiluminescence protocols (ECL; Amersham Biosciences).
Invasion assays
Tissue culture Transwell inserts (8 μm pore size; Falcon, Franklin Lakes, NJ) were coated for 3 hours with 10 μg (1205Lu and WM852) or 5 μg (SKmel28 and 501mel) of Matrigel (Biocoat, BD Biosciences, San Jose, CA) in 100 μl of phosphate-buffered saline at 37 °C. After air-drying the chambers for 16 hours, the Matrigel barrier was reconstituted with 100 μl Dulbecco's minimal essential medium for 24 hours at 37 °C. The chambers were then placed into 24-well dishes containing 500 μl of RPMI medium supplemented with 1% fetal calf serum. 5 × 104 melanoma cells were added to the upper well of each chamber in 250 μl of serum-free RPMI medium. After a 24-hour (1205Lu and WM852) or a 48-hour (SKmel28 and 501mel) incubation period, invading cells were counted by bright field microscopy at × 200 in six random fields. Additional details of the procedure may be found in Alexaki et al. (2010). Experiments were performed at least three times, each with triplicate samples.
Anchorage-independent growth
Mock-, YAP-, and TAZ-knockdown melanoma cells (1 × 104 or 2 × 104) resuspended in RPMI 1640 medium containing 0.3% agar (Sigma-Aldrich) and supplemented with 10% fetal calf serum were seeded into six-well plates on top of a 0.6% soft agar bed in similar medium. Cultures were maintained for 28 days and the total number of colonies in each well was determined using a phase contrast microscope (Nikon France, Rollay, France). Results are expressed as the mean±SEM of three independent experiments performed with triplicate dishes.
Human skin reconstructs
Human skin reconstructs were generated as described previously (Li et al., 2011). Briefly, dermal reconstructs consisted of bovine type I collagen contracted for 4 days with embedded dermal fibroblasts (7.5 × 104 per dish) before seeding control shCTL, or shYAP-infected 1205Lu cells (0.83 × 105) together with keratinocytes (4.17 × 105) on top of the dermal reconstructs over an 4-day period in skin reconstruct medium before air exposure until day 18 to allow epidermal differentiation. At that point, skin reconstructs were harvested and fixed in 10% neutral buffered formalin for 2–3 hours, processed by routine histological methods, embedded into paraffin, sectioned (5 μm), then stained with an S-100 antibody (1:1,000, 30 minutes at room temperature (Z0311, DAKO, Les Ulis, France)) to identify cells of melanocytic origin, and with Ki67 (ready to use, 1 hour at room temperature, (08-0156 Life technologies) as a marker of cell proliferation.
Experimental lung metastasis in nude mice
Female 6-week-old Swiss nu/nu (nude) mice (Janvier, Le-Genest-Saint-Isle, France) were housed at the animal facility of the Curie Institute, under pathogen-free conditions. Their care was in accordance with the principles of the Declaration of Helsinki Principles and with the institutional guidelines of the French Ethical Committee (Ministère de l'Agriculture et de la Forêt, Direction de la Santé et de la Protection Animale, Paris, France). Experiments were performed under supervision of authorized investigators. shCTL, shYAP, or shTAZ 1205Lu melanoma cells in logarithmic growth phase (4 × 105/200 μl phosphate-buffered saline) were slowly injected into the tail vein. Mice were killed 45 days later, as the first signs of distress were noticed. Lungs were rapidly excised and dissected, photographed, and fixed in paraformaldehyde (Sigma-Aldrich) for further immunohistochemical analysis.
Statistical analyses
Differences between groups were determined by one-way analysis of variance followed by Bonferroni's posttest. All data were analyzed using GraphPad Prism v6.0 software (GraphPad Software, La Jolla, CA), and expressed as mean±SEM. Statistical significance was set at P<0.05.
Acknowledgments
We thank Amélie Crozet, Institut Curie, for help with animal experiments, and Corine Bertolotto, INSERM U895, University of Nice, France, for human melanocyte RNA preparations. We are indebted to Anju Agarwal, consultant histopathologist, West Herts NHS Trust, Herts, UK, who helped with histopathological analysis of human samples. This work was supported by Ligue Nationale Contre le Cancer (Equipe Labellisée LIGUE EL2011-AM), INCa (PLBIO08-126), and a donation from Emile and Henriette Goutière (to AM), and institutional funding from Institut Curie, INSERM, and CNRS. MS was supported by PA Breast Cancer Coalition Grants (#60707 and #920093) and the Geisinger Clinic.
Glossary
- CTGF
connective-tissue growth factor
- siRNA
small interfering RNA
The authors state no conflict of interest.
Footnotes
SUPPLEMENTARY MATERIAL
Supplementary material is linked to the online version of the paper at http://www.nature.com/jid
Supplementary Material
References
- Alexaki VI, Javelaud D, Mauviel A. JNK supports survival in melanoma cells by controlling cell cycle arrest and apoptosis. Pigment Cell Melanoma Res. 2008;21:429–438. doi: 10.1111/j.1755-148X.2008.00466.x. [DOI] [PubMed] [Google Scholar]
- Alexaki VI, Javelaud D, Van Kempen LC, et al. GLI2-mediated melanoma invasion and metastasis. J Natl Cancer Inst. 2010;102:1148–1159. doi: 10.1093/jnci/djq257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao Y, Hata Y, Ikeda M, et al. Mammalian Hippo pathway: from development to cancer and beyond. J Biochem. 2011;149:361–379. doi: 10.1093/jb/mvr021. [DOI] [PubMed] [Google Scholar]
- Basu S, Totty NF, Irwin MS, et al. Akt phosphorylates the Yes-associated protein, YAP, to induce interaction with 14-3-3 and attenuation of p73-mediated apoptosis. Mol Cell. 2003;11:11–23. doi: 10.1016/s1097-2765(02)00776-1. [DOI] [PubMed] [Google Scholar]
- Busca R, Berra E, Gaggioli C, et al. Hypoxia-inducible factor 1{alpha} is a new target of microphthalmia-associated transcription factor (MITF) in melanoma cells. J Cell Biol. 2005;170:49–59. doi: 10.1083/jcb.200501067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan SW, Lim CJ, Chen L, et al. The Hippo pathway in biological control and cancer development. J Cell Physiol. 2011;226:928–939. doi: 10.1002/jcp.22435. [DOI] [PubMed] [Google Scholar]
- Chan SW, Lim CJ, Guo K, et al. A role for TAZ in migration, invasion, and tumorigenesis of breast cancer cells. Cancer Res. 2008;68:2592–2598. doi: 10.1158/0008-5472.CAN-07-2696. [DOI] [PubMed] [Google Scholar]
- Chan SW, Lim CJ, Loo LS, et al. TEADs mediate nuclear retention of TAZ to promote oncogenic transformation. J Biol Chem. 2009;284:14347–14358. doi: 10.1074/jbc.M901568200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin L, Garraway LA, Fisher DE. Malignant melanoma: genetics and therapeutics in the genomic era. Genes Dev. 2006;20:2149–2182. doi: 10.1101/gad.1437206. [DOI] [PubMed] [Google Scholar]
- de Winter P, Leoni P, Abraham D. Connective tissue growth factor: structure-function relationships of a mosaic, multifunctional protein. Growth Factors. 2008;26:80–91. doi: 10.1080/08977190802025602. [DOI] [PubMed] [Google Scholar]
- Dhar A, Ray A. The CCN family proteins in carcinogenesis. Exp Oncol. 2010;32:2–9. [PubMed] [Google Scholar]
- Dong J, Feldmann G, Huang J, et al. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell. 2007;130:1120–1133. doi: 10.1016/j.cell.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrigno O, Lallemand F, Verrecchia F, et al. Yes-associated protein (YAP65) interacts with Smad7 and potentiates its inhibitory activity against TGF-beta/Smad signaling. Oncogene. 2002;21:4879–4884. doi: 10.1038/sj.onc.1205623. [DOI] [PubMed] [Google Scholar]
- Gaffney CJ, Oka T, Mazack V, et al. Identification, basic characterization and evolutionary analysis of differentially spliced mRNA isoforms of human YAP1 gene. Gene. 2012;509:215–222. doi: 10.1016/j.gene.2012.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grusche FA, Richardson HE, Harvey KF. Upstream regulation of the hippo size control pathway. Curr Biol. 2010;20:R574–R582. doi: 10.1016/j.cub.2010.05.023. [DOI] [PubMed] [Google Scholar]
- Hergovich A. Mammalian Hippo signalling: a kinase network regulated by protein-protein interactions. Biochem Soc Trans. 2012;40:124–128. doi: 10.1042/BST20110619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javelaud D, Alexaki VI, Mauviel A. Transforming growth factor-beta in cutaneous melanoma. Pigment Cell Melanoma Res. 2008;21:123–132. doi: 10.1111/j.1755-148X.2008.00450.x. [DOI] [PubMed] [Google Scholar]
- Javelaud D, Alexaki VI, Pierrat MJ, et al. GLI2 and M-MITF transcription factors control exclusive gene expression programs and inversely regulate invasion in human melanoma cells. Pigment Cell Melanoma Res. 2011;24:932–943. doi: 10.1111/j.1755-148X.2011.00893.x. [DOI] [PubMed] [Google Scholar]
- Javelaud D, Mohammad KS, McKenna CR, et al. Stable overexpression of Smad7 in human melanoma cells impairs bone metastasis. Cancer Res. 2007;67:2317–2324. doi: 10.1158/0008-5472.CAN-06-3950. [DOI] [PubMed] [Google Scholar]
- Kang Y, Siegel PM, Shu W, et al. A multigenic program mediating breast cancer metastasis to bone. Cancer Cell. 2003;3:537–549. doi: 10.1016/s1535-6108(03)00132-6. [DOI] [PubMed] [Google Scholar]
- Lamar JM, Stern P, Liu H, et al. The Hippo pathway target, YAP, promotes metastasis through its TEAD-interaction domain. Proc Natl Acad Sci USA. 2012;109:E2441–E2450. doi: 10.1073/pnas.1212021109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Fukunaga-Kalabis M, Herlyn M.2011The three-dimensional human skin reconstruct model: a tool to study normal skin and melanoma progression J Vis Expdoi: 10:3791/2937). [DOI] [PMC free article] [PubMed]
- Liu AM, Xu Z, Luk JM. An update on targeting Hippo-YAP signaling in liver cancer. Expert Opin Ther Targets. 2012;16:243–247. doi: 10.1517/14728222.2012.662958. [DOI] [PubMed] [Google Scholar]
- Liu-Chittenden Y, Huang B, Shim JS, et al. Genetic and pharmacological disruption of the TEAD-YAP complex suppresses the oncogenic activity of YAP. Genes Dev. 2012;26:1300–1305. doi: 10.1101/gad.192856.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Li Y, Kim SM, et al. Hippo signaling is a potent in vivo growth and tumor suppressor pathway in the mammalian liver. Proc Natl Acad Sci USA. 2010;107:1437–1442. doi: 10.1073/pnas.0911427107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauviel A, Nallet-Staub F, Varelas X. Integrating developmental signals: a Hippo in the (path)way. Oncogene. 2012;31:1743–1756. doi: 10.1038/onc.2011.363. [DOI] [PubMed] [Google Scholar]
- Michels S, Schmidt-Erfurth U. Photodynamic therapy with verteporfin: a new treatment in ophthalmology. Semin Ophthalmol. 2001;16:201–206. doi: 10.1076/soph.16.4.201.10298. [DOI] [PubMed] [Google Scholar]
- Mohammad KS, Javelaud D, Fournier PG, et al. TGF-beta-RI kinase inhibitor SD-208 reduces the development and progression of melanoma bone metastases. Cancer Res. 2011;71:175–184. doi: 10.1158/0008-5472.CAN-10-2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore R, Champeval D, Denat L, et al. Involvement of cadherins 7 and 20 in mouse embryogenesis and melanocyte transformation. Oncogene. 2004;23:6726–6735. doi: 10.1038/sj.onc.1207675. [DOI] [PubMed] [Google Scholar]
- Ota M, Sasaki H. Mammalian Tead proteins regulate cell proliferation and contact inhibition as transcriptional mediators of Hippo signaling. Development. 2008;135:4059–4069. doi: 10.1242/dev.027151. [DOI] [PubMed] [Google Scholar]
- Overholtzer M, Zhang J, Smolen GA, et al. Transforming properties of YAP, a candidate oncogene on the chromosome 11q22 amplicon. Proc Natl Acad Sci USA. 2006;103:12405–12410. doi: 10.1073/pnas.0605579103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan D. The hippo signaling pathway in development and cancer. Dev Cell. 2010;19:491–505. doi: 10.1016/j.devcel.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodeck U, Nishiyama T, Mauviel A. Independent regulation of growth and SMAD-mediated transcription by transforming growth factor beta in human melanoma cells. Cancer Res. 1999;59:547–550. [PubMed] [Google Scholar]
- Schlegelmilch K, Mohseni M, Kirak O, et al. Yap1 acts downstream of alpha-catenin to control epidermal proliferation. Cell. 2011;144:782–795. doi: 10.1016/j.cell.2011.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song H, Mak KK, Topol L, et al. Mammalian Mst1 and Mst2 kinases play essential roles in organ size control and tumor suppression. Proc Natl Acad Sci USA. 2010;107:1431–1436. doi: 10.1073/pnas.0911409107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staley BK, Irvine KD. Hippo signaling in Drosophila: recent advances and insights. Dev Dyn. 2012;241:3–15. doi: 10.1002/dvdy.22723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudol M. Newcomers to the WW domain-mediated network of the Hippo tumor suppressor pathway. Genes Cancer. 2010;1:1115–1118. doi: 10.1177/1947601911401911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudol M, Shields DC, Farooq A. Structures of YAP protein domains reveal promising targets for development of new cancer drugs. Sem Cell Dev Biol. 2012;23:827–833. doi: 10.1016/j.semcdb.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudol M, Sliwa K, Russo T. Functions of WW domains in the nucleus. FEBS Lett. 2001;490:190–195. doi: 10.1016/s0014-5793(01)02122-6. [DOI] [PubMed] [Google Scholar]
- Varelas X, Sakuma R, Samavarchi-Tehrani P, et al. TAZ controls Smad nucleocytoplasmic shuttling and regulates human embryonic stem-cell self-renewal. Nat Cell Biol. 2008;10:837–848. doi: 10.1038/ncb1748. [DOI] [PubMed] [Google Scholar]
- Varelas X, Wrana JL. Coordinating developmental signaling: novel roles for the Hippo pathway. Trends Cell Biol. 2012;22:88–96. doi: 10.1016/j.tcb.2011.10.002. [DOI] [PubMed] [Google Scholar]
- Vassilev A, Kaneko KJ, Shu H, et al. TEAD/TEF transcription factors utilize the activation domain of YAP65, a Src/Yes-associated protein localized in the cytoplasm. Genes Dev. 2001;15:1229–1241. doi: 10.1101/gad.888601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin M, Zhang L. Hippo signaling: a hub of growth control, tumor suppression and pluripotency maintenance. J Genet Genomics. 2011;38:471–481. doi: 10.1016/j.jgg.2011.09.009. [DOI] [PubMed] [Google Scholar]
- Zeng Q, Hong W. The emerging role of the hippo pathway in cell contact inhibition, organ size control, and cancer development in mammals. Cancer Cell. 2008;13:188–192. doi: 10.1016/j.ccr.2008.02.011. [DOI] [PubMed] [Google Scholar]
- Zhang H, Liu CY, Zha ZY, et al. TEAD transcription factors mediate the function of TAZ in cell growth and epithelial-mesenchymal transition. J Biol Chem. 2009;284:13355–13362. doi: 10.1074/jbc.M900843200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Lei QY, Guan KL. The Hippo-YAP pathway: new connections between regulation of organ size and cancer. Curr Opin Cell Biol. 2008a;20:638–646. doi: 10.1016/j.ceb.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Li L, Lei Q, et al. The Hippo-YAP pathway in organ size control and tumorigenesis: an updated version. Genes Dev. 2010;24:862–874. doi: 10.1101/gad.1909210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Ye X, Yu J, et al. TEAD mediates YAP-dependent gene induction and growth control. Genes Dev. 2008b;22:1962–1971. doi: 10.1101/gad.1664408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D, Conrad C, Xia F, et al. Mst1 and Mst2 maintain hepatocyte quiescence and suppress hepatocellular carcinoma development through inactivation of the Yap1 oncogene. Cancer Cell. 2009;16:425–438. doi: 10.1016/j.ccr.2009.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.