Abstract
Objective
To evaluate the predictive ability of baseline confocal scanning laser ophthalmoscopy (CSLO) Glaucoma Probability Score (GPS) for the development of primary open angle glaucoma (POAG) and to compare it to the Moorfields Regression Analysis (MRA) classification, other topographic optic disc parameters and stereophotograph- based cup-to-disc ratio.
Design
Longitudinal randomized clinical trial
Participants
857 eyes of 438 participants in the CSLO Ancillary Study to the Ocular Hypertension Treatment Study (OHTS) with good quality baseline CSLO images.
Methods
The ability of baseline GPS, MRA and optic disc parameters to predict the development of POAG was evaluated in univariate and multivariable proportional hazard ratio analyses. Likelihood ratios and positive and negative predictive values were compared
Main Outcome Measures
POAG end point as determined by repeatable changes in the visual field or optic disc.
Results
Sixty-four eyes of 50 CSLO Ancillary Study participants developed POAG. Median time to reach a POAG endpoint was 72.3 months . The 93 eyes of 388 participants not reaching endpoint were followed for a median124.9 months. Baseline GPS identified many more eyes as outside normal limits than the MRA. In multivariable analyses, all regional and global baseline GPS indices were significantly associated with the development of POAG; hazard ratios (95% confidence interval [CI]) ranged from 2.92 to 3.74 for an outside normal limit result. MRA indices were also significantly associated with the development of POAG in multivariable analyses. In addition, the predictive ability of baseline GPS, MRA and stereometric parameters were similar to the predictive ability of models using photograph-based horizontal cup-to-disc ratio.
Conclusion
These results suggest that baseline GPS, MRA and stereoparameters alone or when combined with baseline clinical and demographic factors can be used to predict the development of POAG endpoints in OHTS participants and are as effective as stereophotographs for estimating the risk of developing POAG in ocular hypertensive subjects.
Introduction
We previously have shown that many baseline confocal scanning laser ophthalmoscopic (CSLO) topographic optic disc measurements in the CSLO Ancillary Study of the Ocular Hypertension Treatment Study (OHTS) were significantly predictive of the development of primary open angle glaucoma (POAG)1. Several stereometric parameters, as well as their classification as outside normal limits by Heidelberg Retina Tomograph (HRT) analysis, and global and regional measurements outside normal limits by Moorfields' Regression Analysis (MRA) were significantly predictive of the development of POAG. Hazard ratios (of an outside normal limits result compared to not outside normal limits) for these parameters ranged from 2.5 to 5.8. These data suggested that baseline CSLO topographic disc measurements, particularly when combined with other known risk factors such as age, intraocular pressure (IOP), and central corneal thickness, could assist clinicians in assessing the likelihood a patient with ocular hypertension (OH) will develop POAG. Moreover, these results demonstrated for the first time in a prospective cohort that baseline CSLO topographic optic disc measurements were significantly predictive of the development of glaucomatous optic disc or visual field damage in patients with OH.
Although a baseline classification outside normal limits was significantly predictive of the development of POAG among individuals with OH in our earlier report, most of the participants with values outside normal limits at baseline did not develop POAG within the follow-up of 48.4 ± 25.2 months (mean ± standard deviation [SD]).1 Specifically, only 20 (14%) of the 148 participants with an outside normal limits HRT classification and 10 (14%) of the 71 participants with a MRA global classification outside normal limits developed a POAG endpoint during this follow-up period. It was concluded that longer follow-up was required to evaluate the true predictive accuracy of these CSLO measures. Moreover, it was not directly determined whether a prediction model that included baseline CSLO measurements performed better than the OHTS prediction model that included baseline stereophotograph-based cup-to--disc ratio measurements. As CSLO parameters are strongly correlated with stereophotograph-based cup-to--disc ratio measurements, it is difficult to resolve the independent contribution of these measurements. Additional POAG endpoints from longer follow-up were needed to appropriately compare prediction models including CSLO and stereophotograph-based cup-to--disc ratio measurements, and also to evaluate the true predictive accuracy of CSLO measures. The current analysis includes additional OHTS POAG endpoints accrued over a follow-up period 3 years longer than the previous report to obtain better estimates of the positive and negative predictive value and likelihood ratio of an outside normal limits result.
In addition to MRA, the current report also evaluates the Glaucoma Probability Score (GPS), a CSLO topographic parameter that was introduced after our earlier report. GPS is of particular interest, as it does not require outlining the disc margin and facilitates the ease of CSLO topography interpretation in clinical practice. GPS has recently been shown to be predictive of the progression of glaucoma patients with suspected glaucoma.2 To validate the use of GPS, it is important to directly compare it with baseline MRA and stereometric parameters with respect to the ability to predict the development of glaucoma in a cohort of OH subjects.
Methods
Participants met the OHTS inclusion and exclusion criteria3 and were recruited from the 7 CSLO Ancillary Study OHTS clinics: Hamilton Glaucoma Center, University of California, San Diego, California; New York Eye and Ear Infirmary, New York, New York; Devers Eye Institute, Portland, Oregon; Henry Ford Medical Center, Troy Michigan; Jules Stein Eye Institute, University of California, Los Angeles, California: University of California, Davis, California; and Scheie Eye Institute, University of Pennsylvania, Philadelphia, Pennsylvania. The CSLO Ancillary study protocol was approved by the Institutional Review Board of each clinic participating in this ancillary study. Written informed consent for participation in this ancillary study was obtained from OHTS participants.
In brief, participants were eligible for inclusion if their IOP ranged from 24 mm Hg to 32 mm Hg in at least one eye and 21 mm Hg to 32 mm Hg in the fellow eye. Participants were required to have 2 normal, reliable automated achromatic 30-2 visual fields (Carl-Zeiss-Meditec, Dublin, California)3 and normal appearing optic discs based on clinical examination and review of full-frame 35 mm pairs or a split –frame simultaneous stereoscopic optic disc photographs as assessed by two independent, masked, certified graders at the Optic Disc Reading Center.3 The Optic Disc Reading Center (ODRC) graders estimated horizontal and vertical cup-to-disc ratios by contour.
The primary endpoints for OHTS were either the development of a confirmed visual field abnormality or a confirmed clinically significant stereophotograph-based optic disc deterioration attributed to POAG.3 Abnormalities were independently identified by masked, certified readers at the Visual Field and/or Optic Disc Reading Centers. The masked Endpoint Committee then determined whether these confirmed abnormalities were attributable to POAG. In addition, optic disc deterioration had to be clinically significant to be classified as an endpoint. The date for a POAG endpoint was the first date of three consecutive abnormal visual fields or the first date of two consecutive sets of stereo photographs that classified the eye as reaching a POAG endpoint.
As described previously, 1,4,5 the Heidelberg Retina Tomograph (HRT; Heidelberg Engineering, GmbH, Heidelberg, Germany) images were obtained by operators certified by the CSLO Reading Center at the University of California, San Diego. Three 10-degree images were obtained on both eyes and three 15-degree images were obtained on the right eye at the annual OHTS dilated examination. If both 10-degree and 15-degree good quality images were available, the 10-degree images were used in this analysis. Corneal curvature measurements were used to correct images for magnification error. Corrective lenses were used during image acquisition when astigmatism was greater than one diopter. Mean images were used for statistical analyses. The CSLO Reading Center also conducted all quality assessment and image processing.4
As described previously, the CSLO Ancillary Study to the OHTS was funded after the initiation of enrollment in OHTS. As a result, 77% of participants completed their first CSLO examination visit after their baseline, randomization visit.4 For this reason, a few of these participants developed optic disc deterioration or visual field abnormality that was subsequently confirmed and attributed to POAG at or before their first CSLO imaging session. If the first CSLO measurement took place after the OHTS examination that had a suspicious finding later confirmed as POAG, that eye was excluded from this analysis. The sample for the current report consists of all images from the first CSLO visit with good quality images to the closure date for this report (May 2007) or to the first suspicious date of POAG, whichever was first.
Stereometric parameters and Moorfields Regression Analysis (MRA): To calculate the stereometric parameters, it first is necessary for an operator to outline the disc margin. Contour lines outlining the disc margin were drawn by a certified operator while viewing a copy of stereo disc photographs from the Optic Disc Reading Center.4 The scans were obtained using the HRT 1 Classic instrument and analyzed using software version 3.0. Software version 3.0 includes improved alignment algorithms, a larger normative database and the calculation of the Glaucoma Probability Score (GPS).
The following CSLO stereometric parameters are included in this report: disc area, cup area and volume, cup-to-disc area ratio, rim-to-disc area ratio, mean cup depth, retinal nerve fiber layer (RNFL) thickness and cross-sectional area, rim area and volume, mean height contour, reference plane height, cup shape. Some stereometric parameters were measured relative to the standard reference plane; these included cup area, cup-to-disc area ratio, cup volume below the reference plane, RNFL thickness and cross-sectional area, and rim area. Using HRT software (version 2.01 or higher), the “standard” reference plane height is calculated 50 microns posterior to the mean height contour along a small temporal section of the contour line. Other parameters, including mean height contour, cup shape and mean cup depth were measured from the curved surface. The standard deviation of the mean image is a measure of image quality. The MRA compares measured rim area to predicted rim area adjusted for disc size to categorize eyes as outside normal limits, borderline or within normal limits6.
Glaucoma Probability Score: The GPS is available with HRT 3.0 (or higher software). It does not depend on an operator drawn contour line or a reference plane and is therefore operator independent. The GPS uses a geometric model to describe the shape of the optic disc/parapapillary retina (globally and locally) based on 5 parameters (cup size, cup depth, rim steepness, horizontal retinal nerve fiber layer curvature, and vertical retinal nerve fiber layer curvature).7 These parameters are then interpreted by a relevance vector machine classifier,8 and the resulting output describes the probability that the eye is glaucomatous as between 0% and 100% (based on fit to training data from healthy and glaucoma eyes). GPS output is then automatically classified into 3 categories; outside normal limits (GPS > 64%), borderline (GPS between 24% and 64%) and within normal limits (GPS < 24%).
Using the HRT 3.0 software, both the MRA and GPS classify eyes as within normal limits (WNL), borderline (BL) or outside normal limits (ONL) utilizing the same normative database of 700 Caucasian eyes and 200 African American eyes. The comparison to the normative database is provided in six regions (superior temporal, inferior temporal, temporal, superior nasal, inferior nasal and nasal), and as an overall global classification. If any of the six regions are ONL, then the eye is classified as ONL. In addition, if any of the regional or global values are “outside normal limits” then the MRA and GPS overall “result” measurement is defined as “outside normal limits.”
Statistical analysis
The analysis data set included OHTS and CSLO baseline data and POAG endpoints with initial suspicious dates before November 2006 that were confirmed and entered into the database by May 18, 2007. All descriptive tables (means and percents) report the average of the right and left eyes.
The association of each CSLO parameter and the MRA and the GPS for the development of POAG was assessed individually via univariate Cox proportional hazards models using the PHREG procedure of SAS (Software version SAS9.1.3, SAS Institute Inc, Cary, NC). Data for both eyes of the participant were included in the model and the method of Lee, Wei, and Amato was used to adjust for the correlation of the two eyes from the same participant.9 CSLO variables' units were standardized in the Cox proportional hazards models. As implemented previously,1 the units used in the analyses for the continuous variables were chosen so that each variable would have an approximate standard deviation of one. Baseline CSLO parameters, MRA and GPS were also studied in multivariable Cox proportional hazards models that adjusted for the same baseline factors predictive of POAG in the OHTS and European Glaucoma Prevention Study (EGPS) combined analysis.10 These variables included participant status at the time of the baseline HRT for age, pattern standard deviation (PSD), baseline IOP and central corneal thickness. In a separate multivariable analysis, baseline horizontal cup-to-disc ratio as determined by the ODRC from stereophotographs was also included. Medication was included in the univariate and multivariable models as a time-dependent covariate due to the initiation of ocular hypotensive medications for the observation group in 2002.
For the MRA and GPS, the hazard ratios and 95% confidence intervals were calculated by comparing outside normal limits to not outside normal limits (borderline and within normal limits combined). Kaplan-Meier plots for developing POAG for selected individual CSLO measures are included.
Sensitivity, specificity, positive predictive value, negative predictive value and positive likelihood ratios were calculated. Likelihood ratios for WNL, borderline, and ONL categories can be interpreted by the extent to which they affect post-test probability of disease.13 Ratios between 0.5 and 2.0 have an insignificant effect on post-test probability of disease. Likelihood ratios between 0.2 and 0.5 or between 2 and 5 have a “small” effect on post-test probability. Ratios between 0.1 and 0.2 or between 5 and 10 have a “moderate” effect, and ratios 0.1 or 10 have a “large” effect on post-test probability of disease. Confidence intervals for the likelihood ratios were calculated using the Simel method.11
A direct comparison of hazard ratios is not suitable because the magnitude of the ratio depends on the unit of measurement. We therefore used Harrell's12 c-index to compare the predictive ability of the various survival models. The c-index computes the proportion of subjects in which the predictions of the model match the actual outcome. This method has been used to compare predictive models in a Diagnostic Innovations in Glaucoma Study (DIGS) analysis2 and in the EGPS-OHTS combined prediction analysis.13 Similar to the area under the receiver operator characteristic curve, c-index values are between 0 and 1 with 0.5 representing random prediction and 1.0 representing perfect prediction. The c-index was computed for the multivariable Cox proportional hazard models. The R software (version 2.6.2,http://www.r-project.org/ Accessed February 4, 2010) was used to compute the c-index (using the Hmisc library and the rcorr.cens function).
Results
Eight hundred sixty-six eyes from 439 participants had good quality baseline CSLO images and were eligible for inclusion in this analysis. Nine eyes were excluded due to the initial CSLO measurement being on the same day or after the first suspicious date for a POAG endpoint. One participant had both eyes excluded. Therefore, the analysis sample for this report consists of 857 eyes of 438 participants. Baseline demographic, clinical and ocular factors of the 438 CSLO Ancillary Study participants included in this analysis are reported in Tables 1 and 2. Seventy-four (17%) of the CSLO participants were African American, 253 (58%) were female, and 140 (32%) reported a family history of glaucoma (Table 1). The average age of the participants at baseline was 55.4 years, average IOP was 25 mm Hg and average central corneal thickness was 575 μm (Table 2).
Table 1. Baseline demographic and clinical characteristics by POAG status* (n=number of participants).
| Not at POAG endpoint** |
POAG endpoint |
All | ||||
|---|---|---|---|---|---|---|
| N | % | N | % | N | % | |
| Race | ||||||
| Other | 326 | 89.6 | 38 | 10.4 | 364 | 100 |
| African American | 62 | 83.8 | 12 | 16.2 | 74 | 100 |
|
| ||||||
| Sex | ||||||
| Female | 231 | 91.3 | 22 | 8.7 | 253 | 100 |
| Male | 157 | 84.9 | 28 | 15.1 | 185 | 100 |
|
| ||||||
| Parent/sibling family history of glaucoma | ||||||
| No | 256 | 85.9 | 42 | 14.1 | 298 | 100 |
| Yes | 132 | 94.3 | 8 | 5.7 | 140 | 100 |
|
| ||||||
| History of Heart Disease | ||||||
| No | 372 | 89.0` | 46 | 11.0 | 418 | 100 |
| Yes | 16 | 80.0 | 4 | 20.0 | 20 | 100 |
Primary open angle glaucoma (POAG) endpoint confirmed and entered into the database by May 30, 2007
Table 2. Baseline demographic and ocular characteristics (average of the eyes) by POAG Status*.
| Not at POAG endpoint | POAG endpoint | All | |||||||
|---|---|---|---|---|---|---|---|---|---|
| N | Mean | (95% CI) | N | Mean | (95% CI) | N | Mean | (95% CI) | |
| Age | 388 | 55.1 | (54.2, 56.0) | 50 | 57.6 | (55.0, 60.2) | 438 | 55.4 | (54.5, 56.2) |
| IOP mm Hg** | 388 | 25.0 | (24.7, 25.2) | 50 | 25.4 | (24.6, 26.2) | 438 | 25.0 | (24.8, 25.2) |
| Central corneal thickness μm | 354 | 577 | (573, 581) | 49 | 563 | (533, 573) | 404 | 575 | (592, 579) |
| Visual field pattern standard deviation, dB** | 388 | 2.00 | (1.94, 2.06) | 50 | 1.96 | (1.90, 2.01) | 438 | 2.02 | (1.96, 2.08) |
| Visual field mean deviation, dB** | 388 | 0.07 | (-0.09, 0.23) | 50 | -0.29 | (-.66, 0.09) | 438 | 0.02 | (-0.13, 0.17) |
| Photograph Horizontal cup-to-disc ratio closest to HRT baseline*** | 388 | 0.38 | (0.36,.040) | 50 | 0.48 | (0.44, 0.53) | 438 | 0.39 | (0.37, 0.40) |
| Disc area (mm2) | 388 | 1.92 | (1.89, 1.97) | 50 | 1.88 | (1.78, 1.99) | 438 | 1.92 | (1.89, 1.96) |
Abbreviations: POAG= primary open-angle glaucoma; IOP =Intraocular pressure; CI=Confidence Interval
POAG endpoint confirmed and entered into the database by May 30, 2007
From OHTS (Ocular Hypertension Treatment Study) baseline visit
From date closest to the HRT (Heidelberg Retina Tomograph) baseline visit.
POAG Endpoints
Sixty-four eyes of 50 CSLO participants developed POAG after the initial CSLO measurement. Fourteen of these participants developed bilateral POAG and 36 developed unilateral POAG during the follow-up period in this report. Of the 64 POAG eyes, 19 (30%) initially reached endpoint based on visual fields alone, 44 (69%) initially on stereophotographs alone and 1 (1%) based on concurrent visual fields and stereophotographs. Of the 44 eyes that were initially classified as POAG only on the basis of stereophotographs, 8 (18%) went on to develop visual field damage attributable to POAG by May 2007. Seven (37%) of the 19 eyes classified initially as POAG based on only visual fields later developed optic disc changes attributable to POAG. There were 793 eyes from 388 participants that did not develop POAG.
Median follow-up from the first CSLO examination to the first suspicious date for the 64 eyes reaching a POAG endpoint was 72.3 months (mean ± SD was 68.1 ± 38.9 months). Median follow-up from the first CSLO examination to the last useable visual field or disc photograph among eyes not reaching a POAG endpoint was 124.9 months (mean ± SD was 111.8 ± 34.0 months) for eyes that did not develop POAG.
Cox Proportional Hazards Models
In univariate and multivariable analyses, several baseline clinical and ocular factors and CSLO topographic optic disc measurements were associated with the development of POAG (Tables 3 and 4). One multivariable model was completed for each of the CSLO parameters and indices to test the independent relationship of each CSLO parameter with POAG adjusting for baseline age, IOP, central corneal thickness, and visual field PSD, with medication status as a time-dependent covariate.
Table 3. Glaucoma Probability Score: Univariate and Multivariable Hazard Ratios for the development of POAG.
| Univariate | Multivariable* | ||
|---|---|---|---|
| Hazard Ratio (95% CI*) | Hazard Ratio (95% CI) | ||
| Outside normal limits versus not outside normal limits | Result | 3.03 (1.67, 5.50) | 2.84 (1.49 5.40)) |
| Global | 3.99 (2.21 7.22) | 3.51 (1.86 6.90) | |
| Nasal | 3.94 (2.18 7.12) | 3.86 (1.86 6.86) | |
| Nasal Inferior | 3.18 (1.66 6.10) | 2.87 (1.42 5.82) | |
| Nasal Superior | 3.32 (1.76 6.26) | 3.05 (1.51 6.15) | |
| Temporal | 3.69 (2.04 6.66) | 3.25 (1.70 6.20) | |
| Temporal Inferior | 3.38 (1.84 6.22) | 3.07 (1.58 5.95) | |
| Temporal Superior | 3.14 (1.66 5.94) | 2.92 (1.46 5.83) | |
| per 10% greater | Global | 1.22 (1.11 1.34) | 1.20 (1.09 1.33) |
| Nasal | 1.22 (1.11 1.33) | 1.20 (1.09 1.33) | |
| Nasal Inferior | 1.22 (1.12 1.34) | 1.21 (1.09 1.33) | |
| Nasal Superior | 1.22 (1.11 1.34) | 1.20 (1.09 1.33) | |
| Temporal | 1.22 (1.11 1.34) | 1.20 (1.09 1.33) | |
| Temporal Inferior | 1.22 (1.10 1.34) | 1.20 (1.08 1.33) | |
| Temporal Superior | 1.22 (1.11 1.33) | 1.20 (1.09 1.32) | |
| Individual components of GPS | rim steepness normalized (GLOBAL) (per 0.8) | 0.49 (0.32 0.75) | 0.54 (0.37 0.78) |
| GPS Maximum cup depth (per 0.1 mm) | 1.29 (1.10 1.51) | 1.34 (1.14 1.58) | |
| GPS Mean cup depth (per 0.02 mm) | 1.47 (1.17 1.84) | 1.51 (1.17 1.94) | |
| GPS cup area (mm2) | 1.10 (0.99 1.22) | 1.11 (0.98 1.26) | |
| Vertical RNFL curvature (per 0.05) | 0.67 (0.54 0.83) | 0.67 (0.54 0.84) | |
| Horitzontal RNFL curvature (per 0.05) | 0.50 (0.38 0.64) | (0.51 (0.38 0.67) | |
Abbreviations: POAG= primary open-angle glaucoma; CI=Confidence Interval
Multivariable model contains baseline age, intraocular pressure, visual field pattern standard devision, central corneal thickness with medication status as a time dependent covariate. 64 eyes were excluded from the multivariable analyses due to missing central corneal thickness values.
Table 4. Univariate and Multivariable Hazard Ratios and 95% CI for the development of POAG.
| Univariate | Multivariable* | |||
|---|---|---|---|---|
| Hazard Ratio (95% CI) | Hazard Ratio (95% CI) | |||
| Moorfields Regression Analysis (outside normal limits versus not) | Result | 4.34 (2.40 7.84) | 3.90 (2.09 7.28) | |
| Global | 6.92 (3.09 15.49) | 6.29 (2.84 13.99) | ||
| Nasal | 4.10 (2.14 7.87) | 4.35 (2.07 9.15) | ||
| Nasal Inferior | 5.83 (2.93 11.61) | 4.71 (2.26 9.16) | ||
| Nasal Superior | 3.75 (1.67 8.42) | 3.34 (1.54 7.60) | ||
| Temporal | 1.89 (0.26 13.69) | 2.41 (0.36 16.00) | ||
| Temporal Inferior | 5.20 (2.03 13.31) | 3.84 (1.16 12.70) | ||
| Temporal Superior | 14.25 (6.57 30.90) | 11.03 (4.81 25.30) | ||
|
OHTS Predictive Factors |
Age (per decade) | 1.38 (1.03 1.84) | NA | |
| History of heart disease | 2.81 (1.14 6.94) | NA | ||
| IOPˆ (per mm Hg) | 1.12 (1.02 1.24) | NA | ||
| Central Corneal Thickness (per 40 μm thinner) | 1.60 (1.18 2.18) | NA | ||
| PSDˆˆ (per 0.2 dB greater) | 1.04 (1.00 1.07) | NA | ||
| Photograph Horizontal cup-to--disc ratio closest to HRTˆˆˆ baseline (per 0.1 larger) | 1.38 (1.21 1.58) | NA | ||
| CSLO Measures | Disc area (per 0.4 mm2 greater) | 0.94 (0.73 1.23) | 0.99 (0.74 1.32) | |
| Cup area (per 0.3 mm2 greater) | 1.46 (1.22 1.74) | 1.47 (1.21 1.79) | ||
| Cup area-to-disc area (per 0.1 greater) | 1.66 (1.39 1.98) | 1.64 (1.36 1.96) | ||
| Linear cup-to-disc ratio (per 0.1 greater) | 1.74 (1.38 2.19) | 1.71 (1.36 2.15) | ||
| Mean Cup depth (per 0.1 mm greater) | 1.85 (1.45 2.35) | 1.93 (1.50 2.49) | ||
| RNFL thickness (per 0.1 mm greater) | 0.43 (0.27 0.71) | 0.49 (0.29 0.84) | ||
| Standard deviation of mean image (per 6 μm greater) | 1.21 (1.00 1.47) | 1.16 (0.96 1.41) | ||
| Cup shape (per 0.1 greater) | 1.57 (1.13 2.17) | 1.33 (0.94 1.88) | ||
| Cup volume below surface (per 0.1 mm3 greater) | 1.21 (1.09 1.34) | 1.22 (1.10 1.36) | ||
| Rim area (per 0.2 greater) | 0.46 (0.36 0.50) | 0.46 (0.36 0.59) | ||
| Rim area/disc area (per 0.1 greater) | 0.60 (0.50 0.72) | 0.61 (0.51 0.73) | ||
| Reference height (per 0.1 mm greater) | 1.27 (1.00 1.62) | 1.34 (1.02 1.75) | ||
| Corneal curvature (per 0.2 mm greater) | 1.17 (0.95 1.45) | 1.13 (0.91 1.40) | ||
| RNFL cross section (per 0.3 mm2 greater) | 0.58 (0.43 0.77) | 0.62 (0.45 0.85) | ||
| Mean height contour (per 0.1 mm greater) | 2.61 (1.86 3.67) | 2.64 (1.86 3.73) | ||
| Rim volume above ref (per 0.1 mm3 greater) | 0.50 (0.37 0.68) | 0.53 (0.39 0.72) | ||
| Cup volume below ref (per 0.1 mm3 greater) | 1.40 (1.22 1.59) | 1.40 (1.23 1.60) | ||
Abbreviations: POAG= primary open-angle glaucoma; IOP =Intraocular pressure; CI=Confidence Interval; PSD = Pattern Standard Deviation; HRT = Heidelberg Retina Tomograph; RNFL = Retinal Nerve Fiber Layer; CSLO = Confocal Scanning Laser Ophthalmoscopy; OHTS = Ocular Hypertension Treatment Study; NA= not applicable
Multivariable model contains baseline age, intraocular pressure, visual field pattern standard devision, central corneal thickness with medication status as a time dependent covariate. 64 eyes were excluded from the multivariable analyses due to missing central corneal thickness values.
Glaucoma Probability Score
The GPS model did not converge in 7 (1.6%) participants for the GPS “global” and “result” indices and in 9 (2.0%) participants for the regional GPS measurements. None of these excluded participants reached a POAG endpoint. In both univariate and multivariable analyses, baseline GPS indices were significantly associated with the development of POAG (Table 3). The association was significant in all regions and regardless of whether GPS was analyzed as a categorical variable (outside normal limits versus not outside normal limits) or continuous variable (from 0 to 100%). The univariate hazard ratios (95% confidence interval [CI]) for comparing baseline results of outside normal limits to not outside normal limits ranged from 3.03 (1.67, 5.50) (GPS Result) to 3.99 (2.21 to 7.22) (GPS Global), while the multivariable hazard ratios (95% CI) ranged from 2.92 (1.44, 5.92) (GPS Nasal Inferior) to 3.74 (1.93, 7.27) (GPS Nasal). The univariate and multivariable hazard ratios for the regional and global baseline continuous GPS indices (per 10% greater at baseline on a scale from 1% to 100%)) were almost identical for all regions (approximately 1.22). The Kaplan Meier Survival Curve for baseline GPS result as a categorical variable is shown in Figure 1.
FIgure 1.
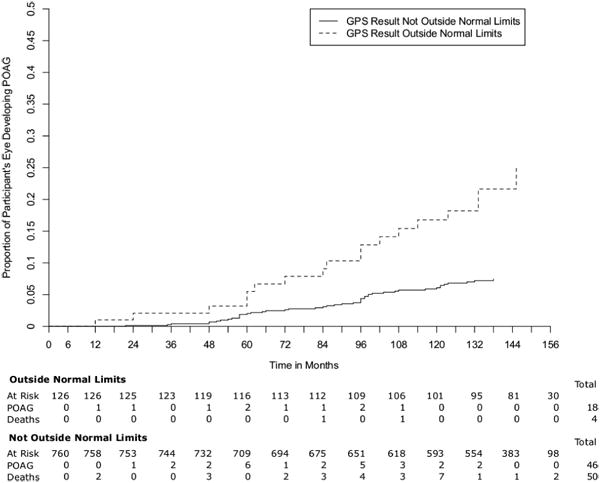
CSLO topographic optic disc measurements and indices
As reported previously, baseline demographic and clinical factors including a history of heart disease, thinner central corneal thickness, and larger stereophotograph-based horizontal and vertical cup-to-disc diameter ratios were significantly predictive of POAG. (Table 4). With longer follow-up and more eyes reaching a POAG endpoint, baseline age, IOP, and visual field PSD were significantly associated in the expected direction with the development of POAG. Baseline assessments of race, gender, parent or sibling history of glaucoma, history of high or low blood pressure, myopia, history of heart disease and baseline visual field mean deviation (MD) were not associated with the development of POAG in this study population in the multivariable models (data not shown).
Significant associations with the development of POAG were found in univariate analyses for MRA and all baseline topographic optic disc parameters except disc area and in multivariable analyses for all parameters and indices except MRA in the temporal region, disc area and cup shape. All associations were in the expected directions. The Kaplan Meier Survival Curve for MRA result is shown in Figure 2.
FIgure 2.
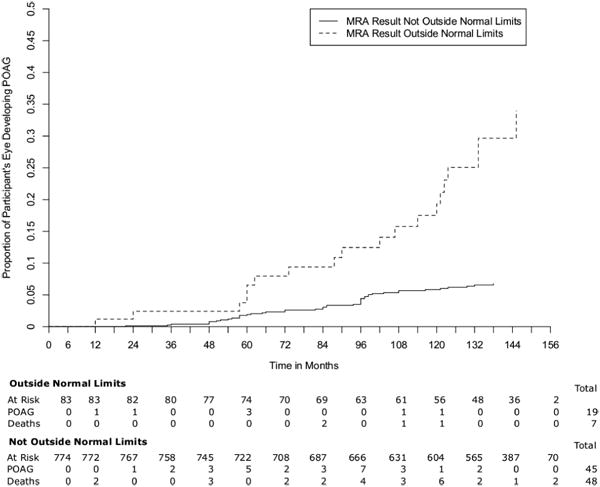
In addition, a multivariable model was evaluated that included stereophotograph-based baseline horizontal cup-to-disc ratio with baseline age, IOP, central corneal thickness, visual field PSD, and medication status as a time-dependent covariate. With photograph-based horizontal cup-to disc ratio in the model, the GPS hazard ratios (both categorical and continuous) were lower and no longer statistically significant. The MRA hazard ratios with photograph-based horizontal cup-to disc ratio added to the multivariable model also were lower, but nasal inferior and temporal superior MRA reached statistical significance. Interestingly, a larger disc area was associated with a reduced likelihood of developing glaucoma when photograph-based horizontal cup-to disc ratio was in the model, hazard ratio (HR) (95%CI) .56 (0.38 0.84).
Diagnostic accuracy, Predictive Values, and Likelihood Ratios
The sensitivity, specificity, positive and negative predictive values and likelihood ratios for GPS are presented in Table 5 and for MRA in Ta ble 6. In general, with the exception of the overall “result”, in each region, the GPS identified many more eyes as outside normal limits than the MRA. For example, GPS identified 81 eyes as outside normal limits for the temporal inferior region while MRA identified 14 eyes. In the temporal superior region, outside normal limits were reported in 79 eyes by GPS and 13 by MRA.
Table 5. Sensitivity*, Specificity**, Positive and Negative Predictive Values, and Likelihood ratios for Glaucoma Probability Score (n=number of eyes).
| Glaucoma Probability Score | Not at POAG endpoint (n) | nonPOAG % | POAG endpoint (n) | POAG % | All (n) | Likelihood Ratio (95% CI) | Negative Predictive Value | Positive Predictive Value | |
|---|---|---|---|---|---|---|---|---|---|
| Result | Within normal limits | 560 | 0.73** | 32 | 0.50 | 592 | 0.68 (0.53, 0.87) | 0.95 | 0.05 |
| Borderline | 146 | 0.19 | 14 | 0.22 | 160 | 1.14 (0.70, 1.86) | 0.91 | 0.09 | |
| Outside normal limits | 58 | 0.08 | 18 | 0.28* | 76 | 3.70 (2.33, 5.89) | 0.76 | 0.24 | |
| Global | Within normal limits | 531 | 0.70** | 30 | 0.47 | 561 | 0.67 (0.52, 0.88) | 0.95 | 0.05 |
| Borderline | 154 | 0.20 | 16 | 0.25 | 170 | 1.24 (0.79, 1.94) | 0.91 | 0.09 | |
| Outside normal limits | 79 | 0.10 | 18 | 0.28* | 97 | 2.72 (1.74, 4.24) | 0.81 | 0.19 | |
| Nasal | Within normal limits | 551 | 0.73** | 32 | 0.50 | 583 | 0.68 (0.53, 0.86) | 0.95 | 0.05 |
| Borderline | 143 | 0.19 | 14 | 0.22 | 157 | 1.15 (0.71, 1.87) | 0.91 | 0.09 | |
| Outside normal limits | 58 | 0.08 | 18 | 0.28* | 76 | 3.65 (2.29, 5.79) | 0.76 | 0.24 | |
| Nasal Inferior | Within normal limits | 557 | 0.74 | 32 | 0.50 | 589 | 0.68 (0.53, 0.87) | 0.95 | 0.05 |
| Borderline | 139 | 0.18 | 17 | 0.27 | 156 | 1.44 (0.93, 2.22) | 0.89 | 0.11 | |
| Outside normal limits | 56 | 0.07 | 15 | 0.23* | 71 | 3.15 (1.89, 5.23) | 0.79 | 0.21 | |
| Nasal Superior | Within normal limits | 557 | 0.74** | 33 | 0.52 | 590 | 0.70 (0.55, 0.89) | 0.94 | 0.06 |
| Borderline | 135 | 0.18 | 15 | 0.23 | 150 | 1.31 (0.82, 2.09) | 0.90 | 0.10 | |
| Outside normal limits | 60 | 0.08 | 16 | 0.25* | 76 | 3.13 (1.92, 5.11) | 0.79 | 0.21 | |
| Temporal | Within normal limits | 551 | 0.73** | 33 | 0.52 | 584 | 0.70 (0.55, 0.90) | 0.94 | 0.06 |
| Borderline | 140 | 0.19 | 13 | 0.20 | 153 | 1.09 (0.66, 1.81) | 0.92 | 0.08 | |
| Outside normal limits | 61 | 0.08 | 18 | 0.28* | 79 | 3.47 (2.19, 5.49) | 0.77 | 0.23 | |
| Temporal Inferior | Within normal limits | 543 | 0.72** | 32 | 0.50 | 575 | 0.69 (0.54, 0.89) | 0.94 | 0.06 |
| Borderline | 145 | 0.19 | 15 | 0.23 | 160 | 1.22 (0.76, 1.94) | 0.91 | 0.09 | |
| Outside normal limits | 64 | 0.09 | 17 | 0.27* | 81 | 3.12 (1.95, 4.99) | 0.79 | 0.21 | |
| Temporal Superior | Within normal limits | 561 | 0.75** | 33 | 0.52 | 594 | 0.69 (0.54, 0.88) | 0.94 | 0.06 |
| Borderline | 128 | 0.17 | 15 | 0.23 | 143 | 1.38 (0.86, 2.20) | 0.90 | 0.10 | |
| Outside normal limits | 63 | 0.08 | 16 | 0.25* | 79 | 2.98 (1.83, 4.85) | 0.80 | 0.20 |
Abbreviations: POAG= primary open-angle glaucoma; CI=Confidence Interval;
The positive predictive value, that is the proportion of eyes with a baseline outside normal limit result that developed a POAG endpoint, was between 20% (16/79, temporal superior) and 24% (18/76, result and nasal) for the GPS regional parameters. The positive predictive value of the MRA was highest for temporal superior (8/13, 62%), followed by global (7/19, 37%) and nasal inferior (11/35, 31%). In general, the negative predictive value was high; between 93% and 95% of those eyes within normal limits at baseline for GPS and MRA did not develop POAG during the follow-up period.
Among the 64 eyes that developed POAG, 18 (28.1%) had values outside normal limits by GPS Result, and 19 (29.7%) had values outside normal limits by MRA Result.
In general, the likelihood ratios for various GPS indices of outside normal limits had a “small” effect on post-test probability ranging from 3.0 (GPS temporal superior) to 3.7 (GPS result) (Table 5). The likelihood ratios for various MRA indices outside normal limits also generally had a small effect, ranging from 2.1 (temporal), to 5.0 (temporal inferior). The likelihood ratio for 3 MRA calculations were greater, 5.7 (Nasal inferior), 7.2 (global) and 39.7 (temporal superior), but the confidence intervals also were larger (Table 6).
Table 6. Sensitivity*, Specificity**, Positive and Negative Predictive Values, and Likelihood ratios for Moorfields Regression Analysis (n=number of eyes).
| Moorfields Regression Analysis | Not at POAG endpoint (n) | Non POAG % | POAG endpoint (n) | POAG % | All (n) | Likelihood Ratio | Negative Predictive Value | Positive Predictive Value | |
|---|---|---|---|---|---|---|---|---|---|
| Result | Within normal limits | 615 | 0.78** | 32 | 0.50 | 647 | 0.64 | 0.95 | 0.05 |
| Borderline | 114 | 0.14 | 13 | 0.20 | 127 | 1.41 | 0.90 | 0.10 | |
| Outside normal limits | 64 | 0.08 | 19 | 0.30* | 83 | 3.68 | 0.77 | 0.23 | |
| Global | Within normal limits | 726 | 0.92** | 42 | 0.66 | 768 | 0.72 | 0.95 | 0.05 |
| Borderline | 55 | 0.07 | 15 | 0.23 | 70 | 3.38 | 0.79 | 0.21 | |
| Outside normal limits | 12 | 0.02 | 7 | 0.11* | 19 | 7.23 | 0.63 | 0.37 | |
| Nasal | Within normal limits | 721 | 0.91** | 45 | 0.70 | 766 | 0.77 | 0.94 | 0.06 |
| Borderline | 44 | 0.06 | 10 | 0.16 | 54 | 2.82 | 0.81 | 0.19 | |
| Outside normal limits | 28 | 0.04 | 9 | 0.14* | 37 | 3.98 | 0.76 | 0.24 | |
| Nasal Inferior | Within normal limits | 704 | 0.89** | 44 | 0.69 | 748 | 0.77 | 0.94 | 0.06 |
| Borderline | 65 | 0.08 | 9 | 0.14 | 74 | 1.72 | 0.88 | 0.12 | |
| Outside normal limits | 24 | 0.03 | 11 | 0.17* | 35 | 5.68 | 0.69 | 0.31 | |
| Nasal Superior | Within normal limits | 718 | 0.91** | 45 | 0.70 | 763 | 0.78 | 0.94 | 0.06 |
| Borderline | 49 | 0.06 | 11 | 0.17 | 60 | 2.78 | 0.82 | 0.18 | |
| Outside normal limits | 26 | 0.03 | 8 | 0.13* | 34 | 3.81 | 0.76 | 0.24 | |
| Temporal | Within normal limits | 758 | 0.96** | 56 | 0.88 | 814 | 0.92 | 0.93 | 0.07 |
| Borderline | 29 | 0.04 | 7 | 0.11 | 36 | 2.99 | 0.81 | 0.19 | |
| Outside normal limits | 6 | 0.01 | 1 | 0.02* | 7 | 2.07 | 0.86 | 0.14 | |
| Temporal Inferior | Within normal limits | 745 | 0.94** | 51 | 0.80 | 796 | 0.85 | 0.94 | 0.06 |
| Borderline | 38 | 0.05 | 9 | 0.14 | 47 | 2.93 | 0.81 | 0.19 | |
| Outside normal limits | 10 | 0.01 | 4 | 0.06* | 14 | 4.96 | 0.71 | 0.29 | |
| Temporal Superior | Within normal limits | 757 | 0.48** | 50 | 0.78 | 807 | 1.64 | 0.94 | 0.06 |
| Borderline | 31 | 0.04 | 6 | 0.09 | 37 | 2.40 | 0.84 | 0.16 | |
| Outside normal limits | 5 | 0.00 | 8 | 0.13* | 13 | 39.65 | 0.38 | 0.62 | |
POAG = Primary open angle glaucoma
Comparing Prediction Models
In general, the c-index, which assesses the predictive ability of survival models, was reasonable (> 0.7) for all multivariable models evaluated. Specifically, the c-index (95% CI) for the GPS global and regional parameters ((all = 0.75 (.69, .82)) were similar to the c-index for the photograph based horizontal cup-to-disc ratio ((0.76 (0.70, 0.81)), the MRA result ((0.76 (0.70, 0.82)) and the regional MRA variables (between 0.73 (nasal superior) to 0.76 (temporal)). The stereometric parameters also had similar predictive ability, with the c-index ranging from 0.73 (0.67, 0.79) for disc area, and standard deviation of the mean image, to 0.78 (0.72, 0.83) for mean height contour and linear cup disc ratio, and rim area=0.80 (0.75, 0.86)).
Discussion
In this analysis, both baseline GPS and MRA were predictive of POAG in patients with ocular hypertension. Further, the predictive ability of models including GPS, MRA and stereometric parameters were similar to the predictive ability of models using stereophotograph-based horizontal cup-to-disc ratio. These results suggest that CSLO disc topographic assessment with GPS and / or MRA can be used as effectively as stereophotographs to estimate the risk of developing POAG in ocular hypertensive subjects. To our knowledge, this is the first report comparing the predictive ability of GPS, and MRA to stereophotograph assessment in a cohort of ocular hypertensive subjects.
Specifically, participants with a GPS categorical parameter outside normal limits at baseline were approximately 3 to 4 times more likely to develop a POAG endpoint than those whose GPS categorical parameter value was within normal limits, while participants with a MRA outside normal limits result were between 3 and 10 times more likely to develop a POAG endpoint. Using the GPS continuous parameters, a 10% greater GPS value at baseline resulted in a 21% higher likelihood of developing a POAG endpoint. A 0.1 larger stereophotograph based horizontal cup-to-disc ratio at baseline resulted in a 38% increased likelihood of developing a POAG endpoint.
As illustrated above by the comparison of continuous versus categorical assessment of the same GPS parameters, it is inappropriate to directly compare hazard ratios as the magnitude of the measured increased risk depends on the unit of analysis. For example, the GPS categorical global parameter multivariable hazard ratio was 3.59 (outside versus not outside normal limits), compared to 1.21 (per 10% greater at baseline) for the continuous variable. To overcome this issue and compare the hazard ratios of the models including GPS, MRA, stereophotograph cup-to--disc ratio, the c-index was used. The c-indices for models that included MRA (.73 to .76), GPS (all .75) and stereophotograph based horizontal cup-to-disc ratio (.76) were similar, and therefore had comparable predictive ability. These results in this ocular hypertensive cohort confirm earlier reports of the comparability of stereophotograph based cup-to-disc ratio measurements and HRT measures in predictive models,.17 Specifically, the HRT linear cup-to-disc ratio was comparable to stereophotograph based cup-to-disc ratio17 and GPS2 indices for the prediction of the development of glaucomatous visual field damage in a cohort of glaucoma suspect eyes. In addition, the magnitude of the c-index reported for the pooled multivariable model (0.74) in OHTS and European Glaucoma Prevention Study combined analysis10 was similar to the c-index reported in the current report. These results suggest that HRT GPS, MRA and cup-to disc ratio can be substituted for stereophotograph based measures to estimate the risk of developing glaucoma in ocular hypertensive eyes.
There are very few studies evaluating the ability of GPS parameters to predict the development or progression of POAG. The current results for GPS confirm previous reports by Alencar et al2 that the predictive ability of GPS parameters were similar to those of stereophotograph assessment. In the current report, hazard ratios were generally lower, ranging from 2.92 to 3.59 for the GPS global and regional parameters outside normal limits compared to not outside normal limits, compared to ranges of 4.08 to 5.43 for the same parameters reported by Alencar.2 These differences are likely due to the differences in study populations and rate of progression. In contrast to the current report of ocular hypertensive patients, in the Alencar study,2 113 glaucoma suspects (with optic disc damage at baseline) and 110 ocular hypertensives from the Diagnostic Innovations in Glaucoma Study (DIGS) were included. They also reported a higher rate of progression (54 of the 223 participants (24.4%) showed POAG changes) than in the OHTS population (50 of 438 (11.5%) participants developed POAG). It should be noted that approximately 75% of eyes with a GPS outside normal result did not develop a POAG endpoint during the current OHTS follow-up. The positive predictive values of GPS outside normal limits were highest for the “GPS result” and nasal region (.24), followed by the temporal (.23), nasal inferior, nasal superior and temporal inferior region (.21).
Our analysis found that GPS tends to identify more eyes than MRA as being outside normal limits. Other cross-sectional studies have also reported that GPS identifies more eyes as outside normal limits than does MRA leading to higher sensitivities and lower specificities for GPS.14,16 The current report also documented that the number of eyes in which the GPS model fails is small, and GPS results were available for at least 98% of participants.14, 16,18, 19
In our previous report, the multivariable models did not adjust for baseline cup-to-disc ratio as determined by the ODRC from stereophotographs because the limited sample size was not sufficient to resolve statistically the independent contribution of cup-to-disc ratio from the independent contribution of CSLO parameters because of their high intercorrelations.5 In the current analysis with longer follow-up and more POAG endpoints, this analysis could be conducted. Specifically, when stereophotograph-based horizontal cup-to-disc ratio is included in the multivariable model, some of the MRA parameters (global, nasal inferior and temporal superior) reached statistical significance. In contrast, the GPS did not add to the model that already included stereophotograph-based horizontal cup-to-disc ratio. These results suggest that at least some topographic parameters add predictive ability to the multivariable model for the development of POAG over and above the information provided when the model includes assessment of stereophotographs.
It is important to differentiate between a risk factor and a predictive factor. As the structural appearance of the optic disc is used to identify glaucoma, structural measures of the optic disc cannot be considered risk factors. However, structural features and measures of the optic disc can be important predictive factors providing essential information to the clinician on the likelihood of developing progressive glaucomatous changes. It can further be argued that larger optic cups and smaller neuroretinal rim measurements are consistent with early glaucomatous disc damage. However, at the baseline visit included in this analysis, all of the eyes had normal appearing visual field and optic discs as classified by the OHTS Visual field Reading Center and Optic Disc Reading Center.
These results would be most generalizable to ocular hypertensive patients with similar clinical characteristics included in this study. As the case with most clinical trials, this study was completed using rigorous quality control systems of the HRT images, photographs and visual fields. Such conditions are unlikely to exist in clinical practice. Using data of lesser quality will increase the variability of the measurements and reduce the predictive accuracy of the model.
Stereophotography of the optic disc has been the “gold standard” for assessing glaucomatous optic disc damage. Photography provides objective documentation for comparison over time. However, stereophotography also requires subjective assessment and it has been well documented that there is wide variation in interpretation of photographs. In contrast to photography, computer based imaging instruments provide real-time objective, quantitative information on the optic disc, with reduced need for pupil dilation and clear media. For these reasons imaging instruments increasingly are becoming the standard method for documenting optic nerve head and retinal nerve fiber layer (RNFL) appearance in clinical practice. The challenge facing clinicians is how to use the information these instruments provide for glaucoma management decisions.
In conclusion, these results suggest that baseline GPS, MRA and stereoparameters alone or when combined with baseline clinical and demographic factors predict the development of POAG endpoints in OHTS participants and they are as effective as stereophotographs for estimating the risk of developing POAG in ocular hypertensive subjects. More research is needed in cohorts with longer follow-up and a larger number of progressing eyes to determine whether the eyes identified by GPS are false positives or early indicators of glaucomatous damage.
Acknowledgments
Support: NIH/NEI grants, EY11158 (RNW), (EY09341, EY09307 (MOG, MAK)), Horncrest Foundation awards, NIH Vision Core Grant P30 EY02687, Merck Research Laboratories, Pfizer Inc, White House Station, New Jersey
Footnotes
Author Disclosure Information: R.N. Weinreb, Heidelberg Engineering, GmbH, S; L.M. Zangwill, Heidelberg Engineering, Heidelberg Germany, S; Carl Zeiss Meditec Inc, Dublin, CA, S; S. Jain, None; L.M. Becerra, None; K. Dirkes, None; J. Piltz-Seymour, Pfizer, Inc., S; Alcon Laboratories, Inc., S; Allergan, Inc., S; Merck, S; G.A. Cioffi, Allergan, Inc., C; Pfizer Inc, C; G.L. Trick, Allergan, Inc., C; A.L. Coleman, Alcon Laboratories, Inc., C; Allergan, Inc., C; Pfizer, Inc., C; J.D. Brandt, Alcon Laboratories, Inc., C; Allergan, Inc., C; Pfizer, Inc., C; Alcon Laboratories, Inc., L; Allergan, Inc., L; Pfizer, Inc., L; J.M. Liebmann, Alcon Laboratories, Inc, C; Allergan, Inc., C; Carl Zeiss Meditec, Inc, S; Diopsys, Inc., C, S; Heidelberg Engineering, GmbH, S; Optovue, Inc., C, S; Pfizer, Inc., C; Topcon Medical Systems, Inc., C, S; M.O. Gordon, None; M.A. Kass, Pfizer Inc, C.
References
- 1.Zangwill LM, Weinreb RN, Beiser JA, et al. Confocal Scanning Laser Ophthalmoscopy Ancillary Study to the Ocular Hypertension Treatment Study Group. Baseline topographic optic disc measurements are associated with the development of primary open-angle glaucoma. Arch Ophthalmol. 2005;123:1188–97. doi: 10.1001/archopht.123.9.1188. [DOI] [PubMed] [Google Scholar]
- 2.Alencar LM, Bowd C, Weinreb RN, et al. Comparison of HRT-3 glaucoma probability score and subjective stereophotograph assessment for prediction of progression in glaucoma. Invest Ophthalmol Vis Sci. 2008;49:1898–906. doi: 10.1167/iovs.07-0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gordon MO, Kass MA Ocular Hypertension Treatment Study Group. The Ocular Hypertension Treatment Study: design and baseline description of the participants. Arch Ophthalmol. 1999;117:573–83. doi: 10.1001/archopht.117.5.573. [DOI] [PubMed] [Google Scholar]
- 4.Zangwill LM, Weinreb RN, Berry CC, et al. OHTS CSLO Ancillary Study Group. The Confocal Scanning Laser Ophthalmoscopy Ancillary Study to the Ocular Hypertension Treatment Sstudy: study design and baseline factors. Am J Ophthalmol. 2004;137:219–27. doi: 10.1016/j.ajo.2003.08.031. [DOI] [PubMed] [Google Scholar]
- 5.Zangwill LM, Weinreb RN, Berry CC, et al. Confocal Scanning Laser Ophthalmoscopy Ancillary Study to the Ocular Hypertension Treatment Study. Racial differences in optic disc topography: baseline results from the Confocal Scanning Laser Ophthalmoscopy Ancillary Study to the Ocular Hypertension Treatment Study. Arch Ophthalmol. 2004;122:22–8. doi: 10.1001/archopht.122.1.22. [DOI] [PubMed] [Google Scholar]
- 6.Wollstein G, Garway-Heath DF, Hitchings RA. Identification of early glaucoma cases with the scanning laser ophthalmoscope. Ophthalmology. 1998;105:1557–63. doi: 10.1016/S0161-6420(98)98047-2. [DOI] [PubMed] [Google Scholar]
- 7.Swindale NM, Stjepanovic G, Chin A, Mikelberg FS. Automated analysis of normal and glaucomatous optic nerve head topography images. Invest Ophthalmol Vis Sci. 2000;41:1730–42. [PubMed] [Google Scholar]
- 8.Tipping ME. Sparse Bayesian learning and the relevance vector machine. J Mach Learn Res. 2001;1:211–44. [Google Scholar]
- 9.Lee EW, Wei LJ, Amato DA. Cox-type regression analysis for large numbers of small groups of correlated failure time observations. In: Klein JP, Goel PK, Amato DA, editors. Survival Analysis: State of the Art. Dordrecht, Netherlands: Kluwer; 1992. pp. 237–47. NATO ASI series, series E: Applied sciences. No. 211. [Google Scholar]
- 10.Ocular Hypertension Treatment Study Group, European Glaucoma Prevention Study Group. Validated prediction model for the development of primary open-angle glaucoma in individuals with ocular hypertension. Ophthalmology. 2007;114:10–9. doi: 10.1016/j.ophtha.2006.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simel DL, Samsa GP, Matchar DB. Likelihood ratios with confidence: sample size estimation for diagnostic test studies. J Clin Epidemiol. 1991;44:763–70. doi: 10.1016/0895-4356(91)90128-v. [DOI] [PubMed] [Google Scholar]
- 12.Harrell FE., Jr . Regression Modeling Strategies: with Applications to Linear Models, Logistic Regression and Survival Analysis. New York: Springer; 2001. p. 493. [Google Scholar]
- 13.Harizman N, Zelefsky JR, Ilitchev E, et al. Detection of glaucoma using operator-dependent versus operator-independent classification in the Heidelberg retinal tomograph-III. Br J Ophthalmol. 2006;90:1390–2. doi: 10.1136/bjo.2006.098111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jaeschke R, Guyatt GH, Sackett DL Evidence-Based Medicine Working Group. Users' guides to the medical literature. III: How to use an article about a diagnostic test. B: what are the results and will they help me in caring for my patients. JAMA. 1994;271:703–7. doi: 10.1001/jama.271.9.703. [DOI] [PubMed] [Google Scholar]
- 15.Coops A, Henson DB, Kwartz AJ, Artes PH. Automated analysis of Heidelberg retinal tomograph optic disc images by glaucoma probability score. Invest Ophthalmol Vis Sci. 2006;47:5348–55. doi: 10.1167/iovs.06-0579. [DOI] [PubMed] [Google Scholar]
- 16.Medeiros FA, Zangwill LM, Bowd C, et al. Agreement between stereophotographic and confocal scanning laser ophthalmoscopy measurements of cup/disc ratio: effect on a predictive model for glaucoma development. J Glaucoma. 2007;16:209–14. doi: 10.1097/IJG.0b013e31802d695c. [DOI] [PubMed] [Google Scholar]
- 17.Ferreras A, Pajarin AB, Polo V, et al. Diagnostic ability of Heidelberg Retina Tomograph 3 classifications: glaucoma probability score versus Moorfields regression analysis. Ophthalmology. 2007;114:1981–7. doi: 10.1016/j.ophtha.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 18.Oddone F, Centofanti M, Rossetti L, et al. Exploring the Heidelberg Retinal Tomograph 3 diagnostic accuracy across disc sizes and glaucoma stages: a multicenter study. Ophthalmology. 2008;115:1358–65. doi: 10.1016/j.ophtha.2008.01.007. [DOI] [PubMed] [Google Scholar]


