Abstract
Using transgenic embryos, we have identified two distinct CNS progenitor cell-specific enhancers, each requiring the cooperation of at least two independent regulatory sites, within the second intron of the rat nestin gene. One enhancer is active throughout the developing CNS while the other is specifically active in the ventral midbrain. These experiments demonstrate that neural progenitor cells in the midbrain constitute a unique subpopulation based upon their ability to activate the midbrain regulatory elements. Our finding of differential enhancer activity from a gene encoding a structural protein reveals a previously unrecognized diversity in neural progenitor cell populations.
Keywords: Midbrain, Neuroepithelium, Nestin, Transgenic Mice, LacZ, Enhancer, CNS, Embryo, Development, Stem Cell, Progenitor Cell, Regulatory Element
INTRODUCTION
In the developing nervous system, multipotential stem cells give rise to neurons and glia (reviewed in McKay, 1997; Morrison et al., 1997; Stemple and Mahanthappa, 1997). An early marker for most, but not all, stem cells in the central nervous system (CNS) is the intermediate filament protein, nestin (Dahlstrand et al., 1995; Frederiksen and McKay, 1988; Hockfield and McKay, 1985; Lendahl et al., 1990; Reynolds and Weiss, 1992; Williams and Price, 1995). Upon neuronal differentiation, a switch in intermediate filament gene expression replaces nestin with the neurofilaments (Lendahl et al., 1990; reviewed in Lee and Cleveland, 1996). In the adult CNS, nestin is re-expressed in reactive astrocytes (Clarke et al., 1994; Frisén et al., 1995) and in CNS neuroepithelial tumors, most notably, gliomas and glioblastomas (Dahlstrand et al., 1992A; Tohyama et al., 1992). During embryonic development, nestin expression is not restricted to the CNS. Nestin is also found in myogenic precursors (Kachinsky et al., 1994; Lendahl et al., 1990; Sejersen and Lendahl, 1993), the peripheral nervous system (Dahlstrand et al., 1995; Hockfield and McKay, 1985, Stemple and Anderson, 1992), the heart (Kachinsky et al., 1995), the developing tooth bud (Terling et al., 1995) and the testis (Fröjdman et al., 1997).
The regulation of nestin gene expression during development has been investigated recently in transgenic mice (Zimmerman et al., 1994). Interestingly, the upstream promoter region of the gene does not possess any regulatory elements specific for neural stem cells. Instead, this work identified a myogenic precursor cell-specific enhancer within the first intron and a CNS specific-enhancer within the second intron of the rat nestin gene. This second intron from the nestin gene has since been used to direct heterologous gene expression to the developing nervous system (Lardelli et al., 1996; Lothian and Lendahl, 1997; Ringstedt et al., 1997).
In order to understand more about the molecular mechanisms that direct gene expression to neurons and their progenitor cells during development, we constructed a comprehensive set of overlapping deletions within the 1.8 kb second intron of the rat nestin gene. By monitoring the expression of a LacZ reporter gene in transgenic embryos, we have tested various DNA fragments for enhancer activity. Based on the evolutionary conservation of the nestin gene (Dahlstrand et al., 1992b), we also cloned the second intron from the human nestin gene and demonstrated that it contained enhancer activity specifically in neuroepithelial cells. Our results define DNA regulatory elements that control spatial and temporal regulation of nestin enhancer activity during embryonic development. In addition, we provide evidence that neuroepithelial cells differ in their ability to activate distinct enhancers. The transcriptional diversity of precursor cells in the developing nervous system implies a previously unrecognized heterogeneity. Our finding of discrete enhancer elements now allows for the identification of the respective regulatory transcription factors which establish heterogeneity in neural progenitor cells. Furthermore, these regulatory elements will be valuable tools to introduce heterologous gene expression to neurons and their precursor cells.
MATERIALS AND METHODS
Constructs
pLacZ
A 4.0 kb EcoRI/XhoI fragment containing the LacZ gene linked to 142 bp of the promoter from the immediate early (IE) gene ICP4 of Herpes Simplex Virus (Yaworsky, et al., 1997) was end-filled and ligated into an EcoRV opened pSafyre vector (Bogarad, unpublished). This minimal IE-promoter-LacZ construct was used to generate all of the subsequent plasmids.
Construct 1
A 1.85 kb BstYI fragment from the plasmid pNesIx (kind gift of Dr. A. McMahon, Harvard University) corresponding to the second intron of the rat nestin gene (Zimmerman et al., 1994) was ligated into the BamHI site of pLacZ. The forward orientation of the insert was confirmed by restriction mapping.
Constructs 2–19
Deletions within the second intron of the rat nestin gene were generated either by restriction endonuclease digestion or by the polymerase chain reaction (PCR). In cases where PCR was used, primers were designed to each contain 16 oligonucleotides of nestin intron sequence. To facilitate directional cloning into the pLacZ plasmid, the PCR primers incorporated restriction enzyme sites. The 5′ primers all contained an XbaI site while the 3′ primers had a PstI site. All primers contained two random bases at the 5′ end for optimal restriction digest of the amplified product. Identical PCR conditions of 28 cycles of 94°C for 30 sec., 62°C for 1 min., 72°C for 30 sec. with 94°C for 5 min. at the first cycle and 72°C for 5 min. at the last cycle were used. Each clone generated by PCR was sequenced to ensure identity to the wild-type sequence. The beginning and end base positions of each deletion fragment is indicated in Fig. 1. The numbering exactly coincides with our GenBank submission (accession number AF004334).
Figure 1. Nestin enhancer deletion constructs and LacZ transgene expression results.
The LacZ gene is linked to the minimal IE gene promoter and is represented schematically as an open box, the solid dark line indicates the nestin enhancer. Numbering for the 1.85 kb BstYI fragment from plasmid pNesIx (for Constructs 1–19) and for the 1.73 kb PCR product cloned in plasmid pHNes (for Construct 20), is identical to the sequence entries in GenBank under accession numbers AF004334 (rat) and AF004335 (human). Four DNA regulatory elements have been identified and are indicated with colored triangles: midbrain-specific element, blue; general transcriptional potentiator, yellow; CNS element I, green; CNS element II, red. All enhancer fragments are in the 5′-3′ orientation unless indicated otherwise by an arrow (Constructs 8 and 12). The number of embryos isolated and examined for enhancer activity at 10.5 dpc is indicated; the transgene positive column shows how many of these embryos carried the LacZ transgene, as determined by PCR screening of yolk sac DNA. Central nervous system β–galactosidase activity was divided into two categories: either the full CNS stained (marked under the column “entire”) or only cells of the midbrain stained (under the column “MB”). Embryos which stained outside of the nervous system were scored as ectopic. The asterisk under the “MB” column with Construct 12 indicates that although the midbrain stained in 1 embryo, staining was also present at non-specific sites such as the heart, limbs, skin and additional neural tissue and was therefore scored as ectopic.
Construct 20
Cloning the second intron from the human nestin gene.
The second intron from the human nestin gene was amplified from human genomic DNA by PCR using primers 5′-AATCTAGACCTGGAGGTGGCCAACG-3′ and 5′-AACTGCAGAGTTCTCAGCCTCCAGG-3′ which anneal in the coding region of the human nestin gene flanking the second intron (Dahlstrand et al., 1992B) and provide XbaI and PstI restriction sites. Cycling conditions were at 94°C, 30 sec.; 60°C, 1 min.; 72°C, 1 min. for 35 cycles with a 5 min. initial denaturation step at 94°C and a final 5 min. extension at 72°C. A 1.73 kb amplification product was agarose gel purified (Qiagen, Chatsworth, CA) and cloned into the pCRScript vector (Stratagene, La Jolla, CA). The plasmid, pHNes, was digested with XbaI and PstI, which generated a short deletion from the 3′-end of the intron, and ligated into pLacZ creating Construct 20. This plasmid contains 1605 bp of the second intron from the human nestin gene.
DNA Sequence Analysis
The 1.85 kb BstYI fragment from the plasmid pNesIx and the 1.73 kb fragment of pHNes were used to sequence the second intron from the rat and human nestin genes, respectively. Automated DNA sequencing was performed on an Applied Biosystems 373A DNA Sequencer (ABI, Foster City, CA). Primers were specifically designed to provide sequence from both DNA strands. Subsequent sequence analyses were carried out using the University of Wisconsin Genetics Computer Group (Madison, WI) software or McMolly Tetra (Soft Gene GmbH, Berlin).
Generation and Identification of Transgenic Embryos
DNA fragments of interest were isolated by agarose gel electrophoresis and purified over Qiagen columns. The purified DNA was diluted to a final concentration of 2.0 μg/ml in a buffer of 10 mM Tris-Cl pH 7.3, 0.25 mM EDTA for microinjection. FVB mice were used as donors for microinjection and CD-1 mice were used as foster mothers following standard protocols of Hogan et al. (1994). The yolk sac of each embryo was used as a source of genomic DNA and the presence of the LacZ transgene was detected by PCR. Using the primers 5′-GACGGGTTGTTACTCGCTCAC-3′ and 5′-GCGTGTACCACAGCGGATGGT-3′, an 868 bp amplification product identifies the LacZ transgene after 35 cycles of 94°C for 30 sec., 62°C for 1 min., 72°C for 30 sec. with an initial denaturation step of 94°C for 5 min. and a final extension step of 72°C for 5 min.
Embryo Isolation and β–galactosidase Staining
Embryos were isolated at various developmental stages with the morning of the microinjection designated as 0.5 dpc. Embryos were fixed in 4% paraformaldehyde in PBS at 4°C for 2 hours at 10.5 dpc and for 3 hours at 13.5 dpc. The fixative was removed with three washes of 15 min. at 37°C in a buffer of 100 mM NaH2PO4 pH7.3, 2 mM MgCl2, 0.1% deoxycholate, 0.2% NP-40. Staining proceeded in the same buffer supplemented with 5 mM K3Fe(CN)6·3H2O, 5 mM K4Fe(CN)6 and 1 mg/ml X-gal. Staining was monitored after 1 hour and allowed to proceed overnight. The reaction was stopped by rinsing with PBS and post-fixing the embryos in 4% paraformaldehyde/0.25% glutaraldehyde in PBS for 4 hours at 4°C. Embryos were dehydrated in 70% ethanol and photographed on a Leica Stereo Zoom M3Z microscope. Histologic sections were obtained by embedding the embryos in paraffin and microtome sectioning at 10 μm thickness. Sections were analyzed and photographed with a Leica DMRB microscope.
Immunohistochemistry on Tissue Sections
Embryos were isolated at 10.5 dpc in ice-cold PBS, fixed for 3 hours in 4% paraformaldehyde in PBS, embed in paraffin wax and microtome cross-sectioned at a thickness of 10 μm. Immunohistochemical staining was carried out using the Vectastain Elite ABC Kit and Vector DAB Substrate Kit (Vector Laboratories, Burlingame, CA). The anti-rat-nestin (Clone Rat-401, Pharmingen, San Diego, CA) primary antibody was used at a concentration of 5 μg/ml; the anti-proliferating-cell-nuclear-antigen (Clone PC10, Boehringer Mannheim, Indianapolis, IN) was used at 50 μg/ml. No staining was observed in the absence of primary antibody. The sections were counterstained briefly with hematoxylin and photographed on a Leica DMRB microscope.
Statistical Analysis
Statistical independence was established using the chi-squared test. In cases where any expected frequency was less than 5, the Fisher exact test was used.
RESULTS
The nestin CNS stem cell enhancer resides in the 3′ region of the second intron
Zimmerman et al. (1994) have previously demonstrated that the second intron of the rat nestin gene contains CNS stem cell enhancer activity. A 1.85 kb DNA fragment, containing the full 1670 bp of the second intron, was shown to direct LacZ expression exclusively within the developing CNS of transgenic embryos. In order to identify which genetic regulatory elements conferred this neuroepithelial expression pattern, we generated deletions of the nestin intron and assayed for enhancer activity using a heterologous promoter. Figure 1 depicts the various deletion constructs tested, each construct’s tissue specificity and the identification of several regulatory elements within the enhancer as indicated by colored triangles. Using a minimal promoter from the herpes simplex virus ICP4 gene with the full 1.85 kb nestin intron fragment, Construct 1 (Fig 1), we were able to reproduce the staining pattern previously observed at 10.5 days post coitum (dpc) (Zimmerman et al., 1994) (Fig 2A). Intense β–galactosidase activity was detected in the telencephalon, diencephalon, mesencephalon, metencephalon, myelencephalon and spinal cord. Ventral spinal cord staining progressed along the entire anterior-posterior axis of the embryo except at the most posterior portions of the spinal cord. Dorsal regions of the telencephalon did not stain, and in the spinal cord, dorsal staining was found only in regions anterior to the forelimb buds.
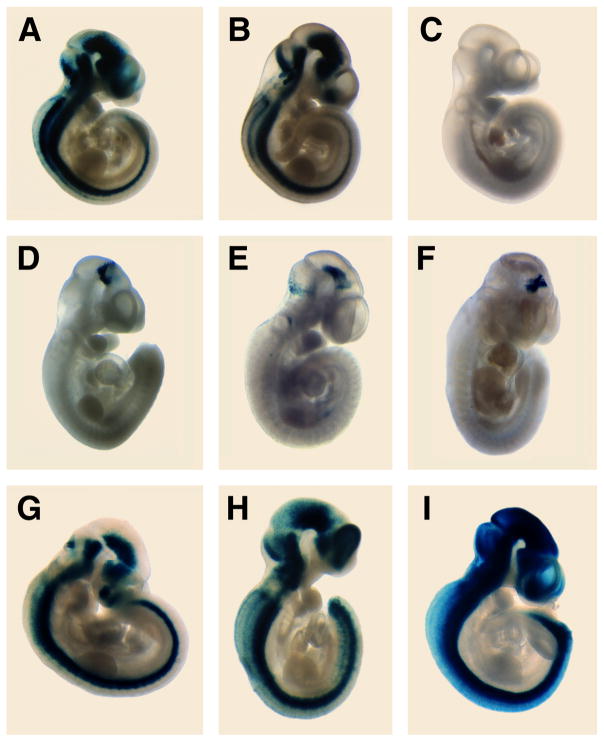
Figure 2. Whole mount β–galactosidase activity in transient transgenic embryos.
Embryos were isolated at 10.5 dpc and assayed for β–galactosidase activity. Reprentative embryos are depicted for A: construct 1; B: construct 4; C: construct 5; D: construct 8; E: construct 10; F: construct 11; G: construct 15; H: construct 19; I: construct 20. Consistently, three staining patterns emerged: the entire developing CNS was positive (A, B, G–I), or ventral midbrain only (D–F), or the neuroepithelium was negative (C).
Deletions of the nestin intron revealed that the CNS-specific enhancer(s) resides downstream of base 1069. Transgenic embryos which expressed Construct 4 displayed a pattern of staining identical to that observed with the full 1.85 kb intron fragment (compare Figs 2A and B). As we observed with Construct 1, β–galactosidase staining was slightly reduced in the most posterior portion of the ventral spinal cord. In addition, staining in the dorsal spinal cord was weak, even at anterior levels. In the developing brain, staining was restricted by boundaries between the telencephalon and the diencephalon and between the mesencephalon and the metencephalon. The staining pattern from Construct 3 was identical to that of both Constructs 1 and 4 (not shown). Construct 2 which lacked the 3′ portion of the intron could not direct LacZ expression in the developing CNS (not shown). These results restrict any relevant enhancers to the interval between bases 1069 and 1850, in the 3′ region of the intron. Constructs 5 and 6, which contained shorter 3′ regions of the intron, were unable to produce LacZ transgene expression within the developing nervous system (Fig 2C). These data suggested that the relevant CNS enhancer element(s) reside within the 332 bp region between base 1069 and base 1401.
Identification of a midbrain-specific enhancer
Constructs 7 and 8 contained the intron fragment between bases 1068 and 1406 in forward and reverse orientation respectively. Unexpectedly, these constructs did not reproduce the full staining pattern but rather contained enhancer activity specific for the developing midbrain (Fig 2D). Only cells of the mesencephalon expressed the transgene with a concentration of staining localized in more ventral regions (Fig 2D). Regardless of enhancer orientation, identical β–galactosidase expression patterns were observed, confirming that the midbrain-specific element functions as a true enhancer (not shown). In order to further localize the midbrain-specific element, we analyzed five additional constructs that overlap this 339 bp fragment. Constructs 9 and 10 represent the last and first two-thirds of the 339 bp midbrain enhancer element, respectively. Construct 9 was unable to specifically direct transgene expression to the nervous system (not shown). However, Construct 10 produced LacZ transgene expression in the developing mesencephalon, narrowing the relevant DNA region down to 204 bp (Fig 2E). The dorsal extension of stained cells along the midbrain-hindbrain border previously observed with the Construct 8 embryo (Fig 2D) was not seen in every midbrain-stained embryo (compare with Fig 2E), possibly due to variability in developmental maturity. In fact, 3 out of a total of 16 embryos with midbrain staining (Constructs 7,8,10,11, combined Figure 1) displayed the pattern shown in Figure 2E, while 13 exhibited staining in more dorsal cells corresponding the the pattern in Figure 2D. Attempts to further shorten the enhancer proved inconclusive. With Construct 11, we observed 3 out of 43 transgenic embryos that exhibited a midbrain expression pattern (Fig 2F), with an additional 7 transgenic embryos displaying independent and different ectopic staining (not shown). This low frequency of specific staining (7.0%) compared with the frequency of midbrain-specific staining observed with Constructs 7, 8 and 10 combined (38%), suggested that either the midbrain enhancer did not reside on this fragment or that the 123 bp enhancer was too weak to overcome DNA integration effects in the transgenic embryos. Alternatively, the spacing of the enhancer relative to the minimal promoter could have been suboptimal to allow consistent expression of enhancer activity. To test the latter possibility, we generated a construct with 3 copies of this 123 bp DNA fragment, Construct 12, that would place the enhancer at varying distances from the transcription start site. None of the 4 transgenic embryos obtained with this multimerized fragment displayed specific staining in the midbrain (not shown). To formally exclude that the midbrain-specific enhancer might reside between bases 1190 and 1199 (difference between Constructs 9 and 11), we generated Construct 13. No midbrain-enhancer activity was observed in these transgenic embryos (not shown). Therefore, we concluded that the midbrain-specific enhancer resides between bases 1068 and 1271 as positively demonstrated with Construct 10 (Fig 2E).
A transcriptional potentiator element is necessary for midbrain-specific enhancer activity
The large number of transgenic embryos produced in this study allowed us to perform statistical analyses on enhancer activity. These results suggested the presence of a general transcriptional potentiator element which is necessary for efficient activity of the midbrain-specific enhancer. In the case of Construct 9 (bases 1200–1406), we observed that 5 of 16 (31%) transgenic embryos exhibited intense β–galactosidase activity at ectopic sites in completely independent staining patterns (not shown). This high frequency of ectopic staining (31% as compared to 7%, 16 out of 222, for all other constructs combined, p=0.008) was suggestive of the presence of an element that alone possesses no tissue specificity, but that enables transcriptional activity when under the influence of another enhancer. We proposed that this transcriptional potentiator resides between bases 1200 and 1271 based on the ratio of the number of LacZ expressing embryos (specific or ectopic) to the total number of transgenic embryos. Reproducible midbrain-specific enhancer activity was detectable only with constructs (Constructs 7–10) that harbored the putative transcriptional potentiator element (Fig 1). A further refinement of this element between bases 1200–1255 was based on Constructs 7–19. Constructs 7–10, all of which contained bases 1200–1271, yielded 20 out of 50 embryos (40%) that had specific or ectopic LacZ expression. Furthermore, Constructs 14–16, also containing bases 1200–1255, produced 15 out of 20 (75%) LacZ expressing embryos. In contrast, constructs which lacked bases 1200–1271 had low numbers of embryos with β–galactosidase activity. Constructs 11–13 generated only 15 out of 69 (22%, p=0.031) stained embryos while Constructs 17–19 yielded 10 out of 39 (26%, p=0.0003) stained embryos. While these observations did not conclusively prove the existence of such an element, the statistical analyses of LacZ expression (see also legend to Table 1) support the presence of the putative potentiator element between bases 1200 and 1255. Taken together, our results demonstrated that tissue-specific enhancer activity in the midbrain was achieved through the interaction/cooperation of a midbrain-specifying element, element A (between 1068 and 1199, blue triangle) and a transcriptional potentiator, element B (between 1200 and 1255, yellow triangle).
Table 1. Temporal regulation of nestin enhancer activity.
Embryos generated with the midbrain enhancer (construct 8) or rat or human the CNS enhancer (constructs 16 and 20) were isolated at various developmental stages and assayed for β-galactosidase activity. The total number of embryos examined and the number of LacZ -transgene positive embryos (as detected by PCR) are presented. The midbrain enhancer is not specific at stages earlier or later than 10.5 days. Both constructs 16 and 20 maintain neuroepithelial-specific staining at later developmental stages. The striking frequency of ectopically-stained embryos from construct 8 at later developmental stages provided further evidence for the presence of a general transcriptional potentiator in this fragment. In the absence of active tissue-specific regulatory elements (down regulation of MB enhancer activity), an increased frequency of transcriptional activity at ectopic sites was obtained. We obtained 14 ectopically-stained embryos out of 34 total transgenics (41%) as compared to 21 out of 2597 (8%) for all other constructs (p<0.0001).
| Construct
|
Stage (dpc)
|
Number of Embryos
|
Transgene Positive
|
CNS
|
Ectopic
|
|
|---|---|---|---|---|---|---|
| Entire
|
Midbrain
|
|||||
| 8 (Midbrain–Enhancer) | 9.5 | 7 | 2 | 0 | 0 | |
| 10.5 | 73 | 18 | 8 | 0 | ||
| 11.5 | 34 | 3 | 0 | 3 | ||
| 12.5 | 37 | 8 | 0 | 2 | ||
| 13.5 | 126 | 21 | 0 | 9 | ||
|
| ||||||
| 16 (CNS–Enhancer) | 10.5 | 43 | 10 | 8 | 0 | |
| 13.5 | 69 | 8 | 2 | 0 | ||
| 17.5 | 32 | 6 | 2 | 0 | ||
|
| ||||||
| 20 (Human) | 10.5 | 21 | 7 | 5 | 0 | |
| 13.5 | 33 | 5 | 4 | 0 | ||
Mapping of elements required for enhancer activity in the entire CNS
The identification of a midbrain-specific enhancer between bases 1068 and 1271 lead us to postulate that at least one other region in the nestin intron would have to be responsible for the CNS staining pattern observed with Construct 4 (Fig 2B). Furthermore, we reasoned that such element(s) must be located between bases 1401 and 1850 since we observed the original CNS activity with Construct 4 (Fig 2B), yet Constructs 7 and 8 were only active in the midbrain (Fig 2D). In addition, Constructs 5 and 6 showed that the most 3′ region of the nestin intron alone (bases 1406–1850) was unable to function as an enhancer (Fig 2C). Thus, CNS activity in regions beyond the midbrain would either require an element in the region around 1401–1406 which would be split in Constructs 5 and 7–9, or the interaction of sequences in the 1068–1406 fragment with those in the 1406–1850 fragment. In order to identify the elements responsible for this interaction, we generated deletions from the 3′ end of the intron. Two independent constructs, 14 and 15, produced staining patterns that were identical to each other as well as indistinguishable from those obtained from Construct 4 (Fig 2G). These results suggested that the regulatory element(s) contributing to spinal cord and brain-specific enhancer activity must reside were encompassed between bases 1068 and 1587.
We then asked if the midbrain enhancer (1068–1271) was required for the full CNS pattern, and generated three additional deletions from the 5′ end through the midbrain enhancer. Constructs 16, 17 and 18 were all able to reproducibly generate the expression pattern observed with Construct 4 (not shown). Furthermore, Construct 18 demonstrated that the CNS enhancer activity was achieved in the absence of the midbrain enhancer. Subsequent deletion from the 3′ end to base 1477 in Construct 19 indicated that a 206 bp enhancer fragment could reproducibly generate the full nestin intron staining pattern (Fig 2H). From these results, we concluded that (a) regulatory element(s) within those 206 bp is/are sufficient for CNS enhancer activity. While we cannot formally exclude the presence of a single element that overlaps with the bases around 1401–1406, we favor the interpretation that elements between bases 1272 and 1400 interact with elements between bases 1401 and 1587 to produce the original pattern of gene expression observed with the intact intron. This proposition is supported by the presence of putative binding sites in the respective sequences (see below, Figure 4). The above results refined the location of the two cooperating elements in the CNS enhancer to element C (between bases 1272 and 1400, green triangles in Fig 1) and to element D (between bases 1401 and 1477, red triangles in Fig 1). The addition of element B to the 206 bp CNS-enhancer (Construct 16) resulted in a high frequency of transcriptionally active transgenes further supporting a role for element B as a transcriptional potentiator.
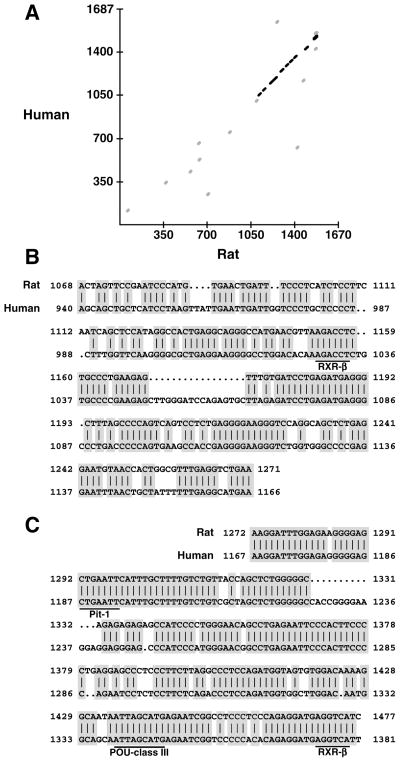
Figure 4. Sequence conservation in the second intron of the rat and human nestin gene.
Sequencing was performed by an ABI automatic sequencer. A: comparison between the two intron sequences was performed using the Complign program of McMolly Tetra (3.5) with minimal match length: 10; mismatches: 0; and gap penalty: 3. The sequences are overall 37% similar with the highest region of similarity clustered in the last third of the intron. B, C: sequence alignments were performed using the Bestfit program of GCG. B: The sequences of the midbrain enhancer from rat (upper) and human (lower) are 72% similar. C: The CNS-enhancer sequences are 85% similar. Sequence identities are shaded, and putative transcription factor binding sites are underlined and labeled. Numbering is identical to the intron sequences deposited in GenBank under accession numbers AF004334 (rat) and AF004335 (human).
The second intron of the human nestin gene possesses CNS - specific enhancer activity
Based on the high degree of conservation between the rat and human nestin gene (Dahlstrand et al., 1992b), we presumed that the human nestin gene would possess similar neural enhancers within its second intron. Therefore, we cloned the second intron from the human nestin gene and assayed for enhancer activity in transgenic embryos. The 1.7 kb human clone contained 21 bp of exon 2, the complete 1687 bp of intron 2, and 23 bp of exon 3. Based on our mapping of enhancers in the rat intron, we generated Construct 20 which included the first 1584 bp of human intron sequence (Fig 1). Five of seven transgenic embryos exhibited intense β–galactosidase staining throughout the entire CNS (Fig. 2I). The expression pattern was similar to that previously observed with the rat enhancer with either Constructs 1, 4 or 19 (Figs. 2A, B, H). These experiments demonstrated that the second intron from the human nestin gene also possesses CNS enhancer activity thereby revealing functional conservation of these intronic enhancer elements during evolution.
Activity of the nestin enhancers follows strict boundaries within the neuroepithelium
In order to characterize the specific neuroepithelial cells targeted by the midbrain- and CNS-enhancers, we examined histologic sections from transgenic embryos. Sections through a Construct 1 embryo confirmed that all of the β–galactosidase activity was restricted to cells of the developing nervous system. In the hindbrain, an intense ventrally localized stripe of expression was visible around the 4th ventricle (Fig 3A). Staining was also observed in lateral stripes around the 3rd ventricle of the ventral midbrain. The anterior spinal cord of these embryos showed β–galactosidase activity in both ventral and dorsal cells, however, the dorsal-most regions of the spinal cord were consistently negative (Fig 3B). The staining in ventral regions was intense and predominantly localized to ventricular cells with activity detected along the pial surface as well. Floor plate cells and the notochord did not stain. In the more posterior region of the spinal cord, the staining became more restricted to ventral, ventricular cells (Fig 3C).
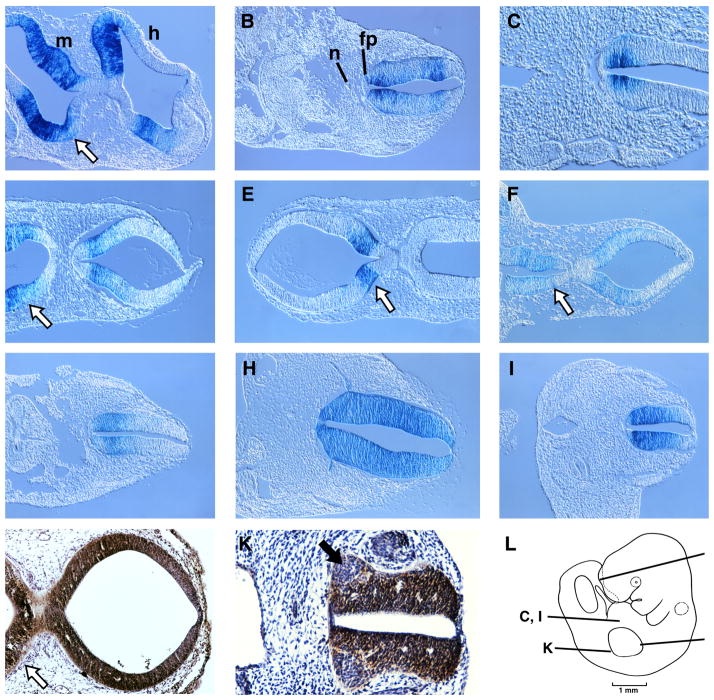
Figure 3. Cellular localization of nestin enhancer activity and nestin immunoreactivity.
Histologic cross and transverse sections were obtained from either LacZ-stained transgenic embryos (A–I) or from normal control embryos (J–K) at a thickness of 10 μm. A schematic of a 10.5 dpc embryo showing the sectioning planes is presented in panel L. Midbrain, m; hindbrain, h; notochord, n; floor plate, fp. Dorsal is to the right. A–C: construct 1; D: construct 4; E: construct 8; F–G: construct 19; H–I: construct 20; J–K: immunohistochemistry for nestin. In the developing brain, β–galactosidase activity is detected in stripes in both the midbrain and hindbrain (A, D, F), however, only midbrain cells were targeted with construct 8 (E). Midbrain cells are indicated with a white arrow (A, D–F, J). In the anterior spinal cord, enhancer activity is observed in both ventral and dorsal cells with strong ventricular cell localization (B, G, H). Staining becomes more ventrally restricted in posterior spinal cord (C, I). Nestin immunoreactivity is uniform throughout the hindbrain and midbrain (J) and the posterior spinal cord (K), except in areas where presumptive motor neurons have differentiated (black arrow). Immunoreactivity was also detected in notochord, dorsal root ganglia, and endothelial walls of blood vessels. Some axonal projections were detected by either enhancer activity (I) or by nestin protein detection (K).
The cellular localization of β–galactosidase activity was identical between Constructs 1 and 4 (compare Figs 3A and D). However, sections through Construct 8 embryos confirmed that only ventral cells of the midbrain were stained (Fig 3E). Positive cells were present bilaterally, on each side of the 3rd ventricle, in tegmental neuroepithelial cells. In contrast, Construct 19, which contained the 206 bp CNS-enhancer, produced stripes of staining in both the hindbrain and midbrain similar to the original expression pattern observed with Constructs 1 and 4 (compare Figs. 3A, D and F). Staining in the anterior spinal cord was also similar although the intensity of staining was weak dorsally (Fig 3G). These experiments have shown that the 206 bp CNS-enhancer contains all of the spatial and temporal regulatory elements necessary for the full (Construct 1) enhancer expression pattern. The human nestin enhancer was the strongest enhancer used in this study. Sections through the anterior spinal cord of these embryos displayed intense β–galactosidase activity throughout the entire dorsal-ventral axis of the spinal cord (Fig 3H). The notochord and floor plate cells again appeared negative. The posterior spinal cord of these embryos was strongly stained, however, as we observed with previous constructs, the dorsal-most region of the spinal cord was only weakly positive (Fig 3I). Together these data revealed that only cells in the developing nervous system were targeted by either nestin enhancer; yet, it is important to note that not every neuroepithelial cell activated the nestin enhancers at this developmental stage.
Heterogeneity of neuronal progenitors revealed by nestin enhancer activity
Using a monoclonal antibody against nestin, we compared nestin protein expression to nestin enhancer activity. While expression from the nestin enhancers observed strict boundaries (Figs 3A–I), nestin immunoreactivity was detected throughout the entire dorsal-ventral axis (Fig 3J) in the hindbrain and midbrain. In the spinal cord, nestin expression was detected both in ventral and dorsal cells (Fig 3K) but, as reported previously (Frederiksen and McKay, 1988; Zimmerman et al., 1994), it was absent in post-mitotic regions of the ventral horns where presumptive motor neurons are differentiating (black arrow Fig 3K). Interestingly, the notochord was weakly immunopositive yet none of the enhancer constructs were active there. The more extensive expression of nestin protein indicated that additional DNA regulatory elements, residing outside of the second intron, possibly participate in the regulation of the endogenous nestin gene locus. The comparison of protein expression and enhancer activity at 10.5 dpc suggested that cells in the neuroepithelium could be subdivided into at least three populations: nestin-positive cells that activate the midbrain-specific enhancer, nestin-positive cells that activate the CNS-specific enhancer, and nestin-positive cells that do not activate either enhancer. This was most apparent when Figs 3A, D–F were compared with Fig 3E. Constructs 1, 4, 8 and 19 targeted midbrain tegmental neuroepithelial cells in the very same location (indicated with a white arrow). Although the enhancer from Construct 8 was activated by cells in the midbrain, other CNS cells were unable to activate this fragment. This observation suggested that these ventral midbrain precursor cells are different from other CNS progenitor cells, such as in the spinal cord and forebrain. Similarly, within the spinal cord, ventral cells expressed the LacZ transgene when presented with the CNS-enhancer while dorsal cells did not. Thus, neuroepithelial cell populations differ in their capability to activate different nestin enhancer elements at a given time point. These data provide molecular evidence that neural progenitor cells are heterogeneous based on differences in their transcription factor repertoires. Thus, the isolation of nestin enhancer elements in transgenic mice reveals a molecular heterogeneity in neural progenitor cells that is not apparent with either in situ hybridization or immunohistochemical approaches.
Temporal regulation of nestin enhancers
To further analyze the specificity of our novel midbrain-enhancer element (Construct 8), we characterized the activity of this enhancer during embryogenesis. The midbrain-enhancer was only active at 10.5 dpc (Table 1) but not at later developmental stages as evidenced by β-galactosidase activity. These data suggested that the midbrain neuroepithelial cells that expressed the transgene at 10.5 dpc are a unique subpopulation of neuroepithelial cells that possess a distinct complement of transcription factors. Such molecular differences enable transient enhancer activity in these specific cells for a short period of time prior to terminal neuronal differentiation. In contrast, activity from the CNS-enhancer, as represented with either Construct 16 or the human enhancer (Construct 20), was maintained in CNS progenitor cells at later developmental stages (Table 1).
Sequence comparison of the rat and human nestin enhancers
In parallel to our functional analyses in transgenic mice, we sequenced the second intron of both the rat and human nestin genes. We expected that DNA sequences of functional enhancer elements would be conserved between rat and human. The rat intron contained 1670 bp while the human had 1687 bp. Both sequences were novel and had no overall similarities to sequences in the databases. A comparison between the two sequences revealed a highly divergent 5′ region and a clustering of conserved regions in the 3′ end of the intron resulting in 37% total similarity (Fig 4A). The stretches of sequence conservation exactly coincided with regions of the intron for which we have demonstrated neural enhancer activity. The 204 bp midbrain enhancer is 72% identical between rat and human (Fig 4B) while the 206 bp CNS-enhancer is 85% identical (Fig 4C). Few consensus sequences for putative transcription factor binding were present in either enhancer region. Two putative retinoid-X receptor beta (RXR-β) half-site consensus sequences (Marks et al., 1992) were conserved: in the midbrain-enhancer at base 1154 of the rat (base 1029 in human); and in the CNS-enhancer at base 1469 in the rat (1373 in human). In addition, the CNS-enhancer had two putative POU-domain transcription factor binding sites which were conserved in both species: a Pit-1 binding site (Peers et al., 1990) at base 1292 in rat (1187 in human) and a POU-class III binding site (Li et al., 1993) at base 1435 in rat (1339 in human). This high degree of sequence similarity and the conservation of putative transcription factor binding sites in regions shown to possess enhancer activity suggested that similar regulatory mechanisms may control nestin expression in both rat and human.
DISCUSSION
In order to understand the molecular mechanisms regulating gene expression in neuroepithelial cells, we localized regulatory sequences in the rat nestin gene that control neuronal enhancer activity. Our results demonstrate that two separable enhancer elements reside within the second intron of the gene. We have identified a 204 bp midbrain-specific enhancer between bases 1068 and 1271, and a 206 bp CNS-enhancer between bases 1272 and 1477. We show that these two enhancers can function independently of each other and that they each require the cooperation of at least two distinct regulatory sites. In the midbrain-enhancer, we postulate the presence of a midbrain-specifying element, between bases 1068 and 1199 (element A), and a general transcriptional potentiator element, between bases 1200 and 1255 (element B), which, in the absence of the tissue-specific elements, promotes transgene expression in a non-specific fashion. In the presence of element A, this potentiator element reproducibly contributes to transgene expression in the ventral midbrain. In the CNS-enhancer, there are likely two sites that must interact to produce enhancer activity throughout the developing nervous system. Our data have refined these sites to regions between bases 1272 and 1400 (element C) and 1401–1477 (element D). Such multiple regulatory elements may be required to ensure that CNS progenitor cells express nestin irrespective of their location along the anterior-posterior or dorsal-ventral axis of the embryo and at appropriate times during embryogenesis.
The sequences from both the rat and human nestin gene second intron are novel and divergent from each other. Significant stretches of similarity are only present in sequences that we define as functional enhancers. Potential transcription factor binding sites, conserved within the enhancer elements, may provide insights into the molecular mechanisms that regulate nestin expression in the nervous system. In both the midbrain-enhancer and the CNS-enhancer, we have identified half-sites for potential retinoid-X receptor-beta (RXR-β) binding (Marks et al., 1992). These half sites may serve as targets for other members of the nuclear receptor family of transcription factors which bind DNA as a monomer at the RXR core recognition motif (Wilson et al., 1993; Glass, 1994; Kastner et al., 1995). In the CNS-enhancer, we also identified two binding sites for POU-domain transcription factors. The conservation of transcription factor consensus sites, together with the expression of these factors in the developing nervous system (He et al., 1989) provides strong support for the possibility that POU-domain or nuclear receptor proteins may be involved in regulating nestin enhancer activity.
Since nestin is a structural gene, widely expressed during embryonic development, the finding of a distinct enhancer with activity only in a small subset of ventral midbrain neuroepithelial cells was unexpected. The biological relevance of this regulatory element is presently unclear since the same midbrain cells can activate the 206 bp CNS-enhancer which lacks bases 1068–1271. However, it is tempting to ascribe a functional role for the midbrain-specific enhancer based on both its sequence conservation and on its activity in neural progenitor cells that give rise to dopaminergic neurons of the ventral mesencephalic tegmentum (Bayer et al., 1995). Most intriguingly, the RXR-β consensus motif at base 1154 (rat), with one mismatch, represents the consensus site for the orphan nuclear receptor, Nurr1 (Law et al., 1992; Murphy et al., 1995). By 10.5 dpc, Nurr1 mRNA is detected in the ventral mesencephalic flexure prior to the expression of markers for dopaminergic neurons, and Nurr1 null mice die early postnatally with profound deficits in the midbrain including the loss of midbrain dopaminergic neurons (Zetterström et al., 1997). Together, these data suggest that Nurr1 may play a role in regulating the expression of nestin in the midbrain. The nestin midbrain enhancer will be useful to dissect cell signaling and transcriptional mechanisms in the midbrain as well as to direct heterologous gene expression to precursors of dopaminergic cells. As these neurons are selectively lost in Parkinson’s Disease, the nestin midbrain-enhancer provides a novel tool in gene therapy approaches for treating neurodegenerative diseases.
Nestin has been previously established as a marker for stem cells of the nervous system (Frederiksen and McKay, 1988; Lendahl et al., 1990; Morshead et al., 1994; Reynolds and Weiss, 1992; Stemple and Anderson, 1992). These experiments documented that nestin is expressed in proliferative cells that can give rise to both neurons and glia as well as self renew. Our data show that nestin enhancer activity is always coincident with the known pattern of nestin protein expression (Fig 3) and that these cells are proliferative based on immunodetection of proliferating cell nuclear antigen (PCNA, not shown). Thus, enhancer activity is restricted to neural progenitor cells.
We propose that the differential activity of enhancer elements in the ventral midbrain and other CNS regions reveals molecular variations among CNS progenitor cell populations. The midbrain-enhancer is only active in the ventral midbrain progenitor cells (Fig 5A), while the CNS-enhancer is active in distinct regions of both the hindbrain and midbrain (Fig 5B), and nestin protein is detected almost uniformly throughout the same regions (Fig 5C). The midbrain cells that activate both enhancers (population 1, Fig 5C) must, a priori, possess a pool of transcription factors different from other CNS cells that can only activate the CNS-specific enhancer (population 2). In addition, there are populations of cells (population 3) which express nestin protein without activating either nestin enhancer. This third population of progenitor cells, too, must differ in its repertoire of transcription factors. Our model thus defines subpopulations of neuroepithelial cells based on their differences in transcription factor repertoires which may play a role in specifying eventual neuronal fates.
Figure 5. Model of neural stem cell heterogeneity.
A schematic representation of a transverse section through the third and fourth ventricles of a 10.5 dpc embryo is shown. Dorsal is on the right. Colors indicate cells that activate the midbrain-enhancer (A) or the CNS enhancer (B). C depicts cells that express nestin protein. The comparison identifies three populations of neuroepithelial cells: Population 1 is nestin+/CNS-enhancer+/MB-enhancer+; population 2 is nestin+/CNS enhancer+/MB-enhancer−; and population 3 is nestin+/enhancer−.
It has recently been proposed that the generation of regional neuronal diversity is conferred by unique combinations of transcription factor expression in the forebrain and anterior neural tube (Rubenstein et al., 1994; Shimamura et al., 1995). An intriguing aspect of our study is that such transcriptional heterogeneity in the developing CNS is now being detected from the standpoint of a transcriptional target. Given that nestin is a structural protein that is transiently expressed in most neural stem cells, the temporal and regional control of specific enhancers may serve as the paradigm to define the functional relevance of transcriptional repertoires in CNS stem cells and to investigate to which extent cell intrinsic and cell extrinsic factors determine the generation of neuronal diversity.
Cell transplantation experiments suggest that environmental influences alone may not be sufficient to account for neuronal diversity (Campbell et al., 1995; Lumsden et al., 1994; Suhonen et al., 1996) revealing early cell-autonomous differences in neural stem cell lineages. However, there are also examples where transplanted neural precursors have been shown to adapt to their new environment (Brüstle et al., 1995; Campbell et al., 1995; Renfranz et al., 1991; Vicario-Abejon et al., 1995). In these models, the transplanted stem cells were capable of interpreting the instructive environmental cues of the ectopic site and follow a differentiation pathway characteristic of cells which normally reside there. Together these variations in CNS stem cell behavior can likely be explained by heterogeneity in neural stem cells with respect to temporal, lineage and regional restrictions.
Temporal heterogeneity between neural stem cells has been well documented for the retina, where the competence of multipotential progenitor cells to produce specific cell types changes over time (Turner and Cepko, 1987; Wetts and Fraser, 1988; Cepko et al., 1996). Analogously, the strict temporal regulation of the midbrain-enhancer implies temporal changes in transcriptional repertoires in ventral midbrain progenitor cells. Heterogeneity of neuroepithelial cells with respect to future neuronal cell lineages is evident at the molecular level by differential expression of the neurogenin family of transcription factors (Sommer et al., 1996). Interestingly, except for perhaps the midbrain dopaminergic precursor cells, we find no evidence for a correlation of enhancer activity with presumptive cell lineage. This further supports the notion that nestin enhancers are activated by progenitor cells prior to cell type specification or lineage restriction. Finally, it has been shown that neuronal progenitor cells are non-uniform based on region. Diffusible factors from the floor plate and notochord are capable of inducing ventral cell types (Hynes et al., 1995a; Yamada et al., 1993). However, the dopaminergic cells of the ventral midbrain require contact-mediated interactions with the floor plate (Hynes et al., 1995b). These data imply that ventral neuronal progenitor cells vary in response to floor plate inductive signals in a region-specific manner. Our identification of a midbrain-specific enhancer provides evidence that ventral midbrain cells also possess transcriptional competence distinct from cells in other ventral regions of the developing CNS.
Therefore, while nestin protein expression has previously been thought to be a general marker of neural stem cells, the distinct regulatory mechanisms for nestin enhancer activation provide compelling evidence for molecular heterogeneity of developing CNS cells. These molecular differences in neural progenitor cells may be functionally relevant in the eventual specification of neuronal subtype. Distinct populations of progenitor cells can now be identified and isolated by virtue of nestin enhancer activity. These enhancers and the future identification of specific transcription factors will have important implications for the development of stem cell transplantation strategies in neurodegenerative diseases.
Acknowledgments
We are grateful to Teresa Tinder for expert technical assistance, M. Anita Jennings for the preparation of histologic sections, Stephanie Munger and Dr. Sergei Ochkur for the production of transgenic mice, Amy Weaver for statistical analyses, and Marv Ruona and Julie Jensen for graphics assistance. We also thank Drs. J. Michael Salbaum and David Zacharias for critical discussion. The plasmid pNesIx was a generous gift from Dr. Andrew McMahon (Harvard University). This work was supported by Mayo Foundation for Medical Education and Research.
References
- Bayer SA, Wills KV, Triarhou LC, Ghetti B. Time of neuron origin and gradients of neurogenesis in midbrain dopaminergic neurons in the mouse. Exp Brain Res. 1995;105:191–199. doi: 10.1007/BF00240955. [DOI] [PubMed] [Google Scholar]
- Brüstle O, Maskos U, McKay RD. Host-guided migration allows targeted introduction of neurons into the embryonic brain. Neuron. 1995;15:1275–1285. doi: 10.1016/0896-6273(95)90007-1. [DOI] [PubMed] [Google Scholar]
- Campbell K, Olsson M, Björklund A. Regional incorporation and site-specific differentiation of striatal precursors transplanted to the embryonic forebrain ventricle. Neuron. 1995;15:1259–1273. doi: 10.1016/0896-6273(95)90006-3. [DOI] [PubMed] [Google Scholar]
- Cepko CL, Austin CP, Yang X, Alexiades M, Ezzeddine D. Cell fate determination in the vertebrate retina. Proc Natl Acad Sci USA. 1996;93:589–595. doi: 10.1073/pnas.93.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke SR, Shetty AK, Bradley JL, Turner DA. Reactive astrocytes express the embryonic intermediate neurofilament nestin. NeuroReport. 1994;5:1885–1888. doi: 10.1097/00001756-199410000-00011. [DOI] [PubMed] [Google Scholar]
- Dahlstrand J, Collins VP, Lendahl U. Expression of the class VI intermediate filament nestin in human central nervous system tumors. Cancer Res. 1992a;52:5334–5341. [PubMed] [Google Scholar]
- Dahlstrand J, Zimmerman LB, McKay RDG, Lendahl U. Characterization of the human nestin gene reveals a close evolutionary relationship to neurofilaments. J Cell Sci. 1992b;103:589–597. doi: 10.1242/jcs.103.2.589. [DOI] [PubMed] [Google Scholar]
- Dahlstrand J, Lardelli M, Lendahl U. Nestin mRNA expression correlates with the central nervous system progenitor cell state in many, but not all, regions of developing central nervous system. Dev Brain Res. 1995;84:109–129. doi: 10.1016/0165-3806(94)00162-s. [DOI] [PubMed] [Google Scholar]
- Frederiksen K, McKay RDG. Proliferation and differentiation of rat neuroepithelial precursor cells in vivo. J Neurosci. 1988;8:1144–1151. doi: 10.1523/JNEUROSCI.08-04-01144.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisén J, Johansson CB, Török C, Risling M, Lendahl U. Rapid, widespread, and longlasting induction of nestin contributes to the generation of glial scar tissue after CNS injury. J Cell Biol. 1995;131:453–464. doi: 10.1083/jcb.131.2.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fröjdman K, Pelliniemi LJ, Lendahl U, Virtanen I, Eriksson JE. The intermediate filament protein nestin occurs transiently in differentiating testis of rat and mouse. Differentiation. 1997;61:243–249. doi: 10.1046/j.1432-0436.1997.6140243.x. [DOI] [PubMed] [Google Scholar]
- Glass CK. Differential recognition of target genes by nuclear receptor monomers, dimers and heterodimers. Endocrine Rev. 1994;15:391–407. doi: 10.1210/edrv-15-3-391. [DOI] [PubMed] [Google Scholar]
- He X, Treacy MN, Simmons DM, Ingraham HA, Swanson LW, Rosenfeld MG. Expression of a large family of POU-domain regulatory genes in mammalian brain development. Nature. 1989;340:35–42. doi: 10.1038/340035a0. [DOI] [PubMed] [Google Scholar]
- Hockfield S, McKay RDG. Identification of major cell classes in the developing mammalian nervous system. J Neurosci. 1985;5:3310–3328. doi: 10.1523/JNEUROSCI.05-12-03310.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan B, Beddington R, Costantini F, Lacy E. Manipulating the mouse embryo: A laboratory manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1994. [Google Scholar]
- Hynes M, Porter JA, Chiang C, Chang D, Tessier-Lavigne M, Beachy PA, Rosenthal A. Induction of midbrain dopaminergic neurons by sonic hedgehog. Neuron. 1995a;15:35–44. doi: 10.1016/0896-6273(95)90062-4. [DOI] [PubMed] [Google Scholar]
- Hynes M, Poulsen K, Tessier-Lavigne M, Rosenthal A. Control of neuronal diversity by the floor plate: contact-mediated induction of midbrain dopaminergic neurons. Cell. 1995b;80:95–101. doi: 10.1016/0092-8674(95)90454-9. [DOI] [PubMed] [Google Scholar]
- Kachinsky AM, Dominov JA, Miller JB. Myogenesis and the intermediate filament protein, nestin. Dev Biol. 1994;165:216–228. doi: 10.1006/dbio.1994.1248. [DOI] [PubMed] [Google Scholar]
- Kachinsky AM, Dominov JA, Miller JB. Intermediate filaments in cardiac myogenesis: nestin in the developing mouse heart. J Histochem Cytochem. 1995;43:843–847. doi: 10.1177/43.8.7542682. [DOI] [PubMed] [Google Scholar]
- Kastner P, Mark M, Chambon P. Nonsteroid nuclear receptors: what are genetic studies telling us about their role in real life? Cell. 1995;83:859–869. doi: 10.1016/0092-8674(95)90202-3. [DOI] [PubMed] [Google Scholar]
- Lardelli M, Williams R, Mitsiadis T, Lendahl U. Expression of the Notch 3 intracellular domain in mouse central nervous system progenitor cells is lethal and leads to disturbed neural tube development. Mech Dev. 1996;59:177–190. doi: 10.1016/0925-4773(96)00589-8. [DOI] [PubMed] [Google Scholar]
- Law SW, Conneely OM, DeMayo FJ, O’Malley BW. Identification of a new brain-specific transcription factor, NURR1. Mol Endocrinol. 1992;6:2129–2135. doi: 10.1210/mend.6.12.1491694. [DOI] [PubMed] [Google Scholar]
- Lee MK, Cleveland DW. Neuronal intermediate filaments. Annu Rev Neurosci. 1996;19:187–217. doi: 10.1146/annurev.ne.19.030196.001155. [DOI] [PubMed] [Google Scholar]
- Lendahl U, Zimmerman LB, McKay RDG. CNS stem cells express a new class of intermediate filament protein. Cell. 1990;60:585–595. doi: 10.1016/0092-8674(90)90662-x. [DOI] [PubMed] [Google Scholar]
- Li P, He X, Gerrero MR, Mok M, Aggarwal A, Rosenfeld MG. Spacing and orientation of bipartite DNA-binding motifs as potential functional determinants for POU domain factors. Genes Dev. 1993;7:2483–2496. doi: 10.1101/gad.7.12b.2483. [DOI] [PubMed] [Google Scholar]
- Lothian C, Lendahl U. An evolutionarily conserved region in the second intron of the human nestin gene directs gene expression to CNS progenitor cells and to early neural crest cells. Eur J Neurosci. 1997;9:452–462. doi: 10.1111/j.1460-9568.1997.tb01622.x. [DOI] [PubMed] [Google Scholar]
- Lumsden A, Clarke JDW, Keynes R, Fraser S. Early phenotypic choices by neuronal precursors, revealed by clonal analysis of the chick embryo hindbrain. Development. 1994;120:1581–1589. doi: 10.1242/dev.120.6.1581. [DOI] [PubMed] [Google Scholar]
- Marks MC, Hallenbeck PL, Nagata T, Segars JH, Appella E, Nikodem VM, Ozato K. H-2RIIBP (RXRβ) heterodimerization provides a mechanism for combinatorial diversity in the regulation of retinoic acid and thyroid hormone responsive genes. EMBO J. 1992;11:1419–1435. doi: 10.1002/j.1460-2075.1992.tb05187.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay R. Stem cells in the central nervous system. Science. 1997;276:66–71. doi: 10.1126/science.276.5309.66. [DOI] [PubMed] [Google Scholar]
- Morshead CM, Reynolds BA, Craig CG, McBurney MW, Staines WA, Morasutti D, Weiss S, Van der Kooy D. Neural stem cells in the adult mammalian forebrain: a relatively quiescent subpopulation of subependymal cells. Neuron. 1994;13:1071–1082. doi: 10.1016/0896-6273(94)90046-9. [DOI] [PubMed] [Google Scholar]
- Morrison SJ, Shah NM, Anderson DJ. Regulatory mechanisms in stem cell biology. Cell. 1997;88:287–298. doi: 10.1016/s0092-8674(00)81867-x. [DOI] [PubMed] [Google Scholar]
- Murphy EP, Dobson ADW, Keller C, Conneely OM. Differential regulation of transcription by the NURR1/NURR77 subfamily of nuclear transcription factors. Gene Expr. 1996;5:169–179. [PMC free article] [PubMed] [Google Scholar]
- Peers B, Voz ML, Monget P, Mathy-Hartert M, Berwaer M, Belayew A, Martial JA. Regulatory elements controlling pituitary-specific expression of the human prolactin gene. Mol Cell Biol. 1990;10:4690–4700. doi: 10.1128/mcb.10.9.4690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renfranz PJ, Cunningham MG, McKay RDG. Region-specific differentiation of the hippocampal stem cell line HiB5 upon implantation into the developing mammalian brain. Cell. 1991;66:713–729. doi: 10.1016/0092-8674(91)90116-g. [DOI] [PubMed] [Google Scholar]
- Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255:1707–1710. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- Ringstedt T, Kucera J, Lendahl U, Ernfors P, Ibanez CF. Limb proprioceptive deficits without neuronal loss in transgenic mice overexpressing neurotrophin-3 in the developing nervous system. Development. 1997;124:2603–2613. doi: 10.1242/dev.124.13.2603. [DOI] [PubMed] [Google Scholar]
- Rubenstein JLR, Martinez S, Shimamura K, Puelles L. The embryonic vertebrate forebrain: the prosomeric model. Science. 1994;266:578–580. doi: 10.1126/science.7939711. [DOI] [PubMed] [Google Scholar]
- Sejersen T, Lendahl U. Transient expression of the intermediate filament nestin during skeletal muscle development. J Cell Sci. 1993;106:1291–1300. doi: 10.1242/jcs.106.4.1291. [DOI] [PubMed] [Google Scholar]
- Shimamura K, Hartigan DJ, Martinez S, Puelles L, Rubenstein JLR. Longitudinal organization of the anterior neural plate and neural tube. Development. 1995;121:3923–3933. doi: 10.1242/dev.121.12.3923. [DOI] [PubMed] [Google Scholar]
- Sommer L, Ma Q, Anderson DJ. neurogenins, a novel family of atonal-related bHLH transcription factors, are putative mammalian neuronal determination genes that reveal progenitor cell heterogeneity in the developing CNS and PNS. Mol Cell Neurosci. 1996;8:221–241. doi: 10.1006/mcne.1996.0060. [DOI] [PubMed] [Google Scholar]
- Stemple DL, Anderson DJ. Isolation of a stem cell for neurons and glia from the mammalian neural crest. Cell. 1992;71:973–985. doi: 10.1016/0092-8674(92)90393-q. [DOI] [PubMed] [Google Scholar]
- Stemple DL, Mahanthappa NK. Neural stem cells are blasting off. Neuron. 1997;18:1–4. doi: 10.1016/s0896-6273(01)80018-0. [DOI] [PubMed] [Google Scholar]
- Suhonen JO, Peterson DA, Ray J, Gage FH. Differentiation of adult hippocampus-derived progenitors into olfactory neurons in vivo. Nature. 1996;383:624–627. doi: 10.1038/383624a0. [DOI] [PubMed] [Google Scholar]
- Terling C, Rass A, Mitsiadis TA, Fried K, Lendahl U, Wroblewski J. Expression of the intermediate filament nestin during rodent tooth development. Int J Dev Biol. 1995;39:947–956. [PubMed] [Google Scholar]
- Tohyama T, Lee VMY, Rorke LB, Marvin M, McKay RDG, Trojanowski JQ. Nestin expression in embryonic human neuroepithelium and in human neuroepithelial tumor cells. Lab Invest. 1992;66:303–313. [PubMed] [Google Scholar]
- Turner DL, Cepko CL. A common progenitor for neurons and glia persists in rat retina late in development. Nature. 1987;238:131–136. doi: 10.1038/328131a0. [DOI] [PubMed] [Google Scholar]
- Vicario-Abejon C, Cunningham MG, McKay RD. Cerebellar precursors transplanted to the neonatal dentate gyrus express features characteristic of hippocampal neurons. J Neurosci. 1995;15:6351–6363. doi: 10.1523/JNEUROSCI.15-10-06351.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetts R, Fraser SE. Multipotent precursors can give rise to all major cell types of the frog retina. Science. 1988;239:1142–1145. doi: 10.1126/science.2449732. [DOI] [PubMed] [Google Scholar]
- Williams BP, Price J. Evidence for multiple precursor cell types in the embryonic rat cerebral cortex. Neuron. 1995;14:1–20. doi: 10.1016/0896-6273(95)90265-1. [DOI] [PubMed] [Google Scholar]
- Wilson TE, Fahrner TJ, Milbrandt J. The orphan receptors NGFI-B and steroidogenic factor 1 establish monomer binding as a third paradigm of nuclear receptor-DNA interaction. Mol Cell Biol. 1993;13:5794–5804. doi: 10.1128/mcb.13.9.5794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada T, Pfaff SL, Edlund T, Jessell TM. Control of cell pattern in the neural tube: motor neuron induction by diffusable factors from notochord and floor plate. Cell. 1993;73:673–686. doi: 10.1016/0092-8674(93)90248-o. [DOI] [PubMed] [Google Scholar]
- Yaworsky PJ, Gardner DP, Kappen C. Transgenic analyses reveal developmentally regulated neuron-and muscle-specific elements in the murine neurofilament light chain gene promoter. J Biol Chem. 1997;272:25112–25120. doi: 10.1074/jbc.272.40.25112. [DOI] [PubMed] [Google Scholar]
- Zetterström RH, Solomin L, Jansson L, Hoffer BJ, Olson L, Perlmann T. Dopamine neuron agenesis in Nurr1-deficient mice. Nature. 1997;276:248–250. doi: 10.1126/science.276.5310.248. [DOI] [PubMed] [Google Scholar]
- Zimmerman LB, Lendahl U, Cunningham M, McKay R, Parr B, Gavin B, Mann J, Vassileva G, McMahon A. Independent regulatory elements in the nestin gene direct transgene expression to neural stem cells or muscle precursors. Neuron. 1994;12:11–24. doi: 10.1016/0896-6273(94)90148-1. [DOI] [PubMed] [Google Scholar]