Version Changes
Updated. Changes from Version 1
In response to the reviewer’s comments, we have updated our research report. We have added a figure (Figure S1) to the manuscript to show the location of sampling sites and also clarified that transects were haphazardly placed within a site in light of the Reviewer 1’s comments regarding replication of the study. We have also added a sentence to the discussion section regarding among-site variability in bleaching patterns, but note that the trend we describe, declining mortality with increasing depth, was consistent among all sites.
Abstract
Coral bleaching caused by rising sea temperature is a primary cause of coral reef degradation. However, bleaching patterns often show significant spatial variability, therefore identifying locations where local conditions may provide thermal refuges is a high conservation priority. Coral bleaching mortality often diminishes with increasing depth, but clear depth zonation of coral communities and putative limited overlap in species composition between deep and shallow reef habitats has led to the conclusion that deeper reef habitats will provide limited refuge from bleaching for most species. Here, we show that coral mortality following a severe bleaching event diminished sharply with depth. Bleaching-induced mortality of Acropora was approximately 90% at 0-2m, 60% at 3-4 m, yet at 6-8m there was negligible mortality. Importantly, at least two-thirds of the shallow-water (2-3 m) Acropora assemblage had a depth range that straddled the transition from high to low mortality. Cold-water upwelling may have contributed to the lower mortality observed in all but the shallowest depths. Our results demonstrate that, in this instance, depth provided a refuge for individuals from a high proportion of species in this Acropora-dominated assemblage. The persistence of deeper populations may provide a critical source of propagules to assist recovery of adjacent shallow-water reefs.
Introduction
Mass bleaching events causing extensive mortality of reef-building corals have become more frequent and widespread in recent decades and have affected almost all coral reef regions 1– 3. Coral bleaching is a generalised stress response resulting from numerous causes including sedimentation, freshwater exposure or disease 4; however, the most geographically extensive and severe events are correlated with sustained periods of elevated sea water temperatures and high light irradiance 5. The bleaching response is caused by the expulsion of a symbiotic dinoflagellate Symbiodinium that occur within the coral tissue and allow corals to harness energy from sunlight, thus providing a significant portion of the energy requirements. The sensitivity of this symbiosis to elevated sea temperature is well-documented 5, 6, suggesting that many coral species will be highly vulnerable to the effects of global warming 7, 8.
Despite this apparent sensitivity, reef corals have persisted through numerous large-magnitude and sometimes rapid changes in sea surface temperatures over the past 240 million years 3, 9. One mechanism by which a species can cope with changing local climate is to move to a more favourable area, and tropical reef corals have repeatedly shifted their distribution to higher latitudes in response to past climate warming 10, 11. Alternatively, populations may persist in microrefugia, defined as small areas of suitable habitat within regionally unfavourable environmental conditions 12, 13. Despite increasing recognition of their importance for conservation planning in terrestrial ecosystems 14– 16, microrefugia are less considered in the marine realm.
The severity of coral bleaching is often spatially heterogeneous due to both historical 17, 18 and environmental 19– 21 factors. Coral bleaching is caused by a synergistic effect between heat and light, and therefore microrefugia from bleaching are likely to occur in regions where oceanographic or atmospheric conditions reduce water temperatures or light irradiance relative to surrounding areas 22. Light irradiance declines with depth and ambient temperatures are often lower in deeper waters, therefore the incidence of bleaching and/or subsequent mortality is likely to be lower at greater water depths 1, 5, 22. Warm-water coral bleaching is occasionally reported to depths of 50 m, however, such observations are rarely followed up in order to estimate bleaching-induced mortality. Typically the incidence of bleaching is substantially lower at greater depths and in the few cases it has been measured, so is bleaching-induced mortality 23– 25. For example, mortality rates of corals at a depth of 6 m were only a third of those in 2 m across several turbid inshore reefs on the Great Barrier Reef (GBR) 24. A transition from high to low mortality with increasing depth was observed at numerous sites in the western Indian Ocean during 1998, the most severe and widespread bleaching event on record 26. This transition often occurred across a fairly sharp depth boundary at intermediate depths of 10–15 m 26, therefore species with depth ranges that straddle this transition from high to low bleaching mortality will have a refuge from bleaching in deeper water. However, most assessments of coral reefs consider only shallow habitats, and reductions in mortality with increasing depth may go unnoticed. Furthermore, recent studies of deep-water reefs have indicated that many corals may occur over a wider depth range than currently thought 27, 28.
In May-June 2010, a sustained increase in seawater temperatures in the Andaman and South China Seas resulted in extensive coral bleaching and caused high mortality of many coral species 29. Six weeks after the peak seawater temperatures, 45% of all corals and 94% of Acropora colonies were dead in shallow waters (1–2 m) around Pulau Weh, Sumatra, Indonesia 29. Here, we assess the effects of this severe thermal bleaching event at Pulau Weh over a depth gradient from 2–27 m to investigate 1) whether severe mortality of reef corals observed in shallow water (0–2 m) extended into deeper habitats; and 2) whether depth provided a refuge from bleaching mortality. We concentrate on the corals of the genus Acropora because they are the most diverse and abundant genus in the Indo-Pacific, and are important ecosystem engineers on most Indo-Pacific coral reefs. They are also often amongst the most susceptible taxa to bleaching-induced mortality, and bleaching events often result in shifts from Acropora – dominated communities towards communities dominated by more bleaching resistant taxa (e.g. Porites and the family Merulinidae) 26, 30. Change in Acropora cover before and after a bleaching event is therefore a useful indicator of bleaching severity.
Materials and methods
Pulau Weh (5° 50’N, 95° 20’E) is located in the province of Aceh off the northwest coast of Sumatra, Indonesia. The region’s reefs have received little attention from scientists, but support similarly diverse coral communities to the rest of the Indo-Australian Archipelago 31. Northwest Sumatra was the epicentre of the December 2004 Indian Ocean tsunami, and although Pulau Weh’s coral communities were relatively unaffected by this event 32, they suffered substantial mortality in the 2010 Andaman Sea bleaching 29. To examine the influence of depth on bleaching mortality, we compared both total coral cover and Acropora cover collected before (November 2009 to February 2010) and after (July 2011) the bleaching event at three depths (0–2 m, 3–4 m and 6–8 m) at four sites on the northern and western sides of Pulau Weh (Batee Gla, Ba Kopra, Rubiah Sea Garden, Rubiah Channel – Figure S1). Coral cover was estimated along 6–10 replicate 10 m line intercept transects, which were haphazardly placed at 0–2 m, and 3–6 replicate 50 m point intercept transects at 3–4 and 6–8 m (see Data File). Any live hard coral (i.e. scleractinian or hydrozoan coral) underlying each survey point was recorded to genus level. Changes in total live coral cover and Acropora cover between 2009 and 2011 were compared using two-factor ANOVA’s. Assumptions of the ANOVA’s were examined using residual analysis and no transformation was necessary. The analyses were based on the proportion of total coral or Acropora cover per 50 m transect.
To determine the proportion of the Acropora assemblage afforded a depth refuge from this bleaching event, we conducted species-level surveys of Acropora assemblages in 0 to 2 m and then at 5 m intervals from 3–27 m in February 2012 at five sites on the northern and western sides of Pulau Weh (Batee Gla, Ba Kopra, Rubiah Sea Garden, Rubiah Channel and Tokong - Figure S1. Sites were chosen based on their bathymetry profiles, with accessible deep sites only present on the steeply-sloping, ocean-facing northern and western coasts. Data were collected at 5 m depth intervals using replicate 10-minute timed swims, where the species identity of every living Acropora colony was recorded. Post-bleaching surveys were compared to shallow-water (0–2 m) surveys conducted in November 2009 before the bleaching event using 40 min timed swims 31 at these same sites. Corals were identified using taxonomic references provided in “Staghorn Corals of the World” by Wallace CC and “Corals of the World”, by Veron JEN 33, 34. Analysis of Similarities (ANOSIM), a multivariate approximation of ANOVA 35, was performed on a square root-transformed Bray-Curtis similarity matrix to determine any significant difference in the Acropora assemblage among sites.
“Bleaching” denotes whether the survey was pre-bleaching (2009) or post-bleaching (2011). “Depth” is the depth of the survey in metres, “Transect” is the transect number, “Acropora” denotes the % cover of Acropora in each transect, and “Total Hard Coral” is total coral cover for each transect.
Results and discussion
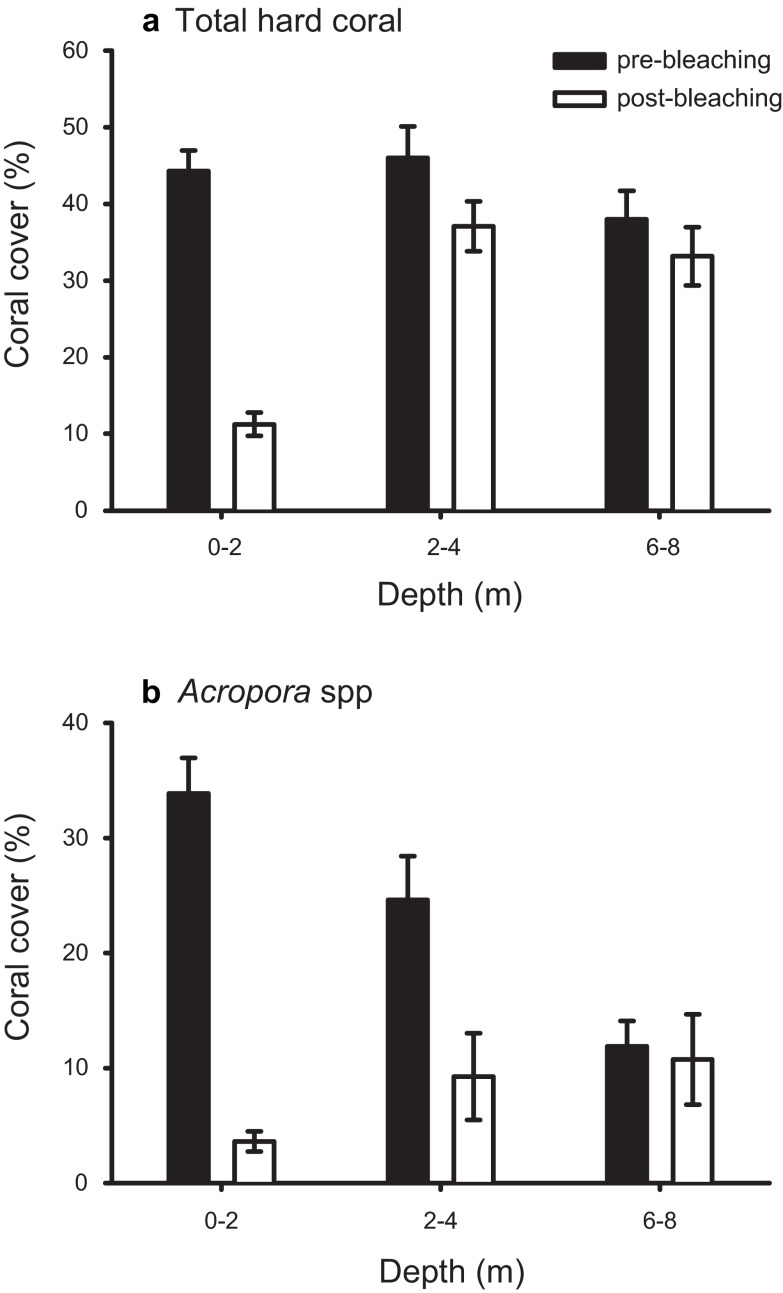
A total of 40 Acropora species were observed during the study, confirming the high diversity previously reported on Acehnese reefs 31. ANOSIM revealed no significant difference in assemblage structure among sites, which were therefore pooled for further analysis. Bleaching mortality was very high in the shallows, however, mortality diminished rapidly with increasing depth ( Figure 1). Total coral cover declined by 75% at 0–2 m but only 20% at 3–4 m, while there was no significant change at 6–8 m ( Figure 1a; 2-way ANOVA depth by year interaction; F 2,123 = 21.2, p < 0.001). The decline in mortality was even more pronounced in the Acropora, with cover declining by approximately 90% at 0–2 m and 60% at 3–4 m, with no change detected at 6–8 m ( Figure 1b; 2-way ANOVA depth x year; F 2,123 = 17.9, p < 0.001).
Figure 1. Change in live coral cover on Pulau Weh following the 2010 bleaching event at depths of 0–2, 2–4, and 6–8 metres.
( a) total live coral cover; and ( b) live Acropora cover.
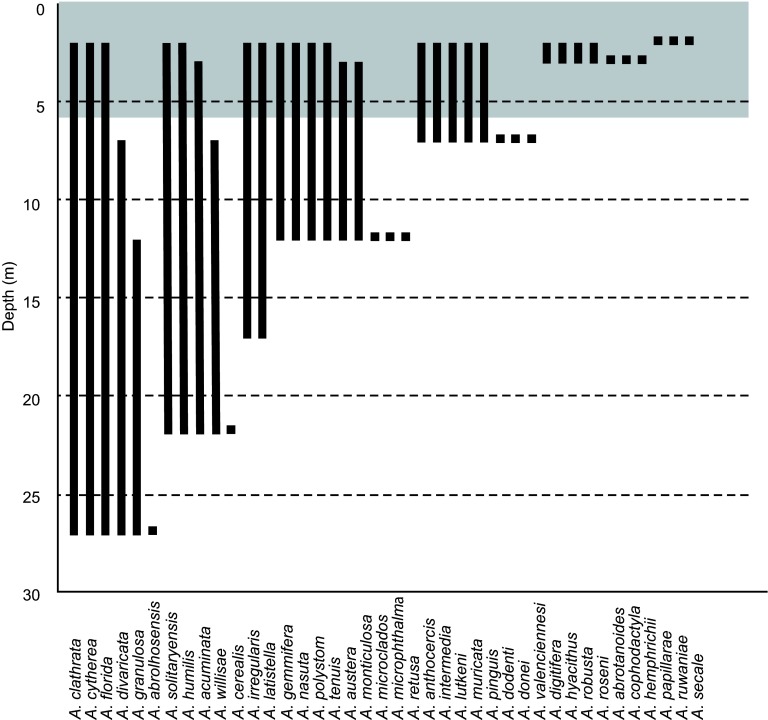
A high proportion of this diverse Acropora assemblage was afforded a refuge from bleaching mortality by depth. Of the 29 Acropora species occurring in shallow waters < 7 m, 19 (66%) also occurred below the approximate depth of transition from high to low mortality ( Figure 2). However, the refuge effect would be diminished if mortality had reached into deeper waters. If, for example, the transition between high and low bleaching mortality had occurred at 12 m, 14 (48%) of the species affected would have had a refuge in depth. Similarly, if bleaching mortality extended to 22 m, only 6 species from the shallow assemblage (21%) would have had colonies persisting below the transition depth.
Figure 2. Depth distribution of 41 Acropora species recorded on coral reefs around Pulau Weh.
Shaded (grey) panel indicates the depth range where bleaching mortality was high. Of the 29 species occurring in 0–7 m depth, 19 (66%) also occurred below 7 m.
Doubts regarding the potential significance of depth as a refuge for corals from warm-water bleaching have previously been raised because (1) bleaching has been observed in the deeper areas of reefs, (2) there is limited overlap of species between deep and shallow reef areas, and (3) genetic partitioning within species among depths suggests that deeper population cannot provide an effective source of recruits for shallow populations 36, 37. Firstly, while bleaching often extends to the lower depth limits of some shallow water species, both bleaching frequency and, most importantly, mortality, is often strongly depth dependent ( Figure 3) 24, 26. Indeed, a transition from high to low mortality occurred at depths of ≤ 15 m ~50% of sites surveyed in the Indian Ocean in 1998 26– see Table 1). Secondly, our results indicate that even with a pronounced depth zonation in the Acropora assemblage, two-thirds of species occurring in shallow depths had a depth range that straddled the transition in bleaching mortality. The depth zonation of coral assemblages is one of the most consistent and predictable patterns in nature 38, 39 and therefore our results are not an anomaly. Thirdly, the genetic divergence between populations above and below the transition in mortality at between 4 and 8 m is unlikely to be sufficient to prevent larval migration in either direction. For example, larvae of the coral Seriatopota hystrix migrate among sub-populations over a 30 m depth range 40. Furthermore, connectivity modelling in two Caribbean coral species indicates demographically significant larval subsidy from deep to shallow reef habitats over a much greater depth range (5–40 m) even when deep-water fertilisation rates and post-settlement survival are greatly reduced 41.
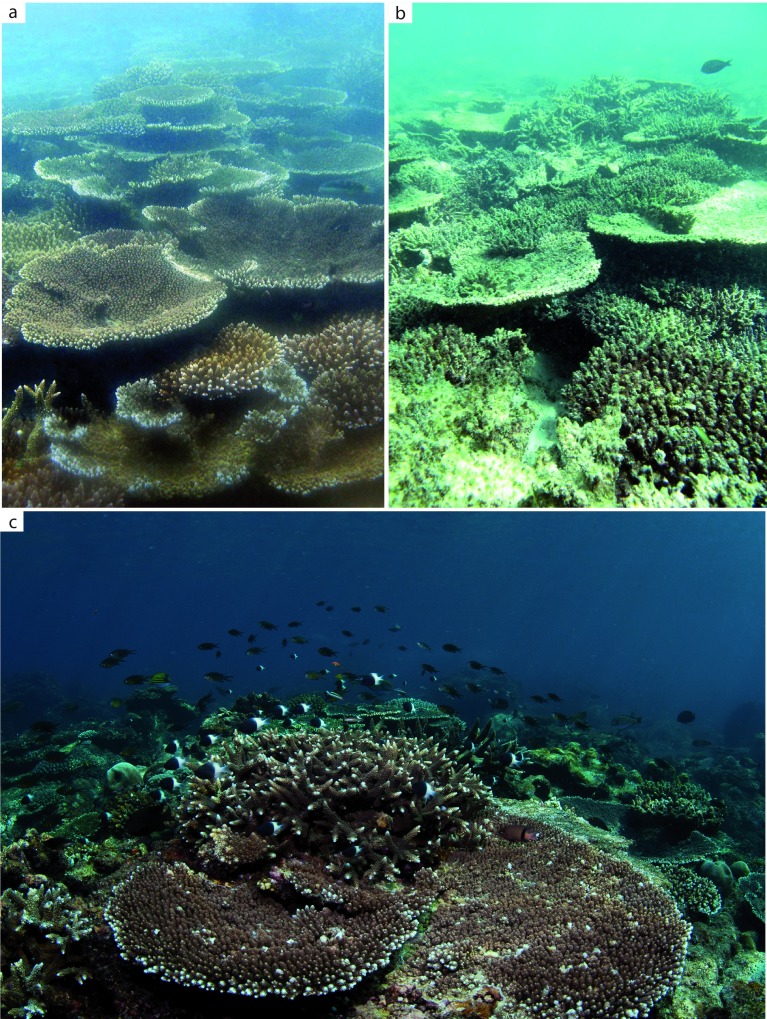
Figure 3. Acropora-dominated communities at Pulau Weh.
( a) Reef crest at 2 m depth prior to bleaching (16 November 2009); ( b) the same reef crest six weeks after the peak of bleaching (26 July 2010); ( c) upper reef slope community at 6 m depth largely unaffected by the bleaching event, 25 February 2012.
Our results indicate that bleaching mortality can vary considerably over a small depth range. Consequently, surveys conducted only in shallow waters may greatly overestimate the proportion of coral populations killed by coral bleaching 29. Conversely, surveys conducted in deeper areas are likely to underestimate the effects of bleaching. For example, long-term, large-scale monitoring of coral cover on reef slopes (6–9 m depth) on the GBR suggests that bleaching has been a comparatively minor source of coral mortality over the last few decades 42, 43, despite two mass bleaching events in 1998 and 2002 44. However, in the 1998 bleaching event on the inshore GBR, bleaching mortality was on average 3-times higher at 2–4 m when compared to 5–8 m 24. Clearly, ecosystem assessments considering only a single depth may provide a biased view of the relative importance of the many different agents of coral mortality, and should therefore be conducted over a range of depths to accurately assess the relative importance of multiple stressors.
Identifying areas or conditions that consistently provide refuges for corals from thermal stress is critically important for coral reef conservation under future climate change. In 1998, lower mortality and a shallower transition depth was often associated with sites that experienced episodic upwelling of cold water 26, 45, 46. Although environmental data are not available from Pulau Weh, pulses of cold water were regularly experienced during data collection, and rapid upwelling-driven temperature plunges of up to 10°C are recorded from the west coast of the nearby Similan Islands 47. Interestingly, Acehnese reefs appeared unaffected by the 1998 bleaching event 29, despite the coral bleaching extending across virtually the entire Indian Ocean from east Africa and north-western Australia 26, 48, 49. These cold-water upwelling events may explain the lack of mortality in 1998 and the shallow transition depth during 2010 despite very high sea surface temperatures. Depth-dependent mortality was most evident at Ba Kopra, on the western side of Pulau Weh ( Figure S1), supporting the hypothesis that these upwelling events may create small-scale refugia from thermal anomalies. If so, this region may provide a consistent refuge for many corals against rising sea temperatures. In summary, our results show that coral bleaching mortality can diminish rapidly even where shallow-water corals experience severe mortality, and modest depths can provide a refuge for a significant proportion of coral species. Identifying sites where oceanographic conditions reduce the effects of thermal anomalies should be a priority for coral reef conservation.
Acknowledgements
The activities for this study were conducted under a Memorandum of Understanding (MoU) between the Wildlife Conservation Society (WCS) and the Indonesian Ministry of Forestry, and a MoU between the ARC Centre of Excellence for Coral Reef Studies, James Cook University, Australia and Syiah Kuala University, Banda Aceh, Indonesia. No flora or fauna were collected or manipulated during this research and all surveys were conducted on public land. We thank Ismayudi Dodent and Rubiah Tirtah Divers for their field support.
This paper is dedicated to the late Dr Edi Rudi, a pioneer of coral research in Aceh and a great friend.
Funding Statement
Funding for this study was provided by the Australian Research Council Centre of Excellence for Coral Reef Studies, the Wildlife Conservation Society Indonesia Marine Program, and the Kerzner Marine Foundation.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
v2; ref status: indexed
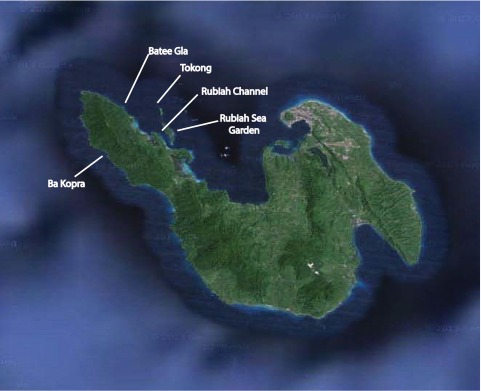
Supplementary figure
Figure S1. Map of Pulau Weh showing location of study sites.
References
- 1.Hughes TP, Baird AH, Bellwood DR, et al. : Climate change, human impacts, and the resilience of Coral Reefs. Science. 2003;301(5635):929–933 10.1126/science.1085046 [DOI] [PubMed] [Google Scholar]
- 2.Baker AC, Glynn PW, Riegl B: Climate change and coral reef bleaching: an ecological assessment of long-term impacts, recovery trends, and future outlook. Estuar Coast Shelf Sci. 2008;80:435–471 10.1016/j.ecss.2008.09.003 [DOI] [Google Scholar]
- 3.Pandolfi JM, Connolly SR, Marshall DJ, et al. : Projecting coral reef futures under global warming and ocean acidification. Science. 2011;333(6041):418–422 10.1126/science.1204794 [DOI] [PubMed] [Google Scholar]
- 4.Baird AH, Bhagooli R, Ralph PJ, et al. : Coral bleaching: The role of the host. Trends Ecol Evol. 2009;24(1):16–20 10.1016/j.tree.2008.09.005 [DOI] [PubMed] [Google Scholar]
- 5.Glynn PW: Coral reef bleaching: facts, hypothesies and implications. Glob Chang Biol. 1996;2:495–509 10.1111/j.1365-2486.1996.tb00063.x [DOI] [Google Scholar]
- 6.Brown BE: Coral bleaching: causes and consequences. Coral Reefs. 1997;16:129–138 10.1007/s003380050249 [DOI] [Google Scholar]
- 7.Carpenter KE, Abrar M, Aeby G, et al. : One-third of reef-building corals face elevated extinction risk from climate change and local impacts. Science. 2008;321(5888):560–563 10.1126/science.1159196 [DOI] [PubMed] [Google Scholar]
- 8.Foden WB, Butchart SHM, Stuart SN, et al. : Identifying the world’s most climate change vulnerable species: a systematic trait-based assessment of all birds, amphibians and corals. PLoS One. 2013;8(6):e65427 10.1371/journal.pone.0065427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Veron JEN: Corals in space and time: the biogeography and evolution of the Scleractinia. Comstock/Cornell University Press, Ithaca, NY.1995;321pp Reference Source [Google Scholar]
- 10.Wallace CC, Rosen BR: Diverse staghorn corals (Acropora) in high-latitude Eocene assemblages: implications for the evolution of modern diversity patterns of reef corals. Proc Biol Sci. 2006;273(1589):975–982 10.1098/rspb.2005.3307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greenstein BJ, Pandolfi JM: Escaping the heat: range shifts of reef coral taxa in coastal Western Australia. Glob Chang Biol. 2008;14:513–528 10.1111/j.1365-2486.2007.01506.x [DOI] [Google Scholar]
- 12.Rull V: Microrefugia. J Biogeogr. 2009;36:481–484 10.1111/j.1365-2699.2008.02023.x [DOI] [Google Scholar]
- 13.Dobrowski SZ: A climatic basis for microrefugia: the influence of terrain on climate. Glob Chang Biol. 2011;17:1022–1035 10.1111/j.1365-2486.2010.02263.x [DOI] [Google Scholar]
- 14.Ashcroft MB: Identifying refugia from climate change. J Biogeogr. 2010;37:1407–1413 10.1111/j.1365-2699.2010.02300.x [DOI] [Google Scholar]
- 15.Keppel G, Van Niel KP, Wardell-Johnson GW, et al. : Refugia: identifying and understanding safe havens for biodiversity under climate change. Global Ecol Biogeogr. 2012;21:393–404 10.1111/j.1466-8238.2011.00686.x [DOI] [Google Scholar]
- 16.Groves CR, Game ET, Anderson MG, et al. : Incorporating climate change into systematic conservation planning. Biodivers Conserv. 2012;21:1651–1671 10.1007/s10531-012-0269-3 [DOI] [Google Scholar]
- 17.Thompson DM, van Woesik R: Corals escape bleaching in regions that recently and historically experienced frequent thermal stress. Proc Biol Sci. 2009;276(1669):2893–2901 10.1098/rspb.2009.0591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oliver TA, Palumbi SR: Many corals host thermally resistant symbionts in high-temperature habitat. Coral Reefs. 2011;30:241–250 10.1007/s00338-010-0696-0 [DOI] [Google Scholar]
- 19.Dunne RP, Brown BE: The influence of solar radiation on bleaching of shallow water reef corals in the Andaman Sea 1993–1998. Coral Reefs. 2001;20:201–210 10.1007/s003380100160 [DOI] [Google Scholar]
- 20.Kleypas JA, Danabasoglu G, Lough JM: Potential role of the ocean thermostat in determining regional differences in coral reef bleaching events. Geophys Res Lett. 2008;35: L03613. 10.1029/2007GL032257 [DOI] [Google Scholar]
- 21.Woesik R, Houk P, Isechal AL, et al. : Climate-change refugia in the sheltered bays of Palau: analogs of future reefs. Ecol Evol. 2012;2(10):2474–2484 10.1002/ece3.363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Riegl B, Piller WE: Possible refugia for reefs in times of environmental stress. Int J Earth Sci. 2003;92:520–531 10.1007/s00531-003-0328-9 [DOI] [Google Scholar]
- 23.Rowan R, Knowlton N, Baker AC, et al. : Landscape ecology of algal symbionts creates variation in episodes of coral bleaching. Nature. 1997;388(6639):265–269 [DOI] [PubMed] [Google Scholar]
- 24.Marshall PA, Baird AH: Bleaching of corals on the Great Barrier Reef: differential susceptibilities among taxa. Coral Reefs. 2000;19:155–163 10.1007/s003380000086 [DOI] [Google Scholar]
- 25.Glynn PW, Mate JL, Baker AC, et al. : Coral bleaching and mortality in Panama and Ecuador during the 1997–1998 El Nino-Southern Oscillation event: spatial/temporal patterns and comparisons with the 1982–1983 event. Bull Mar Sci. 2001;69:79–109 Reference Source [Google Scholar]
- 26.Sheppard CRC, Obura D: Corals and reefs of the Cosmoledo and Aldabra atolls: Extent of damage, assemblage shifts and recovery following the severe mortality of 1998. J Nat Hist. 2005;39:103–121 10.1080/00222930310001657900 [DOI] [Google Scholar]
- 27.Bridge TCL, Fabricius KE, Bongaerts P, et al. : Diversity of Scleractinia and Octocorallia in the mesophotic zone of the Great Barrier Reef, Australia. Coral Reefs. 2012;31:179–189 10.1007/s00338-011-0828-1 [DOI] [Google Scholar]
- 28.Bridge TCL, Hughes TP, Guinotte JM, et al. : Call to protect all coral reefs. Nat Clim Chang. 2013;3:528–529 10.1038/nclimate1879 [DOI] [Google Scholar]
- 29.Guest JR, Baird AH, Maynard JA, et al. : Contrasting patterns of coral bleaching susceptibility in 2010 suggest adaptive response to the thermal stress. PLoS One. 2012;7(3):e33353 10.1371/journal.pone.0033353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Loya Y, Sakai K, Yamazato K, et al. : Coral bleaching: the winners and losers. Ecol Lett. 2001;4:122–131 10.1046/j.1461-0248.2001.00203.x [DOI] [Google Scholar]
- 31.Rudi E, Campbell SJ, Hoey AS, et al. : The Coral Triangle Initiative: What are we missing? A case study from Aceh, Indonesia. Oryx. 2012;46,482–485 10.1017/S0030605312000178 [DOI] [Google Scholar]
- 32.Baird AH, Campbell SJ, Anggoro AW, et al. : Acehnese reefs in the wake of the Asian tsunami. Curr Biol. 2005;15(21):1926–1930 10.1016/j.cub.2005.09.036 [DOI] [PubMed] [Google Scholar]
- 33.Wallace CC: Staghorn corals of the world. CSIRO Publishing, Collingwood, Victoria.1999. Reference Source [Google Scholar]
- 34.Veron JEN: Corals of the world. Australian Institute of Marine Science, Townsville.2000. Reference Source [Google Scholar]
- 35.Clarke KR, Gorley RN: PRIMER v6: user manual/tutorial. PRIMER-E: Plymouth.2006. Reference Source [Google Scholar]
- 36.Bongaerts P, Riginos C, Ridgway T, et al. : Genetic divergence across habitats in the widespread coral Seriatopora hystrix and its associated Symbiodinium. PLoS One. 2010;5(5):e10871 10.1371/journal.pone.0010871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brazeau DA, Lesser MP, Slattery M: Genetic Structure in the Coral, Montastraea cavernosa: Assessing Genetic Differentiation among and within Mesophotic Reefs. PLoS One. 2013;8(5):e65845 10.1371/journal.pone.0065845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Done TJ: Coral zonation: its nature and significancePerspectives on Coral Reefs. Brian Clouston, Manuka, A.C.T.1983;pp95–147 Reference Source [Google Scholar]
- 39.Hughes TP, Baird AH, Dinsdale EA, et al. : Assembly rules of reef corals are flexible along a steep climatic gradient. Curr Biol. 2012;22(8):736–741 10.1016/j.cub.2012.02.068 [DOI] [PubMed] [Google Scholar]
- 40.van Oppen MJ, Bongaerts P, Underwood JN, et al. : The role of deep reefs in shallow reef recovery: an assessment of vertical connectivity in a brooding coral from west and east Australia. Mol Ecol. 2011;20(8):1647–1660 10.1111/j.1365-294X.2011.05050.x [DOI] [PubMed] [Google Scholar]
- 41.Holstein DM: Vertical connectivity in mesophotic coral ecosystems. Open Access Dissertations. Paper 1064.2013. Reference Source [Google Scholar]
- 42.Osborne K, Dolman AM, Burgess SC, et al. : Disturbance and the dynamics of coral cover on the Great Barrier Reef (1995–2009). PLoS One. 2009;6(3):e17516 10.1371/journal.pone.0017516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.De’ath G, Fabricius KE, Sweatman H, et al. : The 27-year decline of coral cover on the Great Barrier Reef and its causes. Proc Natl Acad Sci U S A. 2012;109(44):17995–9 10.1073/pnas.1208909109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Berkelmans R, De’ath G, Kininmonth S, et al. : A comparison of the 1998 and 2002 coral bleaching events on the Great Barrier Reef: spatial correlation, patterns and predictions. Coral Reefs. 2004;23:74–83 10.1007/s00338-003-0353-y [DOI] [Google Scholar]
- 45.Teleki KA, Spencer T: Reef systems of the islands of the southern Seychelles. In: Souter D, Obura D, Linden O, editors. Coral Reef Degradation in the Indian Ocean. Kalmar, Sweden: CORDIO.2000;p 87–93 Reference Source [Google Scholar]
- 46.Goreau T, McClanahan T, Hayes R, et al. : Conservation of coral reefs after the 1998 global bleaching event. Conserv Biol. 2000;14:5–15 10.1046/j.1523-1739.2000.00011.x [DOI] [Google Scholar]
- 47.Schmidt GM, Phongsuwan N, Jantzen C, et al. : Coral community composition and reef development at the Similan Islands, Andaman Sea in response to strong environmental variations. Marine Ecolology Progress Series. 2012;456:113–126 10.3354/meps09682 [DOI] [Google Scholar]
- 48.Sheppard CR: Predicted recurrences of mass coral mortality in the Indian Ocean. Nature. 2003;425(6955):294–297 10.1038/nature01987 [DOI] [PubMed] [Google Scholar]
- 49.Gilmour JP, Smith LD, Heyward AJ, et al. : Recovery of an isolated coral reef system following severe disturbance. Science. 2013;340(6128):69–71 10.1126/science.1232310 [DOI] [PubMed] [Google Scholar]