Abstract
Recent scientific advances suggest that slowing the aging process (senescence) is now a realistic goal. Yet most medical research remains focused on combating individual diseases. Using the Future Elderly Model—a microsimulation of the future health and spending of older Americans in the United States—we compared optimistic “disease specific” scenarios with a hypothetical “delayed aging” scenario in terms of their impact on longevity, disability, and major entitlement program costs. Delayed aging could increase life expectancy by an additional 2.2 years, most of which would be spent in good health. The economic value of delayed aging is estimated to be $7.1 trillion over fifty years. In contrast, addressing heart disease and cancer separately would yield diminishing improvements in health and longevity by 2060—mainly due to competing risks. Delayed aging would substantially increase entitlement outlays, especially for Social Security, but these changes could be offset by increasing the Medicare eligibility age and the normal retirement age for Social Security. Overall, greater investment in research to delay aging appears to be a highly efficient way to forestall disease, extend healthy life, and improve public health.
Over the past fifty years, life expectancy has increased dramatically, and there have been major improvements in the functional capacity of the elderly. These gains have been driven by advances in public health and nutrition. They have also been driven by physicians’ and scientists’ use of a highly focused “disease model” to improve the diagnosis and treatment of various major fatal conditions. The goal of the model is to reduce mortality rates by the earlier detection of these conditions and by the reduction of risk factors and the development of effective new treatments for them.
But the longer lives that people now enjoy come with social and fiscal side effects. Today more people are qualifying for old-age entitlement programs, and they remain in these programs longer. Medicare spending alone is projected to almost double as a share of gross domestic product, from 3.7 percent in 2012 to 7.3 percent in 2050.(1)
Although attacking diseases has extended life for younger and middle-aged people, evidence suggests it may not extend healthy life once people reach older ages. Increased disability rates are now accompanying increases in life expectancy, leaving the length of a healthy life span unchanged (2-4) or even shorter than in the past.(5) The evidence is not completely one-sided, however: in one study of health status among successive birth cohorts in Denmark, those born in the more recent era experienced better health.(6)
In any case, as people age, they are now much less likely to fall victim to a single isolated disease than was previously the case. Instead, competing causes of death more directly associated with biological aging (e.g., heart disease, cancer, stroke, Alzheimer's, etc.) cluster within individuals as they reach older ages. These conditions elevate mortality risk, as well as create the frailty and disabilities that can accompany old age.
Delayed Aging
Fortunately, new research is emerging that has the potential to extend life while reducing the prevalence of comorbidities over the entire lifetime.(7, 8) Scientists have been asking whether we can decelerate the process by which the cluster of conditions described above arises, making people healthier at older ages and even lowering spending on health care.(7, 9-14) Simply put, can we age more slowly—thereby delaying the onset and progression of all fatal and disabling diseases simultaneously?
At the practical level, delayed aging means having the body and mind of someone who is years younger than the majority of today's population at one's chronological age and spending a larger proportion of one's life in good health and free from frailty and disability.(9, 11, 15) Experimental studies involving animal models have already succeeded in accomplishing this in the laboratory.(12) In addition, there is evidence that centenarians (whose longevity is at least partially heritable) often have delayed onset of age-related diseases and disabilities, which suggests that they senesce (grow old biologically) more slowly than the rest of the population.(16)
By manipulating genes, altering reproduction, reducing caloric intake, modulating the levels of hormones that affect growth and maturation, and altering insulin-signaling pathways, it has been possible to extend the lifespan—and the healthy lifespan—of invertebrates and mammals.(8, 17, 18) These specific manipulations are unlikely to be directly applicable in humans, but they may lead scientists in the right direction. However, some compounds, such as rapamycin (used to prevent organ rejection in transplant patients), may eventually be shown to extend healthy life, even when used in older individuals.(19)
In addition, clinical interventions to delay aging have been proposed that involve interfering with chronic inflammation. In mice the selective removal of senescent cells has been documented to lead to significant improvements in health, an intervention that many researchers believe could be clinically effective in people.(7) Some scientists contend that such interventions are sufficiently close to fruition that people alive today will benefit from them.(7, 8, 11-14) Should we continue on this path of discovery?
In deciding whether and how much society should continue to invest in delayed aging, two specific questions arise. First, what are the social returns—in terms of health and spending—on continued investments in a “disease model” versus the returns on investments in delayed aging? Second, can society afford to invest in the accelerated development of interventions that extend healthy life, given fiscal uncertainties? In this article, we compare the future health and economic benefits—as well as the costs—of continuing to prioritize the “disease model” with the benefits and costs of placing a new emphasis on delayed aging.
Study Data And Methods
To estimate the potential benefits and costs, we used the Future Elderly Model (FEM), a microsimulation that tracks older cohorts of people and projects their health and economic outcomes. Prior work with the FEM has examined the impact of new medical technologies, changes in disability, improved prevention of diseases, and other health policy changes.(20-23) We describe the model and methods briefly here; more detail is provided in the online Appendix.(24)
The FEM models representative cohorts of people age fifty-one or fifty-two based on the Health and Retirement Survey, a biennial survey of Americans age fifty-one or older that began in 1992.(25) For each individual, the FEM predicts medical spending, health conditions, functional status, and employment for the next two years, given initial demographic characteristics and health states. Medical spending is predicted using the Medical Expenditure Panel Survey for the non-Medicare population and the Medicare Current Beneficiary Survey for Medicare beneficiaries, in each case adjusted to 2010 dollars using the medical Consumer Price Index.
Health states are derived from survey questions. Disability is measured by any limitations in activities of daily living or in instrumental activities of daily living, or by nursing home residency. Both functional status and the likelihood of developing a health condition depend on age, sex, education, race, ethnicity, body mass index, smoking status, and health at the time of entry into the study. All health conditions, functional states, and risk factors are modeled using first-order Markov processes that control for baseline unobserved factors, using health variables collected at baseline. These turn out to be effective controls as revealed by goodness-of-fit tests.
New Cohorts
Because of evidence of worsening health in younger cohorts, the FEM accounts for these trends for future populations.(2, 3) Specifically, the FEM includes trends in disability, obesity, smoking, and chronic disease among younger populations, based on projections from the National Health Interview Survey, the Current Population Survey, and other work of the Census Bureau. For instance, in the FEM the prevalence of diabetes among people age fifty-one or fifty-two in 2030 is 27 percent higher than the prevalence for that age group in 2004.
Fiscal Outcomes
We examined the costs of major entitlement programs– specifically, federal and state spending for Medicare and Medicaid, and federal income support through Old-Age, Survivors, and Disability Insurance and Supplemental Security Income. Economic outputs were aggregated into fiscally relevant variables using benefit rules for particular programs. Annual costs are given in constant 2010 dollars. All cumulative costs are discounted using a 3 percent annual discount rate.(26)
Scenarios
We developed four scenarios (one representing the status quo, or baseline) and compared the health and medical spending they would involve. For each scenario, we conducted the simulation fifty times and averaged the outcomes.
We assumed that all changes were accomplished at no additional cost relative to baseline, to allow us to focus on population benefits. Each scenario assumed that changes in mortality and disease processes occurred in the period 2010–30. The scenarios also assumed that progress ceased after 2030, but that the effects of earlier changes continued to play out.
Two disease-specific scenarios were meant to represent optimistic developments in medical research, disease treatment, and improvements in behavioral risk factors. In other words, these scenarios assumed that by attacking diseases individually through treatments or systemically through behavior modification, the incidence of disease and the impact of cases of disease would be reduced.
The fourth scenario (assuming delayed aging) is a hypothetical assessment of a successful effort to translate research on the biology of aging into therapeutic interventions that would reduce and compress both morbidity and mortality into a shorter period of time at the end of life.(27) Unlike the delayed disease interventions in the two disease-specific scenarios—which face diminishing returns because of competing causes of sickness and death in aging populations—the delayed aging scenario assumed that all fatal and disabling diseases were influenced simultaneously. Thus, this scenario represented what might best be thought of as a superefficient method of attacking the fatal and disabling diseases that are most prevalent at older ages.
Status Quo Scenario
In the status quo—or baseline—scenario, we used the mortality forecasts for all-cause mortality in the intermediate projections of the Social Security Administration.(28) We did not change the incidence of disease. Heuristically, in this scenario mortality improvements can be seen as the result of improved treatments for people with disease.
Delayed Cancer Scenario
In the first disease-specific scenario, we modified the status quo scenario by reducing the incidence of cancer over time. From 2010 to 2030, we phased in a linear 25 percent reduction in cancer incidence. We assumed that this change was accomplished at no additional Medicare cost relative to the baseline.
Historical evidence suggests that there was a reduction of 1.3 percent in overall cancer incidence rates for men per year from 2000 to 2006 and a reduction of 0.5 percent for women per year from 1998 to 2006.(29) Averaged over twenty years, these trends yield a range of reductions in cancer incidence of 10–26 percent. Thus, our assumptions in this scenario were within the bounds of the observed trends. We assumed that the reduced incidence rate continued until the end of the simulation.
To account for improvements in health prior to age fifty-one, the prevalence of chronic conditions in the incoming cohorts of fifty-one-year-olds was adjusted to match the prevalence for forty-four-year olds in the target year, as measured in the National Health Interview Survey.
Delayed Heart Disease Scenario
In the second disease-specific scenario, we modified the status quo scenario by reducing the incidence of heart disease over time. As was the case with cancer, we assumed a linear reduction in the incidence of 25 percent between 2010 and 2030, and no change thereafter.(30) And, again as in the delayed cancer scenario, we assumed that there was no additional Medicare cost, and we adjusted the prevalence of chronic conditions in the incoming cohorts.
Delayed Aging Scenario
In the delayed aging scenario, we assumed that improvements in mortality and health started earlier in life than they did in the disease-specific scenarios. We assumed that the slope of the intrinsic mortality curve—that is, mortality from factors such as age, as opposed to exposure to external risks such as trauma or smoking—observed in 2000 for both men and women ages 15–50 would decline by 20 percent by 2050. These hypothesized changes are consistent with research on the biology of aging, which suggests that the health benefits of delayed aging would begin at puberty—the time when mortality begins rising exponentially.(31, 32)
Although this scenario altered the effects of getting disease, it was not the same as scenarios of disease prevention because it addressed the underlying biology of aging. The scenario reduced mortality and the probability of onset of both chronic conditions (heart disease, cancer, stroke or transient ischemic attack, diabetes, chronic bronchitis and emphysema, and hypertension), and disability by 1.25 percent for each year of life lived above age fifty (the period in life when most of these diseases emerge). This reduction was phased in over twenty years, starting with a 0 percent reduction in 2010 and increasing linearly until the full 1.25 percent reduction was achieved in 2030.
The impact of the changing rates of transition of disease and functional status can be seen in the change in average life cycle characteristics. Life expectancy at age fifty-one in 2030 was 35.8 years in the status quo scenario, based on current Social Security Administration projections.(28) It improved by about one year in both the delayed cancer (36.9 years) and delayed heart disease (36.6 years) scenarios. In the delayed aging scenario, however, it increased to 38.0 years, an improvement of 2.2 years (see Appendix Exhibit S1).(24)
As in the delayed cancer and delayed heart disease scenarios, in the delayed aging scenario we adjusted the prevalence of chronic conditions in the incoming cohorts.
Delayed Aging With An Eligibility Fix
We modeled a variant of the delayed aging scenario that included an adjustment to the eligibility age for Medicare and the normal retirement age for Social Security. Social Security provides a strong precedent for such a policy fix: The normal retirement age was raised in 1983 from sixty-five to sixty-six, and the age will increase to sixty-seven for people born in 1960 and later. Our “eligibility fix” consisted of a gradual increase in the eligibility age for Medicare from sixty-five to sixty-eight, and for Social Security from sixty-seven to sixty-eight (extending the Social Security Amendments of 1983 – which mandated gradual increases in the retirement over a 22-year period starting in 2000 – for about ten years).
In this scenario, people enrolled in Medicare Part A as soon as they were eligible to do so. Medicare Part B take-up was modeled as an age-independent probit, so the scenario assumed that take-up was at the same rate regardless of age and depended directly on health and functional status (see Appendix Table 22).(24) Part D take-up was modeled in a similar way (see Appendix Table 24).(24)
Starting Social Security benefits was also modeled as a probit, but we used the normal retirement age (see Appendix Table 13).(24) This yielded a conservative estimate of the eligibility change required to counterbalance the fiscal impact of extended life. The delayed aging scenario with the eligibility fix – because of the later official retirement age – would result in more taxes collected during working years than in the original delayed aging scenario without an eligibility change, and less lifetime benefits paid because of the later start of retirement.
Limitations
There are several limitations involved in this approach. First, this is a simulation of various scenarios of biomedical innovation. All of the usual caveats about simulations apply, including assumptions about no changes in the underlying parameters that model health-related behavior and economic outcomes.
Most importantly, the proposed eligibility fix was intended as a useful metric to see what changes would be necessary to fiscally accommodate delayed aging. Obviously, more study would be required before the implementation of any policy change—including an official scoring of such a change by the Congressional Budget Office, fuller consideration of distributional and health outcomes beyond the major entitlement programs, and consideration of the impact of any financing reforms such as those in the Affordable Care Act.
Finally, our model demonstrates the hypothetical benefits of scientific progress in various areas, but not the costs of that progress. People who decide what research to invest in need to consider the costs of the research and the likelihood of success. To identify medical breakthroughs, we have attempted to evaluate such issues in previous work, with reasonable success.(23) However, a full accounting of the costs and probabilities is beyond the scope of this research.
Study Results
In the status quo scenario, the number of elderly people—those age sixty-five or older—in the United States more than doubled, increasing from 43 million in 2010 to 106 million in 2060. The scenarios of delayed cancer and delayed heart disease diverged little from the first scenario, leading to only 0.8 percent and 2.0 percent more elderly people in 2060, respectively. In contrast, the delayed aging scenario added 6.9 percent more elderly people. These demographic gains would occur quickly, with 6.1 percent more elderly Americans than in the status quo scenario after only twenty years. When we conducted a sensitivity analysis after adjusting the scenarios to include relative changes in incidence over a wider range, the effects were similar (see Appendix Exhibit S7).(24)
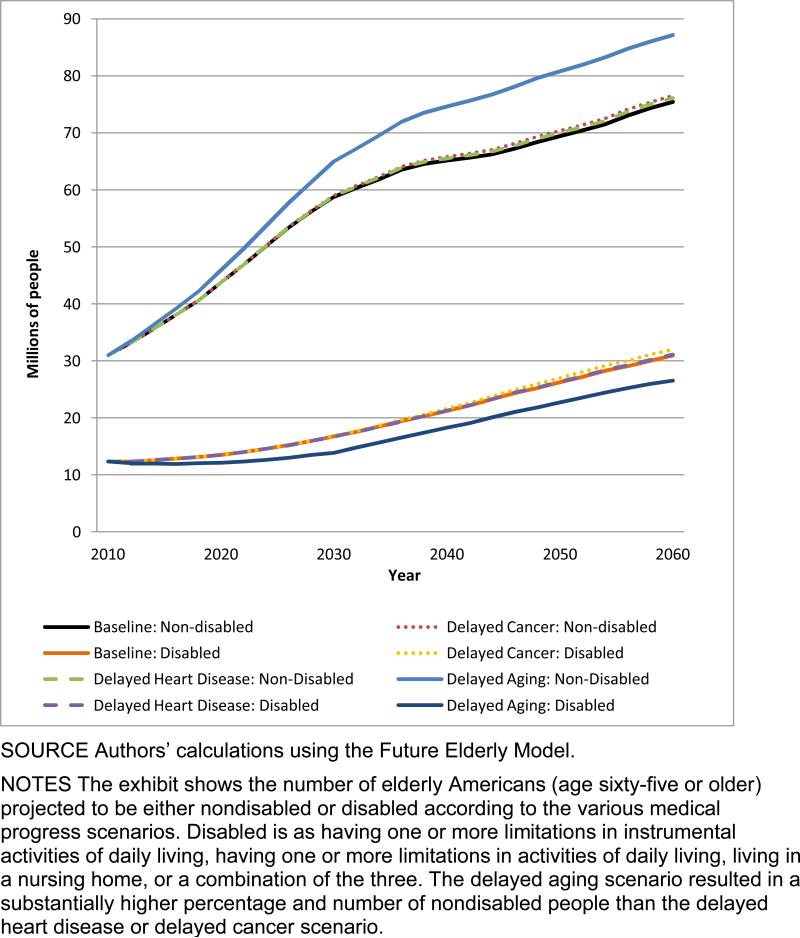
Of course, it matters whether these survivors would be healthy or disabled. In the status quo scenario, 31.0 million people age sixty-five or older were not disabled in 2010; the number was 75.5 million in 2060 (Exhibit 1).(33, 34) In the disease-specific scenarios there were very small increases in the number of nondisabled elderly people compared to the delayed aging scenario, in which there was a 15 percent increase from the status quo scenario.
EXHIBIT 1. Millions Of Nondisabled And Disabled Elderly Americans In Various Scenarios, 2010–60.
SOURCE Authors’ calculations using the Future Elderly Model.
NOTES The exhibit shows the number of elderly Americans (age sixty-five or older) projected to be either nondisabled or disabled according to the various medical progress scenarios. Disabled is as having one or more limitations in instrumental activities of daily living, having one or more limitations in activities of daily living, living in a nursing home, or a combination of the three. The delayed aging scenario resulted in a substantially higher percentage and number of nondisabled people than the delayed heart disease or delayed cancer scenario.
These absolute numbers can also be translated into disability rates. Today the share of the elderly US population without disabilities is around 72 percent. In the status quo scenario, this share increased to 78 percent in 2026 but then declined to 71 percent in 2060 (see Appendix Exhibit S2).(24) This decline was due to the lower all-cause mortality rates projected for the future, and the growing prevalence of health risks (such as obesity) among people entering the elderly group.
As Appendix Exhibit S2 shows,(24) the disease-specific scenarios both had an effect nearly identical to the status quo scenario. In comparison, the delayed aging scenario yielded a larger share of nondisabled seniors in every year between 2010 and 2026, compared to the status quo scenario. Although the size of the difference declined from 2030 to 2060, during that thirty-year period an additional 5 percent of elderly people were nondisabled in the delayed aging scenario. Per capita Medicare spending was also lower in the delayed aging scenario than in the status quo scenario.
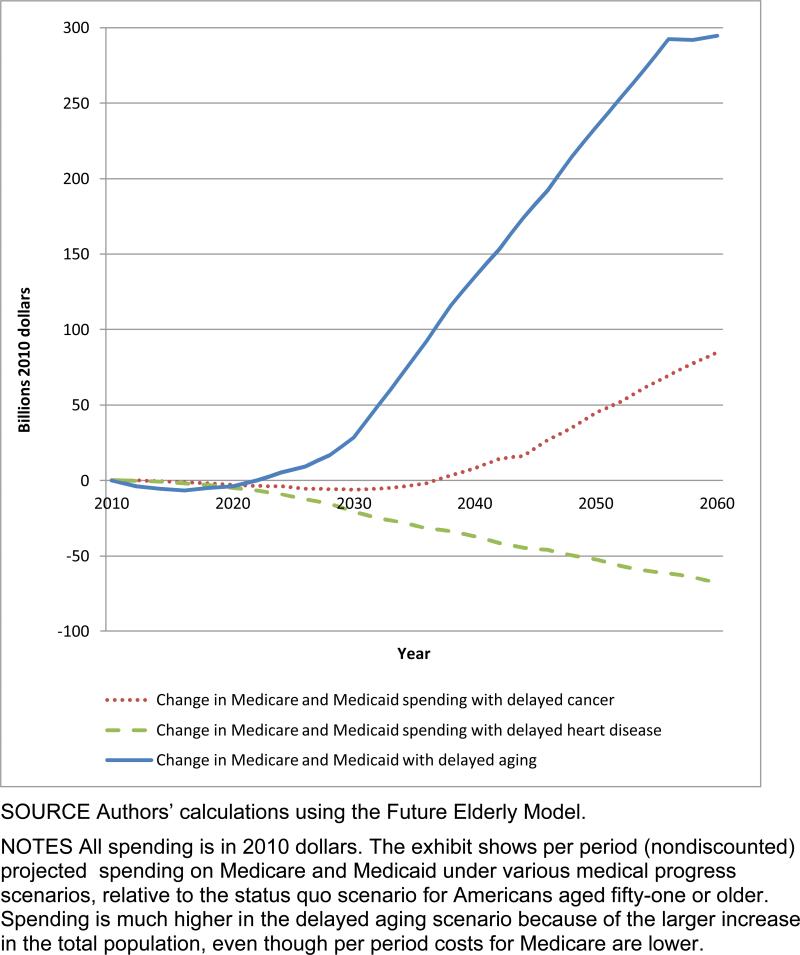
On the population level, the aggregate costs demonstrate the fiscal strain imposed by delayed aging (Exhibit 2). In that scenario, more elderly people were alive. Consequently, more people qualified for entitlement programs, and costs were higher. In 2060 spending in the delayed aging scenario was $295 billion more than in the status quo scenario. In contrast, the delayed cancer scenario led to only a modest increase, and the delayed heart disease scenario brought spending below the level in the status quo scenario.
EXHIBIT 2. Change In Medicare and Medicaid Spending On Health Care In Various Scenarios Compared To Status Quo, Billions Of Dollars, 2010–60.
SOURCE Authors’ calculations using the Future Elderly Model.
NOTES All spending is in 2010 dollars. The exhibit shows per period (nondiscounted) projected spending on Medicare and Medicaid under various medical progress scenarios, relative to the status quo scenario for Americans aged fifty-one or older. Spending is much higher in the delayed aging scenario because of the larger increase in the total population, even though per period costs for Medicare are lower.
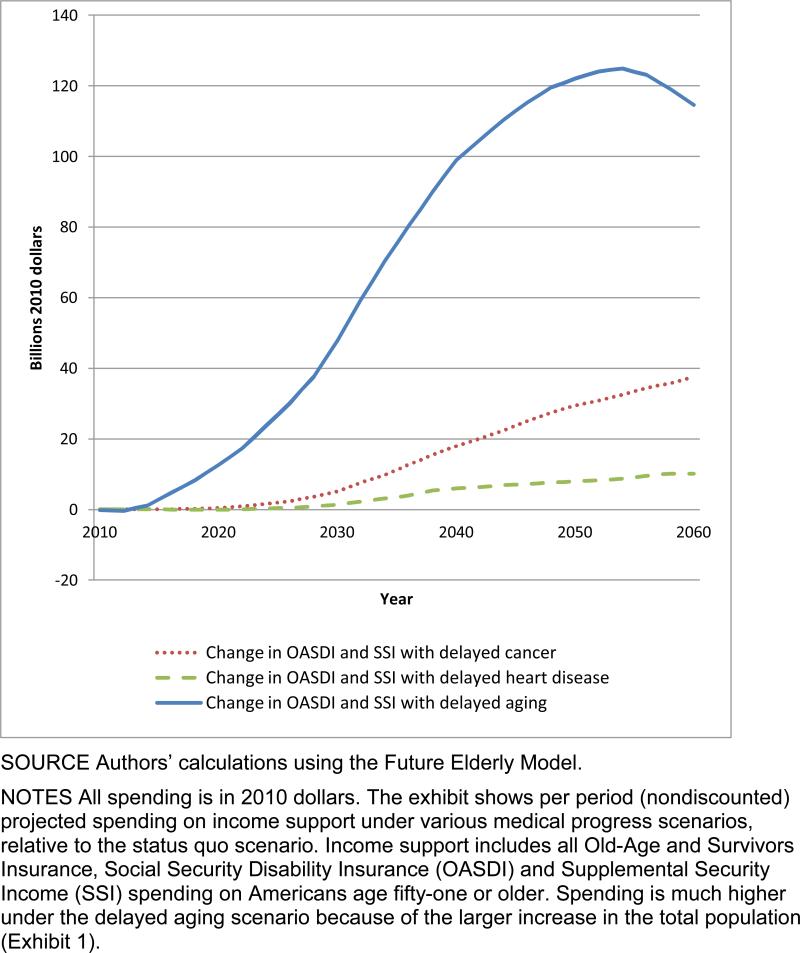
The gap in income support was also considerable. Spending beyond that in the status quo scenario was relatively low in the disease-specific scenarios (Exhibit 3). In comparison, it climbed to around $125 billion in the delayed aging scenario by 2055. Delayed aging would add nearly $420 billion to the entitlement deficit in the status quo scenario in 2060, 70 percent of which would come from increased outlays for Medicare and Medicaid.
EXHIBIT 3. Change In Income Support Spending In Various Scenarios Compared To Status Quo, Billions Of Dollars, 2010–60.
SOURCE Authors’ calculations using the Future Elderly Model.
NOTES All spending is in 2010 dollars. The exhibit shows per period (nondiscounted) projected spending on income support under various medical progress scenarios, relative to the status quo scenario. Income support includes all Old-Age and Survivors Insurance, Social Security Disability Insurance (OASDI) and Supplemental Security Income (SSI) spending on Americans age fifty-one or older. Spending is much higher under the delayed aging scenario because of the larger increase in the total population (Exhibit 1).
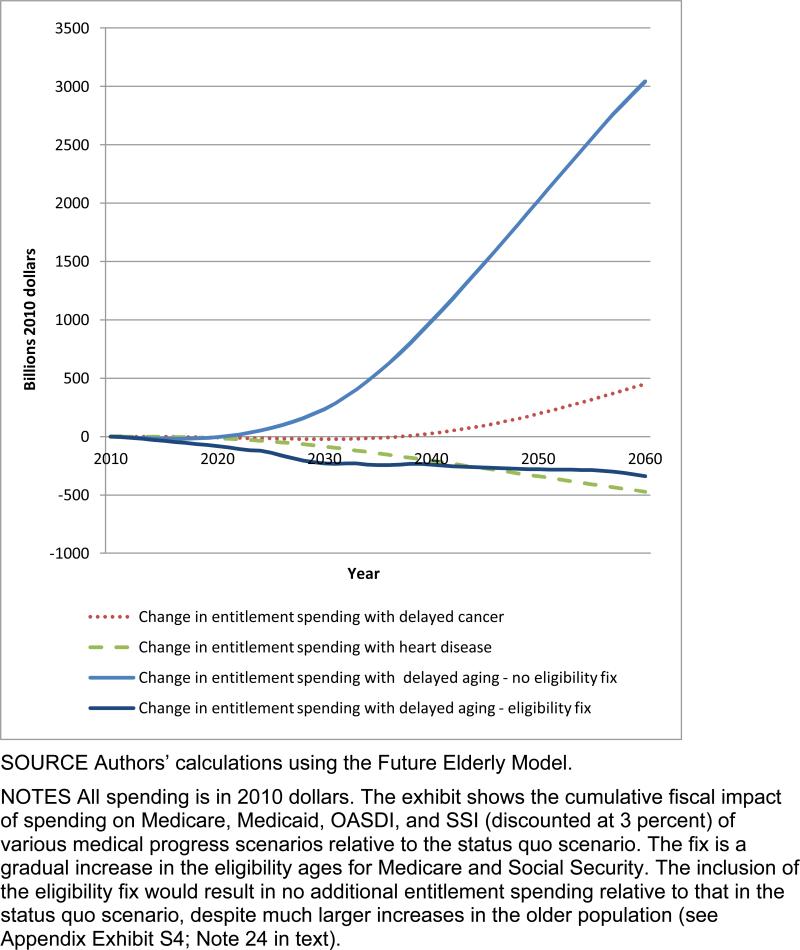
Exhibit 4 shows the fiscal effects of the four main scenarios as well as the effect of delayed aging with the eligibility fix to Medicare and Social Security described above. The eligibility fix would more than offset the additional costs of delayed aging relative to the costs of the status quo scenario.
EXHIBIT 4. Change In Major Entitlement Spending In Various Scenarios Compared To Status Quo, Billions Of Dollars, 2010–60.
SOURCE Authors’ calculations using the Future Elderly Model.
NOTES All spending is in 2010 dollars. The exhibit shows the cumulative fiscal impact of spending on Medicare, Medicaid, OASDI, and SSI (discounted at 3 percent) of various medical progress scenarios relative to the status quo scenario. The fix is a gradual increase in the eligibility ages for Medicare and Social Security. The inclusion of the eligibility fix would result in no additional entitlement spending relative to that in the status quo scenario, despite much larger increases in the older population (see Appendix Exhibit S4; Note 24 in text).
Discussion
Our results demonstrate that shifting the focus of medical investment to delayed aging would lead to a set of desirable, but economically challenging, circumstances. The potential gains are significant. Although the disease model has reduced mortality from lethal conditions dramatically in the past century, its influence is now waning because of competing risks. As people live longer, they are more likely to fall victim to multiple diseases. Our simulations of reduced incidence of heart disease and cancer suggested incrementally smaller gains in longevity going forward. The medical costs of treating these diseases independently would rise but, for example, would produce only a 3.2-year increase in life expectancy for sixty-five-year olds from 2010 to 2060.(28)
Recent research has shown that the decades-long improvement in the functional status of older Americans halted in 2002.(2, 3) This suggests that many of the historical drivers of better health in the elderly may no longer work. Declining disability buttresses the case for research on slowing aging by compressing morbidity and extending healthy life, which would provide an adequate workforce for producing the goods and services that the future aging society would use and would yield direct benefits to those older people who remain socially engaged.
Still, the fact remains that longer lives would mean greater fiscal burdens for Social Security and other income support programs and increased Medicare and Medicaid expenditures, even as per capita medical costs declined. An unequivocal answer to the question of whether the current focus of medical research and investment should be shifted from the disease model to delayed aging depends on whether the potential gains could be realized and the adverse consequences allayed. One way to think about the future gains is to look at the presented discounted value of all the additional, quality-adjusted life years that arise from delayed aging relative to the status quo. These can then be valued using a conservative metric such as $100,000 per life-year. Doing so, yields a social benefit of approximately $7.1 trillion–without even considering the cognitive benefits that could arise from these interventions.(6)
Given the large social return, the question then becomes how we accommodate these changes fiscally. Several policy measures might achieve fiscal balance–we demonstrate one involving eligibility changes–but a full evaluation of the options is beyond the scope of this research. However, we note here one benefit of delayed aging that might enlarge the set of possibilities: with people staying healthy until a much later age, it might be more feasible to justify raising the eligibility age for public programs for seniors. Arguments against doing so often note that life expectancy increases in lower socioeconomic groups have lagged far behind those in better-off groups.(35, 36) A future in which delayed aging increased the health of all socioeconomic groups would make these increases in eligibility ages more palatable.
Conclusion
It is clear that competing health risks limit the impact of major clinical breakthroughs for specific diseases—in other words, making progress against one disease means that another one will eventually emerge in its place. However, evidence suggests that when aging is delayed, all fatal and disabling disease risks are lowered simultaneously. Not surprisingly, we see extremely large population health benefits in our delayed aging scenario.
The major challenges of delayed aging appear to be of a fiscal nature, but they are manageable. The benefits to society of delayed aging would accrue rapidly and would extend to all future generations. Investing in research to delay aging should become a priority.
Supplementary Material
Acknowledgement
Drs. Cutler and Goldman are grateful to MetLife Foundation for supporting this research through the MetLife Foundation Silver Scholar Award, administered by the Alliance for Aging Research. Additional support came from the Ellison Medical Foundation. Development of the Future Elderly Model was supported by the National Institute on Aging through the Roybal Center for Health Policy Simulation (P30AG024968) and grant number RC4AG039036; and the MacArthur Research Network on an Aging Society. No sponsors had any input into the research.
Notes
- 1.Congressional Budget Office . The 2012 long-term budget outlook [Internet] CBO; Washington (DC): Jun, 2012. [2013 Aug 27]. (Pub. No. 4507). Available from: www.cbo.gov/sites/default/files/cbofiles/attachments/06-05-Long-Term_Budget_Outlook.pdf. [Google Scholar]
- 2.Crimmins EM, Beltrán-Sánchez H. Mortality and morbidity trends: is there compression of morbidity? J Gerontol B Psychol Sci Soc Sci. 2011;66(1):75–86. doi: 10.1093/geronb/gbq088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lakdawalla DN, Bhattacharya J, Goldman DP. Are the young becoming more disabled? Health Aff (Millwood) 2004;23(1):168–76. doi: 10.1377/hlthaff.23.1.168. [DOI] [PubMed] [Google Scholar]
- 4.Bhattacharya J, Cutler DM, Goldman DP, Hurd MD, Joyce GF, Lakdawalla DN, et al. Disability forecasts and future Medicare costs. Front Health Policy Res. 2004;7:75–94. doi: 10.2202/1558-9544.1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hulsegge G, Picavet HS, Blokstra A, Nooyens AC, Spijkerman AM, van der Schouw YT, et al. Today's adult generations are less healthy than their predecessors: generation shifts in metabolic risk factors: the Doetinchem Cohort Study. Eur J Prev Cardiol. 2013 Apr 15; doi: 10.1177/2047487313485512. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 6.Christensen K, Thinggaard M, Oksuzyan A, Steenstrup T, Andersen-Ranberg K, Jeune B, et al. Physical and cognitive functioning of people older than 90 years: a comparison of two Danish cohorts born 10 years apart. Lancet. 2013 Jul 10; doi: 10.1016/S0140-6736(13)60777-1. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tchkonia T, Zhu Y, van Deursen J, Campisi J, Kirkland JL. Cellular senescence and the senescent secretory phenotype: therapeutic opportunities. J Clin Invest. 2013;123(3):966–72. doi: 10.1172/JCI64098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kirkland JL. Translating advances from the basic biology of aging into clinical application. Exp Gerontol. 2013;48(1):1–5. doi: 10.1016/j.exger.2012.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fries JF. Aging, natural death, and the compression of morbidity. N Engl J Med. 1980;303(3):130–5. doi: 10.1056/NEJM198007173030304. [DOI] [PubMed] [Google Scholar]
- 10.Fries JF, Koop CE, Beadle CE, Cooper PP, England MJ, Greaves RF, et al. Reducing health care costs by reducing the need and demand for medical services. N Engl J Med. 1993;329(5):321–5. doi: 10.1056/NEJM199307293290506. [DOI] [PubMed] [Google Scholar]
- 11.Butler RN, Miller RA, Perry D, Carnes BA, Williams TF, Cassel C, et al. New model of health promotion and disease prevention for the 21st century. BMJ. 2008;337:a399. doi: 10.1136/bmj.a399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller RA. Extending life: scientific prospects and political obstacles. Milbank Q. 2002;80(1):155–74. doi: 10.1111/1468-0009.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martin GM, Bergman A, Barzilai N. Genetic determinants of human health span and life span: progress and new opportunities. PLoS Genet. 2007;3(7):e125. doi: 10.1371/journal.pgen.0030125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sierra F, Hadley E, Suzman R, Hodes R. Prospects for life span extension. Annu Rev Med. 2009;60:457–69. doi: 10.1146/annurev.med.60.061607.220533. [DOI] [PubMed] [Google Scholar]
- 15.Vergara M, Smith-Wheelock M, Harper JM, Sigler R, Miller RA. Hormone-treated snell dwarf mice regain fertility but remain long lived and disease resistant. J Gerontol A Biol Sci Med Sci. 2004;59(12):1244–50. doi: 10.1093/gerona/59.12.1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lipton RB, Hirsch J, Katz MJ, Wang C, Sanders AE, Verghese J, et al. Exceptional parental longevity associated with lower risk of Alzheimer's disease and memory decline. J Am Geriatr Soc. 2010;58(6):1043–9. doi: 10.1111/j.1532-5415.2010.02868.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sebastiani P, Perls TT. The genetics of extreme longevity: lessons from the New England Centenarian Study. Front Genet. 2012;3:277. doi: 10.3389/fgene.2012.00277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tatar M, Bartke A, Antebi A. The endocrine regulation of aging by insulin-like signals. Science. 2003;299(5611):1346–51. doi: 10.1126/science.1081447. [DOI] [PubMed] [Google Scholar]
- 19.Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460(7253):392–5. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lakdawalla DN, Eber MR, Forma FM, Sullivan J, Michaud PC, Bradley LA, et al. Measuring the value of better diabetes management. Am J Manag Care. 2013;19:E11. (Special No 2) [PubMed] [Google Scholar]
- 21.Goldman DP, Zheng Y, Girosi F, Michaud PC, Olshansky SJ, Cutler D, et al. The benefits of risk factor prevention in Americans aged 51 years and older. Am J Public Health. 2009;99(11):2096–101. doi: 10.2105/AJPH.2009.172627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goldman DP, Shang B, Bhattacharya J, Garber AM, Hurd M, Joyce GF, et al. Consequences of health trends and medical innovation for the future elderly. Health Aff (Millwood) 2005;24:w5–r5–17. doi: 10.1377/hlthaff.w5.r5. DOI: 10.1377/hlthaff.w5.r5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shekelle PG, Ortiz E, Newberry SJ, Rich MW, Rhodes SL, Brook RH, et al. Identifying potential health care innovations for the future elderly. Health Aff (Millwood) 2005;242:w5–r67–76. doi: 10.1377/hlthaff.w5.r67. DOI: 10.1377/hlthaff.w5.r67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.To access the Appendix, click on the Appendix link in the box to the right of the article online.
- 25.Wallace RB, Herzog AR. Overview of the health measures in the Health and Retirement Study. J Hum Resour. 1995;30(Suppl):S84–107. [Google Scholar]
- 26.Gold M, Siegel J, Russell L, Weinstein M, editors. Cost-effectiveness in health and medicine. Oxford University Press; New York (NY): 1996. [Google Scholar]
- 27.Olshansky SJ, Goldman DP, Zheng Y, Rowe JW. Aging in America in the twenty-first century: demographic forecasts from the MacArthur Foundation Research Network on an Aging Society. Milbank Q. 2009;87(4):842–62. doi: 10.1111/j.1468-0009.2009.00581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Board of Trustees of the Federal Old-Age and Survivors Insurance and Federal Disability Insurance Trust Funds. 2011 annual report [Internet] Government Printing Office; Washington (DC): May 13, 2011. [2013 Aug 27]. Available from: www.socialsecurity.gov/oact/TR/2011/tr2011.pdf. [Google Scholar]
- 29.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60(5):277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 30.Kubo M, Kiyohara Y, Kato I, Tanizaki Y, Arima H, Tanaka K, et al. Trends in the incidence, mortality, and survival rate of cardiovascular disease in a Japanese community: the Hisayama study. Stroke. 2003;34(10):2349–54. doi: 10.1161/01.STR.0000090348.52943.A2. [DOI] [PubMed] [Google Scholar]
- 31.De Magalhães JP, Cabral JA, Magalhães D. The influence of genes on the aging process of mice: a statistical assessment of the genetics of aging. Genetics. 2005;169(1):265–74. doi: 10.1534/genetics.104.032292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Edlin AS, Stiglitz JE, editors. The economists’ voice 2.0: the financial crisis, health care reform, and more. Columbia University Press; New York (NY): 2012. [Google Scholar]
- 33.Manton KG, Corder L, Stallard E. Chronic disability trends in elderly United States populations: 1982-1994. Proc Natl Acad U S A. 1997;94(6):2593–8. doi: 10.1073/pnas.94.6.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Freedman VA, Crimmins E, Schoeni RF, Spillman BC, Aykan H, Kramarow E, et al. Resolving inconsistencies in trends in old-age disability: report from a technical working group. Demography. 2004;41(3):417–41. doi: 10.1353/dem.2004.0022. [DOI] [PubMed] [Google Scholar]
- 35.Ketcham JD, Simon KI. Medicare Part D's effects on elderly patients’ drug costs and utilization. Am J Manag Care. 2008;14(11 Suppl):SP14–22. [PubMed] [Google Scholar]
- 36.Kindig DA, Cheng ER. Even as mortality fell in most US counties, female mortality nonetheless rose in 42.8 percent of counties from 1992 to 2006. Health Aff (Millwood) 2013;32(3):451–8. doi: 10.1377/hlthaff.2011.0892. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.