Abstract
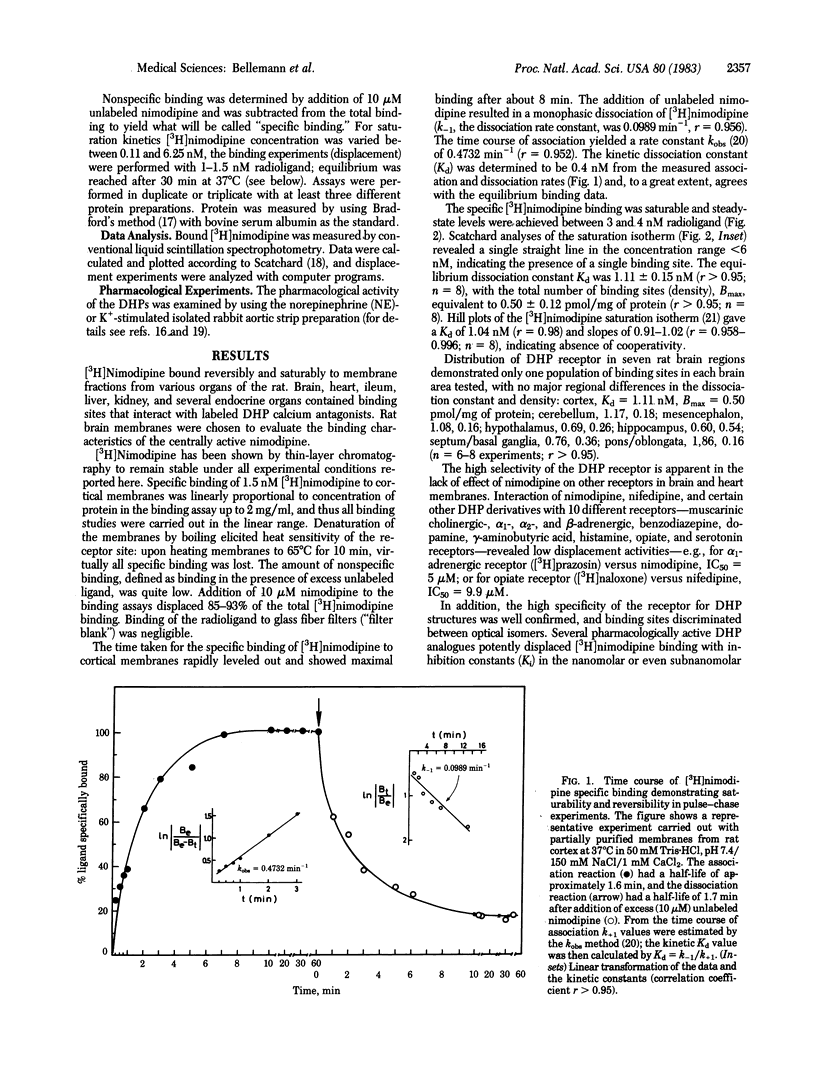
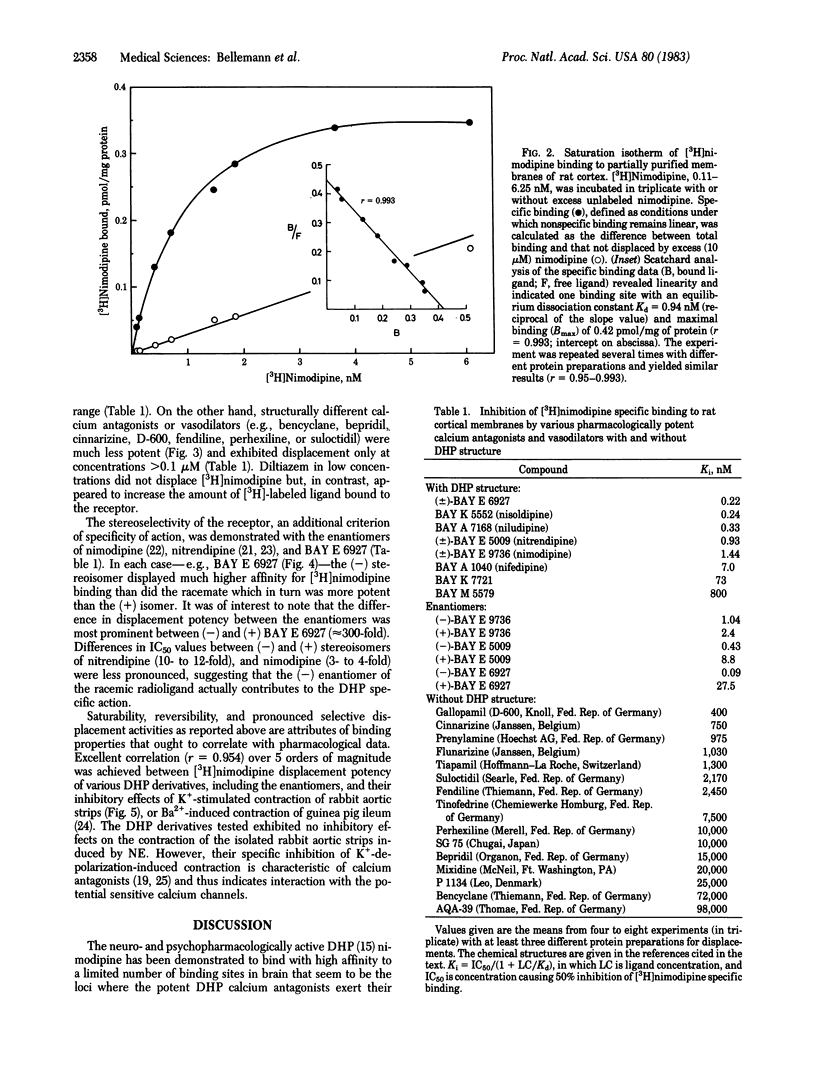
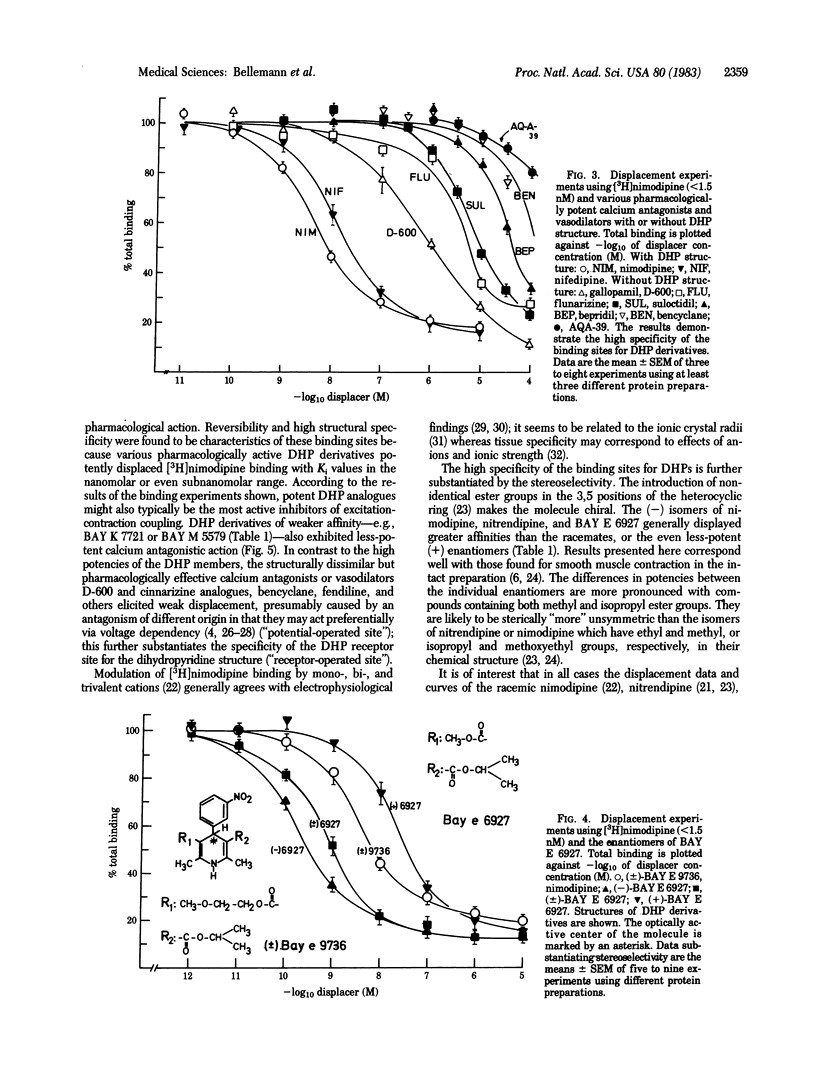
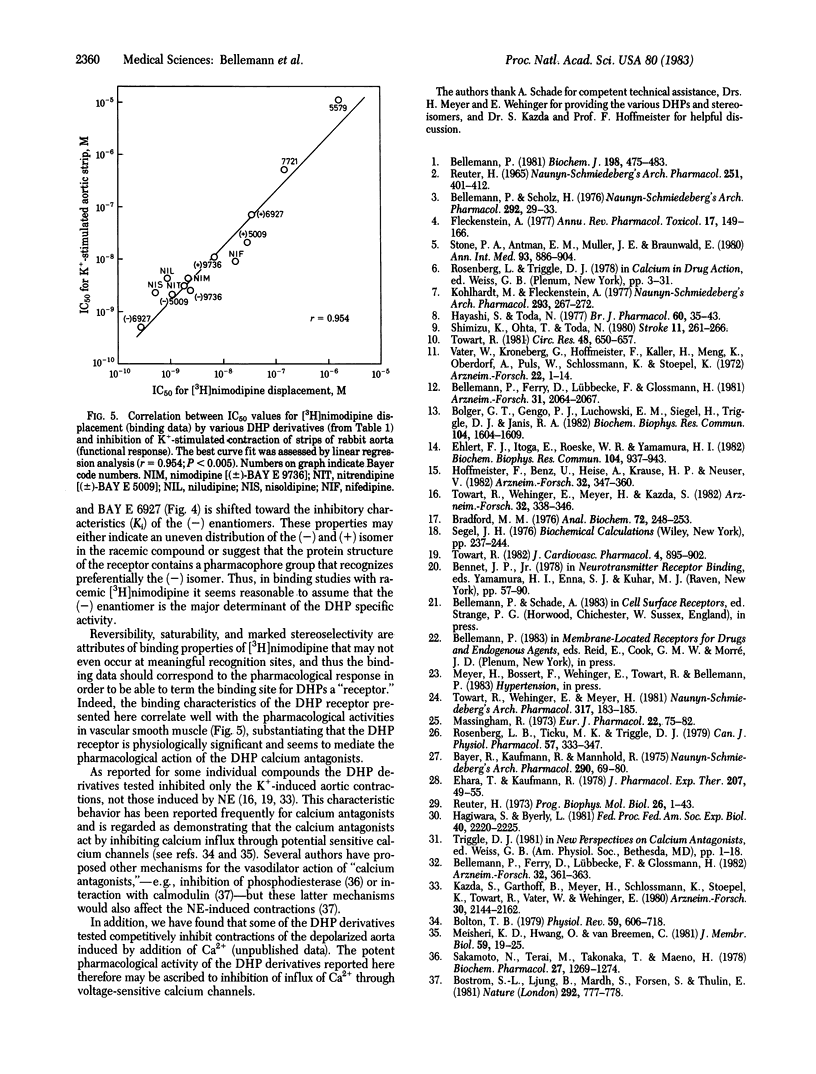
Receptor binding sites for 1,4-dihydropyridine (DHP) calcium antagonists have been characterized by using [3H]-nimodipine, a potent analogue of nifedipine with cerebrovascular and neuro- and psychopharmacological properties. [3H]Nimodipine exhibited reversible and saturable binding to partially purified brain membranes. The equilibrium dissociation constant, Kd, was 1.11 nM and the maximal binding capacity, Bmax, was 0.50 pmol/mg of protein. The DHP receptor proved to be highly specific for various potently displacing DHP derivatives and discriminated between their optical isomers (stereoselectivity) with inhibition constants (Ki) in the nanomolar or even subnanomolar range. Structurally different calcium antagonists such as gallopamil (D-600), on the other hand, displayed much lower affinities, further substantiating the specificity of the receptor for DHP structures. Furthermore, the displacement potency of a series of DHP derivatives correlated well with that determined for inhibition of mechanical response in the intact smooth muscle over 5 orders of magnitude. [3H]Nimodipine binding thus may provide a molecular probe to elucidate the nature of the interaction of calcium entry blockers with specific membrane-located receptor sites that may be associated with the putative calcium channel. These receptor sites might well represent the loci of signaling events where the potent DHPs exert their pharmacological action.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bayer R., Kaufmann R., Mannhold R. Inotropic and electrophysiological actions of verapamil and D 600 in mammalian myocardium. II. Pattern of inotropic effects of the optical isomers. Naunyn Schmiedebergs Arch Pharmacol. 1975;290(1):69–80. doi: 10.1007/BF00499990. [DOI] [PubMed] [Google Scholar]
- Bellemann P. Amino acid transport and rubidium-ion uptake in monolayer cultures of hepatocytes from neonatal rats. Biochem J. 1981 Sep 15;198(3):475–483. doi: 10.1042/bj1980475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellemann P., Ferry D., Lübbecke F., Glossman H. [3H]-Nitrendipine, a potent calcium antagonist, binds with high affinity to cardiac membranes. Arzneimittelforschung. 1981;31(12):2064–2067. [PubMed] [Google Scholar]
- Bellemann P., Ferry D., Lübbecke F., Glossmann H. [3H]-Nimodipine and [3H]-nitrendipine as tools to directly identify the sites of action of 1,4-dihydropyridine calcium antagonists in guinea-pig tissues. Tissue-specific effects of anions and ionic strength. Arzneimittelforschung. 1982;32(4):361–363. [PubMed] [Google Scholar]
- Bellemann P., Scholz H. Effect of theophylline on calcium exchangeability in ventricular myocardium. Naunyn Schmiedebergs Arch Pharmacol. 1976;292(1):29–33. doi: 10.1007/BF00506486. [DOI] [PubMed] [Google Scholar]
- Bolger G. T., Gengo P. J., Luchowski E. M., Siegel H., Triggle D. J., Janis R. A. High affinity binding of a calcium channel antagonist to smooth and cardiac muscle. Biochem Biophys Res Commun. 1982 Feb 26;104(4):1604–1609. doi: 10.1016/0006-291x(82)91436-x. [DOI] [PubMed] [Google Scholar]
- Bolton T. B. Mechanisms of action of transmitters and other substances on smooth muscle. Physiol Rev. 1979 Jul;59(3):606–718. doi: 10.1152/physrev.1979.59.3.606. [DOI] [PubMed] [Google Scholar]
- Bostróm S. L., Ljung B., Mårdh S., Forsen S., Thulin E. Interaction of the antihypertensive drug felodipine with calmodulin. Nature. 1981 Aug 20;292(5825):777–778. doi: 10.1038/292777a0. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Ehara T., Daufmann R. The voltage- and time-dependent effects of (-)-verapamil on the slow inward current in isolated cat ventricular myocardium. J Pharmacol Exp Ther. 1978 Oct;207(1):49–55. [PubMed] [Google Scholar]
- Ehlert F. J., Itoga E., Roeske W. R., Yamamura H. I. The interaction of [3H]nitrendipine with receptors for calcium antagonists in the cerebral cortex and heart of rats. Biochem Biophys Res Commun. 1982 Feb 11;104(3):937–943. doi: 10.1016/0006-291x(82)91339-0. [DOI] [PubMed] [Google Scholar]
- Fleckenstein A. Specific pharmacology of calcium in myocardium, cardiac pacemakers, and vascular smooth muscle. Annu Rev Pharmacol Toxicol. 1977;17:149–166. doi: 10.1146/annurev.pa.17.040177.001053. [DOI] [PubMed] [Google Scholar]
- Hagiwara S., Byerly L. Membrane biophysics of calcium currents. Fed Proc. 1981 Jun;40(8):2220–2225. [PubMed] [Google Scholar]
- Hayashi S., Toda N. Inhibition by Cd2+ verapamil and papaverine of Ca2+-induced contractions in isolated cerebral and peripheral arteries of the dog. Br J Pharmacol. 1977 May;60(1):35–43. doi: 10.1111/j.1476-5381.1977.tb16744.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmeister F., Benz U., Heise A., Krause H. P., Neuser V. Behavioral effects of nimodipine in animals. Arzneimittelforschung. 1982;32(4):347–360. [PubMed] [Google Scholar]
- Kazda S., Garthoff B., Meyer H., Schlossmann K., Stoepel K., Towart R., Vater W., Wehinger E. Pharmacology of a new calcium antagonistic compound, isobutyl methyl 1,4-dihydro-2,6-dimethyl-4(2-nitrophenyl)-3,5-pyridinedicarboxylate (Nisoldipine, Bay k 5552). Arzneimittelforschung. 1980;30(12):2144–2162. [PubMed] [Google Scholar]
- Kohlhardt M., Fleckenstein A. Inhibition of the slow inward current by nifedipine in mammalian ventricular myocardium. Naunyn Schmiedebergs Arch Pharmacol. 1977 Jul;298(3):267–272. doi: 10.1007/BF00500899. [DOI] [PubMed] [Google Scholar]
- Massingham R. A study of compounds which inhibit vascular smooth muscle contraction. Eur J Pharmacol. 1973 Apr;22(1):75–82. doi: 10.1016/0014-2999(73)90186-6. [DOI] [PubMed] [Google Scholar]
- Meisheri K. D., Hwang O., van Breemen C. Evidence for two separated Ca2+ pathways in smooth muscle plasmalemma. J Membr Biol. 1981 Mar 15;59(1):19–25. doi: 10.1007/BF01870817. [DOI] [PubMed] [Google Scholar]
- Reuter H. Divalent cations as charge carriers in excitable membranes. Prog Biophys Mol Biol. 1973;26:1–43. doi: 10.1016/0079-6107(73)90016-3. [DOI] [PubMed] [Google Scholar]
- Reuter H. Uber die Wirkung von Adrenalin auf den cellulären Ca-Umsatz des Meerschweinchenvorhofs. Naunyn Schmiedebergs Arch Exp Pathol Pharmakol. 1965 Aug 20;251(4):401–412. [PubMed] [Google Scholar]
- Rosenberger L. B., Ticku M. K., Triggle D. J. The effect of Ca2+ antagonists on mechanical responses and Ca2+ movements in guinea pig ileal longitudinal smooth muscle. Can J Physiol Pharmacol. 1979 Apr;57(4):333–347. doi: 10.1139/y79-052. [DOI] [PubMed] [Google Scholar]
- Sakamoto N., Terai M., Takenaka T., Maeno H. Inhibition of cyclic AMP phosphodiesterase by 2,6-dimethyl-4-(3-nitrophenyl)-1,4-dihydropyridine-3,5-dicarboxylic acid 3-[2-(N-benzyl-N-methylamino)] ethyl ester 5-methyl ester hydrochloride (YC-93), a potent vasodilator. Biochem Pharmacol. 1978;27(8):1269–1274. doi: 10.1016/0006-2952(78)90462-8. [DOI] [PubMed] [Google Scholar]
- Shimizu K., Ohta T., Toda N. Evidence for greater susceptibility of isolated dog cerebral arteries to Ca antagonists than peripheral arteries. Stroke. 1980 May-Jun;11(3):261–266. doi: 10.1161/01.str.11.3.261. [DOI] [PubMed] [Google Scholar]
- Stone P. H., Antman E. M., Muller J. E., Braunwald E. Calcium channel blocking agents in the treatment of cardiovascular disorders. Part II: Hemodynamic effects and clinical applications. Ann Intern Med. 1980 Dec;93(6):886–904. doi: 10.7326/0003-4819-93-6-886. [DOI] [PubMed] [Google Scholar]
- Towart R. Effects of nitrendipine (BAY e 5009), nifedipine, verapamil, phentolamine, papaverine, and minoxidil on contractions of isolated rabbit aortic smooth muscle. J Cardiovasc Pharmacol. 1982 Nov-Dec;4(6):895–902. [PubMed] [Google Scholar]
- Towart R. The selective inhibition of serotonin-induced contractions of rabbit cerebral vascular smooth muscle by calcium-antagonistic dihydropyridines. An investigation of the mechanism of action of nimodipine. Circ Res. 1981 May;48(5):650–657. doi: 10.1161/01.res.48.5.650. [DOI] [PubMed] [Google Scholar]
- Towart R., Wehinger E., Meyer H. Effects of unsymmetrical ester substituted 1,4-dihydropyridine derivatives and their optical isomers on contraction of smooth muscle. Naunyn Schmiedebergs Arch Pharmacol. 1981 Sep;317(2):183–185. doi: 10.1007/BF00500079. [DOI] [PubMed] [Google Scholar]
- Towart R., Wehinger E., Meyer H., Kazda S. The effects of nimodipine, its optical isomers and metabolites on isolated vascular smooth muscle. Arzneimittelforschung. 1982;32(4):338–346. [PubMed] [Google Scholar]
- Vater W., Kroneberg G., Hoffmeister F., Saller H., Meng K., Oberdorf A., Puls W., Schlossmann K., Stoepel K. Zur Pharmakologie von 4-(2'-Nitrophenyl)-2,6-dimethyl-1,4-dihydropyridin-3,5-dicarbonsäuredimethylester (Nifedipine), BAY a 1040. Arzneimittelforschung. 1972 Jan;22(1):1–14. [PubMed] [Google Scholar]


