Abstract
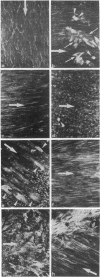
The distribution of actin stress fibers in normal and regenerating (after endothelial denudation by means of a balloon catheter) rabbit aortic endothelial cells has been studied by means of immunofluorescence with human actin autoantibodies on en face endothelial cell preparations. Our results show that: (i) under normal conditions actin is accumulated as a network at the periphery of endothelial cells. Stress fibers are present only in endothelial cells located immediately below intercostal artery branches; (ii) stress fibers develop in endothelial cells early during regeneration and persist after the end of endothelial mitotic and motile activities; and (iii) the orientation of stress fibers within the cytoplasm follows the direction of blood flow, with the exception of stress fibers situated in cells at the edge of the wound, when endothelial cell progression toward the denuded area as well as mitotic activity have ceased. We conclude that stress fibers are an organelle present in endothelial cells in vivo and that they reorganize during endothelial cell adaptation to unfavorable or pathological situations.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ausprunk D. H., Berman H. J. Spreading of vascular endothelial cells in culture: spatial reorganization of cytoplasmic fibers and organelles. Tissue Cell. 1978;10(4):707–724. doi: 10.1016/0040-8166(78)90057-5. [DOI] [PubMed] [Google Scholar]
- Baumgartner H. R., Studer A. Folgen des Gefässkatheterismus am normo- und hypercholesterinaemischen Kaninchen. Pathol Microbiol (Basel) 1966;29(4):393–405. [PubMed] [Google Scholar]
- Burridge K. Are stress fibres contractile? Nature. 1981 Dec 24;294(5843):691–692. doi: 10.1038/294691a0. [DOI] [PubMed] [Google Scholar]
- Byers H. R., Fujiwara K. Stress fibers in cells in situ: immunofluorescence visualization with antiactin, antimyosin, and anti-alpha-actinin. J Cell Biol. 1982 Jun;93(3):804–811. doi: 10.1083/jcb.93.3.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clowes A. W., Collazzo R. E., Karnovsky M. J. A morphologic and permeability study of luminal smooth muscle cells after arterial injury in the rat. Lab Invest. 1978 Aug;39(2):141–150. [PubMed] [Google Scholar]
- Fry D. L. Acute vascular endothelial changes associated with increased blood velocity gradients. Circ Res. 1968 Feb;22(2):165–197. doi: 10.1161/01.res.22.2.165. [DOI] [PubMed] [Google Scholar]
- Fujiwara K., Pollard T. D. Fluorescent antibody localization of myosin in the cytoplasm, cleavage furrow, and mitotic spindle of human cells. J Cell Biol. 1976 Dec;71(3):848–875. doi: 10.1083/jcb.71.3.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbiani G., Badonnel M. C., Rona G. Cytoplasmic contractile apparatus in aortic endothelial cells of hypertensive rats. Lab Invest. 1975 Feb;32(2):227–234. [PubMed] [Google Scholar]
- Gabbiani G., Chaponnier C., Zumbe A., Vassalli P. Actin and tubulin co-cap with surface immunoglobulins in mouse B lymphocytes. Nature. 1977 Oct 20;269(5630):697–698. doi: 10.1038/269697a0. [DOI] [PubMed] [Google Scholar]
- Gabbiani G., Elemer G., Guelpa C., Vallotton M. B., Badonnel M. C., Hüttner I. Morphologic and functional changes of the aortic intima during experimental hypertension. Am J Pathol. 1979 Aug;96(2):399–422. [PMC free article] [PubMed] [Google Scholar]
- Harris A. K., Stopak D., Wild P. Fibroblast traction as a mechanism for collagen morphogenesis. Nature. 1981 Mar 19;290(5803):249–251. doi: 10.1038/290249a0. [DOI] [PubMed] [Google Scholar]
- Herman I. M., Crisona N. J., Pollard T. D. Relation between cell activity and the distribution of cytoplasmic actin and myosin. J Cell Biol. 1981 Jul;90(1):84–91. doi: 10.1083/jcb.90.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isenberg G., Rathke P. C., Hülsmann N., Franke W. W., Wohlfarth-Bottermann K. E. Cytoplasmic actomyosin fibrils in tissue culture cells: direct proof of contractility by visualization of ATP-induced contraction in fibrils isolated by laser micro-beam dissection. Cell Tissue Res. 1976 Feb 27;166(4):427–443. doi: 10.1007/BF00225909. [DOI] [PubMed] [Google Scholar]
- Korn E. D. Actin polymerization and its regulation by proteins from nonmuscle cells. Physiol Rev. 1982 Apr;62(2):672–737. doi: 10.1152/physrev.1982.62.2.672. [DOI] [PubMed] [Google Scholar]
- Kreis T. E., Birchmeier W. Stress fiber sarcomeres of fibroblasts are contractile. Cell. 1980 Nov;22(2 Pt 2):555–561. doi: 10.1016/0092-8674(80)90365-7. [DOI] [PubMed] [Google Scholar]
- Lazarides E., Burridge K. Alpha-actinin: immunofluorescent localization of a muscle structural protein in nonmuscle cells. Cell. 1975 Nov;6(3):289–298. doi: 10.1016/0092-8674(75)90180-4. [DOI] [PubMed] [Google Scholar]
- Lazarides E. Tropomyosin antibody: the specific localization of tropomyosin in nonmuscle cells. J Cell Biol. 1975 Jun;65(3):549–561. doi: 10.1083/jcb.65.3.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarides E., Weber K. Actin antibody: the specific visualization of actin filaments in non-muscle cells. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2268–2272. doi: 10.1073/pnas.71.6.2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reidy M. A., Schwartz S. M. En face morphology of endothelial junctions. J Ultrastruct Res. 1981 Jun;75(3):363–367. doi: 10.1016/s0022-5320(81)80092-5. [DOI] [PubMed] [Google Scholar]
- Reidy M. A., Schwartz S. M. Endothelial regeneration. III. Time course of intimal changes after small defined injury to rat aortic endothelium. Lab Invest. 1981 Apr;44(4):301–308. [PubMed] [Google Scholar]
- Reidy M. A., Standaert D., Schwartz S. M. Inhibition of endothelial cell regrowth. Cessation of aortic endothelial cell replication after balloon catheter denudation. Arteriosclerosis. 1982 May-Jun;2(3):216–220. doi: 10.1161/01.atv.2.3.216. [DOI] [PubMed] [Google Scholar]
- Schwartz S. M., Benditt E. P. Clustering of replicating cells in aortic endothelium. Proc Natl Acad Sci U S A. 1976 Feb;73(2):651–653. doi: 10.1073/pnas.73.2.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz S. M., Gajdusek C. M., Reidy M. A., Selden S. C., 3rd, Haudenschild C. C. Maintenance of integrity in aortic endothelium. Fed Proc. 1980 Jul;39(9):2618–2625. [PubMed] [Google Scholar]
- Schwartz S. M., Stemerman M. B., Benditt E. P. The aortic intima. II. Repair of the aortic lining after mechanical denudation. Am J Pathol. 1975 Oct;81(1):15–42. [PMC free article] [PubMed] [Google Scholar]
- Selden S. C., 3rd, Rabinovitch P. S., Schwartz S. M. Effects of cytoskeletal disrupting agents on replication of bovine endothelium. J Cell Physiol. 1981 Aug;108(2):195–211. doi: 10.1002/jcp.1041080210. [DOI] [PubMed] [Google Scholar]
- Willingham M. C., Yamada K. M., Yamada S. S., Pouysségur J., Pastan I. Microfilament bundles and cell shape are related to adhesiveness to substratum and are dissociable from growth control in cultured fibroblasts. Cell. 1977 Mar;10(3):375–380. doi: 10.1016/0092-8674(77)90024-1. [DOI] [PubMed] [Google Scholar]