Abstract
An evolutionary perspective suggests that iron deficiency may have opposing effects on infectious disease risk, decreasing susceptibility by restricting iron availability to pathogens, and increasing susceptibility by compromising cellular immunocompetence. In some environments, the trade-off between these effects may result in optimal iron intake that is inadequate to fully meet body iron needs. Thus, it has been suggested that moderate iron deficiency may protect against acute infection, and may represent a nutritional adaptation to endemic infectious disease stress. To test this assertion, we examined the association between infection, reflected by C-reactive protein, a biomarker of inflammation, and iron status, reflected by transferrin receptor (TfR) and zinc protoporphyrin to heme ratio (ZPP:H), among school-age Kenyan children, and evaluated the hypothesis that moderate iron deficiency is associated with lower odds of infectious disease. TfR > 5.0 mg/l, with sensitivity and specificity for iron deficiency (ZPP:H > 80 μmol/mol) of 0.807 and 0.815, was selected as the TfR definition of iron deficiency. Controlling for age and triceps skinfold thickness (TSF), the odds ratio (OR) for acute viral or bacterial infection associated with iron deficiency (compared to normal/replete) was 0.50 (P = 0.11). Controlling for age and TSF, the OR for infection associated with an unequivocally iron replete state (compared to all others) was 2.9 (P = 0.01). We conclude that iron deficiency may protect against acute infection in children.
The field of evolutionary medicine has drawn attention to the fact that certain manifestations of infectious disease are not pathological, but rather adaptive defenses against infection (Ewald, 1994; Williams and Nesse, 1991). Just as fever has been identified as a subtle and well-regulated nonspecific response to infection, so, too, has the iron withholding response. In 1932, it was noted that patients experiencing infection had depressed plasma iron concentrations (hypoferremia) (Locke et al., 1932). It is now known that this hypoferremia is an important aspect of the nonspecific immune response: microbial invasion stimulates an “iron withholding” defense, in which acute phase reactants sequester circulating iron and decrease iron absorption, restricting the availability of growth essential iron to pathogens and inhibiting pathogen proliferation (Kent et al., 1994; Kluger and Rothenberg, 1979; Nemeth and Ganz, 2006; Weinberg, 1984).
Evolutionary medicine has further emphasized that pathogens, with their rapid rate of reproduction in comparison to their host, are under intense selective pressure to overcome host defenses. Unsurprisingly, bacterial and eukaryotic pathogens have evolved a myriad of mechanisms to extract iron from the host in the presence of iron withholding (reviewed by Schaible and Kaufmann, 2004; Weinberg, 1999). For this reason, low dietary intake of iron may be a particularly effective method of depriving pathogens of iron and mitigating infectious disease stress. Nutritional anthropologists have long recognized that dietary choice provides an avenue not only for meeting basic nutritional needs, but also for adapting to local disease ecology, as in the case of malarial protection from cyanide-rich cassava (Jackson, 1990; Katz, 1987). Similarly, dietary iron deficiency may represent a nutritional adaptation to infectious disease (Denic and Agarwal, 2007; Scrimshaw and SanGiovanni, 1997; Strauss, 1978).
DEFINING OPTIMAL BODY IRON: AN ECOLOGICAL APPROACH
Despite the enormous body of literature devoted to the assessment and treatment of iron deficiency, little attention has been given to understanding and critically evaluating the concept of “optimal” iron status. Clinicians often define optimal iron status as the absence of any functional impairment, which is usually not evident short of iron deficiency anemia (IDA); others define optimal iron status as adequate cellular supply and body stores of iron (Cook, 1999).
An ecological approach defines optimal iron status in a particular environmental context. Iron intake that meets or exceeds total body iron needs and allows the accumulation of iron stores can also support high levels of pathogen proliferation and may be dangerous in the face of frequent infectious disease. Laboratory studies have demonstrated that the progressive development of hypoferremia with restricted iron intake can inhibit microbial proliferation and protect against infectious disease (Kluger and Bullen, 1987; Kochan, 1973). Thus, in environments with high levels of endemic infectious disease, restricted iron intake may protect against infection, and inadequate intake may be optimal.
An ecological approach further considers both the costs and benefits of iron intake in defining optimal iron status. Of greatest relevance to infectious disease stress, iron deficiency can increase susceptibility to infection by compromising immune function. Although antibody-mediated immunity is largely unaffected by iron status, cell-mediated immunity (CMI) is impaired by iron deficiency (Chandra and Newberne, 1977; Field et al., 2002; Joynson et al., 1972). The extent of deficiency necessary to impact CMI is unclear: some argue that even mild and moderate levels of iron deficiency cause some CMI deficit (Joynson et al., 1972; Thibault et al., 1993), while others question the existence and importance of such an effect short of anemia (Field et al., 2002; Oppenheimer, 2001).
These opposing effects of iron deficiency suggest that a trade-off in infectious disease susceptibility exists between hypoferremia-related resistance to infection and compromised cellular immunocompetence. If this is the case, optimal iron levels for resisting infectious disease will represent a compromise between these two effects of iron deficiency on susceptibility, and will depend on the local disease ecology. Where infectious disease morbidity and transmission are substantial, optimal iron intake for avoiding infection may fall short of meeting the body’s total iron needs.
Population-based evidence supporting this hypothesis comes from iron supplementation trials. Recent systematic reviews of randomized, controlled trials of iron supplementation conclude that supplementation moderately increases the risk of malaria (significantly in five of nine trials conducted in malarious areas, with risk estimates ranging from 1.4 to 15; Oppenheimer, 2001) and diarrheal disease (incidence rate ratio upon meta-analysis = 1.11, 95% CI = 1.01, 1.23, P = 0.04; Gera and Sachdev, 2002). A recent iron supplementation trial on Pemba Island (Zanzibar, Tanzania), was terminated early due to excess hospitalization and mortality, largely attributable to malaria, in the groups receiving iron supplement (Sazawal et al., 2006). Additionally, a recent study conducted in the absence of iron supplementation found lower malaria risk among iron-deficient children in coastal Kenya (Nyakeriga et al., 2004). These studies constitute strong evidence that some degree of iron deficiency is protective against infection with the malaria parasite.
It is also important to note the heterogeneity in the results of iron supplementation trials: some report no effect of iron supplementation on infectious disease outcomes, some increased risk with supplementation, and some protective effects of supplementation (Gera and Sachdev, 2002; Oppenheimer, 2001). This heterogeneity may be a consequence of the varied infectious disease environments in which supplementation trials have been conducted (Oppenheimer, 2001), suggesting that iron supplementation programs may benefit from an ecological approach to defining optimal iron status.
POPULATION-BASED ASSESSMENT OF IRON STATUS
Testing the hypothesis that iron deficiency can protect against infectious disease requires the use of sensitive biomarkers of iron status that identify iron deficiency across the range of mild to severe deficiency, and that differentiate anemia caused by dietary iron deficiency from other causes of anemia. Iron deficiency progresses through three stages, from an iron replete state to iron deficiency severe enough to cause anemia (Table 1). The first stage is characterized by depletion of iron stores, as they are mobilized to meet basic metabolic needs when iron intake is inadequate or iron loss increases. The exhaustion of iron stores is followed by the onset of actual iron deficiency, when physiological manifestations of iron stress become perceivable. This second stage is characterized by iron-deficient erythropoiesis (IDE), when biochemical evidence of iron deficiency, especially in bone marrow tissue, becomes evident, but the deficiency is not manifest in reductions in hemoglobin concentrations. In the third stage, IDA, iron delivery to the bone marrow is inadequate to support sufficient hemoglobin synthesis and both biochemical evidence of iron deficiency and reduced hemoglobin concentrations (anemia) are evident.
TABLE 1.
Progressive development of iron deficiency anemia
| Iron deficiency stage | Description | Biomarker |
|---|---|---|
| Stage 1: Iron depletion | Iron stores are mobilized when iron intake is inadequate to meet body iron needs | |
| Stage 2: Iron deficient erythropoiesis (IDE) | Iron delivery to tissues is restricted and evidence of iron stress is apparent | ↑ ZPP:H ↑ TfR |
| Stage 3: Iron deficiency anemia (IDA) | Reduced iron delivery to bone marrow is manifest in inadequate hemoglobin synthesis | ↑ ZPP:H ↑ TfR ↓ Hb |
ZPP:H, zinc protoporphyrin to heme ratio; TfR, transferrin receptor; Hb, hemoglobin.
Assessment of iron status is complicated by the fact that dietary iron deficiency is only one of many conditions that can result in anemia. Others include malaria and malaria-protective hemoglobinopathies, which destroy red blood cells; schistosomiasis and hookworm infection, which cause blood loss; and chronic inflammatory disease and cancer malignancy, which can induce long-term iron sequestration. Anemia that is attributable to chronic activation of the iron withholding response is referred to as anemia of chronic disease (ACD).
Many of the above causes of anemia can also artificially alter some iron status indicators, mimicking IDA. For this reason, no single biomarker is sufficient to characterize individuals’ iron status, as each available indicator is vulnerable to one or more sources of misclassification. As a result, a suite of laboratory measures are typically employed (Cook et al., 1992; Worwood, 1997). A multiple criteria model for assessing iron status from capillary blood has been developed by Shell-Duncan and McDade (2004) using three biomarkers: the zinc protoporphyrin to heme ratio (ZPP:H) and transferrin receptor (TfR) to identify iron deficiency, including preanemic IDE, and hemoglobin (Hb) to detect anemia. The addition of TfR to the suite of markers used to classify iron deficiency is desirable because, unlike most other iron status biomarkers, TfR is not altered in the presence of inflammation (Harthoorn-Lasthuizen et al., 2000; Labbé and Dewanji, 2004; but see Zimmerman et al., 2005). However, assessment of TfR is characterized by lack of comparability due to differences in assay standard, measurement units, and reference range (Ahluwalia, 1998); there is no single, widely accepted TfR cut off for identifying iron deficiency.
These biomarkers have been used to investigate the iron status of children (5–10 years of age) in northern Kenya (Shell-Duncan and McDade, 2004). Among the children sampled, iron deficiency and anemia were quite common, while malaria and urinary schistosomiasis were rare and unimportant as sources of anemia (Shell-Duncan and McDade, 2004). Markers of iron deficiency (TfR and ZPP:H) demonstrated a strong and significant negative correlation with hemoglobin, and dietary recall data revealed diets low in bioavailable iron, strongly suggesting that dietary iron deficiency was the primary cause of anemia among these children (Shell-Duncan and McDade, 2004, 2005). The absence of confounding infectious causes of anemia (malaria and urinary schistosomiasis) and the presence of substantial morbidity due to acute infectious disease render these data ideal for evaluating the association between dietary iron intake and infection. To do this, we (1) used ZPP:H to assess TfR’s sensitivity and specificity and select a cut off for identifying iron deficiency, (2) used combined markers of iron status to estimate the prevalence of moderate and severe iron deficiency among the children sampled, and (3) tested the hypothesis that moderate iron deficiency is protective against acute viral and bacterial infection.
MATERIALS AND METHODS
Setting and subjects
This study was conducted in July 1999, in Marsabit District in northern Kenya, Kenya’s largest and least-populated district. Data were collected from a cross-section of children 5–10 years of age among the Rendille, in two villages of settled, formerly nomadic pastoralists. Data collection was carried out in Korr, a lowland desert community, and Karare, a highland community. Following the construction of community maps and a complete census of the 5- to 10-year-old population in each village, 300 children were selected in a 30-strata sampling design, with each stratum being a menyatta (a household compound of extended family members).
Data collection
Interviews were conducted with each child’s primary caregiver. Caregivers were asked to describe their relative socioeconomic status (SES) using binary categories (poor or not). Children’s ages were determined by reports from primary caretakers using a local event history calendar, and by the dates of birth recorded on clinic cards. Discrepancies were resolved by relative ranking with other children in the community. Children’s heights were measured to the nearest mm with an anthropometer while standing on a level platform; weights were measured to the nearest 100 g with a digital scale. Mid-upper arm circumference was measured to the nearest mm with a plastic insertion tape. Triceps skinfold thickness (TSF) was measured with calibrated calipers. Sterile, disposable lancets were used to collect free-flowing capillary blood by methods previously described (Shell-Duncan and McDade, 2004). Briefly, capillary blood collected with a disposable cuvette was immediately inserted into a HemoCue B-Hemoglobin system (HemoCue, Mission Viejo, CA) and blood Hb level recorded. For laboratory analyses, capillary blood drops were also collected on glass slides for thick and thin smears, in heparinized capillary tubes, and on filter paper as dried blood spots (DBS). The presence of urinary schistosomiasis was assessed by applying urine samples to Hemastix reagent strips to identify hematuria (blood in the urine). The study protocol was reviewed and approved by the Human Subjects Division at the University of Washington and the Ethics Committee at Kenyatta Hospital in Nairobi.
Laboratory analysis
Thick and thin smears were fixed and stained with Giemsa stain and screened for malaria parasites and hemoglobinopathies at the Laboratory of Medicine at the University of Nairobi. Capillary tubes were sealed and transported to the Clinical Nutrition Laboratory at the University of Washington and analyzed for ZPP:H using a ProtoFluor-Z Hematofluorometer (Helena Laboratories, Beaumont, TX). DBS were transported to the Laboratory for Human Biology Research at Northwestern University and concentrations of TfR and C-reactive protein (CRP) were measured using enzyme immunoassay protocols previously validated for use with DBS samples (McDade and Shell-Duncan, 2002; McDade et al., 2004). CRP, an acute phase reactant and widely used biomarker of inflammation, was used to identify acute infection (Pepys and Hirschfield, 2003). In the absence of substantial obesity, injury, or malaria incidence, elevations in CRP are most likely due to viral or bacterial infection and can be used to identify both symptomatic and subclinical infection (Gendrel et al., 1999; Gillespie et al., 1991; LaMonte et al., 2002; Mimoz et al., 1998; Tchernof et al., 2002; Toikka et al., 2000; Visser et al., 1999).
Data analysis
Hemoglobin values were adjusted following World Health Organization (WHO) recommendations for all individuals of African extraction, and anemia was defined by WHO age-specific guidelines (Nestel, 2002). Recommended adjustments for altitude (Nestel, 2002) were not made for children in the highland village of Karare, as Beall et al. (2002) found such adjustments unnecessary among Ethiopian highlanders. Elevated ZPP:H, indicative of iron deficiency, was defined as ZPP:H > 80 μmol/mol after the recommendation of Rettmer et al. (1999). Sensitivity and specificity of TfR definitions of iron deficiency were assessed with ZPP:H > 80 μmol/mol as the gold standard. Infection was defined as CRP > 2 mg/l, as CRP values above this threshold have been consistently observed among children reporting recent symptoms of infectious disease (McDade et al., 2008).
The hypothesis that iron status was associated with odds of infection was tested using maximum-likelihood logistic regression to estimate odds ratios (ORs) for infection across iron status groups. As factors potentially associated with both risk of infection and iron status, age, sex, anthropometric measurements, self-reported SES, and community were evaluated as confounders and controlled as appropriate.
Significance was defined as P ≤ 0.05; “suggestive” results were defined as 0.05 < P ≤ 0.20; and confounding was defined as ≥10% change in the coefficient of the predictor of interest with the inclusion of a potential confounder. Weight for height and height for age Z-scores were calculated with ANTHRO software (CDC/WHO). Sensitivity and specificity of TfR cut offs were assessed with Microsoft Office Excel 2007 software (Microsoft Corporation). Regression analyses were conducted with StataSE 9.2 software (Statacorp, College Station, TX).
RESULTS
Data were collected for a total of 314 children. Complete information for biomarkers of iron status and infection was available for 270 children. Table 2 summarizes sample characteristics and associations with biomarkers of iron status and infection. For iron status markers, mean (± standard deviation) values were 13.0 (±1.6) g/dl Hb, 75.6 (±47.7) μmol/mol ZPP:H, and 4.9 (±2.1) mg/l TfR; there was a positive correlation between TfR and ZPP:H (r = 0.767, P < 0.01) (Shell-Duncan and McDade, 2004). The expected associations between iron status markers and age were present, as were suggestive associations with anthropometric measurements (indicators of general nutritional status). Differences between the study communities, sexes, and SES categories in iron status indicators were small and nonsignificant.
TABLE 2.
Sample characteristics and associations with markers of iron status and infection
| Variable | Mean (Range) | Correlation with Hb | Correlation with TfR | Correlation with ZPP:H | Correlation with CRP |
|---|---|---|---|---|---|
| Age | 7 y (5, 10) | 0.27a | −0.14b | −0.15b | −0.01 |
| HAZ | −1.4 (−5.6, 2.4) | 0.15b | −0.11c | −0.08d | −0.04 |
| WHZ | −1.4 (−3.2, 1.6) | −0.05 | 0.09d | 0.05 | −0.02 |
| TSF | 5.9 mm (2.4, 11) | −0.06 | 0.05 | 0.04 | 0.10d |
| Variable | Percent of sample | Differencee in Hb | Differencee in TfR | Differencee in ZPP:H | Differencee in CRP |
|---|---|---|---|---|---|
| Sex | 52% male | 0.03 | 0.13 | 4.1 | 0.23 |
| Village | 50% in each | 0.18d | 0.32d | 9.6c | 0.32d |
| SES | 57% “poor” | 0.24c | 0.11 | 2.9 | 0.84 |
P < 0.001;
P < 0.05;
P < 0.10;
P < 0.20.
Absolute value of the difference in means between two groups.
HAZ, height for age Z-score; WHZ, weight for height Z-score; TSF, triceps skin fold thickness; SES, socioeconomic status; TfR, transferrin receptor; ZPP:H, zinc protoporphyrin to heme ratio; Hb, hemoglobin; CRP, C-reactive protein.
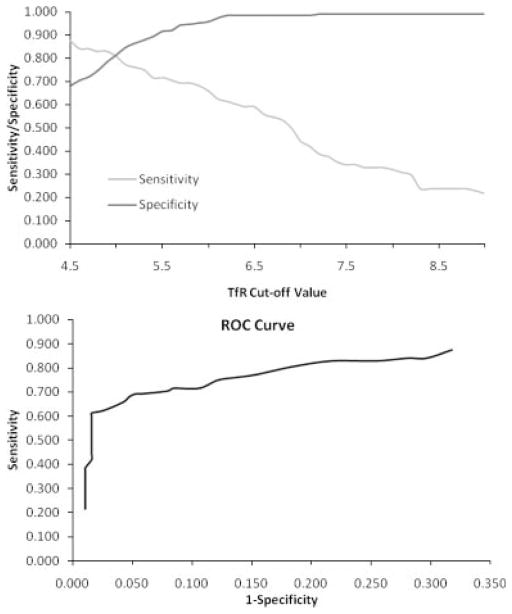
The sensitivity and specificity of TfR for identifying elevated ZPP:H (>80 μmol/mol) are presented Figure 1. The suggested TfR cut off for identifying iron deficiency is 8.5 mg/l (corresponding to 6.7 mg/l in DBS) for this assay, based on healthy, US adults (Ramco Laboratory; Flowers et al., 1989). Olivares et al. (2000) suggest a cut off of 13.8 mg/l (corresponding to 10.0 mg/l in DBS), based on the range observed among healthy children in Chile using this assay. Kasvosve et al. (2007), using the same assay, suggest a cut off of 9.5 mg/l (corresponding to 7.3 mg/l in DBS) based on the range observed among healthy children in Zimbabwe. Among the nonanemic children in this sample with normal ZPP:H (≤80 μmol/mol), the TfR range was 2.3–9.4 mg/l; the 95th percentile value was 5.8 mg/l. The cut offs of 10.0 and 9.4 mg/l identified iron deficiency in only 18 (6.5%) of the children, with very low sensitivity for identifying elevated ZPP:H. TfR > 7.3 mg/l had a sensitivity for elevated ZPP:H of 0.375, TfR > 6.7 mg/l had a sensitivity of 0.545, and TfR > 5.8 mg/l had a sensitivity of 0.693. TfR > 5.0 mg/l (corresponding to a plasma concentration of 6.1 mg/l) showed better sensitivity (0.807), with acceptable specificity (0.815). This cut off demonstrated the best combination of sensitivity and specificity for elevated ZPP:H; thus, elevated TfR was defined as a value >5.0 mg/l. The concordance between elevated ZPP:H and elevated TfR in identifying iron deficiency is shown in Table 3 (concordance = 0.81, κ = 0.58).
Fig. 1.
Sensitivity, specificity, and ROC curve for TfR. Above: Sensitivity (fraction of true positives exceeding the cut off) and specificity (fraction of true negatives falling below the cut off) of transferrin receptor (TfR) for identifying iron deficiency, as defined by elevated zinc protoporphyrin to heme ratio (ZPP:H > 80 μmol/mol). Below: Receiver operating characteristic (ROC) curve (across the range of reasonable cut offs) for transferrin receptor (TfR) in identifying iron deficiency, as defined by elevated zinc protoporphyrin to heme ratio.
TABLE 3.
Concordance between ZPP:H and TfR in identifying iron deficiency
ZPP:H > 80 μmol/mol;
TfR > 5 mg/l.
Concordance = 0.81; κ = 0.58.
These cut offs for iron deficiency were used to characterize iron status groups in the sample. 55% of the children had normal Hb, TfR, and ZPP:H values, the normal, or replete, condition. 35% of the children had normal Hb and elevated TfR or ZPP:H or both, the IDE condition. 11% of the children had low Hb and elevated TfR or ZPP:H or both, the IDA condition. We did not identify any anemia in the absence of iron deficiency (e.g., ACD) among the children we sampled, further supporting the finding that dietary iron deficiency is the primary cause of anemia in this population (Shell-Duncan and McDade, 2004, 2005). Children with missing values for either TfR or ZPP:H were included in the above categories where their iron status was clear because elevation in the other marker was observed, indicating iron deficiency. Adequate information to categorize iron status was available for 280 children.
In addition to these iron status groups, an unequivocally iron replete (UIR) group was also defined. Nonanemic children with both TfR ≤ 4.5 mg/l and ZPP:H ≤ 60 μmol/mol were included in this group. In western populations, iron replete individuals predominantly have ZPP:H values less than 40 μmol/mol, and the cut off of 80 μmol/mol has been used to successfully discriminate between IDE and IDA (Hastka et al., 1994; Rettmer et al., 1999). However, ZPP:H ≤ 40 μmol/mol identified only five children as iron replete, and was judged to be too stringent. The criterion ZPP:H ≤ 60 μmol/mol was substituted; virtually all children with IDA exceeded this threshold. Similarly, TfR ≤ 4.5 mg/l was selected because all children with IDA exceeded this value. So defined, 82 children (29%) fell into the UIR category.
The prevalence of acute infection for the whole sample was 0.104. The prevalence of acute infection was 0.124 in the normal group, 0.083 in the IDE group, and 0.067 in the IDA group (see Fig. 2). Logistic regression results for odds of acute infection by iron status group dummy variables alone are shown in Table 4(a). The variables age and triceps skin fold (TSF) were identified as confounders and were controlled in all further logistic regression analyses. When age and TSF were controlled [Table 4(b)], the addition of other potential confounders (including other anthropometric measurements, sex, and hematuria) did not alter the estimated OR for IDE or IDA beyond the threshold for confounding (10%).
Fig. 2.
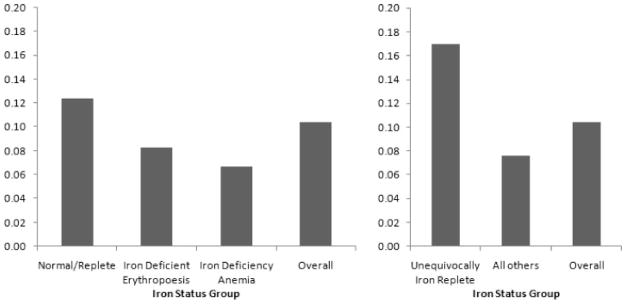
Prevalence of acute infection by iron status. Prevalence of acute infection, defined by elevated CRP, across variously defined iron status groups.
TABLE 4.
| Variable | OR | 95% CI | P-value |
|---|---|---|---|
| (a) | |||
| IDE | 0.63 | 0.27, 1.5 | 0.30 |
| IDA | 0.50 | 0.11, 2.3 | 0.37 |
| (b) | |||
| IDE | 0.51 | 0.20, 1.3 | 0.14 |
| IDA | 0.39 | 0.08, 1.8 | 0.23 |
| TSF | 1.2 | 0.96, 1.6 | 0.10 |
| Age | 0.77 | 0.58, 1.0 | 0.07 |
| (c) | |||
| IDE and IDA | 0.50 | 0.21, 1.2 | 0.11 |
| TSF | 1.2 | 0.96, 1.6 | 0.10 |
| Age | 0.78 | 0.59, 1.0 | 0.09 |
| (d) | |||
| IDEc | 0.47 | 0.19, 1.2 | 0.11 |
| TSF | 1.3 | 1.0, 1.7 | 0.03 |
| Age | 0.73 | 0.54, 0.99 | 0.04 |
CRP > 2.0 mg/l.
Normal/replete is the reference group.
30 children with IDA were excluded.
IDE, iron deficient erythropoiesis; IDA, iron deficiency anemia.
Controlling for age and TSF, the OR for acute infection associated with IDE was 0.51 (95% CI = 0.20, 1.3, P = 0.14) and the OR for acute infection associated with IDA was 0.39 [95% CI = 0.08, 1.8, P = 0.23, Table 4(b)]. OR estimates for IDE and IDA were similar, providing no evidence that odds of infection change with increasing degree of iron deficiency. When these groups were collapsed into one, the suggestive (P = 0.11) OR for infection associated with any degree of iron deficiency was 0.50 [95% CI = 0.21, 1.2, Table 4(c)]. When anemic children were excluded, the estimated OR for infection associated with IDE was similar in magnitude and significance [0.47, 95% CI = 0.19, 1.2, P = 0.11, Table 4(d)].
The prevalence of acute infection among the UIR children was 0.170 (see Fig. 2). Logistic regression results for odds of acute infection associated with an UIR state are shown in Table 5. Controlling for age and TSF, UIR was associated with an OR for infection of 2.9 [95% CI = 1.3, 6.6, P = 0.01, Table 5(b)]. Results were similar when the regression was restricted to nonanemic children [OR = 2.9, 95% CI = 1.3, 6.8, P = 0.01, Table 5(c)].
TABLE 5.
| Variable | OR | 95% CI | P-value |
|---|---|---|---|
| (a) | |||
| UIR | 2.5 | 1.2, 5.5 | 0.02 |
| (b) | |||
| UIR | 2.9 | 1.3, 6.6 | 0.01 |
| TSF | 1.2 | 0.95, 1.5 | 0.12 |
| Age | 0.77 | 0.58, 1.0 | 0.07 |
| (c) | |||
| UIRc | 2.9 | 1.3, 6.8 | 0.01 |
| TSF | 1.3 | 1.0, 1.6 | 0.03 |
| Age | 0.73 | 0.54, 0.99 | 0.04 |
CRP > 2.0 mg/l.
Compared to all other children.
Compared to all other non-anemic children; 30 children with IDA were excluded.
UIR, unequivocally iron replete.
DISCUSSION
Using data from 5- to 10-year-old children living in northern Kenya, we show that for TfR measured in whole blood stored on filter paper, 5.0 mg/l is a sensitive and specific cut off for identifying iron deficiency. We present evidence that the iron replete condition increases odds of infection (OR = 2.9, P = 0.01) and that clinical iron deficiency may protect against infection (OR = 0.50, P = 0.11); we found no evidence that the odds of infection change with degree of iron deficiency.
TfR has many of the characteristics desirable in a biomarker of iron status: it is responsive to iron deficiency and accurately reflects tissue iron stress (Skikne et al., 1990); it is unaffected by the acute phase response or chronic inflammation (Baynes et al., 1994; Cook et al., 1993); and it can be accurately assessed in capillary whole blood stored on filter paper, facilitating its use in a wide range of field situations (Cook et al., 1998; Shell-Duncan and McDade, 2004). However, the utility of this biomarker is somewhat limited by a lack of standardization in assay methods, measurement units, and reference range (Ahluwalia, 1998). Zinc protoporphyrin is also a field-friendly biomarker of iron stress: ZPP:H can be easily and cheaply assessed using capillary whole blood, it is well-characterized, and the cut off for iron deficiency of 80 μmol/mol is widely employed (Rettmer et al., 1999). However, ZPP:H is a less accurate marker of iron deficiency, as it is elevated by chronic infection and inflammation (Harthoorn-Lasthuizen et al., 2000; Labbé and Dewanji, 2004).
We used the conventional ZPP:H cut off for iron deficiency of 80 μmol/mol to assess TfR cut offs. For ZPP:H > 80 μmol/mol as the gold standard, TfR > 5.0 mg/l demonstrated the best combination of sensitivity and specificity. 5.0 mg/l is lower than the cutoff value suggested by the kit manufacturer and others (Flowers et al., 1989; Kasvosve et al., 2007; Olivares et al., 2000). This could be due to population variation in TfR expression, as has been documented by others (Allen et al., 1998; Zimmerman et al., 2005); population-specific cut offs for TfR may be appropriate. The difference may also reflect artificial elevation of the cut offs suggested by others due to inclusion of individuals with undetected iron deficiency in the subjects used to establish the reference range.
To counteract the arbitrariness inherent in the choice of cut off, we chose to define the replete condition not only as the lack of definitive evidence of deficiency, but also as a group that captured only UIR individuals. Review of the literature, primarily assessing the iron status of western adults, suggested that UIR individuals should have ZPP:H values under 40 μmol/mol (Hastka et al., 1994; Rettmer et al., 1999). However, as virtually none of the Kenyan children met this criterion, we chose to use ZPP:H ≤ 60 μmol/mol as a criterion for the UIR condition. Review of the literature did not provide a corresponding TfR value to characterize UIR; we chose to use the criterion TfR ≤ 4.5 mg/l, as all children with IDA fell above this value. Further characterization of these biomarkers, especially in nonwestern populations, will hopefully provide a better basis for defining cut offs for iron replete and iron deficient states.
Because dietary iron deficiency was the primary cause of anemia among children sampled, while malaria and urinary schistosomiasis were rare and not significantly associated with anemia (Shell-Duncan and McDade, 2004, 2005), we were able to evaluate the relationship between iron status and infection (as reflected by elevated CRP). We tested the hypothesis that moderate iron deficiency (short of anemia) protects against acute infection and found suggestive but nonsignificant support: controlling for age and TSF thickness, the OR for infection associated with IDE was 0.51 (P = 0.14). We further hypothesized that antagonism between the immune system’s need for iron and the antimicrobial effects of hypoferremia would obliterate this protective effect with increasing degree of iron deficiency, and thus predicted elevated odds of infection in the IDA group (compared to IDE). However, we found no evidence of this pattern: the estimated protective effect for iron deficiency groups was similar in magnitude and significance when the IDA group was assessed separately, included with the IDE group, or excluded from the analysis (Table 4). Further, we found significant evidence that the most iron replete children are at increased risk of infection: controlling for age and TSF, the OR for infection associated with UIR was 2.9 (P = 0.01; Table 5). This is consistent with the suggestive protective effect of iron deficiency described above. The larger magnitude and significance obtained in this analysis we interpret to suggest that dietary iron restriction is protective against infection at levels that do not constitute clinical iron deficiency. Again, this protection does not seem to diminish with increasing iron stress: the estimated OR for infection associated with UIR was unchanged by the exclusion of IDA children from the analysis.
The key limitations of this research, namely small sample size and cross-sectional design, are important to note. Future investigations of the relationship between infectious disease susceptibility and iron status in populations experiencing a high infectious disease burden should be designed to address both of these limitations, by incorporating monitoring of incident infections and by expanding the sample size. Further, a better understanding of the detrimental impact of iron deficiency, especially mild or moderate deficiency, on CMI, cognitive development, and growth is needed to understand its overall fitness impact.
Implications
Our results are consistent with the hypothesis that mild or moderate iron deficiency in children protects against acute infection. Thus, dietary iron restriction may represent a nutritional adaptation to infection under conditions of high transmission or morbidity. We did not find evidence that more severe iron deficiency or anemia are not also protective against infectious disease, although the small number of anemic children included limits the power of this study to draw conclusions about acute infection among those with severe iron deficiency and anemia.
Our finding that iron deficiency may represent a nutritional adaptation to combat infection exemplifies evolutionary medicine’s potential to provide insight into patterns of disease. While a purely clinical perspective views iron intake that is inadequate to meet the body’s needs as uniformly undesirable, an evolutionary perspective recognizes both its costs and potential benefits, and further, renders the high degree of iron deficiency we observed a predictable response to the infectious disease ecology of northern Kenya. This perspective also foresees an important potential consequence of interventions aimed at correcting iron deficiency in such environments, namely, increased acute bacterial and viral infectious disease morbidity.
An evolutionary perspective also requires that the overall impact on fitness across the lifecourse be considered in assessing dietary iron deficiency as a nutritional adaptation to infectious disease stress. In addition to potentially protecting against acute infection, iron deficiency can compromise childhood cognitive development (reviewed by Beard, 2003) and adult work capacity (endurance, efficiency, and productivity; Haas and Brownlie, 2001). These costs may (or may not) outweigh the benefit iron deficiency confers to overall fitness, depending on specific ecological circumstances.
These results should be considered in planning iron supplementation of children living in environments characterized by frequent exposure to infectious disease, such as northern Kenya. In such settings, widespread iron supplementation or fortification risks moving many children from a protective level of iron restriction or clinical iron deficiency to a definitively iron replete state, increasing their risk of infectious disease. Augmenting iron supplementation with periodic chemoprophylaxis against malaria seems to be capable of improving iron status without increasing malaria incidence (Desai et al., 2003; Ekvall et al., 2000; Massaga et al., 2003; Verhoef et al., 2002). Accompanying iron supplementation with similar prevention measures, surveillance, or enhanced access to treatment for acute infections should be considered.
Acknowledgments
Contract grant sponsor: National Science Foundation Program in Physical Anthropology; Contract grant number: BCS-0200767; Contract grant sponsors: University of Washington Royalty Research Fund, National Science Foundation Graduate Research Fellowship.
The authors thank Jonathan D. Mayer, as well as the editor and anonymous reviewers, for their help in the preparation of this manuscript.
LITERATURE CITED
- Ahluwalia N. Diagnostic utility of serum transferrin receptors measurement in assessing iron status. Nutr Rev. 1998;56:133–141. doi: 10.1111/j.1753-4887.1998.tb01738.x. [DOI] [PubMed] [Google Scholar]
- Allen J, Backstrom KR, Cooper JA, Cooper MA, Detwiler TC, Essex DW, Fritz RP, Means RT, Jr, Meier PB, Pearlman SR, Rottman-Johnson BR, Seligman PA. Measurement of soluble transferrin receptor in serum of healthy adults. Clin Chem. 1998;44:35–39. [PubMed] [Google Scholar]
- Baynes RD, Skikne BS, Cook JD. Circulating transferrin receptors and assessment of iron status. J Nutr Biochem. 1994;5:322–330. [Google Scholar]
- Beall CM, Decker MJ, Brittenham GM, Kushner I, Gebremedhin A, Strohl KP. An Ethiopian pattern of human adaptation to high-altitude hypoxia. Proc Natl Acad Sci USA. 2002;99:17215–17218. doi: 10.1073/pnas.252649199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beard JL. Iron deficiency alters brain development and functioning. J Nutr. 2003;133:1468S–1472S. doi: 10.1093/jn/133.5.1468S. [DOI] [PubMed] [Google Scholar]
- Chandra RK, Newberne PM. Nutrition, immunity, and infection: mechanisms of interactions. New York: Plenum Press; 1977. p. 246. [Google Scholar]
- Cook JD. Defining optimal body iron. Proc Nutr Soc. 1999;58:489–495. doi: 10.1017/s0029665199000634. [DOI] [PubMed] [Google Scholar]
- Cook JD, Baynes RD, Skikne BS. Iron deficiency and the measurement of iron status. Nutr Res Rev. 1992;5:189–202. doi: 10.1079/NRR19920014. [DOI] [PubMed] [Google Scholar]
- Cook JD, Flowers CH, Skikne BS. An assessment of dried blood-spot technology for identifying iron deficiency. Blood. 1998;92:1807–1813. [PubMed] [Google Scholar]
- Cook JD, Skikne BS, Baynes RD. Serum transferrin receptor. Annu Rev Med. 1993;44:63–74. doi: 10.1146/annurev.me.44.020193.000431. [DOI] [PubMed] [Google Scholar]
- Denic S, Agarwal MM. Nutritional iron deficiency: an evolutionary perspective. Nutrition. 2007;23:603–614. doi: 10.1016/j.nut.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Desai MR, Mei JV, Kariuki SK, Wannemuehler KA, Phillips-Howard PA, Nahlen BL, Kager PA, Vulule JM, ter Kuile FO. Randomized, controlled trial of daily iron supplementation and intermittent sulfadoxine-pyrimethamine for the treatment of mild childhood anemia in western Kenya. J Infect Dis. 2003;187:658–666. doi: 10.1086/367986. [DOI] [PubMed] [Google Scholar]
- Ekvall H, Premji Z, Björkman A. Micronutrient and iron supplementation and effective anti-malarial treatment synergistically improve childhood anaemia. Trop Med Int Health. 2000;5:696–705. doi: 10.1046/j.1365-3156.2000.00626.x. [DOI] [PubMed] [Google Scholar]
- Ewald PW. Evolution of infectious disease. New York: Oxford University Press; 1994. p. 320. [Google Scholar]
- Field CJ, Johnson IR, Schley PD. Nutrients and their role in host resistance to infection. J Leukoc Biol. 2002;71:16–32. [PubMed] [Google Scholar]
- Flowers CH, Skikne BS, Covell AM, Cook JD. The clinical measurement of serum transferrin receptor. J Lab Clin Med. 1989;114:368–377. [PubMed] [Google Scholar]
- Gendrel D, Raymond J, Coste J, Moulin F, Lorrot M, Guerin S, Ravilly S, Lefevre H, Royer C, Lacombe C, Palmer P, Bohuon C. Comparison of procalcitonin with C-reactive protein, interleukin-6 and interferon-α for differentiation of bacterial vs. viral infections. Pediatr Infect Dis J. 1999;18:875–881. doi: 10.1097/00006454-199910000-00008. [DOI] [PubMed] [Google Scholar]
- Gera T, Sachdev HPS. Effects of iron supplementation on incidence of infectious illness in children: systematic review. Br Med J. 2002;325:1142–1151. doi: 10.1136/bmj.325.7373.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie SH, Dow C, Raynes JG, Behrens RH, Chiodini PL, McAdam KP. Measurement of acute phase proteins for assessing severity of Plasmodium falciparum malaria. J Clin Pathol. 1991;44:228–231. doi: 10.1136/jcp.44.3.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas JD, Brownlie T. Iron deficiency and reduced work capacity: A critical review of the research to determine a causal relationship. J Nutr. 2001;131:676S–690S. doi: 10.1093/jn/131.2.676S. [DOI] [PubMed] [Google Scholar]
- Harthoorn-Lasthuizen EJ, van’t Sant P, Lindemans J, Langenhuijsen MMAC. Serum transferrin receptor and erythrocyte zinc protoporphyrin in patients with anemia. Clin Chem. 2000;46:719–722. [PubMed] [Google Scholar]
- Hastka J, Lasserre J-L, Schwarzbeck A, Hehlmann R. Central role of zinc protoporphyrin in staging iron deficiency. Clin Chem. 1994;40:768–773. [PubMed] [Google Scholar]
- Jackson FLC. Two evolutionary models for the interactions of dietary organic cyanogens, hemoglobins, and falciparum malaria. Am J Hum Biol. 1990;2:521–532. doi: 10.1002/ajhb.1310020508. [DOI] [PubMed] [Google Scholar]
- Joynson DH, Walker DM, Jacobs A, Dolby AE. Defect of cell-mediated immunity in patients with iron-deficiency anaemia. Lancet. 1972;2:1058–1059. doi: 10.1016/s0140-6736(72)92340-9. [DOI] [PubMed] [Google Scholar]
- Kasvosve I, Gomo ZAR, Nathoo KJ, Matibe P, Mudenge B, Gordeuk VR. Reference intervals of serum transferrin receptors in pre-school children in Zimbabwe. Clin Chim Acta. 2007;382:138–141. doi: 10.1016/j.cca.2007.03.023. [DOI] [PubMed] [Google Scholar]
- Katz SH. Food and biocultural evolution: A model for the investigation of modern nutritional problems. In: Johnston FD, editor. Nutritional anthropology. New York: Alan R Liss; 1987. pp. 41–63. [Google Scholar]
- Kent S, Weinberg ED, Stuart-Macadam P. The etiology of anemia of chronic disease and infection. J Clin Epidemiol. 1994;47:23–33. doi: 10.1016/0895-4356(94)90030-2. [DOI] [PubMed] [Google Scholar]
- Kluger MJ, Bullen JJ. Clinical and physiological aspects. In: Bullen JJ, Griffiths E, editors. Iron and infection. New York: John Wiley; 1987. pp. 243–282. [Google Scholar]
- Kluger MJ, Rothenberg BA. Fever and reduced iron: their interaction as a host defense response to bacterial infection. Science. 1979;203:374–376. doi: 10.1126/science.760197. [DOI] [PubMed] [Google Scholar]
- Kochan I. The role of iron in bacterial infections, with special consideration of host-tubercle bacillus interaction. Curr Top Microbiol Immunol. 1973;60:1–30. doi: 10.1007/978-3-642-65502-9_1. [DOI] [PubMed] [Google Scholar]
- Labbé RF, Dewanji A. Iron assessment tests: transferrin receptor vis-à-vis zinc protoporphyrin. Clin Biochem. 2004;37:165–174. doi: 10.1016/j.clinbiochem.2003.10.006. [DOI] [PubMed] [Google Scholar]
- LaMonte MJ, Durstine JL, Yanowitz FG, Lim T, Dubose KD, Davis P, Ainsworth BE. Cardiorespiratory fitness and C-reactive protein among a tri-ethnic sample of women. Circulation. 2002;106:403–406. doi: 10.1161/01.cir.0000025425.20606.69. [DOI] [PubMed] [Google Scholar]
- Locke A, Main ER, Rosbach DU. The copper and nonhemoglobinous iron contents of the blood serum in disease. J Clin Invest. 1932;11:527–542. doi: 10.1172/JCI100432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massaga JJ, Kitua AY, Lemnge MM, Akida JA, Malle LN, Rønn AM, Theander TG, Bygbjerg IC. Effect of intermittent treatment with amodiaquine on anemia and malarial fevers in infants in Tanzania: a randomized placebo-controlled trial. Lancet. 2003;361:1853–1860. doi: 10.1016/s0140-6736(03)13504-0. [DOI] [PubMed] [Google Scholar]
- McDade TW, Burhop J, Dohnal J. High-sensitivity enzyme immunoassay for C-reactive protein in dried blood spots. Clin Chem. 2004;50:652–654. doi: 10.1373/clinchem.2003.029488. [DOI] [PubMed] [Google Scholar]
- McDade TW, Reyes-García V, Tanner S, Huanca T, Leonard WR. Maintenance vs. growth: investigating the costs of immune activation among children in lowland Bolivia. Am J Phys Anthropol. 2008;136:478–484. doi: 10.1002/ajpa.20831. [DOI] [PubMed] [Google Scholar]
- McDade TW, Shell-Duncan B. Whole blood collected on filter paper provides a minimally invasive method for assessing human transferrin receptor level. J Nutr. 2002;132:3760–3763. doi: 10.1093/jn/132.12.3760. [DOI] [PubMed] [Google Scholar]
- Mimoz O, Benoist JF, Edouard AR, Assicot M, Bonhuan C, Samii K. Procalcitonin and C-reactive protein during the early posttraumatic systemic inflammatory response syndrome. Intensive Care Med. 1998;24:185–188. doi: 10.1007/s001340050543. [DOI] [PubMed] [Google Scholar]
- Nemeth E, Ganz T. Regulation of iron metabolism by hepcidin. Annu Rev Nutr. 2006;26:323–342. doi: 10.1146/annurev.nutr.26.061505.111303. [DOI] [PubMed] [Google Scholar]
- Nestel P. Adjusting hemoglobin values in program surveys. Washington, DC: International Nutritional Anemia Consultative Group; 2002. [Google Scholar]
- Nyakeriga AM, Troye-Blomberg M, Dorfman JR, Alexander ND, Bäck R, Kortok M, Chemtai AK, Marsh K, Williams TN. Iron deficiency and malaria among children living on the coast of Kenya. J Infect Dis. 2004;190:439–447. doi: 10.1086/422331. [DOI] [PubMed] [Google Scholar]
- Olivares M, Walter T, Cook JD, Hertrampf E, Pizzaro F. Usefulness of serum transferrin receptor and serum ferritin in diagnosis of iron deficiency in infancy. Am J Clin Nutr. 2000;72:1191–1195. doi: 10.1093/ajcn/72.5.1191. [DOI] [PubMed] [Google Scholar]
- Oppenheimer SJ. Iron and its relation to immunity and infectious disease. J Nutr. 2001;131:616S–635S. doi: 10.1093/jn/131.2.616S. [DOI] [PubMed] [Google Scholar]
- Pepys MB, Hirschfield GM. C-reactive protein: a critical update. J Clin Invest. 2003;111:1805–1812. doi: 10.1172/JCI18921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rettmer RL, Carlson TH, Origenes ML, Jack RM, Labbé RF. The zinc protoporphyrin/heme ratio for the diagnosis of pre-anemic iron deficiency. Pediatrics. 1999;104:e37. doi: 10.1542/peds.104.3.e37. [DOI] [PubMed] [Google Scholar]
- Sazawal S, Black RE, Ramsan M, Chwaya HM, Stoltzfus RJ, Dutta A, Dhingra U, Kabole I, Deb S, Othman MK, Kabole FM. Effects of routine prophylactic supplementation with iron and folic acid on admission to hospital and mortality in preschool children in a high malaria transmission setting: community-based, randomized, placebo-controlled trial. Lancet. 2006;367:133–143. doi: 10.1016/S0140-6736(06)67962-2. [DOI] [PubMed] [Google Scholar]
- Schaible UE, Kaufmann SHE. Iron and microbial infection. Nat Rev Microbiol. 2004;2:946–953. doi: 10.1038/nrmicro1046. [DOI] [PubMed] [Google Scholar]
- Scrimshaw NS, SanGiovanni JP. Synergism of nutrition, infection, and immunity: an overview. Am J Clin Nutr. 1997;66:464S–477S. doi: 10.1093/ajcn/66.2.464S. [DOI] [PubMed] [Google Scholar]
- Shell-Duncan B, McDade TW. Use of combined measures from capillary blood to assess iron deficiency in rural Kenyan children. J Nutr. 2004;134:384–387. doi: 10.1093/jn/134.2.384. [DOI] [PubMed] [Google Scholar]
- Shell-Duncan B, McDade TW. Cultural and environmental barriers to adequate iron intake among northern Kenyan schoolchildren. Food Nutr Bull. 2005;26:39–48. doi: 10.1177/156482650502600105. [DOI] [PubMed] [Google Scholar]
- Skikne BS, Flowers CH, Cook JD. Serum transferrin receptor: A quantitative measure of tissue iron deficiency. Blood. 1990;75:1870–1876. [PubMed] [Google Scholar]
- Strauss RG. Iron deficiency, infections, and immune function: a reassessment. Am J Clin Nutr. 1978;31:660–666. doi: 10.1093/ajcn/31.4.660. [DOI] [PubMed] [Google Scholar]
- Tchernof A, Nolan A, Sites CK, Ades PA, Poehlman ET. Weight loss reduced C-reactive protein levels in obese postmenopausal women. Circulation. 2002;105:564–569. doi: 10.1161/hc0502.103331. [DOI] [PubMed] [Google Scholar]
- Thibault H, Galan P, Selz F, Preziosi P, Olivier C, Badoual J, Hercberg S. The immune response in iron-deficient young children: effect of iron supplementation on cell-mediated immunity. Eur J Pediatr. 1993;152:120–124. doi: 10.1007/BF02072487. [DOI] [PubMed] [Google Scholar]
- Toikka P, Irjala K, Juven T, Virkki R, Mertsola J, Leinonen M, Ruuskanen O. Serum procalcitonen, C-reactive protein and interleukin-6 for distinguishing bacterial and viral pneumonia in children. Pediatr Infect Dis J. 2000;19:598–602. doi: 10.1097/00006454-200007000-00003. [DOI] [PubMed] [Google Scholar]
- Verhoef H, West CE, Nzyuko SM, de Vogel S, van der Valk R, Wanga MA, Kuijsten A, Veenemans J, Kok FJ. Intermittent administration of iron and sulfadoxine-pyrimethamine to control anaemia in Kenyan children: a randomized controlled trial. Lancet. 2002;360:908–914. doi: 10.1016/S0140-6736(02)11027-0. [DOI] [PubMed] [Google Scholar]
- Visser M, Bouter LM, McQuillan GM, Wener MH, Harris TB. Elevated C-reactive protein levels in overweight and obese adults. JAMA. 1999;282:2131–2135. doi: 10.1001/jama.282.22.2131. [DOI] [PubMed] [Google Scholar]
- Weinberg ED. Iron withholding: a defense against infection and neoplasia. Physiol Rev. 1984;64:65–102. doi: 10.1152/physrev.1984.64.1.65. [DOI] [PubMed] [Google Scholar]
- Weinberg ED. The role of iron in protozoan and fungal infectious diseases. J Eukaryot Microbiol. 1999;46:231–238. doi: 10.1111/j.1550-7408.1999.tb05119.x. [DOI] [PubMed] [Google Scholar]
- Williams GC, Nesse RM. The dawn of Darwinian medicine. Q Rev Biol. 1991;66:1–22. doi: 10.1086/417048. [DOI] [PubMed] [Google Scholar]
- Worwood M. The laboratory assessment of iron status-an update. Clin Chim Acta. 1997;259:3–23. doi: 10.1016/s0009-8981(96)06488-1. [DOI] [PubMed] [Google Scholar]
- Zimmerman MB, Molinari L, Staubli-Asobayire F, Hess SY, Chaouki N, Adou P, Hurrell RF. Serum transferrin receptor and zinc protoporphyrin as indicators of iron status in African children. Am J Clin Nutr. 2005;81:615–623. doi: 10.1093/ajcn/81.3.615. [DOI] [PubMed] [Google Scholar]