Abstract
How cells communicate during development and regeneration is a critical question. One mechanism of intercellular communication is via exosomes, extracellular vesicles that originate by the fusion of multivesicular endosomes with the plasma membrane [1-8]. To model exosome-based intercellular communication, we used MDCK cell cysts grown in 3D gels of extracellular matrix, which form tubules in response to Hepatocyte Growth Factor (HGF). We report that GPRC5B, an orphan G protein coupled receptor, is in exosomes produced by HGF-treated cysts and released into the cyst lumen. Exosomal GPRC5B is taken up by nearby cells, and together with HGF, promotes ERK1/2 activation and tubulogenesis, even under conditions where tubulogenesis would otherwise not occur. Recovery from injury, such as acute kidney injury (AKI), often recapitulates developmental processes. Here, we show that GPRC5B is elevated in urinary exosomes from patients with AKI. Our results elucidate how GPRC5B is carried by exosomes and augments HGF-induced morphogenesis. The unexpected role of exosomes in transporting GPRC5B between cells during morphogenesis and the ability of GPRC5B to predict the disease state of AKI elucidate a novel mechanism for intercellular communication during development and repair.
RESULTS AND DISCUSSION
As a model system to study mammalian organogenesis, we grow MDCK cells in 3D organotypic culture in thick gels of extracellular matrix (ECM), so that the cells form cysts with a central lumen lined by a monolayer of polarized epithelial cells. When stimulated with HGF, these cells proliferate and undergo tubulogenesis, which is reminiscent of tubulogenesis found in several developmental processes. We previously identified genes that are regulated during HGF-induced tubulogenesis [9]. We focused now on GPRC5B, which is highly upregulated early in in vitro tubulogenesis and is particularly highly expressed in the ureteric bud during embryonic kidney development [10]. GPRC5B is a poorly characterized G protein coupled receptor, which was originally cloned as a retinoic acid- induced gene (also called RAIG-2) [11]. It has been implicated in neuronal cell fate determination [12] and obesity-associated inflammation [13]. However, its molecular signaling mechanisms and the identity of any potential ligand activator or interacting partners, with the exception of the Fyn kinase, remain largely unknown. We noticed that ~9 percent of the genes that we previously identified as undergoing temporal regulation during in vitro tubulogenesis, including GPRC5B, are present in urinary exosomes (data not shown) when compared with exosome databases [14, 15], suggesting a potential role for exosomes in tubulogenesis. We first confirmed by quantitative PCR that GPRC5B was induced in MDCK cells that have been exposed to conditioned medium from MRC5 cells, a fibroblast cell line providing tubulogenic factors, including HGF (Figure 1A). HGF alone can cause tubulogenesis, although not as well as MRC5 conditioned medium, and we found that HGF alone was also sufficient for GPRC5B induction.
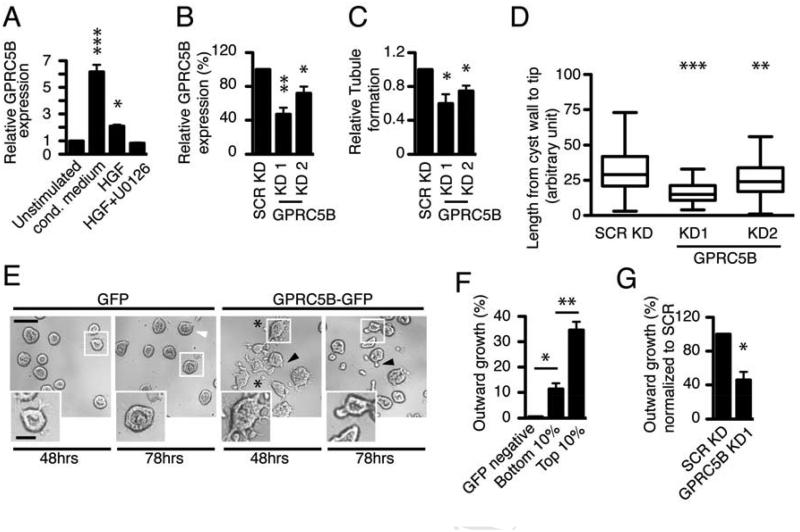
Figure 1. HGF-induced GPRC5B contributes to outward growth in ECM microenvironments.
-
(A)GPRC5B expression of MDCK cells treated with conditioned (cond.) medium from MRC5, 12.5 ng/ml HGF, or 12.5 ng/ml HGF + 10 µM U0126, for 24 hrs.
-
(B)Knockdown efficiency of lentiviral shRNAs targeting GPRC5B in MDCK cysts. The control with scrambled shRNA (SCR KD) is normalized to 100%. GPRC5B expression (A, B) was determined by qRT-PCR.
-
(C)Quantification of in vitro tubule formation in collagen I. Fraction of tubule formation was calculated from tubules counted upon HGF stimulation, and normalized to control knockdown (SCR).
-
(D)Quantification of tubule length, the length from cyst wall to the leading edge of growing tubule. Data are presented as whisker-tukey plots, where boxes encompass values between 25th and 75th percentiles, centered lines represent median values, and whiskers span minimum to maximum values.
-
(E)Representative images of outward growth of cysts in Matrigel upon HGF treatment. GPRC5B-GFP allows cysts to invade as tubules (filled arrowheads) or scattered cells (asterisk) while GFP causes cells to accumulate in the lumen (open arrowheads). These effects were seen at both 48 and 78h. Scale bar 100 µm. Smaller panels are higher magnifications of regions indicated. Scale bar in panel 50 µm.Quantification of outward growth in cysts expressing higher vs lower level of GPRC5B-GFP via overexpression (F) and shRNA-mediated knockdown (G). The transcript level of GPRC5B in cells with bottom 10% intensity shows 3.38 ± 0.424-fold increase when compared with that of endogenous GPRC5B from cells stimulated with 12.5 ng/ml HGF. Significance was calculated using a Student’s t-test and labeled as *p < 0.05, ** < 0.001, *** < 0.0001 in figures.
Induction of GPRC5B was blocked by U0126, a MEK inhibitor, indicating the induction is downstream of the MAP kinase pathway, a key signaling pathway in tubulogenesis [16] (Figure 1A). Next, we tested if GPRC5B induction is required for tubulogenesis in MDCK cysts grown in collagen. Two functional shRNAs targeting GPRC5B significantly decreased the fraction of cysts making tubules upon HGF treatment, compared to control cells expressing an irrelevant shRNA (Figures 1B, C). Additionally, the average length of the tubules that did form was significantly shorter in shRNA-mediated knockdown cells (Figures 1D, S1A), suggesting that GPRC5B controls both the number and length of tubules.
Because GPRC5B is required for tubulogenesis, we tested if exogenous expression of GPRC5B fused to green fluorescent protein (GPRC5B-GFP) could confer outward growth to MDCK cells grown in Matrigel, in which (unlike collagen I gels) wildtype (WT) MDCK cysts are unable to invade in response to HGF [17]. This provides a more stringent model to examine cell movement and tubulogenesis. Unexpectedly, cysts expressing GPRC5B-GFP invaded Matrigel as tubules or scattered cells upon HGF treatment (referred to here as outward growth), while control cysts expressing GFP alone showed virtually no outward growth into the surrounding Matrigel. Instead, cells accumulated in the lumen, indicating the lack of outward growth is not due to the absence of HGF signaling (Figure 1E). We further examined cysts that expressed GPRC5B-GFP at various sub-threshold levels and found that, in response to HGF, high expression resulted in outward growth, while low expression resulted in accumulation of cells in the lumen (Figure S1B), suggesting that the expression level of GPRC5B determines outward growth. Indeed, when cells were sorted on the basis of GFP fluorescence using fluorescence activated cell sorting, cysts formed from cells with the highest 10% of expression level of GPRC5B-GFP had significantly more outward growth in response to HGF, compared with those from the lowest 10% (Figure 1F). Finally, shRNA directed against GPRC5B also decreased outward growth induced by GPRC5B-GFP (Figure 1G). The observed outgrowth is specific for GPRC5B, because overexpression of CD82, an irrelevant exosome protein [18], did not exert a similar effect (Figure S1C). These data suggest that GPRC5B induced by HGF promotes outward growth of cysts.
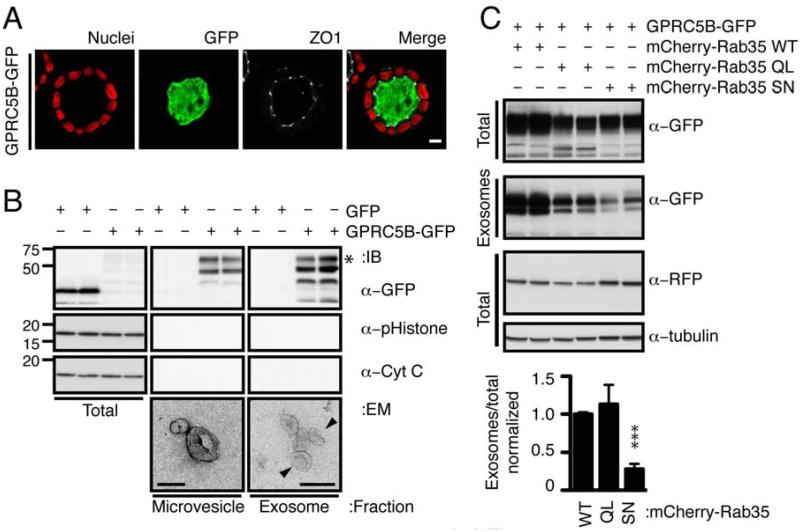
We found GPRC5B-GFP is localized at the apical membrane and/or in the intraluminal space of cysts (Figure 2A) suggesting that GPRC5B may be exported through extracellular vesicles from the apical surface. Differential centrifugation of conditioned medium in contact with the apical surface of monolayer-grown MDCK cells was performed to fractionate extracellular vesicles into fractions enriched in microvesicles (released by blebbing of the plasma membrane) or exosomes (released by fusion of multivesicular endosomes with the plasma membrane) (Figure 2B). Electron micrographs confirmed that each fraction contained a majority of vesicles (111.5 ± 45.6 nm for microvesicles and 63.0 ±19.4 nm for exosomes), consistent with the known diameters of microvesicles and exosomes, respectively [19, 20]. Marker analysis with phospho-Histone and cytochrome C showed that the exosome fraction was not contaminated with apoptotic bodies. Hsp70, a known exosome marker in MDCK was present in the exosome fraction (not shown). Thus, released GPRC5B-GFP was primarily on exosomes.
Figure 2. GPRC5B is apically released on exosomes through a Rab35-dependent pathway.
-
(A)Luminal localization of GPRC5B-GFP. Cysts expressing GPRC5B-GFP (green) were immunostained with Hoeschst 33342 (nuclei, blue) and ZO-1 (tight junction, white). Scale bar, 20 µm.
-
(B)Immunoblots showing indicated proteins in total lysates, and microvesicle and exosome fractions from cells expressing GFP or GPRC5B-GFP. Asterisk indicates glycosylated GPRC5B-GFP, as determined by deglycosylation reactions with PNGases F, O-Glycosidase, Neuraminidase, N-acetylgucosamidase and Neuraminidase (data now shown).Representative transmission electron micrographs of fractionated extracellular vesicles shown below. Vesicles <100nm are enriched in exosome fraction, while vesicles >100nm are enriched in microvesicle fraction. Scale bars 100 nm.
-
(C)Representative immunoblots and quantification showing GPRC5B-GFP in total lysates and exosomes from the tissue culture supernatants of stable cell lines doubly-transfected with indicated constructs. To minimize toxicity associated with overexpression of Rab35, a minimal CMV promoter was used to drive the expression of mCherry-Rab35 WT, QL (constitutively active) or SN (dominant negative).
The release of exosomes in other systems has been shown to depend on the small GTPases Rab7, Rab11, Rab27 and Rab35 [21-24]. We tested if these co-localize with GPRC5B-GFP. Rab35 had the highest colocalization with GPRC5B-GFP (Figure S2) though the possibility of involvement of the other Rabs remains. We grew MDCK cells as a monolayer and tested the effect on release of GPRC5B-GFP in exosomes of constitutively active (QL), dominant negative (SN) and wildtype (WT) alleles of Rab35 fused to the fluorescent protein mCherry (Figure 2C). In contrast to Rab35-WT and Rab35-QL, expression of Rab35-SN decreased release of GPRC5B-GFP. These data suggest GPRC5B is apically released in exosomes via intracellular trafficking controlled by Rab35.
The cis-acting signals that direct proteins into exosomes are poorly understood [25]. We used a series of truncation mutations in GPRC5B-GFP, to determine the signal within GPRC5B that directs the protein into exosomes (Figure S3A, left). Removal of the entire 101 amino acid (aa) cytoplasmic, C-terminal tail (Δ101) spanning residues 286-384, abolished exosomal release of GPRC5B-GFP. This is unlikely due to misfolding, as Δ101 reached the cell surface. In sharp contrast, removal of the C-terminal 80 aa did not block exosomal release. Using alanine scanning, a triple alanine mutant, 286-288A, showed impairment of release of GPRC5B-GFP comparable to Δ101, without loss of surface expression (Figures S3A, 3A). In addition, 286-288A accumulated in small vesicles, possibly endosomes, which were much more prominent compared with WT GPRC5B-GFP (Figure 3B). The correlation of reduction in exosomal release with intracellular vesicular accumulation suggests potential interference in endosome traffic.
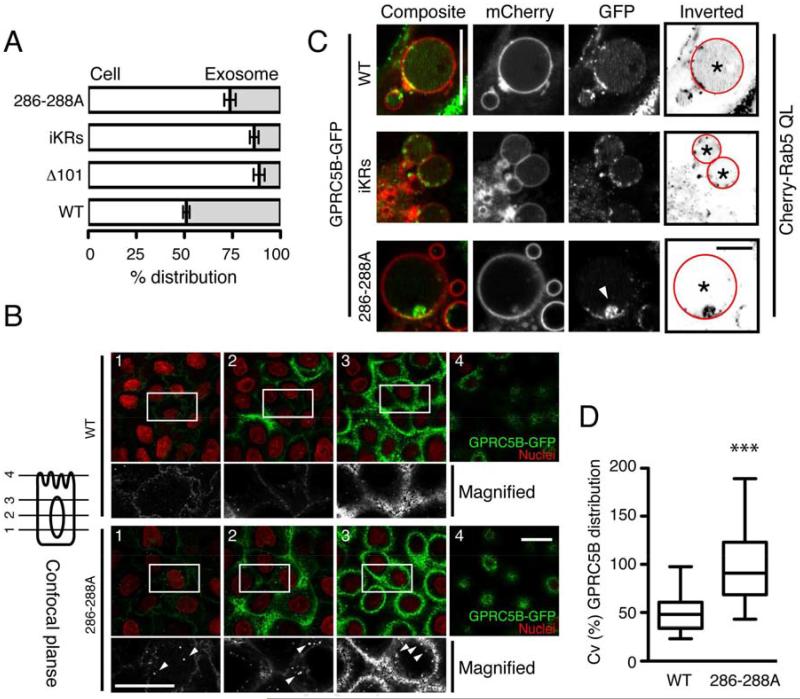
Figure 3. The juxtamembrane residues at the C-terminal tail determine the transfer of GPRC5B from the limiting membrane to the lumen of late endosomes, thereby leading to its exosomal release.
-
(A)Percentage distribution of GPRC5B-GFP in total cell lysate and exosomes stably expressing WT (wild type), Δ101 (no C-terminal tail), iKRs (arginine-to-lysine substitution of all intracellular lysines), or 286-288A (alanine substitution in residues 286-288).
-
(B)Representative GPRC5B-GFP localization of cells expressing WT or 286-288A. A series of z-axis confocal images captured as in scheme at left. Arrowheads indicate intracellular accumulation of 286-288A. Scale bar 20 µm.
-
(C)Representative GPRC5B-GFP localization in endosomes from cells expressing WT, iKRs, 286-288A, together with mCherry-Rab5QL. To better visualize GPRC5B-GFP distribution in endosome lumens, inverted images are shown. Arrowhead indicates an aggregate of 286-288A on the endosome limiting membrane. Scale bar 10 µm.
-
(D)Quantification of the distribution of GPRC5B-GFP in enlarged endosomes. By measuring pixel intensities over the luminal area of endosomes marked with asterisk, the distribution of GPRC5B-GFP in endosome lumens was calculated as percent coefficient variation (CV). Data are presented as whisker-tukey plots, as used in Fig. 1D. See Supl. Information.
We next analyzed budding of GPRC5B-GFP into endosomes. Because GPCRs often use ubiquitination of cytoplasmic-exposed lysines for intra-endosomal transport [26], we mutated all such lysines in GPRC5B-GFP to arginine (iKRs), which had reduced exosomal release compared to WT GPRC5B-GFP (Figures 3A, S3B). To improve visualization of the location of GPRC5B-GFP, we enlarged endosomes by overexpressing constitutively active Rab5(QL) [27] (Figures S2, 3C). WT GPRC5B-GFP was diffusely distributed throughout the lumen of enlarged endosomes, presumably on intraluminal vesicles that are below resolution of the microscope. In contrast, iKRs and 286-288A often appeared as aggregates on the limiting membrane, rather than diffuse in the lumen (Figure 3C). 286-288A was often in a single aggregate on the membrane. Quantitation showed a significant reduction of 286-288A in the lumen and redistribution to the limiting membrane (Figure 3D). These data suggest that the cytoplasmic lysines and juxtamembrane residues 286-288 determine transport of GPRC5B into the lumen of the endosome, presumably by budding of vesicles into the lumen of the endosome, thereby leading to its eventual exosomal release. Finally, cysts expressing 286-288A exhibited significantly decreased outward growth (Figure 4A), providing a direct link between endosomal release of GPRC5B and its biological function.
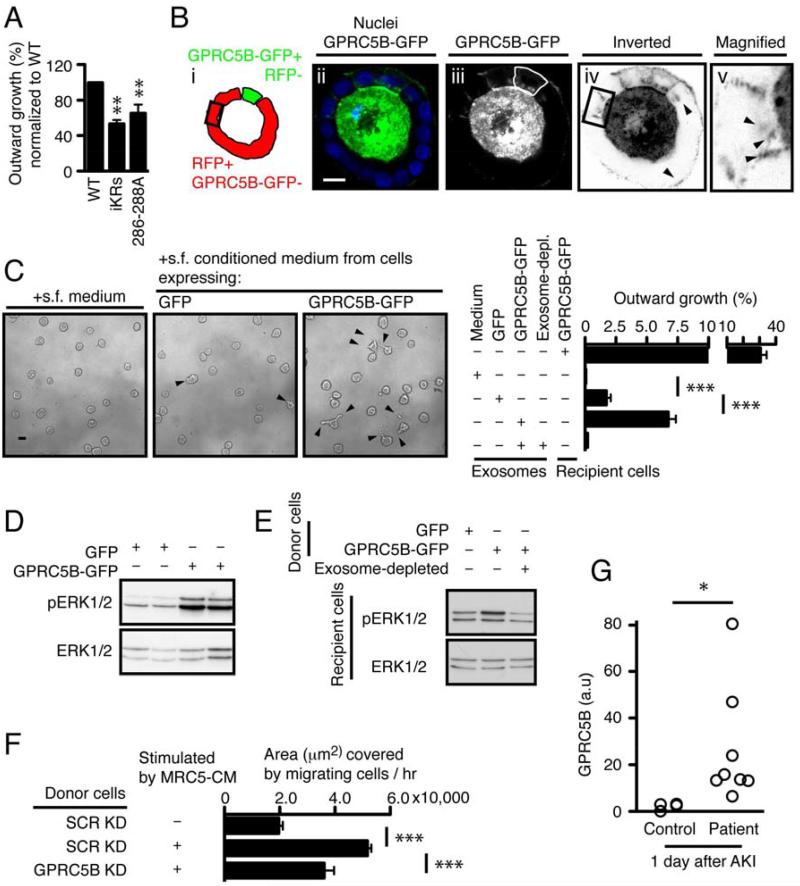
Figure 4. Intercellular transfer of GPRC5B via exosomes enhances HGF-induced morphogenesis.
-
(A)Quantification of outward growth in cysts expressing GPRC5B-GFP WT, iKRs, or 286-288A. The percentage of outward growth was measured as described in Fig. 1.
-
(B)Representative confocal images showing a mosaic cyst with MDCK cells expressing either GPRC5B-GFP or RFP. In panel iii, The GPRC5B-GFP expressing cell is outlined by a hand-drawn green line. Box outlined in black in panels i and iv marks an RFP-expressing cell that is not in contact with cells expressing GPRC5B-GFP, as determined by confocal sectioning (not shown). Panels iv and v are inverted (black to white) to better visualize the GPRC5B-GFP that is taken up by the RFP-expressing target cells. Arrowheads in panels iv and v indicate GPRC5B-GFP+ puncta taken up by RFP+ cells. Scale bar 20µm.
-
(C)Representative images and quantification showing the effect of exosome transfer from cells expressing GPRC5B-GFP. 4-day-old cysts of WT MDCK cells in Matrigel were stimulated with indicated serum free (s.f.) medium or purified exosomes, together with 12.5 ng/ml HGF.
-
(D)Representative immunoblots showing phosphorylated and total ERK1/2 in cysts stimulated as in (C).
-
(E)Representative immunoblots showing augmented ERK1/2 activation in cysts that received exosomes from cells expressing GFP or GPRC5B-GFP after HGF stimulation. Note that exosome depletion abolished ERK1/2 potentiation observed in recipient cells stimulated with HGF, together with exosome transfer from cells expressing GPRC5B-GFP.
-
(F)Wound healing due to endogenous GRPC5B carried in exosomes. MRC5 cells were grown in serum free medium and the resultant conditioned medium was centrifuged to remove any exosomes. This MRC5 conditioned medium was then added to MDCK cells grown as monolayers and expressing either shRNA for GPRC5B (GPRC5B KD) or a scrambled control (SCR KD). The conditioned medium from these MDCK cells was then subjected to centrifugation to purify exosomes. The exosomes, either GPRC5B KD or SCR KD, were then added to a second set of cultures of WT MDCK cells grown as monolayers; these cultures had an ibidi® silicone barrier to prevent growth in a gap region. The silicone barrier was removed immediately before addition of the purified exosomes. Migration was measured as the area of the gap that was covered by migrating cells over 6h.
-
(G)Quantification of GPRC5B in human urinary exosomes. Urine was collected at the first day after critically ill subjects met pre-defined criteria for AKI and provided informed consent. Samples were processed as blinded samples to measure GPRC5B in exosomes, using immunoblotting.
Though little is known about how exosomes are transferred to target cells [28, 29], several membrane-anchored ligands involved in development, such as amphiregulin and Wnt proteins, have been shown to be released in exosomes and act on target cells [3, 8]. C-Met, is released from melanoma cells via exosomes and causes bone marrow cells to have an increased level of c-Met [6], though physical transfer of c-Met between these cell types has not been shown. We therefore tested if an exosomally-released receptor, GPRC5B-GFP, is taken up by other cells and promotes outward growth. We generated mosaic cysts with two populations of MDCK cells: target cells expressed cytoplasmic red fluorescence protein (RFP), but were otherwise WT; and donor cells expressed GPRC5BGFP. Confocal microscopy confirmed that when grown in mosaic cysts, GPRC5B-GFP was observed at the apical membranes of both cell types (Figure S4A), and in the interior of the RFP-expressing cells that were not in contact with cells producing GPRC5B-GFP (Figure 4B). Furthermore when exosomes purified from conditioned medium of cells expressing GPRC5B-GFP were added to the exterior of cysts comprised of WT MDCK, the GPRC5B-GFP was internalized into the WT MDCK (Figure S4B). A similar process of uptake from the basolateral surface may occur when polarity is disrupted due to wounding, HGF stimulation or cancer [30].
Next, we asked if exogenously added exosomes from cells expressing GPRC5B-GFP could induce outward growth in WT MDCK cysts grown in Matrigel (Figure 4C). Upon HGF treatment, cysts underwent outward growth when exosomes purified from cells expressing GPRC5B-GFP was added, while exosomes from cells expressing only GFP, or exosome-depleted medium from cells expressing GPRC5B-GFP did not cause outward growth.
The MAP kinase pathway is downstream of c-MET and is essential for tubulogenesis [16, 31]. We tested if GPRC5B-GFP modulates the MAP kinase pathway as determined by phosphorylation status of ERK1/2 in lysates from cysts. Upon HGF stimulation, ERK1/2 activation was elevated in cysts expressing GPRC5B-GFP, compared with cysts expressing GFP alone (Figure 4D). We next tested if transfer of exosomes containing GPRC5B-GFP was responsible for the increased ERK1/2 activation. We grew MDCK cells expressing either GFP or GPRC5B-GFP as monolayers and collected the conditioned medium. Exosomes were purified from this by centrifugation, resuspended in culture medium, and added to WT MDCK cysts, which were assayed for ERK1/2 activation. (As an additional control, resuspended exosomes from the GPRC5B-GFP conditioned medium were recentrifuged to give an “exosome depleted” condition.) Exosomes containing GPRC5B-GFP potentiated ERK1/2 activation in recipient cysts of WT MDCK cells (Figure 4E), suggesting that GPRC5B augments ERK1/2 activation in recipient cells through exosomal transfer. In the light of our findings, we propose that induced GPRC5B potentiates MAP kinase activation in HGF-stimulated cells. Hyperactivation of MAP kinase signaling leads to cell scattering rather than tubulogenesis. Exosomal release of GPRC5B likely acts to 1) downregulate and avoid inappropriate overactivation of ERK1/2 in exosome releasing cells, and 2) laterally propagate ERK1/2 activation in nearby exosome recipient cells. Thus exosomes are proposed to be part of a signaling mechanism that controls a collective response to HGF for invasive growth.
Developmental processes, such as tubulogenesis, are often recapitulated during wound repair and regeneration. Because our data suggest that exosomes can carry a signal from donor cells to recipient cells during tubulogenesis, we tested if GPRC5B in exosomes from MDCK cells stimulated with MRC5-conditioned medium can increase collective cell migration in a model of wound healing. In this assay, MDCK cells are grown as a monolayer on a solid support. A silicone rubber barrier forms a rectangular, cell-free gap, 500 ± 50 µm wide, in the middle of the monolayer. When the barrier is removed, cells on both sides begin to migrate to fill in the cell-free region. This is similar to a scratch wound assay, but more reproducible. The monolayer showed markedly increased migration when incubated with isolated exosomes from MDCK cells stimulated with MRC5-conditioned medium (Figures S4C, 4F). Importantly, this enhanced migration was significantly decreased when exosomes were isolated from MDCK cells expressing shRNAs targeting GPRC5B, suggesting endogenous GPRC5B transferred via exosomes stimulates migration.
Recovery from injury and regeneration often recapitulates developmental processes, although the role of exosomes in injury repair has been little explored. Such processes are very important clinically. For instance, acute kidney injury (AKI), where the epithelial cells lining the tubules of the kidney are injured or die, is a major source of morbidity and mortality in hospitalized patients, associated with upwards of 10 billion dollars in costs each year in the United States [32]. Furthermore, the consequences of incomplete recovery leading to chronic kidney disease are a major clinical concern that has only recently been appreciated [33, 34]. So far, methods to predict which patients will suffer long-term sequelae from AKI are lacking. The involvement of exosome-mediated transfer of GPRC5B in tubulogenesis and wound healing in culture led us to examine if this protein is involved in AKI. HGF and c-Met play an important role in AKI. In AKI, both serum HGF and renal c-Met expression are increased, and c-Met is activated concomitantly [35-37]. Indeed, HGF enhances renal repair in animal models of AKI [38, 39]. First, we asked where GPRC5B was expressed in human kidney. Marker analysis showed GPRC5B was present predominantly in collecting ducts, to a much lesser extent in proximal tubules, and, rarely, in the loop of Henle (Figure S4D). To determine the involvement of GPRC5B in AKI, we isolated exosomes from the urine of patients who met pre-defined criteria for AKI [40]. Immunoblotting showed a significant increase of urinary GPRC5B in patients with AKI at day 1 (Figure 4G). Collectively, these data suggest urinary exosomal GPRC5B might potentially serve as a much-needed biomarker for AKI.
Together, our study illuminates the surprising roles of exosomes and GPRC5B in development and regeneration, and is an important step in understanding and improving the limited regenerative capacity of the kidney.
EXPERIMENTAL PROCEDURES
Invasive growth assay in extracellular matrices
In vitro tubule formation assays were previously described. Briefly, for HGF-induced tubule formation assay, 4 day-old cysts overlaid on Matrigel and grown in 2% Matrigel, were simulated for 48h or 78h with 12.5 ng/ul recombinant HGF, together with collagen I as supporting 3D matrix. For outward growth in non-permissive ECM microenvironment, 2% Matrigel was used as supporting matrix when 4 days-old cysts were stimulated with 12.5 ng/ul HGF. For counting tubule length, digital images were taken from the center of culture chamber well with DinoXcope microscope (Dino-Lite). The distance between leading edge of tubule and cyst wall was marked with Freehand selection, and was then measured using ImageJ. Other methods are described in Supplemental Information.
Supplementary Material
HIGHLIGHTS.
GPRC5B is induced during in vitro tubulogenesis and potentiates ERK1/2 activation
Blockade of apical release of GPRC5B via exosomes decreases tubule growth
Exosomal transfer of GPRC5B stimulates tubule growth via ERK1/2 activation
During acute kidney injury, increased GPRC5B levels are found in urinary exosomes
ACKNOWLEDGEMENTS
We are grateful to Drs. M. von Zastrow and P. Brakeman (UCSF) for reading and members of the Mostov lab for discussion. We thank Dr. Ho min Kim (KAIST) for help in analyzing EM data and the late Dr. R. Schwall, Genentech, for gift of recombinant human HGF. This work was supported by an NIH Ruth Kirschstein postdoctoral fellowship (DK082115) to S.-H.K., an CTSI-GEMS Grants (69069) to S.-H.K., and NIH grants DK074398, DK091530 and AI53194 to K.M.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
SUPPLEMENTAL INFORMATION
Supplemental information includes Supplemental Experimental Procedures and four figures.
REFERENCES
- 1.Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J. Cell Biol. 2013;200:373–383. doi: 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.El Andaloussi S, Mager I, Breakefield XO, Wood MJ. Extracellular vesicles: biology and emerging therapeutic opportunities. Nat Rev Drug Discov. 2013;12:347–357. doi: 10.1038/nrd3978. [DOI] [PubMed] [Google Scholar]
- 3.Gross JC, Chaudhary V, Bartscherer K, Boutros M. Active Wnt proteins are secreted on exosomes. Nat Cell Biol. 2012;14:1036–1045. doi: 10.1038/ncb2574. [DOI] [PubMed] [Google Scholar]
- 4.Kucharzewska P, Christianson HC, Welch JE, Svensson KJ, Fredlund E, Ringner M, Morgelin M, Bourseau-Guilmain E, Bengzon J, Belting M. Exosomes reflect the hypoxic status of glioma cells and mediate hypoxia-dependent activation of vascular cells during tumor development. Proc. Natl. Acad. Sci. U. S. A. 2013;110:7312–7317. doi: 10.1073/pnas.1220998110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luga V, Zhang L, Viloria-Petit AM, Ogunjimi AA, Inanlou MR, Chiu E, Buchanan M, Hosein AN, Basik M, Wrana JL. Exosomes mediate stromal mobilization of autocrine Wnt-PCP signaling in breast cancer cell migration. Cell. 2012;151:1542–1556. doi: 10.1016/j.cell.2012.11.024. [DOI] [PubMed] [Google Scholar]
- 6.Peinado H, Aleckovic M, Lavotshkin S, Matei I, Costa-Silva B, Moreno-Bueno G, Hergueta-Redondo M, Williams C, Garcia-Santos G, Ghajar C, et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat. Med. 2012;18:883–891. doi: 10.1038/nm.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Putz U, Howitt J, Doan A, Goh CP, Low LH, Silke J, Tan SS. The tumor suppressor PTEN is exported in exosomes and has phosphatase activity in recipient cells. Sci Signal. 2012;5:ra70. doi: 10.1126/scisignal.2003084. [DOI] [PubMed] [Google Scholar]
- 8.Higginbotham JN, Demory Beckler M, Gephart JD, Franklin JL, Bogatcheva G, Kremers GJ, Piston DW, Ayers GD, McConnell RE, Tyska MJ, et al. Amphiregulin exosomes increase cancer cell invasion. Curr. Biol. 2011;21:779–786. doi: 10.1016/j.cub.2011.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kwon SH, Nedvetsky PI, Mostov KE. Transcriptional profiling identifies TNS4 function in epithelial tubulogenesis. Curr. Biol. 2011;21:161–166. doi: 10.1016/j.cub.2010.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brunskill EW, Aronow BJ, Georgas K, Rumballe B, Valerius MT, Aronow J, Kaimal V, Jegga AG, Yu J, Grimmond S, et al. Atlas of gene expression in the developing kidney at microanatomic resolution. Dev Cell. 2008;15:781–791. doi: 10.1016/j.devcel.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Robbins MJ, Michalovich D, Hill J, Calver AR, Medhurst AD, Gloger I, Sims M, Middlemiss DN, Pangalos MN. Molecular cloning and characterization of two novel retinoic acid-inducible orphan G-protein-coupled receptors (GPRC5B and GPRC5C) Genomics. 2000;67:8–18. doi: 10.1006/geno.2000.6226. [DOI] [PubMed] [Google Scholar]
- 12.Kurabayashi N, Nguyen MD, Sanada K. The G protein-coupled receptor GPRC5B contributes to neurogenesis in the developing mouse neocortex. Development. 2013;140:4335–4346. doi: 10.1242/dev.099754. [DOI] [PubMed] [Google Scholar]
- 13.Kim YJ, Sano T, Nabetani T, Asano Y, Hirabayashi Y. GPRC5B activates obesity-associated inflammatory signaling in adipocytes. Sci Signal. 2012;5:ra85. doi: 10.1126/scisignal.2003149. [DOI] [PubMed] [Google Scholar]
- 14.Pisitkun T, Shen RF, Knepper MA. Identification and proteomic profiling of exosomes in human urine. Proc. Natl. Acad. Sci. U. S. A. 2004;101:13368–13373. doi: 10.1073/pnas.0403453101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gonzales PA, Pisitkun T, Hoffert JD, Tchapyjnikov D, Star RA, Kleta R, Wang NS, Knepper MA. Large-scale proteomics and phosphoproteomics of urinary exosomes. J. Am. Soc. Nephrol. 2009;20:363–379. doi: 10.1681/ASN.2008040406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ishibe S, Joly D, Zhu X, Cantley LG. Phosphorylation-dependent paxillin-ERK association mediates hepatocyte growth factor-stimulated epithelial morphogenesis. Mol. Cell. 2003;12:1275–1285. doi: 10.1016/s1097-2765(03)00406-4. [DOI] [PubMed] [Google Scholar]
- 17.Santos OF, Nigam SK. HGF-induced tubulogenesis and branching of epithelial cells is modulated by extracellular matrix and TGF-beta. Dev. Biol. 1993;160:293–302. doi: 10.1006/dbio.1993.1308. [DOI] [PubMed] [Google Scholar]
- 18.Chairoungdua A, Smith DL, Pochard P, Hull M, Caplan MJ. Exosome release of beta-catenin: a novel mechanism that antagonizes Wnt signaling. J. Cell Biol. 2010;190:1079–1091. doi: 10.1083/jcb.201002049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thery C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol. 2009;9:581–593. doi: 10.1038/nri2567. [DOI] [PubMed] [Google Scholar]
- 20.Thery C, Amigorena S, Raposo G, Clayton A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol. 2006:22. doi: 10.1002/0471143030.cb0322s30. Chapter 3, Unit 3. [DOI] [PubMed] [Google Scholar]
- 21.Ostrowski M, Carmo NB, Krumeich S, Fanget I, Raposo G, Savina A, Moita CF, Schauer K, Hume AN, Freitas RP, et al. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat Cell Biol. 2010;12:19–30. doi: 10.1038/ncb2000. sup pp 11-13. [DOI] [PubMed] [Google Scholar]
- 22.Vanlandingham PA, Ceresa BP. Rab7 regulates late endocytic trafficking downstream of multivesicular body biogenesis and cargo sequestration. J. Biol. Chem. 2009;284:12110–12124. doi: 10.1074/jbc.M809277200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hsu C, Morohashi Y, Yoshimura S, Manrique-Hoyos N, Jung S, Lauterbach MA, Bakhti M, Gronborg M, Mobius W, Rhee J, et al. Regulation of exosome secretion by Rab35 and its GTPase-activating proteins TBC1D10A-C. J. Cell Biol. 2010;189:223–232. doi: 10.1083/jcb.200911018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Savina A, Fader CM, Damiani MT, Colombo MI. Rab11 promotes docking and fusion of multivesicular bodies in a calcium-dependent manner. Traffic. 2005;6:131–143. doi: 10.1111/j.1600-0854.2004.00257.x. [DOI] [PubMed] [Google Scholar]
- 25.Yang JM, Gould SJ. The cis-acting signals that target proteins to exosomes and microvesicles. Biochem. Soc. Trans. 2013;41:277–282. doi: 10.1042/BST20120275. [DOI] [PubMed] [Google Scholar]
- 26.Marchese A, Paing MM, Temple BR, Trejo J. G protein-coupled receptor sorting to endosomes and lysosomes. Annu. Rev. Pharmacol. Toxicol. 2008;48:601–629. doi: 10.1146/annurev.pharmtox.48.113006.094646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wegner CS, Malerod L, Pedersen NM, Progida C, Bakke O, Stenmark H, Brech A. Ultrastructural characterization of giant endosomes induced by GTPase-deficient Rab5. Histochem. Cell Biol. 2010;133:41–55. doi: 10.1007/s00418-009-0643-8. [DOI] [PubMed] [Google Scholar]
- 28.Svensson KJ, Christianson HC, Wittrup A, Bourseau-Guilmain E, Lindqvist E, Svensson LM, Morgelin M, Belting M. Exosome uptake depends on ERK1/2-heat shock protein 27 signaling and lipid Raft-mediated endocytosis negatively regulated by caveolin-1. J. Biol. Chem. 2013;288:17713–17724. doi: 10.1074/jbc.M112.445403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parolini I, Federici C, Raggi C, Lugini L, Palleschi S, De Milito A, Coscia C, Iessi E, Logozzi M, Molinari A, et al. Microenvironmental pH is a key factor for exosome traffic in tumor cells. J. Biol. Chem. 2009;284:34211–34222. doi: 10.1074/jbc.M109.041152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Balkovetz DF, Pollack AL, Mostov KE. Hepatocyte growth factor alters the polarity of Madin-Darby canine kidney cell monolayers. J. Biol. Chem. 1997;272:3471–3477. doi: 10.1074/jbc.272.6.3471. [DOI] [PubMed] [Google Scholar]
- 31.O’Brien LE, Tang K, Kats ES, Schutz-Geschwender A, Lipschutz JH, Mostov KE. ERK and MMPs sequentially regulate distinct stages of epithelial tubule development. Dev Cell. 2004;7:21–32. doi: 10.1016/j.devcel.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 32.Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J. Am. Soc. Nephrol. 2005;16:3365–3370. doi: 10.1681/ASN.2004090740. [DOI] [PubMed] [Google Scholar]
- 33.Chawla LS, Kimmel PL. Acute kidney injury and chronic kidney disease: an integrated clinical syndrome. Kidney Int. 2012;82:516–524. doi: 10.1038/ki.2012.208. [DOI] [PubMed] [Google Scholar]
- 34.Hsu CY, Chertow GM, McCulloch CE, Fan D, Ordonez JD, Go AS. Nonrecovery of kidney function and death after acute on chronic renal failure. Clin J Am Soc Nephrol. 2009;4:891–898. doi: 10.2215/CJN.05571008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu Y, Tolbert EM, Lin L, Thursby MA, Sun AM, Nakamura T, Dworkin LD. Up-regulation of hepatocyte growth factor receptor: an amplification and targeting mechanism for hepatocyte growth factor action in acute renal failure. Kidney Int. 1999;55:442–453. doi: 10.1046/j.1523-1755.1999.00267.x. [DOI] [PubMed] [Google Scholar]
- 36.Joannidis M, Spokes K, Nakamura T, Faletto D, Cantley LG. Regional expression of hepatocyte growth factor/c-met in experimental renal hypertrophy and hyperplasia. Am. J. Physiol. 1994;267:F231–236. doi: 10.1152/ajprenal.1994.267.2.F231. [DOI] [PubMed] [Google Scholar]
- 37.Zhou D, Tan RJ, Lin L, Zhou L, Liu Y. Activation of hepatocyte growth factor receptor, c-met, in renal tubules is required for renoprotection after acute kidney injury. Kidney Int. 2013;84:509–520. doi: 10.1038/ki.2013.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nagano T, Mori-Kudo I, Tsuchida A, Kawamura T, Taiji M, Noguchi H. Ameliorative effect of hepatocyte growth factor on glycerol-induced acute renal failure with acute tubular necrosis. Nephron. 2002;91:730–738. doi: 10.1159/000065037. [DOI] [PubMed] [Google Scholar]
- 39.Homsi E, Janino P, Amano M, Saraiva Camara NO. Endogenous hepatocyte growth factor attenuates inflammatory response in glycerol-induced acute kidney injury. Am. J. Nephrol. 2009;29:283–291. doi: 10.1159/000159275. [DOI] [PubMed] [Google Scholar]
- 40.Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, Levin A. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.